Published online Apr 24, 2025. doi: 10.5306/wjco.v16.i4.103651
Revised: December 20, 2024
Accepted: February 8, 2025
Published online: April 24, 2025
Processing time: 120 Days and 13.9 Hours
Ampullary adenocarcinomas are a rare disease. They can be classified anatomically or according to their histology into intestinal, pancreatobiliary, and mixed subtypes, with different subtypes having distinct prognoses and potential treatments. We report a clinical case of a patient with mixed type adenocarcinoma of the ampulla of Vater, with predominantly intestinal histology, associated with an isolated and synchronous peritoneal carcinomatosis. It is the only case reported in the literature of duodenal ampulla cancer with synchronous peritoneal meta
A 53-year-old male patient with non-insulin-dependent diabetes presented with acute abdominal pain in the right hypochondrium. Images revealed dilatation of the biliary tract and the duct of Wirsung, without a clear obstructive factor. Upper gastrointestinal endoscopy revealed a tumor in the duodenal papilla. Biopsies confirmed an adenocarcinoma. In the first surgical step, a biliodigestive bypass was performed in association with resection of the carcinomatosis. Peritoneal metastases was found during the intraoperative period. Subsequently, chemo
We conclude that an important value of this work is showing individualized treatment for a patient with cancer.
Core Tip: Ampullary adenocarcinomas are a rare disease. We present a patient with intestinal ampulla adenocarcinoma subtype with synchronic peritoneal metastases resected, and a subsequent favorable response to chemotherapy. He underwent surgery, achieving R0 resection and long-term disease-free survival. Resection of the peritoneal lesion, without the addition of concomitant hyperthermic intraperitoneal chemotherapy, is in accordance with the last evidence about this topic in digestive tumors. Therefore, we do not suggest initially classifying these cases as out of therapeutic reach.
- Citation: Núñez JC, Rivera MT, Stevens MA. Adenocarcinoma of the duodenal papilla with synchronous peritoneal metastases-5 years of overall survival: A case report. World J Clin Oncol 2025; 16(4): 103651
- URL: https://www.wjgnet.com/2218-4333/full/v16/i4/103651.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i4.103651
Ampullary adenocarcinomas are a rare disease, comprising only 0.2% of gastrointestinal cancers[1,2]. The ampulla of Vater is composed of three anatomical components: The ampulla, the intraduodenal portion of the bile duct, and the intraduodenal portion of the pancreatic duct. Ampullary adenocarcinomas can be classified according to the anatomic location within the ampulla of Vater as well as their histologic features[3-6].
From an anatomic point of view, tumors of the ampulla of Vater can arise from different locations. One possibility is that a tumor can appear in the ampulla (intra-ampullary), arising from an intra-ampullary papillary tubular neoplasm, or as an ampullary ductal (pancreaticobiliary type). The second possibility is that a tumor could be periampullary/ampul
It is possible to precisely determine three differential diagnoses of ampullary adenocarcinoma: Carcinoma of the distal common bile duct, carcinoma of the main pancreatic duct, and duodenal tumors of the second segment (other than periampullary or ampullary adenocarcinoma), as these cancers can originate in different, although closely located, anatomical regions within the gastrointestinal tract. These differential diagnoses are strictly linked to subtypes of adenocarcinoma of ampulla of Vater. Periampullary/ampullary duodenal tumors that arise from the duodenal epithe
From a histological point of view, most ampullary adenocarcinomas are gland-forming (tubular) and classically divided into intestinal, pancreatobiliary, and mixed types, with the last group constituting 40% of cases. It is important to consider the physiopathology of these tumors and note that the ampulla is a transition region between the intestinal epithelium of the duodenum and the pancreatobiliary epithelium from the pancreatic and biliary ducts, and with different etiopathogenic factors. It is a variation zone, where the intestinal epithelium in this location is similar but not identical to the colon. A comparable phenomenon occurs with the ampullary pancreatobiliary epithelium, which is not exactly like that of the pancreatobiliary ducts[4].
In addition to the abovementioned tumor types, there are other non-glandular patterns of carcinoma that can be isolated or mixed. The followings patterns have been described: Mucinous adenocarcinoma, medullary carcinoma, poorly cohesive carcinoma (signet ring cell), adenosquamous carcinoma, invasive papillary adenocarcinoma, neuroendocrine carcinoma, and undifferentiated carcinoma[4,5].
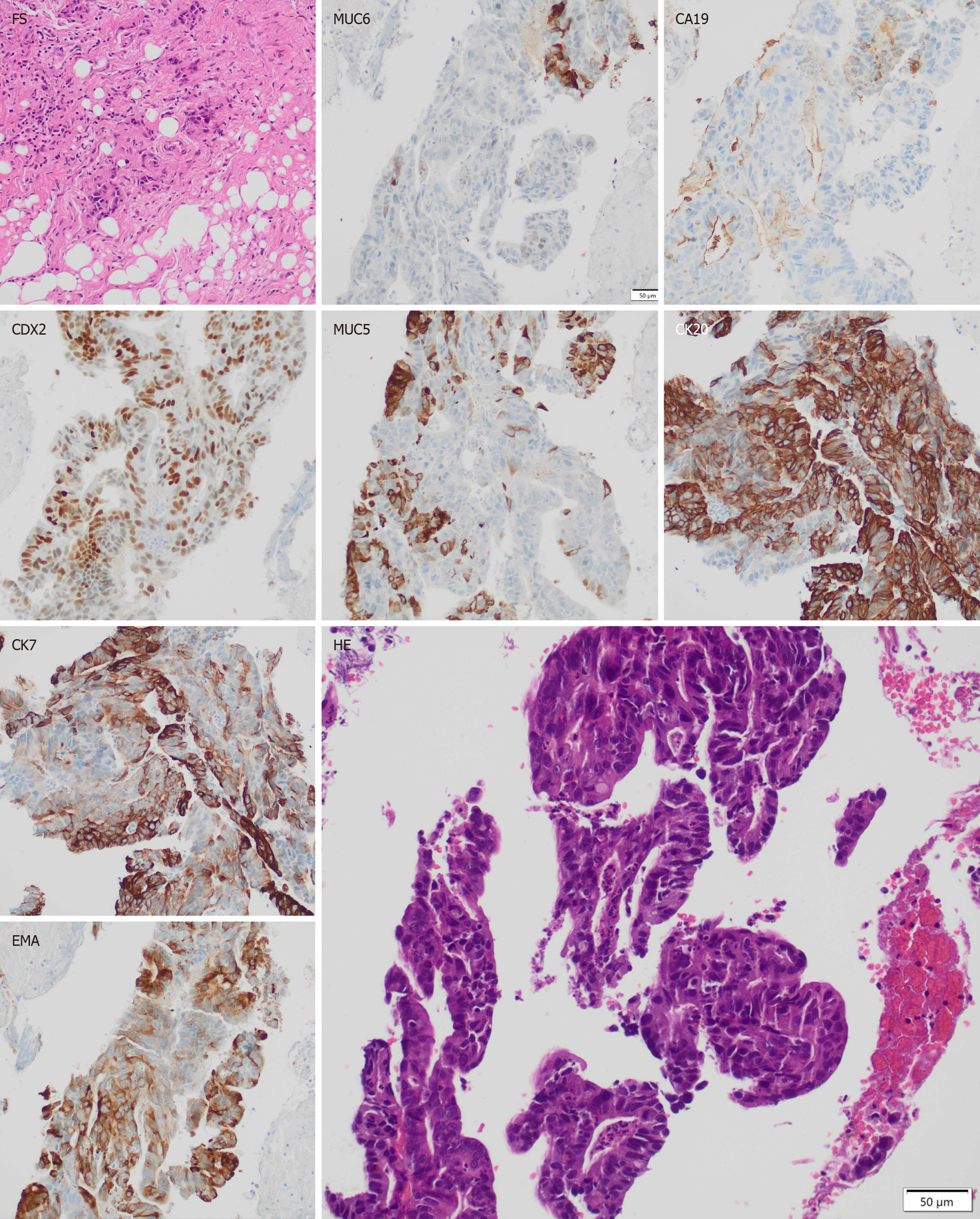
The use of immunohistochemistry is widely used for the adequate characterization of these tumors. Tumors of the intestinal type express high levels of the biomarkers cytokeratin 20 (CK20), caudal-related homeobox transcription factor 2 (CDX2), and mucin 2 (MUC2), regardless of mucin 1 (MUC1) expression level, also known as epithelial membrane antigen. Tumors of the pancreatobiliary type are positive for MUC1 and negative for CDX2 and MUC2, regardless of CK20 expression level, and may also highly express cytokeratin 7 (CK7) and mucin 5AC (MUC5AC)[1,5]. It is also described in literature that a high expression of carbohydrate antigen 19-9 (CA19-9), MUC1, and mucin 6 (MUC6) de
This histological differentiation has prognostic and therapeutic implications. Tumors with intestinal histology have a better prognosis than other histological types and a favorable response to chemotherapy regimens against colon cancer. On the other hand, tumors classified histologically as pancreaticobiliary have a worse prognosis than other cancer types in the same region and may show a better response to therapies against cancers of the biliary tract and pancreas[1,2,3,7].
We present a clinical case of an adenocarcinoma of the ampulla of Vater with intestinal predominance, associated with a synchronous peritoneal carcinomatosis. It is the only case reported in the literature of duodenal ampulla cancer with synchronous peritoneal metastases, with long-term survival.
A 53-year-old male patient with non-insulin-dependent diabetes presented with acute abdominal pain in the right hypochondrium in August 2019.
The patient was afebrile, with no nausea or weight loss.
The patient had no history of past illnesses.
The patient had no personal or family history of cancer.
Physical examination revealed mild pain in the right hypochondrium. The patient did not present jaundice.
Laboratory tests showed alterations of liver enzymes including alkaline phosphatase (218 U/L), gamma-glutamyl transferase (687 U/L), total bilirubin (0.55 mg/dL), and direct bilirubin (0.23 mg/dL). In addition, elevated white blood cells (leukocytosis) of 14100 μL was found.
Computed tomography (CT) scan with intravenous contrast, and magnetic resonance imaging with contrast plus magnetic resonance cholangiopancreatography, were performed. These images revealed dilatation of the biliary tract and the duct of Wirsung, without a clear obstructive factor.
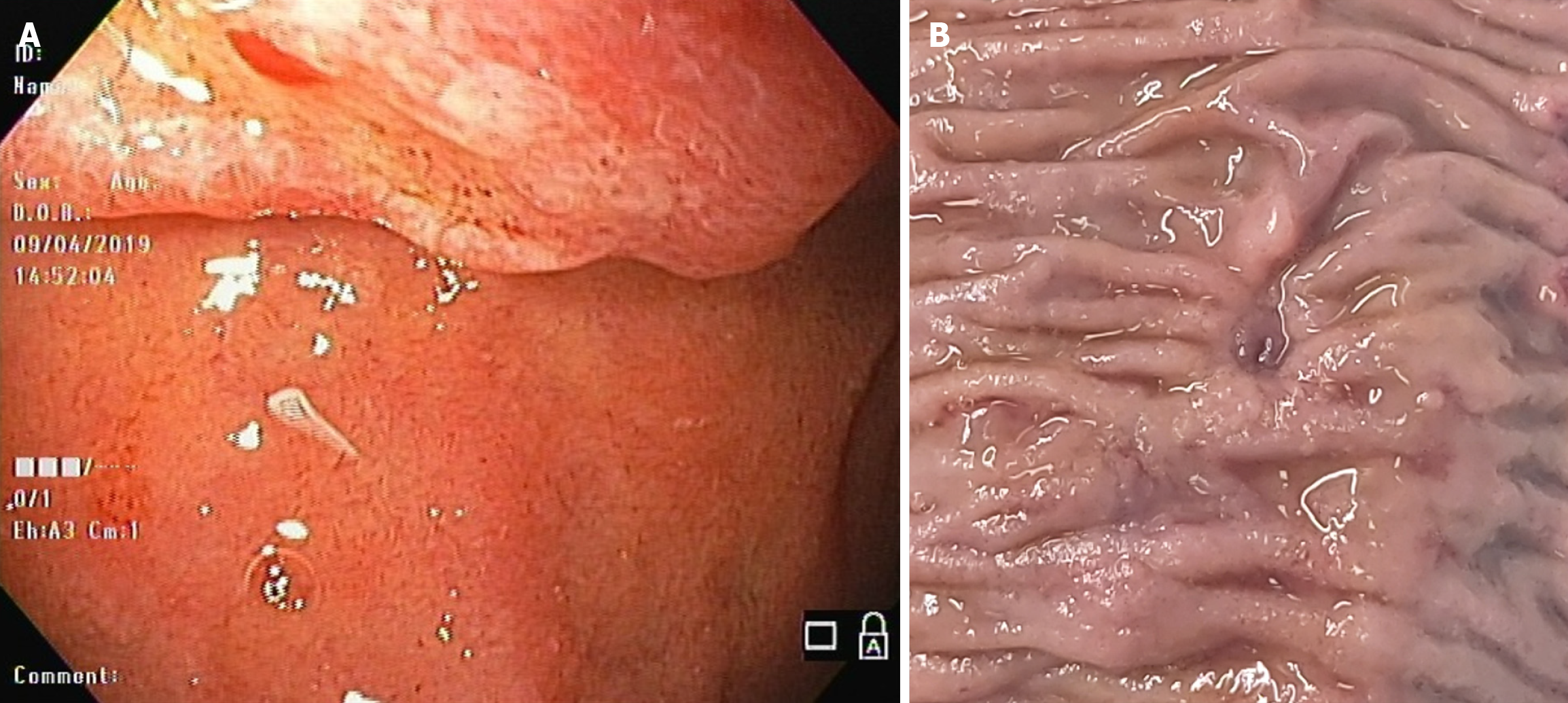
Based on the above findings, upper gastrointestinal endoscopy (UGE) was realized and revealed a tumoral lesion in the duodenal papilla. Biopsies confirmed an adenocarcinoma. Subsequently, an endoscopic ultrasound was made, which revealed a 15 mm lesion. The imaging test showed no evidence of tumor infiltration to the common bile duct or pancreas, and also did not show choledocholithiasis. Two regional lymph nodes were observed, of which the largest was 9 mm.
Biopsies revealed a moderately differentiated tubular adenocarcinoma. Immunohistochemistry showed expression of the following biomarkers: High CDX2 expression in the nuclei of neoplastic cells (NCs), CA19-9 expression in isolated NCs, MUC1 and MUC5AC expression in a proportion of NCs, and MUC6 expression in isolated cells. Additional staining showed intense CK20 and less intense CK7 labeling. The findings revealed a mixed type of duodenal papillary adenocarcinoma, with intestinal predominance (Figure 1).
The Whipple procedure was programmed in December 2019. During the procedure itself, a lesion of 5 mm was found and resected in the mesentery. The intraoperative biopsy result was positive for adenocarcinoma in the peritoneum. Therefore, instead of the planned surgery, Roux-en-Y choledochojejunostomy anastomosis and a cholecystectomy were performed. Subsequently, the patient received 12 cycles of the folinic acid, fluorouracil, and oxaliplatin (FOLFOX) regimen, which were administered between January and July 2020. Control examinations consisted of CT scans and UGE. Treatment led to a decrease in tumor size with no detectable distant lesions. In September 2020, the patient underwent a new surgical intervention. During the procedure, localized disease was observed without peritoneal implants, so a pancreatoduodenectomy was achieved. The gastrointestinal transit was reconstructed with a pancreato-gastro anastomosis, with a new choledochojejunostomy anastomosis. The previous Roux-en-Y was respected, and a gastro-jejunum anastomosis was created. The delayed biopsy report showed a moderately differentiated infiltrating tubular adenocarcinoma located in the ampulla of Vater, with focal infiltration of the muscularis propria. The tumor size was 0.8 cm × 0.2 cm, with no vascular or perineural involvement; 0 of 7 peripancreatic lymph nodes were positive for neoplasia (Figure 2).
The patient was followed up every 3 months for the first 2 years, and then every 6 months. During this period, physical examinations, laboratory tests, CT scans, and positron emission tomography scans were performed. Colonoscopies were performed and detected hyperplastic polyps, which were resected. A left tonsillectomy was done during his follow-up with a benign biopsy result. During 2023, the patient developed pancreatic insufficiency requiring supplementation with exogenous pancreatic enzymes and beginning with insulin. He achieved 5 years of overall survival (OS) in August 2024, and 4 years of disease-free survival (DFS) in September 2024.
This review describes the only case in the literature of ampullary cancer with synchronous peritoneal metastases, with OS of 5 years and DFS of 4 years.
The anatomy of the ampulla of Vater is complex. It is a transition zone between the intestinal epithelium of the duodenum and the pancreatobiliary epithelium from the pancreatic and biliary ducts. When tumors are restricted to the ampulla, compromising until the muscularis propria of the duodenum (T2) without lymph node and distant metastasis, an ampullectomy (endoscopic or open, depending on the features of the patient) could be recommended. In cases of more extensive tumors, the Whipple procedure with lymph node dissection is recommended. Regardless of the histological subtype of adenocarcinoma of the ampulla, the surgical recommendation is the same. For tumors other than those located in the ampulla of Vater, the indication is also the Whipple procedure. Thereby, the anatomic classification defines which procedure the patient needs. To summarize, an ampullectomy is only recommended in the initial stage. In more advanced stages and with the possibility of the abovementioned differential diagnoses, the Whipple procedure should be performed. The latter procedure was performed during a second surgical session, after resection of synchronous peritoneal metastases in the first surgical session. The presence of peritoneal metastases was an indicator of distant dissemination and the reason for performing the Whipple procedure[2,8].
The therapeutic systemic strategy employed, based on the histological study, is another advantage. It facilitates an extrapolation to the possible conduct that would have been taken in another cancer of the gastrointestinal tract. There is biological plausibility, described in the literature, of our selection of systemic therapy and the favorable response to it. The finding of peritoneal carcinomatosis was an indirect sign of disseminated disease, which required control with systemic agents. The FOLFOX regimen was chosen based on the predominant intestinal tumor type, according to immunohistochemical analysis. Studying the presence of mutations such as KRAS allows the identification of patients who would likely benefit from targeted therapies using monoclonal antibodies. In addition, studying microsatellite instability opens the door to treatment with immunotherapy. Neither of those treatments were used in the current case report. Nowadays, the histological and immunohistochemical features of a tumor are more relevant for the indication of a specific systemic treatment than the anatomical location of the neoplasia[7]. In the current case, reduction of the tumor mass after chemotherapy and the absence of neoplasia in lymph nodes, as evidenced by a favorable response to the treatment, is in accordance with studies in other types of gastrointestinal adenocarcinomas in which neoadjuvant therapy reduced tumor size and node positivity[9-11].
Resection of the peritoneal lesion, without the addition of concomitant hyperthermic intraperitoneal chemotherapy (HIPEC), is in accordance with the last evidence about this topic in digestive tumors. This strategy was supported by the results shown in the French multicenter trial, Prodige 7, which enrolled 265 patients with metastatic colorectal cancer with peritoneal metastases. All patients received systemic chemotherapy. They were randomized into two arms: Cytoreduction surgery (CRS) + HIPEC (133 patients) and CRS alone (132 patients). The trial concluded that HIPEC did not provide additional survival advantage when combined with CRS in patients with colon cancer[12]. Other studies with a similar group of patients either had limitations that affect the validity and generalizability of the findings, or did not conclude a benefit in adding HIPEC after CRS[13-15]. There are many studies with controversial results on gastric metastatic peritoneal cancer. In January 2024, the results of the GASTRIPEC-I trial, a multicenter German study, was published. The trial failed to demonstrate a significant OS benefit with the addition of HIPEC after CRS compared to CRS alone. Similar to Prodige 7, a subgroup with intermediate extension of peritoneal carcinomatoses could show improvement in OS after the addition of HIPEC after CRS. OS might be influenced by the introduction of new checkpoint inhibitors as systemic therapy. Indeed, the participation in the effect of new systemic therapies on the outcome of resected synchronous peritoneal metastases is unclear in both groups[16]. In hepatocellular carcinoma (HCC), a French multicenter trial analyzed 135 patients with ruptured HCC; 12% of them had peritoneal recurrence, and 6 of 11 patients with exclusive peritoneal recurrence were operated and had an OS of 36.6 months. In selected cases, the combination of resection plus systemic treatment showed better survival results than systemic treatment alone[17]. For pancreatic adenocarcinoma, there is no evidence supporting CRS with/without HIPEC. There is only evidence of conversion therapy in some selected cases with metastatic disease[18-20]. Patients with biliary tract cancer and synchronous peritoneal metastases receive palliative chemotherapy and best supportive care[21]. At present, the most widely accepted indication for adding HIPEC to CRS in digestive tumors with peritoneal metastases is in cases of peritoneal mucinous carcinoma arising from an appendiceal carcinoma[22].
In summary, we presented a patient with adenocarcinoma of the ampulla with intestinal predominance, and with synchronic peritoneal metastases resected, who showed a favorable response to chemotherapy. After discussion in a multidisciplinary committee, he underwent surgery, achieving R0 resection and long-term DFS. Based on the above findings, we do not suggest initially classifying these cases as out of therapeutic reach. Finally, an important value of this work is the individualized treatment of a patient with cancer, based on adequate preoperative study, and the continuous control of the patient.
Dr. Marta Palma, Former Head of the Medical Oncology Department at Hospital del Salvador, was involved in the strategic planning of the treatment of the patient. She authorized the administration of chemotherapy, and she was the first oncologist to treat the patient. She ceded her position of authorship to the following oncologist. Dr. Jorge Muñoz, an endoscopic surgeon at Hospital del Salvador, provided some conceptual ideas and contributed to clinical follow-up of the patient. Dr. Carlos León, an endoscopic surgeon at Clínica Dávila-Vespucio, is appreciated for implementing the first upper digestive endoscopy and capturing the first endoscopic images, which are presented in this article. Dr Ricardo Urtubia, an anesthesiologist at Clínica Dávila – Vespucio and experienced reviewer for scientific journals, helped with the grammar and spelling correction of the manuscript.
| 1. | Nappo G, Funel N, Laurenti V, Stenner E, Carrara S, Bozzarelli S, Spaggiari P, Zerbi A. Ampullary Cancer: Histological Subtypes, Markers, and Clinical Behaviour-State of the Art and Perspectives. Curr Oncol. 2023;30:6996-7006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Rizzo A, Dadduzio V, Lombardi L, Ricci AD, Gadaleta-Caldarola G. Ampullary Carcinoma: An Overview of a Rare Entity and Discussion of Current and Future Therapeutic Challenges. Curr Oncol. 2021;28:3393-3402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Núñez Villegas JC, Rubel Cohen SM, Cavada Chacón GA, Segovia González L, Matus Floody CL, Sa Cunha A, Rodriguez V, Abularach Cuellar R. Caracterización de lesiones duodenales y su sobrevida. Rev Cirugia. 2020;72. [DOI] [Full Text] |
| 4. | Adsay NV, Reid MD. Ampullary adenocarcinoma. In: Lokuhetty D, White V, Watanabe R, Cree I, editors. WHO Classification of Tumours, 5th Edition, Digestive System Tumours. Villeurbanne, France: Maestro Gestion Edition; 2019; 127-130. Available from: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Digestive-System-Tumours-2019. |
| 5. | Burgart L, Chopp W, Jain D. Protocol for the Examination of Specimens from Patients with Carcinoma of the Ampulla of Vater. College of American Pathologists (CAP), Pathology Electronic Reporting Committee. 2021: 1-14. Available from: https://www.cap.org/protocols-and-guidelines/cancer-reporting-tools/cancer-protocol-templates. |
| 6. | Basturk O, Esposito I, Fukushima N, Furukawa T. Pancreatic intraductal papillary mucinous neoplasm. In: Lokuhetty D, White V, Watanabe R, Cree I, editors. WHO Classification of Tumours, 5th Edition, Digestive System Tumours. Villeurbanne, France: Maestro Gestion Edition; 2019; 313. Available from: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Digestive-System-Tumours-2019. |
| 7. | Chiorean EG, Chiaro MD, Tempero MA, Malafa MP, Benson AB, Cardin DB, Christensen JA, Chung V, Czito B, Dillhoff M, Donahue TR, Dotan E, Fountzilas C, Glazer ES, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Masood A, Moravek C, Nakakura EK, Narang AK, Nardo L, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Truty MJ, Vollmer C, Wolff RA, Wolpin BM, Rn BM, Lubin S, Darlow SD. Ampullary Adenocarcinoma, Version 1.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023;21:753-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 8. | Scroggie DL, Mavroeidis VK. Surgical ampullectomy: A comprehensive review. World J Gastrointest Surg. 2021;13:1338-1350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (9)] |
| 9. | Fong C, Johnston E, Starling N. Neoadjuvant and Adjuvant Therapy Approaches to Gastric Cancer. Curr Treat Options Oncol. 2022;23:1247-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Gosavi R, Chia C, Michael M, Heriot AG, Warrier SK, Kong JC. Neoadjuvant chemotherapy in locally advanced colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2021;36:2063-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 11. | Morton D, Seymour M, Magill L, Handley K, Glasbey J, Glimelius B, Palmer A, Seligmann J, Laurberg S, Murakami K, West N, Quirke P, Gray R; FOxTROT Collaborative Group. Preoperative Chemotherapy for Operable Colon Cancer: Mature Results of an International Randomized Controlled Trial. J Clin Oncol. 2023;41:1541-1552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 207] [Article Influence: 103.5] [Reference Citation Analysis (0)] |
| 12. | Quénet F, Elias D, Roca L, Goéré D, Ghouti L, Pocard M, Facy O, Arvieux C, Lorimier G, Pezet D, Marchal F, Loi V, Meeus P, Juzyna B, de Forges H, Paineau J, Glehen O; UNICANCER-GI Group and BIG Renape Group. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:256-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 517] [Article Influence: 129.3] [Reference Citation Analysis (2)] |
| 13. | Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737-3743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1512] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 14. | Elias D, Delperro JR, Sideris L, Benhamou E, Pocard M, Baton O, Giovannini M, Lasser P. Treatment of peritoneal carcinomatosis from colorectal cancer: impact of complete cytoreductive surgery and difficulties in conducting randomized trials. Ann Surg Oncol. 2004;11:518-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Cashin PH, Mahteme H, Spång N, Syk I, Frödin JE, Torkzad M, Glimelius B, Graf W. Cytoreductive surgery and intraperitoneal chemotherapy versus systemic chemotherapy for colorectal peritoneal metastases: A randomised trial. Eur J Cancer. 2016;53:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 16. | Rau B, Lang H, Koenigsrainer A, Gockel I, Rau HG, Seeliger H, Lerchenmueller C, Reim D, Wahba R, Angele M, Heeg S, Keck T, Weimann A, Topp S, Piso P, Brandl A, Schuele S, Jo P, Pratschke J, Wegel S, Rehders A, Moosmann N, Gaedcke J, Heinemann V, Trips E, Loeffler M, Schlag PM, Thuss-Patience P. Effect of Hyperthermic Intraperitoneal Chemotherapy on Cytoreductive Surgery in Gastric Cancer With Synchronous Peritoneal Metastases: The Phase III GASTRIPEC-I Trial. J Clin Oncol. 2024;42:146-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 51] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 17. | Roussel E, Bubenheim M, Le Treut YP, Laurent A, Herrero A, Muscari F, Mabrut JY, Savier E, Boleslawski E, Ayav A, Lermite E, Doussot A, Regimbeau JM, Riboud R, Cherqui D, Schwarz L; FRENCH Network. Peritoneal Carcinomatosis Risk and Long-Term Survival Following Hepatectomy for Spontaneous Hepatocellular Carcinoma Rupture: Results of a Multicenter French Study (FRENCH-AFC). Ann Surg Oncol. 2020;27:3383-3392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Hashimoto D, Satoi S, Fujii T, Sho M, He J, Hackert T, Del Chiaro M, Jang JY, Gulla A, Yoon YS, Shan YS, Lou W, Valente R, Furuse J, Oba A, Nagai M, Terai T, Tanaka H, Sakai A, Yamamoto T, Yamaki S, Matsumoto I, Murakami Y, Takaori K, Takeyama Y. Is surgical resection justified for pancreatic ductal adenocarcinoma with distant abdominal organ metastasis? A position paper by experts in pancreatic surgery at the Joint Meeting of the International Association of Pancreatology (IAP) & the Japan Pancreas Society (JPS) 2022 in Kyoto. Pancreatology. 2023;23:682-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M, Donahue TR, Dotan E, Ferrone CR, Fountzilas C, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Moravek C, Nakakura EK, Narang AK, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Vollmer C, Wolff RA, Wolpin BM, Lynn B, George GV. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:439-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 692] [Article Influence: 173.0] [Reference Citation Analysis (0)] |
| 20. | Larentzakis A, Anagnostou E, Georgiou K, Vrakopoulou GZ, Zografos CG, Zografos GC, Toutouzas KG. Place of hyperthermic intraperitoneal chemotherapy in the armament against pancreatic adenocarcinoma: A survival, mortality and morbidity systematic review. Oncol Lett. 2021;21:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Rijken A, Bakkers C, Klümpen HJ, van der Geest LG, de Vos-Geelen J, van Erning FN, de Hingh IHJT. Insights into synchronous peritoneal metastases from hepatobiliary origin: Incidence, risk factors, treatment, and survival from a nationwide database. Eur J Surg Oncol. 2023;49:1436-1443. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Govaerts K, Lurvink RJ, De Hingh IHJT, Van der Speeten K, Villeneuve L, Kusamura S, Kepenekian V, Deraco M, Glehen O, Moran BJ; PSOGI. Appendiceal tumours and pseudomyxoma peritonei: Literature review with PSOGI/EURACAN clinical practice guidelines for diagnosis and treatment. Eur J Surg Oncol. 2021;47:11-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (1)] |