Published online Apr 24, 2025. doi: 10.5306/wjco.v16.i4.102958
Revised: January 18, 2025
Accepted: February 24, 2025
Published online: April 24, 2025
Processing time: 143 Days and 15.3 Hours
Highly expressed in the gastrointestinal mucosa, huntingtin-associated protein 1 (HAP1) is closely associated with tumor development and prognosis.
To investigate the clinical utility of HAP1 expression in gastric cancer (GC).
We randomly selected 124 GC patients had not undergone preoperative radio
HAP1 protein and mRNA levels were lower in fresh GC tissues than in normal mucosal tissues (P < 0.001, respectively). Immunohistochemistry also revealed lower HAP1 expression in GC tissues and metastatic lymph nodes than in normal mucosal tissues (P < 0.05). HAP1 expression in GC was closely associated with differentiation, lymph node metastasis, lymph node ratio, remote metastasis, clinical stage, tumor location, and survival time (P < 0.05). Furthermore, HAP1 expression independently predicted GC (P < 0.05) and was more accurate in advanced GC than in early GC (P < 0.05).
HAP1 is an important prognostic biomarker for GC, with low HAP1 expression positively correlating with poor overall survival, especially in advanced clinical stages.
Core Tip: The clinical utility of huntingtin-associated protein 1 (HAP1) expression in gastric cancer (GC) was assessed. HAP1 expression was significantly lower in GC tissues than in normal gastric mucosa, and was strongly associated with GC progression and metastasis. Downregulation of HAP1 correlates with poor overall survival in patients with GC, especially in advanced clinical stages. Thus, HAP1 is a promising prognostic marker for GC, with important implications for advancing treatment.
- Citation: Wang XY, Yang FH, Yuan ZY, Wang ZJ, Zhang HF, Xu ZH. Downregulation of huntingtin-associated protein 1 predicts poor prognosis in gastric cancer. World J Clin Oncol 2025; 16(4): 102958
- URL: https://www.wjgnet.com/2218-4333/full/v16/i4/102958.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i4.102958
Gastric cancer (GC) is a malignant tumor with a high incidence in the digestive system and the third most common cancer worldwide[1]. Its occurrence, development, and metastasis involves multiple genetic pathways[2]. Generally, symptoms appear during the middle and late stages, with surgical resection rates decreasing significantly in the late stage. Major treatment methods (i.e., radiotherapy, chemotherapy, targeted therapy, and immunotherapy) yield unsatisfactory outcomes on advanced GC. Therefore, early detection is important for effective therapy. Serum tumor markers are relatively noninvasive and simple to use. Thus, identifying novel biomarkers will improve screening, disease monitoring, and prognosis, while also providing new therapeutic targets.
Huntingtin-associated protein 1 (HAP1) interacts with huntingtin, a protein associated with Huntington’s disease (HD)[3]. While primarily found in the central nervous system, HAP1 is also present in the digestive system, expressed at varying levels throughout the gastrointestinal mucosa. In particular, gastric mucosa highly expresses HAP1[4-6]. HAP1 is crucial to gene transcriptional regulation, membrane endocytosis, inclusion body formation, vesicle transport, and signal transduction[7]. These functions explain why HAP1 is associated with the biological characteristics, radiosensitivity, and drug resistance of malignancies such as pancreatic and breast cancers[4,8]. Its expression is closely related to tumor development and prognosis, suggesting potential as a cancer biomarker.
Cell proliferation, migration and invasion were inhibited in GC cells overexpressing HAP1. HAP1 also triggered apoptosis during glucose deprivation, further reduced adenosine triphosphate production and elevates reactive oxygen species levels, which disrupting cellular redox and increasing the likelihood of tumor cell death[9]. Furthermore, the application of HAP1 as a therapeutic target has already been empirically demonstrated in several diseases[10-14]. For example, HAP1 expression negatively correlated with the sensitivity of acute lymphoblastic leukemia cells to L-asparaginase[15].
However, no empirical data are available regarding whether HAP1 expression is indeed correlated with GC clinical features and prognosis. Therefore, this study investigated HAP1 expression in patients with GC to understand the relationship between HAP1 levels and clinicopathological characteristics. Our findings should clarify the clinical utility of HAP1 expression in GC progression and prognosis.
Between May 2013 and October 2018, 124 paraffin-embedded GC samples were obtained. All individuals with GC [56 male and 68 female patients; age, 28-88 years (mean = 61 years)] were diagnosed at the Central Hospital of Wuhan and had not undergone preoperative radiotherapy or chemotherapy. Clinical data were collected from all participants. Follow up was 5 years; during this period, 104 patients died and 30 patients presented with distant metastases. Informed consent was waived for by the Institutional Review Board (No. WHZXKYL2024-207) of the Central Hospital of Wuhan.
During August to October 2023, 20 matched pairs of fresh GC specimens were collected. Fresh clinical specimens comprised tumor tissues and normal adjacent mucosa. Specimens were stored in liquid nitrogen immediately after surgery. This study was approved by the Ethics Committee of Central Hospital of Wuhan (No. WHZXKYL2024-207). Written informed consent was obtained from all 20 patients.
Total RNA was extracted from 20 matched pairs of frozen GC tissues using TRIzol reagent (Invitrogen), following manufacturer protocol. Reverse transcription (RT) was performed using a high-capacity cDNA RT kit (Applied Bio
Frozen GC and normal mucosal tissues were crushed into powder, placed on ice, and lysed in radioimmunoprecipitation assay buffer (Pierce, IL, United States) containing protease inhibitors (Pierce, IL, United States). Proteins were quantified using a bicinchoninic acid assay. Proteins were loaded into their respective lanes, electrophoresed, and transferred onto polyvinylidene difluoride membranes (Millipore, Burlington, MA, United States). Membranes were blocked in 5% non-fat dry milk, followed by overnight incubation at 4 °C with anti-HAP1 polyclonal antibodies (1:1000 dilution; Santa Cruz Biotechnology, CA, United States), anti-β-actin antibody (1:10000 dilution; Santa Cruz Biotechnology, CA, United States), and their corresponding horseradish peroxidase-conjugated secondary antibodies (1:5000 dilution; Invitrogen, CA, United States). Signals were detected using enhanced chemiluminescence (Pierce, IL, United States).
Tissue sections of patients with GC were oven-dried at 68 °C for 30 minutes. Samples were deparaffinized and hydrated in graded xylene and ethanol. Subsequently, they were incubated in 0.3% hydrogen peroxide for 15 minutes, placed into boiling sodium citrate buffer (potential of hydrogen = 6.0) for 15 minutes, and blocked for 30 minutes using anti-HAP1 polyclonal antibodies at room temperature. Tissue sections were incubated with horseradish peroxidase-conjugated secondary antibodies (1:1000 dilution; Invitrogen, CA, United States) at 4 °C for 30 minutes. Sections were then placed sequentially in diaminobenzidine solution, hematoxylin, 1% ethyl alcohol, 1% ammonium hydroxide, graded xylene, and ethanol. Finally, slides were sealed with neutral gum.
Two pathologists scored the slides on staining range and intensity; their scores were averaged for the final result. Staining range scores were assigned as follows: 1 point (1%-25%), 2 points (26%-50%), 3 points (51%-75%), and 4 points (76%-100%). Staining intensity scores were assigned as follows: 0 (negative), 1 (weakly positive), 2 (moderately positive), and 3 (strongly positive). Final staining scores were classified into four levels: (-), < 3; (+), 3; (++), 4; and (+++), ≥ 5. Scores of 0-3 and 4-7 were considered low and high HAP1 expression, respectively.
Paired-sample or two-sample t-tests were used to analyze HAP1 expression in GC, normal adjacent mucosa, and metastatic lymphatic tissues. HAP1 mRNA and protein levels in GC and normal adjacent mucosa were compared with a paired-sample t-test. The correlation between HAP1 and GC clinicopathological features was analyzed by Pearson’s χ2 test. Uni- and multivariate Cox proportional hazards model was applied to determine the relationships between HAP1, clinical characteristics, and survival. Overall survival was calculated as duration from operation day to death or last follow-up. Kaplan-Meier plots and log-rank tests were used for survival analyses. All statistics were performed in statistical product and service solutions 19.0. Significance was set at P < 0.05.
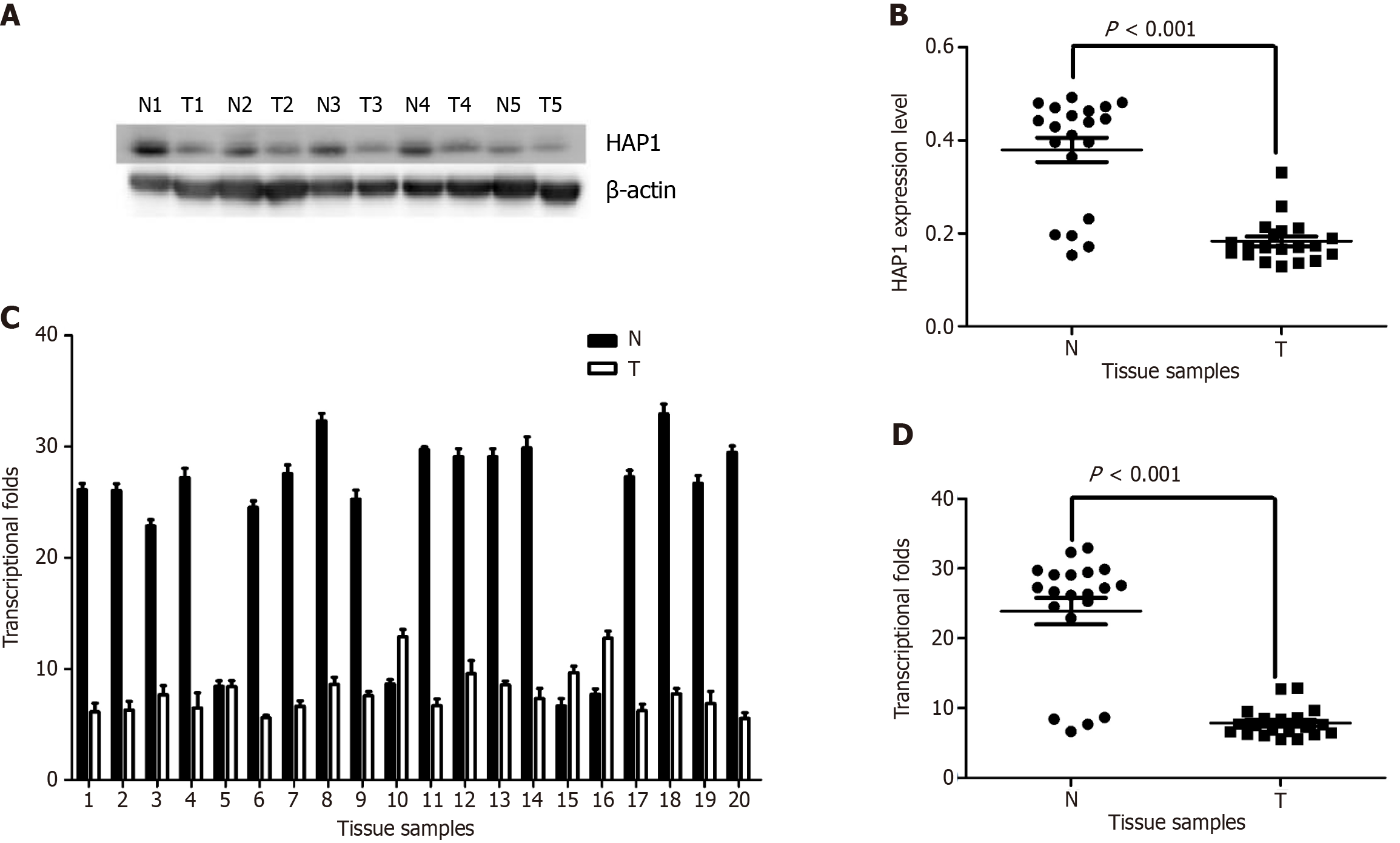
Western blotting on 20 matched pairs of frozen GC specimens and adjacent normal mucosa revealed HAP1 protein downregulation in 15 GC samples, while the remaining five had concentrations nearly equal to or higher than concentrations in mucosa (Figure 1A and B). Results from qPCR demonstrated that HAP1 mRNA was downregulated from mucosa levels in 16 GC tissues, whereas four had nearly equal or higher expression (Figure 1C and D). Overall, mucosa HAP1 protein and mRNA levels were nearly two and three times higher, respectively, than levels in matched GC tissues (P < 0.001).
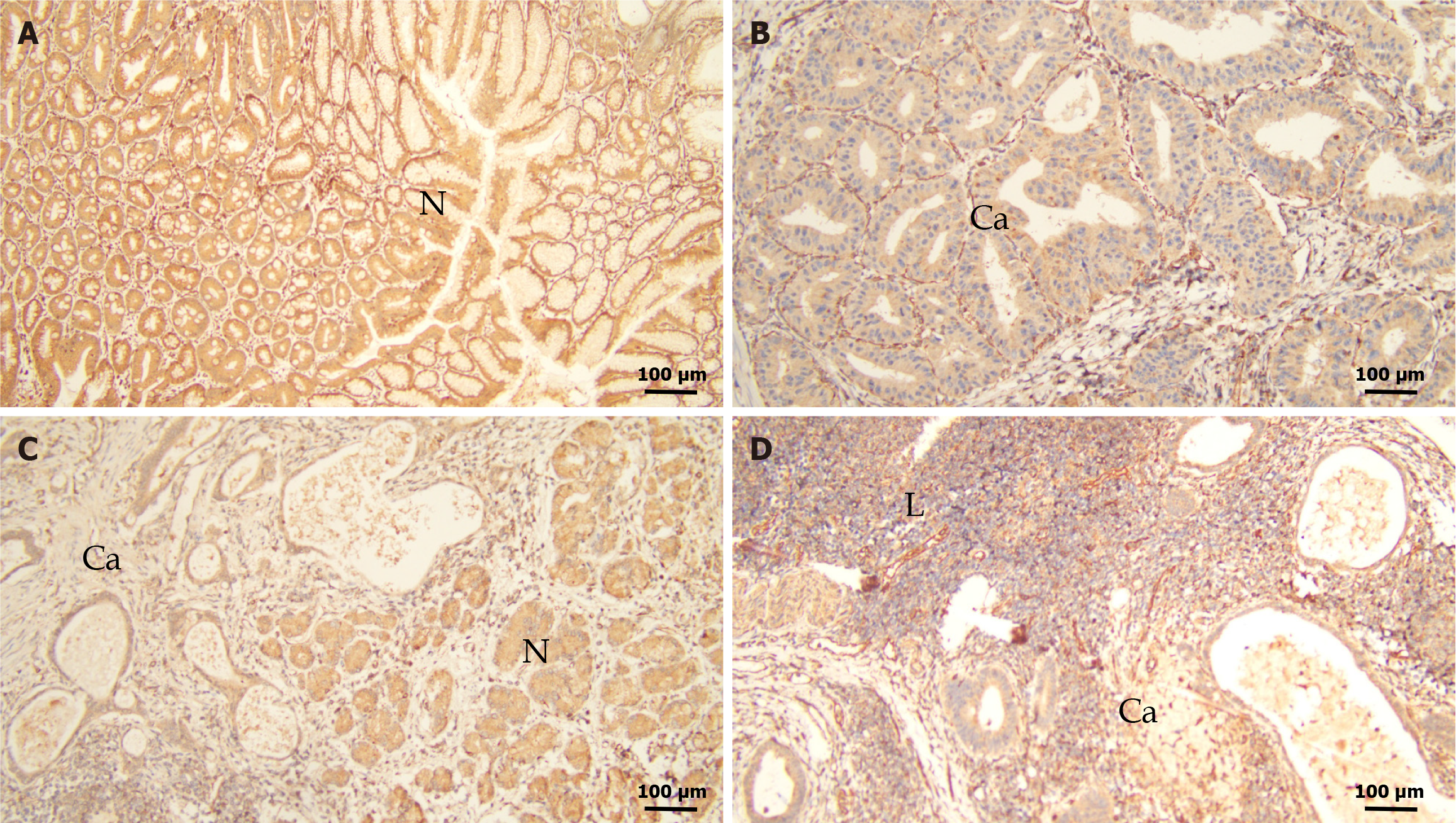
HAP1 expression in GC tissues were lower than those in normal mucosa (Figure 2A-C). Weak or negative signals were detected in metastatic lymph nodes (Figure 2D). Among the 124 GC samples, 85 (68.5%) lowly expressed (0 to 1 +) and 39 (31.5%) highly expressed (2 + to 3 +) HAP1 (Table 1). Both GC (χ2 = 35.631, P < 0.001) and lymphatic metastatic (χ2 = 39.376, P < 0.001) tissues had significantly lower HAP1 expression than adjacent normal mucosa (Table 1). Additionally, HAP1 expression was lower in GC tissues with lymphatic metastasis than in those without lymphatic metastasis (χ2 = 5.494, P = 0.019; Table 1). HAP1 expression did not differ between GC tissues with lymphatic metastasis and lymphatic metastasis tissues (χ2 = 0.036, P = 0.85; Table 1).
In GC tissues, HAP1 expression was clearly correlated with clinicopathological features. Specifically, HAP1 expression was related to lymphatic metastasis (P = 0.019), tumor differentiation (P = 0.014), remote metastasis (P = 0.035), lymph node ratio (P = 0.009), tumor location (P = 0.038), and clinical stage (P = 0.021) (Table 2). In contrast, HAP1 expression was not associated with sex (P = 0.354), age (P = 0.733), tumor size (P = 0.599), or serosal invasion (P = 0.117) (Table 2).
| Features | Low | High | P value | χ2 |
| Cases | 85 | 39 | ||
| Age | ||||
| < 55 years | 30 | 15 | 0.733 | 0.116 |
| ≥ 55 years | 55 | 24 | ||
| Sex | ||||
| Male | 36 | 20 | 0.354 | 0.861 |
| Female | 49 | 19 | ||
| Tumor size | ||||
| < 3 cm | 37 | 16 | 0.599 | 0.276 |
| ≥ 3 cm | 48 | 23 | ||
| Differentiation | ||||
| Low | 40 | 10 | 0.014 | 8.506 |
| Moderate | 28 | 12 | ||
| High | 17 | 17 | ||
| Serosal invasion | ||||
| No | 33 | 21 | 0.117 | 2.454 |
| Yes | 52 | 18 | ||
| Lymphatic metastasis | ||||
| No | 27 | 21 | 0.019 | 5.494 |
| Yes | 58 | 18 | ||
| LNR | ||||
| < 27 | 33 | 25 | 0.009 | 6.862 |
| ≥ 27 | 52 | 14 | ||
| Remote metastasis | ||||
| No | 60 | 35 | 0.035 | 4.458 |
| Yes | 25 | 4 | ||
| Clinical stage | ||||
| Early | 18 | 16 | 0.021 | 5.293 |
| Advanced | 67 | 23 | ||
| Tumor location | ||||
| Proximal | 58 | 19 | 0.038 | 4.327 |
| Distal | 27 | 20 |
Independent prognostic factors in patients with GC was evaluated by the Cox proportional hazards model. In the single-variable analysis, HAP1 levels (P = 0.011), clinical stage (P = 0.012), lymph node metastasis (P = 0.031), remote metastasis (P = 0.009), and tumor location (P = 0.034) were significant predictors (Table 3). Multivariate analysis suggested that the independent prognostic factors for overall survival were HAP1 expression (P = 0.029), clinical stage (P = 0.014), and remote metastasis (P = 0.012) (Table 3).
| Variables1 | Univariate | Multivariate | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| HAP1 (low) | 2.367 (1.876-4.012) | 0.011 | 1.932 (1.142-2.867) | 0.029 |
| Age (≥ 55 years) | 1.358 (0.768-1.152) | 0.934 | ||
| Sex (female) | 0.736 (0.864-1.784) | 0.869 | ||
| Tumor size (≥ 3 cm) | 0.805 (0.653-1.251) | 0.211 | ||
| Differentiation (low) | 0.854 (0.671-1.126) | 0.217 | ||
| Clinical stage (advanced) | 2.675 (1.765-3.467) | 0.012 | 1.878 (1.342-3.247) | 0.014 |
| Lymph metastases (yes) | 2.348 (1.854-2.637) | 0.031 | 1.641 (0.856-2.536) | 0.091 |
| LNR (≥ 27) | 0.843 (0.743-1.987) | 0.451 | ||
| Remote metastasis (yes) | 2.986 (1.897-4.567) | 0.009 | 2.657 (1.564-3.782) | 0.012 |
| Tumor location (distal) | 2.283 (1.891-3.147) | 0.034 | 2.154 (1.965-3.012) | 0.061 |
| Serosal invasion (yes) | 1.786 (1.056-2.435) | 0.091 | ||
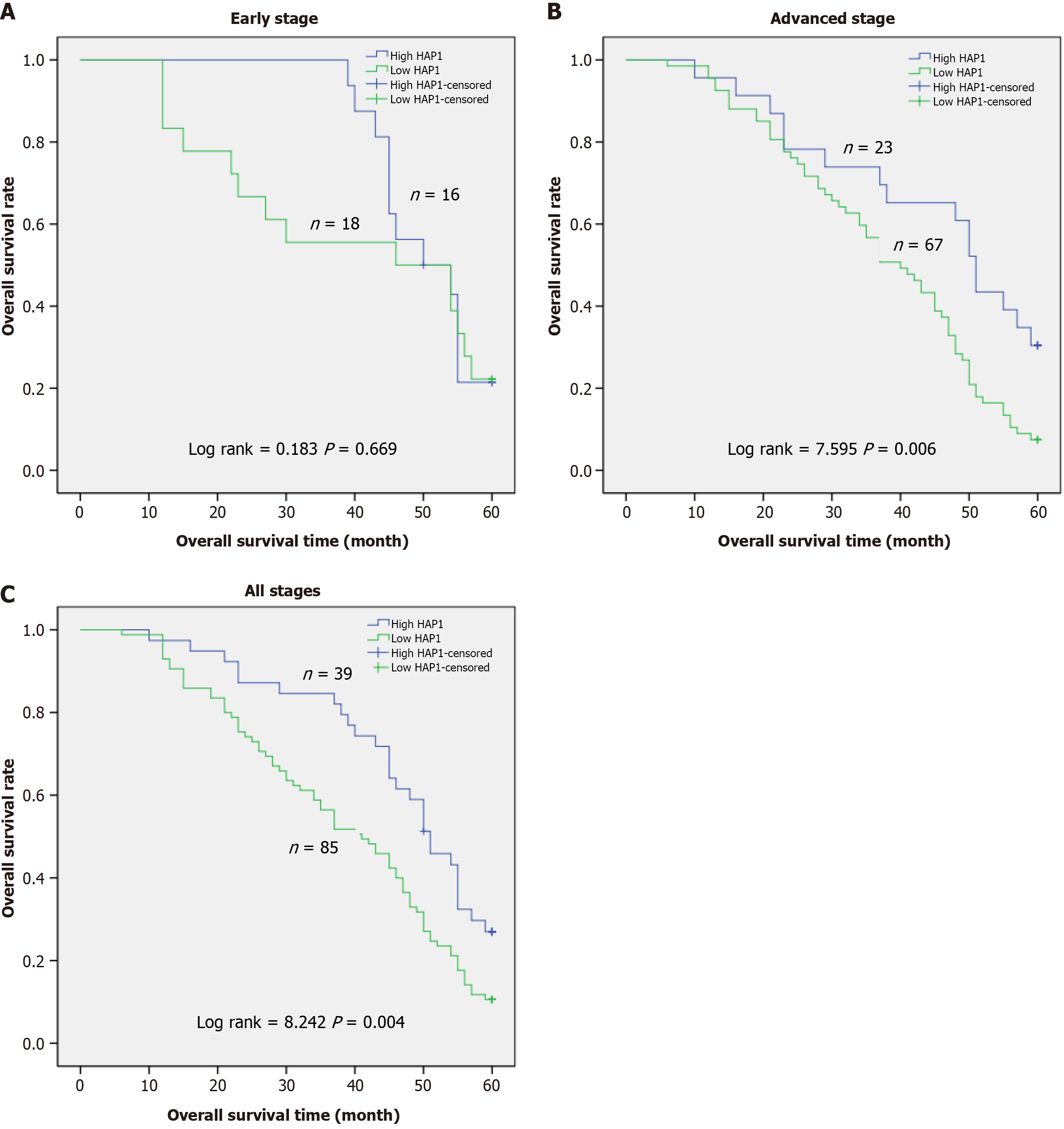
Next, results from Kaplan-Meier curve analysis indicated that in patients with early-stage GC (n = 34), overall survival was not related to HAP1 levels in GC tissues (P = 0.669; Figure 3A). However, overall survival was related to HAP1 levels in patients at advanced-stage (n = 90, P = 0.006; Figure 3B) and all-stage (n = 124, P = 0.004; Figure 3C) GC. Patients with low HAP1 levels had significantly lower 5-year survival rates than those with high HAP1 expression. Hence, HAP1 downregulation may be related to worse prognosis in patients with advanced GC.
Our findings indicate that HAP1 is an independent prognostic factor for overall survival in patients with GC. Patients with advanced-stage GC had lower 5-year survival rates if HAP1 was lowly expressed than if HAP1 was highly expressed. Survival analysis indicated that HAP1 played a significant role in the prognosis of advanced-stage GC, implying that HAP1 may be an important biomarker for such patients.
Despite widespread scientific investigation and massive efforts to develop effective targeted therapies, GC has a poor prognosis and is incurable. Therefore, new treatments and biomarkers are urgently needed to improve therapeutic efficacy for GC. Molecular-targeted therapies exploited to suppress malignant proliferation has potent antitumor activity and remarkably reduces the risk of mortality and recurrence of patients.
Several studies have shown that patients with HD have a relatively low incidence of cancer[16,17]. The nosogenesis of HD involves abnormal repeat amplification of polyglutamine in huntingtin[18]. HAP1 is linked to HD through its interaction with huntingtin[3,19,20]. HAP1 is a multifunctional protein participated in many biological pathways. Current research suggests that HAP1 is associated with the biological characteristics and drug resistance of certain cancers. HAP1 stimulates apoptosis of breast cancer cells, indicating its potential use as a cancer biomarker[8]. However, the potential molecular mechanism of HAP1 affecting cancer remains unclear. HAP1 is also implicated in various cancer types; its downregulation promotes tumor occurrence and development[3]. These studies suggest that HAP1 is a tumor suppressor gene. Our findings support it and demonstrate that HAP1 protein and mRNA are lower in GC tissues than in normal adjacent mucosae. Low HAP1 expression was also related to poor prognosis, consistent with previous results in breast cancer[8].
Results from immunohistochemical confirmed that GC and metastatic lymphatic tissues had lower HAP1 expression than normal mucosa. Additionally, HAP1 expression was lower in GC tissues with lymphatic metastasis than in those without lymphatic metastasis. Furthermore, HAP1 expression was associated with major clinicopathological features, including tumor differentiation, lymphatic metastasis, lymph node ratio, remote metastasis, clinical stage, and tumor location. Overall, our data indicated that HAP1 inhibited GC progression and metastasis, consistent with previous reports indicating that HAP1 upregulation limited breast cancer cell growth in vitro, suppressing cell migration and invasion[8]. Notably, we observed that HAP1 expression was lower in proximal tumors than in distal tumors. Therefore, proximal GC may be more likely to metastasize and has a lower survival rate than distal GC[21].
This study had certain limitations. First, patients came from a single center, meaning our data were inherently biased and may not be applicable to a larger population. Prospective multicenter studies are required to validate our findings. In addition, the lack of a standardized HAP1 expression assessment may introduce bias in clinical practice. Finally, validation using animal models is necessary to better assess the potential for clinical use.
HAP1 is strongly associated with GC progression and metastasis. Moreover, HAP1 downregulation correlates with poor overall survival in patients with GC, especially at advanced clinical stages. HAP1 is thus a promising candidate for a diagnostic and prognostic marker of GC. Importantly, it may also serve as a novel therapeutic target for GC. Further studies are required to elucidate the molecular mechanisms underlying the role of HAP1 in GC.
| 1. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 960] [Article Influence: 320.0] [Reference Citation Analysis (0)] |
| 2. | Usui G, Matsusaka K, Mano Y, Urabe M, Funata S, Fukayama M, Ushiku T, Kaneda A. DNA Methylation and Genetic Aberrations in Gastric Cancer. Digestion. 2021;102:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 3. | Zhao X, Chen A, Wang Z, Xu XH, Tao Y. Biological functions and potential therapeutic applications of huntingtin-associated protein 1: progress and prospects. Clin Transl Oncol. 2022;24:203-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Li T, Li S, Gao X, Cai Q, Li XJ. Expression and Localization of Huntingtin-Associated Protein 1 (HAP1) in the Human Digestive System. Dig Dis Sci. 2019;64:1486-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Tarif AMM, Islam MN, Jahan MR, Afrin M, Meher MM, Nozaki K, Masumoto KH, Yanai A, Shinoda K. Neurochemical phenotypes of huntingtin-associated protein 1 in reference to secretomotor and vasodilator neurons in the submucosal plexuses of rodent small intestine. Neurosci Res. 2023;191:13-27. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Yanai A, Islam MN, Hayashi-Okada M, Jahan MR, Tarif AMM, Nozaki K, Masumoto KH, Shinoda K. Immunohistochemical relationships of huntingtin-associated protein 1 with enteroendocrine cells in the pyloric mucosa of the rat stomach. Acta Histochem. 2020;122:151650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Wu Y, Wang Y, Lu Y, Yan J, Zhao H, Yang R, Pan J. Research advances in huntingtin-associated protein 1 and its application prospects in diseases. Front Neurosci. 2024;18:1402996. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Zhu L, Song X, Tang J, Wu J, Ma R, Cao H, Ji M, Jing C, Wang Z. Huntingtin-associated protein 1: a potential biomarker of breast cancer. Oncol Rep. 2013;29:1881-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Qu YM, Chen A, Zhao X, Wang Z, Guo D, Shao SL, Tao YY, Li QJ, Wang MY, Ma WS. Huntingtin-associated protein 1 is a potential tumor suppressor for gastric cancer. Mol Biol Rep. 2023;50:1517-1531. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Wu J, Zhang JY, Yin L, Wu JZ, Guo WJ, Wu JF, Chen M, Xia YY, Tang JH, Ma YC, He X. HAP1 gene expression is associated with radiosensitivity in breast cancer cells. Biochem Biophys Res Commun. 2015;456:162-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Lee JK, Kang S, Wang X, Rosales JL, Gao X, Byun HG, Jin Y, Fu S, Wang J, Lee KY. HAP1 loss confers l-asparaginase resistance in ALL by downregulating the calpain-1-Bid-caspase-3/12 pathway. Blood. 2019;133:2222-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Gong YJ, Feng Y, Cao YY, Zhao J, Wu W, Zheng YY, Wu JR, Li X, Yang GZ, Zhou X. Huntingtin-associated protein 1 plays an essential role in the pathogenesis of type 2 diabetes by regulating the translocation of GLUT4 in mouse adipocytes. BMJ Open Diabetes Res Care. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Yang GZ, Yang M, Lim Y, Lu JJ, Wang TH, Qi JG, Zhong JH, Zhou XF. Huntingtin associated protein 1 regulates trafficking of the amyloid precursor protein and modulates amyloid beta levels in neurons. J Neurochem. 2012;122:1010-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Li R, Wu B, He M, Zhang P, Zhang Q, Deng J, Yuan J, Chen Y. HAP1 Modulates Epileptic Seizures by Regulating GABA(A)R Function in Patients with Temporal Lobe Epilepsy and in the PTZ-Induced Epileptic Model. Neurochem Res. 2020;45:1997-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Vervliet T, Parys JB. L-asparaginase-induced apoptosis in ALL cells involves IP(3) receptor signaling. Cell Calcium. 2019;83:102076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Hasholt LF. Upregulated Chaperone-Mediated Autophagy May Perform a Key Role in Reduced Cancer Incidence in Huntington's Disease. J Huntingtons Dis. 2023;12:371-376. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Bragina EY, Gomboeva DE, Saik OV, Ivanisenko VA, Freidin MB, Nazarenko MS, Puzyrev VP. Apoptosis Genes as a Key to Identification of Inverse Comorbidity of Huntington's Disease and Cancer. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Chongtham A, Isas JM, Pandey NK, Rawat A, Yoo JH, Mastro T, Kennedy MB, Langen R, Khoshnan A. Amplification of neurotoxic HTTex1 assemblies in human neurons. Neurobiol Dis. 2021;159:105517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Chen X, He E, Su C, Zeng Y, Xu J. Huntingtin-associated protein 1-associated intracellular trafficking in neurodegenerative diseases. Front Aging Neurosci. 2023;15:1100395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 20. | Liu Q, Cheng S, Yang H, Zhu L, Pan Y, Jing L, Tang B, Li S, Li XJ. Loss of Hap1 selectively promotes striatal degeneration in Huntington disease mice. Proc Natl Acad Sci U S A. 2020;117:20265-20273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Xue J, Yang H, Huang S, Zhou T, Zhang X, Zu G. Comparison of the overall survival of proximal and distal gastric cancer after gastrectomy: a systematic review and meta-analysis. World J Surg Oncol. 2021;19:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |