Published online Apr 24, 2025. doi: 10.5306/wjco.v16.i4.104413
Revised: January 15, 2025
Accepted: February 25, 2025
Published online: April 24, 2025
Processing time: 97 Days and 2.4 Hours
Pulmonary lymphoepithelioma-like carcinoma (PLELC) is a rare primary epi
A 38-year-old man was diagnosed with right PLELC. Chest computed tomo
A combination of toripalimab and anlotinib may benefit patients with advanced diseases who have not received systematic antitumor therapy.
Core Tip: We report a case of a 38-year-old man who was diagnosed with right pulmonary lymphoepithelioma-like car
- Citation: Huang FL, Luo M, He ZM, Shen YQ, Liu GD. Diagnosis and treatment of pulmonary lymphoepithelioma-like carcinoma: A case report. World J Clin Oncol 2025; 16(4): 104413
- URL: https://www.wjgnet.com/2218-4333/full/v16/i4/104413.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i4.104413
Pulmonary lymphoepithelioma-like carcinoma (PLELC) is a rare primary epithelial lung cancer associated with the Epstein-Barr virus (EBV). Its incidence rate is less than 1% of non-small cell lung cancer (NSCLC)[1]. Bégin et al[2] first reported a case of malignant tumor originating from the lung and resembling lymphoepithelioma-like carcinoma in 1987, which was subsequently named PLELC. The diagnosis of PLELC presents significant challenges owing to its histological similarity to undifferentiated nasopharyngeal carcinoma (NPC) and its pathological resemblance to lung squamous cell carcinoma. However, PLELC has unique epidemiological, molecular and clinical features. No standardized treatment guidelines exist for PLELC and common driver genes in NSCLC rarely occur in PLELC[1]. Moreover, existing treatment methods are ineffective. Identifying new treatments is essential for enhancing the survival and prognosis of these patients. Surgery is the primary treatment for early-stage PLELC, and platinum chemotherapy is the most common first-line treatment for advanced PLELC[3]. Targeted therapy and immunotherapy are a new era of precision medicine in cancer treatment. Herein, we conducted a retrospective analysis of a patient with PLELC to explore its clinical characteristics, diagnosis and treatment methods.
A 38-year-old man presented with a complaint of a chronic recurrent dry cough over a month. He denied fever, sputum or blood production and chest pain. A chest computed tomography (CT) revealed pulmonary masses at an external hospital. The patient was admitted to the oncology department for further evaluation and treatment.
Complaint of a chronic recurrent dry cough over a month.
The patient had no history of hypertension, coronary heart disease, diabetes, cerebral venous thrombosis or hemorrhage, tuberculosis, chronic hepatitis B virus infection and other diseases.
The patient had no family history of hereditary diseases.
Physical examinations revealed a temperature of 37.2 °C, a pulse rate of 118 beats/minute, a respiratory rate of 20 breaths/minute and a blood pressure reading of 106/69 mmHg. The patient was conscious, with clear respiration and pulmonary sounds; no dry or wet rales were detected. The abdomen appeared flat and soft, with no palpable mass detected. Renal and liver percussive pain were negative. No edema was observed in the lower extremities.
Laboratory examinations were as follows: White blood cells: 7.97 × 109/L (reference value: 4.0-10.0 × 109/L), hemoglobin: 142 g/L (reference value: 115-150 g/L), platelets: 287 × 109/L (reference value: 100-300 × 109/L) and neutrophil percentage 46.7% (reference value: 40%-75%). Urinalysis, routine stool and coagulation function showed no significant abnormalities. Total prostate-specific antigen: 5.77 ng/mL (reference value: < 4 ng/mL), cytokeratin 19 fragment: 13.39 ng/mL (reference value: 0-3.3 ng/mL) and neuron-specific enolase: 17.5 μg/L (reference value: 0-16.3 μg/L). Thyroid function showed no significant abnormalities. Electrolytes, liver and kidney function, myocardial enzymes and inorganic ions were normal. Treponema pallidum-specific antibody, hepatitis B surface antigen, hepatitis C antibody, human immunodeficiency virus antibody and tuberculosis immunoglobulin (Ig) M and IgG antibodies were all negative. EBV antibody was weakly positive.
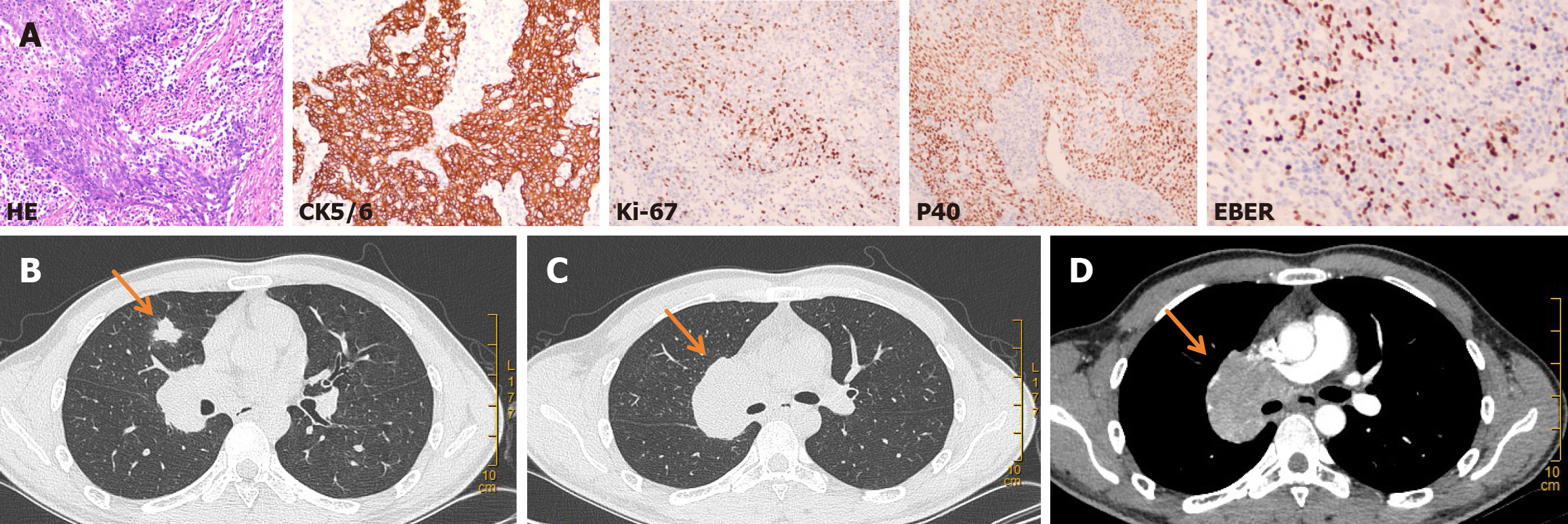
Immunological examination: Bronchoscopy examination showed chronic mucosa inflammation and focal alveolar epithelial hyperplasia, and a tendency towards atypical adenomatous hyperplasia. No cancer cells were found in the bronchoalveolar lavage fluid. CT-guided lung tumor biopsy was performed on March 23, 2020. The postoperative pathological examination combined with immune phenotype and in situ hybridization (ISH) confirmed PLELC. Immunohistochemistry results were as follows: Cytokeratin 5/6 (CK5/6) (+), Ki67 (+), P40 (+). ISH results were as follows: Epstein-Barr encoding region (EBER) (+) (Figure 1A). Standard molecular testing for patients with NSCLC was negative and programmed cell death ligand-1 (PD-L1) was about 2%.
Chest CT revealed a mass in the medial segment of the middle lobe of the right lung (approximately 1.6 cm × 1.7 cm), with lymph node metastasis in the mediastinum and right hilum of the lung (the largest lymph node, approximately 5.4 cm × 4.2 cm, was located in the right hilum) (Figure 1B-D). Notably, this condition affected the superior vena cava, right pulmonary artery and superior right pulmonary vein. The head magnetic resonance imaging (MRI) revealed a coronal ischemic lesion. Abdominal CT, nasopharyngeal MRI and bone emission CT (ECT) showed no abnormalities.
The patient was diagnosed with right PLELC (T1bN2M0 stage IIIA).
The patient underwent wedge resection of the right middle lobe of the lung on March 31, 2020. The postoperative pathological examination demonstrated the diagnosis of PLELC. However, the patient and his family declined ra
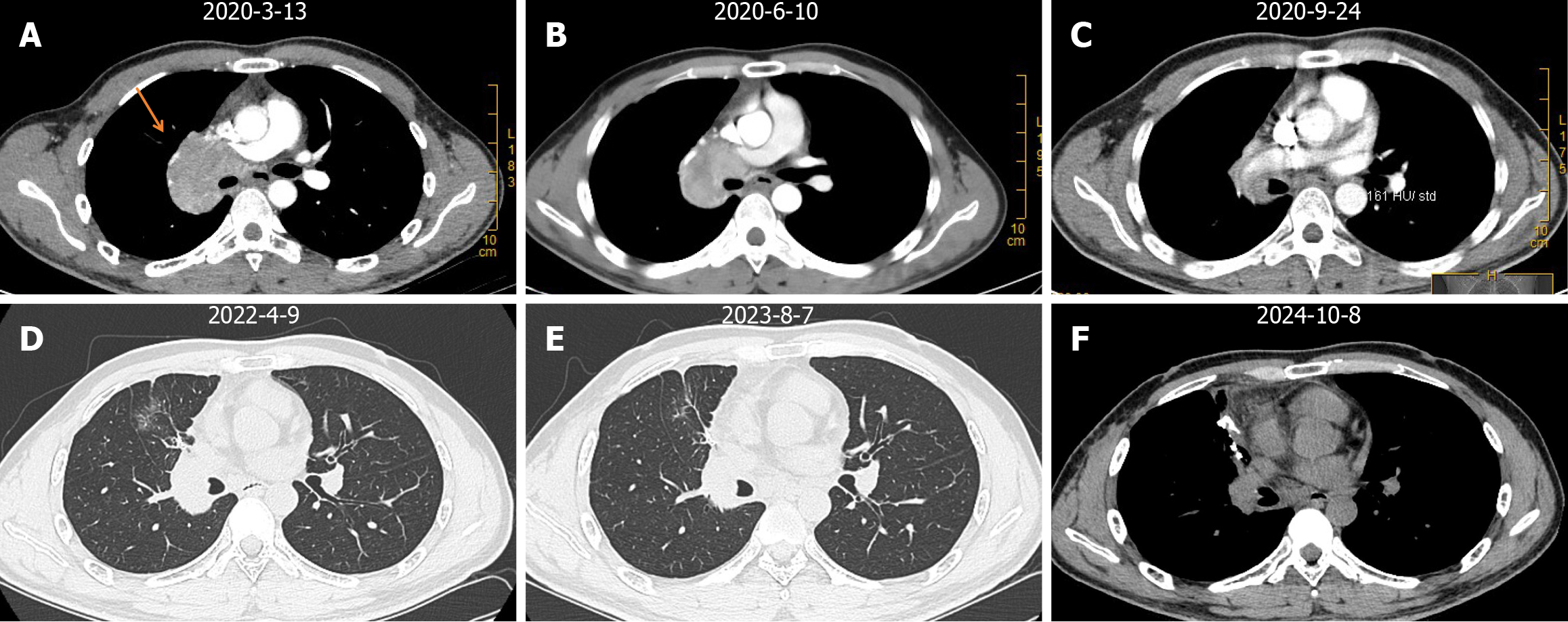
The patient has undergone 10 cycles of toripalimab at a dosage of 240 mg and anlotinib at a dosage of 10 mg daily, administered every 3 weeks as maintenance treatment, initiated after surgery and continuing to date. (Figure 2A-C). On April 9, 2022, a re-examination of the chest CT revealed no significant changes compared with postoperative imaging results. Soft tissue nodules were observed in the surgical area and enlarged lymph nodes in the mediastinum and right hilum of the lung. However, no nodules were found in the anterior segment of the right upper lobe. The therapeutic effect was evaluated as stable disease (SD) (Figure 2D). The follow-up examination between August 7, 2023 and October 8, 2024, showed no significant changes in the soft tissue shadows, pulmonary hilum and mediastinal lymph nodes in the surgical area compared with postoperative imaging results (Figure 2E and F). The therapeutic effect was also evaluated as SD. The patient has been disease-free for approximately 48 months.
PLELC is a subtype of NSCLC that frequently manifests in young, non-smoking individuals of Asian descent. PLELC is characterized by atypical clinical manifestations, including respiratory symptoms, such as cough with or without hemoptysis, chest pain and shortness of breath, weight loss, night sweats, fever, etc.[4]. The patient in this case only presented with recurrent cough symptoms, without obvious chest pain, shortness of breath, fever, night sweats or other associated symptoms. Chest serves as a foundational tool for the diagnosis of PLELC. Pulmonary CT typically reveals PLELC as nodular formations or masses, often appearing as isolated pulmonary nodules or masses near the pleura, measuring < 3.5 cm in diameter and usually not involving lymph nodes. Advanced-stage PLELC is frequently marked by the presence of peribronchial lymph node metastasis, as well as vascular wrapping and dilation[5]. The chest CT of this patient showed a solitary nodule in the middle lobe of the right lung with clear edges, with lymph node metastasis in the mediastinum and right hilum of the lung (the largest lymph node, measuring about 5.4 cm × 4.2 cm, was located in the right hilum). Notably, this condition affected the superior vena cava, right pulmonary artery and superior right pulmonary vein. Additionally, because it is challenging to differentiate PLELC from undifferentiated NPC in histo
PLELC is a rare condition that currently lacks established treatment guidelines. Surgical resection remains the primary treatment for patients with early-stage PLELC; however, there are currently no established recommendations for those with locally advanced and metastatic PLELC. In the next 5 years or even longer in the future, exploring the treatment of PLELC will be the focus and difficulty in this field. A comprehensive treatment regimen, encompassing both che
While immunotherapy has emerged as an effective way to treat various malignant tumors, including lung cancer, reports on PLELC are relatively scarce. Toripalimab is a unique PD-1 inhibitor with high affinity and selectivity. It effectively stimulates the patient’s immune system, strengthens anti-tumor responses, increases tumor cell destruction, and improves the tumor microenvironment[11]. However, PD-1 inhibitors alone have limited effectiveness and a low rate of objective remission; therefore, they are frequently combined with other medications. Anlotinib is a targeted therapy drug that can inhibit tumor angiogenesis, cut off the nutritional supply of tumors and inhibit tumor growth[12]. Standard molecular testing for patients with NSCLC was negative and PD-L1 was about 2%. Therefore, our patient received a combination of immunotherapy and targeted therapy, which included 240 mg of toripalimab on day 1 and 10 mg of anlotinib from days 1 to 14 for 10 cycles, followed by a maintenance treatment of 10 mg of anlotinib daily every 3 weeks. Currently, studies have shown that anlotinib reshapes the immune microenvironment by downregulating PD-L1 expression and increasing the cluster of differentiation 8/forkhead box P3 ratio. Anlotinib combined with PD-1 inhibitors can promote the infiltration of autoimmune cells, including natural killer cells, M1-like tumor-associated macrophages and dendritic cells, to combat tumors[9].
So far, the patient exhibits no recurrence or metastasis. The therapeutic effect was evaluated as SD. The patient has been disease-free for about 48 months. The observed results may arise from various factors: First, anlotinib can inhibit immune suppressive signals by lowering the expression of immune checkpoints. This action modifies the tumor microenvironment's immunosuppressive state, enhancing the effectiveness of toripalimab. Furthermore, toripalimab treatment can activate T cells, improving their ability to destroy tumor cells while also promoting vascular normalization. This process enhances drug delivery and minimizes the chances of adverse events. A combination of anlotinib and toripalimab can produce a synergistic effect, enhancing treatment outcomes.
In short, PLELC challenges doctors in terms of epidemiological characteristics, clinical diagnosis, pathological characteristics and treatment plans. Due to the similarity between PLELC and NPC, perhaps the treatment of NPC can provide new ideas for the future diagnosis and treatment direction of PLELC. For example, early screening of EBV-DNA can also be applied to PLELC. Immunotherapy shows obvious feasibility in advanced NSCLC and NPC, and most PLELCs have PD-L1 positive expression. Immunotherapy combined with or without chemotherapy may become the preferred first-line treatment for advanced PLELC. In addition, due to the low incidence of the disease and the limited number of available samples, there are some limitations in comparing the correlation between PD-L1 expression and treatment response and survival rate, and exploring the difference in efficacy between gene signaling pathways and tumor microenvironments. PLELC's global case reports are mostly presented in the form of case studies and small cohort studies. Large-scale clinical research and forward-looking real-world research are needed to provide the best treatment for PLELC.
A combination of toripalimab and anlotinib may benefit PLELC patients with advanced diseases who have not received systematic antitumor therapy. However, its long-term efficacy requires further comprehensive research and validation.
| 1. | Jiang RR, Feng XL, Zhu WT, Guo MX, Tan XL, Jiang XJ, Dou XM, Liu L. A Rare Subtype of Non-small Cell Lung Cancer: Report of 159 Resected Pathological Stage I-IIIA Pulmonary Lymphoepithelioma-Like Carcinoma Cases. Front Surg. 2021;8:757085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 2. | Bégin LR, Eskandari J, Joncas J, Panasci L. Epstein-Barr virus related lymphoepithelioma-like carcinoma of lung. J Surg Oncol. 1987;36:280-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 157] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Lin Z, Fu S, Zhou Y, Zhang X, Chen C, He LN, Li H, Wang Y, Chen T, Zhang L, Hong S. First-line platinum-based chemotherapy and survival outcomes in locally advanced or metastatic pulmonary lymphoepithelioma-like carcinoma. Lung Cancer. 2019;137:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Hu Y, Ren S, Liu Y, Han W, Liu W. Pulmonary Lymphoepithelioma-Like Carcinoma: A Mini-Review. Onco Targets Ther. 2020;13:3921-3929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Lei Y, Zhou J, Liu J, Xia X, Wang P, Peng Y, Xie X, Liao Y, Wan Q, Li X. The CT and PET/CT findings in primary pulmonary lymphoepithelioma-like carcinoma with pathological correlation: a study of 215 cases. Clin Radiol. 2022;77:e201-e207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Lin Z, Situ D, Chang X, Liang W, Zhao M, Cai C, Liu Y, He J. Surgical treatment for primary pulmonary lymphoepithelioma-like carcinoma. Interact Cardiovasc Thorac Surg. 2016;23:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Li QW, Qiu B, Hu WM, Guo SP, Wu YJ, Zhu YJ, Hu N, Ai XL, Chen NB, Guo JY, Hu YH, Liu MZ, Zeng MS, Liu H. Plasma Epstein-Barr Virus-Deoxyribonucleic Acid Copy Number Predicts Disease Progression in Stage I-III Pulmonary Lymphoepithelioma-Like Carcinoma. Front Oncol. 2020;10:1487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Wu Q, Wang W, Zhou P, Fu Y, Zhang Y, Shao YW, Jiang L. Primary pulmonary lymphoepithelioma-like carcinoma is characterized by high PD-L1 expression, but low tumor mutation burden. Pathol Res Pract. 2020;216:153043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Wu Z, Xian X, Wang K, Cheng D, Li W, Chen B. Immune Checkpoint Blockade Therapy May Be a Feasible Option for Primary Pulmonary Lymphoepithelioma-like Carcinoma. Front Oncol. 2021;11:626566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Fang W, Hong S, Chen N, He X, Zhan J, Qin T, Zhou T, Hu Z, Ma Y, Zhao Y, Tian Y, Yang Y, Xue C, Tang Y, Huang Y, Zhao H, Zhang L. PD-L1 is remarkably over-expressed in EBV-associated pulmonary lymphoepithelioma-like carcinoma and related to poor disease-free survival. Oncotarget. 2015;6:33019-33032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Chen GF, Wang J, Yan Y, Xu S, Chen J. Metastatic stomach lymphoepithelioma-like carcinoma and immune checkpoint inhibitor therapy: A case report. World J Gastrointest Surg. 2024;16:1436-1442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Lei S, Tian S, Lu S, Qing Z, Long J, Li L, Yang D. Primary Pulmonary Lymphoepithelioma-like Carcinoma: A Case Report Utilizing Camrelizumab and Anlotinib for Prolonged Survival. Anticancer Agents Med Chem. 2024;24:954-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |