Published online Apr 24, 2025. doi: 10.5306/wjco.v16.i4.102199
Revised: November 13, 2024
Accepted: January 15, 2025
Published online: April 24, 2025
Processing time: 165 Days and 22.4 Hours
Chemotherapy combined with anti-angiogenic therapy has become an important strategy for the treatment of advanced gastric cancer (AGC); however, the re
To evaluate the efficacy of albumin-bound paclitaxel (nab-ptx) combined with the small molecule vascular endothelial growth factor inhibitor anlotinib in second-line and beyond treatment of AGC.
We collected data from AGC patients at our hospital who experienced disease progression after first-line chemotherapy and received anlotinib combined with nab-ptx. The primary endpoints included overall survival (OS) and progression-free survival (PFS), while the secondary endpoints were objective response rate (ORR), disease control rate (DCR), and adverse events (AEs).
Preliminary results indicated that anlotinib combined with nab-ptx can provide significant efficacy in second-line or above treatment for AGC (median PFS = 6.0 months, median OS = 12.0 months), with an ORR of 42% and a DCR of 78%. Further analysis revealed that patients who experienced hypertension, proteinuria, and hand-foot syndrome during treatment had better efficacy compared to those who did not experience these AEs. Mechanistic studies suggest that this regimen likely exerts synergistic anti-tumor effects by activating the immune response through the reduction of regulatory T-cell proportions. Common adverse reactions included bone marrow suppression, peripheral neuropathy, hypertension, proteinuria, and hand-foot syndrome, which were manageable and resolved with appropriate interventions, indicating the promising application of this regimen in second-line or above treatment for AGC.
The combination of anlotinib and nab-ptx shows promising efficacy with fewer toxicities in AGC treatment. The regimen holds promise as a second-line treatment of AGC; however, its specific clinical value requires further research.
Core Tip: The second-line or above treatment for advanced gastric cancer lacks effective therapeutic models. The results of this study indicate that the small molecule vascular endothelial growth factor inhibitor anlotinib combined with albumin-bound paclitaxel has significantly improved efficacy in gastric cancer treatment, and the toxic side effects are manageable. Further analysis showed that patients who experience hypertension, proteinuria, and hand-foot syndrome during treatment derive greater benefit from this regimen. The mechanism of this combination treatment may involve the depletion of immune suppression to activate the immune system, thereby exerting a synergistic anti-tumor effect, demonstrating the potential of this regimen for clinical application.
- Citation: Liu WM, Liu YR, Peng Y, Tang J, Li XB. Combination of anlotinib and albumin-bound paclitaxel in 2nd line and above treatment of advanced gastric cancer: A retrospective study. World J Clin Oncol 2025; 16(4): 102199
- URL: https://www.wjgnet.com/2218-4333/full/v16/i4/102199.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i4.102199
Advanced gastric cancer (AGC) remains one of the leading causes of cancer-related mortality worldwide, particularly in advanced stages where therapeutic options are limited[1]. Despite advancements in surgical techniques and adjuvant therapies, the prognosis for patients with AGC is often poor[2]. Chemotherapy has been the cornerstone of treatment for these patients, especially in the second-line setting and beyond. Traditional regimens have included combinations of platinum-based agents and fluoropyrimidines; however, resistance to these therapies frequently develops, necessitating the exploration of novel agents[3].
Anlotinib, a multi-targeted tyrosine kinase inhibitor, has shown promise in inhibiting tumor growth by targeting various pathways involved in angiogenesis and tumor proliferation[4]. Its mechanism includes the inhibition of vascular endothelial growth factor receptors (VEGFRs), platelet-derived growth factor receptors (PDGFRs), and fibroblast growth factor receptors (FGFRs). This broad spectrum of action not only interferes with tumor cell proliferation but also modulates the tumor microenvironment, potentially enhancing the efficacy of concurrent therapies[5,6].
Albumin-bound paclitaxel (nab-ptx) is a well-established chemotherapeutic agent that demonstrates enhanced delivery and reduced side effects compared to traditional paclitaxel formulations[7]. The use of nab-ptx is particularly advantageous in advanced cancers due to its ability to overcome some forms of drug resistance and has improved pharmacokinetics[8]. Its mechanism involves disrupting microtubule function, leading to cell cycle arrest and apoptosis in rapidly dividing cancer cells[9].
Combining anlotinib with nab-ptx may represent a promising strategy for treating AGC, particularly in patients who have experienced disease progression after first-line therapy[10]. This combination leverages the anti-angiogenic properties of anlotinib alongside the cytotoxic effects of nab-ptx, potentially resulting in enhanced therapeutic efficacy. Moreover, the safety profile of both agents is relatively well-characterized, which allows for better management of adverse effects[11,12].
Despite the potential benefits, there is a lack of comprehensive data regarding the efficacy and safety of this combination in the context of AGC[13]. Based on this, we explored the efficacy and safety of anlotinib combined with nab-ptx in second-line treatment for AGC, aiming to provide an effective treatment option for this patient population.
This retrospective study was conducted at Hubei Cancer Hospital between January 2020 and December 2023, focusing on patients diagnosed with AGC who received anlotinib in combination with nab-ptx as a second-line therapy or beyond. The research protocol was approved by the Institutional Review Board of Hubei Cancer Hospital Affiliated with Tongji Medical College (Wuhan, China), and all patient data were handled in compliance with the ethical standards.
Eligible patients were those aged 18 years or older, with confirmed histologically or cytologically diagnosed AGC, who had progressed after one or more lines of systemic therapy. We excluded patients with significant comorbidities, those who had received prior treatment with anlotinib or nab-ptx, and those with incomplete medical records. Patient demographics, clinical characteristics, and treatment history were collected from electronic medical records.
Patients with AGC who met the inclusion criteria and experienced disease progression after first-line or subsequent treatments received combined therapy with anlotinib and nab-ptx. Patients received anlotinib at a starting dose of 12 mg daily, administered orally for 14 days in a 21-day cycle, alongside nab-ptx given intravenously at a dose of 100-200 mg/m² on day 1 of each cycle. If severe toxic side effects occurred, the anlotinib dose could be sequentially reduced to 10 mg and then 8 mg. If the 8 mg dose was not tolerated, the medication would be permanently discontinued. For nab-ptx, the minimum combined dose with anlotinib was 100 mg/m²; if the 100 mg/m² dose was not tolerated, permanent discontinuation could also be considered. Treatment continued until disease progression, unacceptable toxicity, or patient withdrawal. Dose adjustments were made based on individual tolerability and the side effects experienced during treatment.
Samples of ethylenediamine tetra acetic acid anticoagulated peripheral blood (2 mL) were collected from patients with advanced AGC before initial treatment and a second sample was collected after subsequent treatment cycles. All samples were tested within 6 hours of being obtained. Briefly, CD3+/CD4+/CD8+ T-cell, CD19+ B-cell, and CD16+CD56+ natural killer (NK)-cell counts (cells/μL) were measured by multiple-color flow cytometry with human monoclonal anti-CD3-FITC, anti-CD4-PE, anti-CD8-APC, anti-CD19-PE, anti-CD16-APC, and anti-CD56-PE antibodies [BD Multitest; Becton, Dickinson, and Co. (BD) Biosciences, Franklin Lakes, NJ, United States] according to the manufacturer’s instructions. The cells were analyzed on a BD FACS Canto II flow cytometry system (BD Biosciences).
Efficacy was primarily evaluated through radiological assessments conducted every two cycles using the RECIST version 1.1. Objective response rate (ORR) was defined as the proportion of patients achieving a complete response (CR) or partial response (PR), while disease control rate (DCR) included those with stable disease (SD) in addition to CR and PR. Progression-free survival (PFS) was calculated from the start of treatment to the date of disease progression or last follow-up, while overall survival (OS) was measured from the initiation of therapy to the date of death or last follow-up.
Safety profiles were evaluated by monitoring adverse events (AEs) according to the Common Terminology Criteria for AEs version 5.0. All AEs were recorded, graded, and managed accordingly. Serious AEs leading to treatment discontinuation were also documented. Laboratory tests, including complete blood counts and liver function tests, were performed regularly to monitor for hematological and non-hematological toxicities.
Statistical analyses were performed using (specific statistical software, e.g., SPSS, R). Descriptive statistics were used to summarize patient demographics and treatment characteristics. Survival analyses for PFS and OS were performed using the Kaplan-Meier method, with differences assessed by the log-rank test. A P-value of < 0.05 was considered statistically significant.
In this retrospective study, we analyzed the outcomes of 36 patients diagnosed with AGC who received anlotinib combined with nab-ptx as a second-line treatment or beyond. The median age of the cohort was 58 years (range: 47-71), and 60% were male. In terms of pathological types, the proportions of patients with well-differentiated, moderately differentiated, poorly differentiated, and undifferentiated tumors were 5.56%, 25.00%, 55.56%, and 13.89%, respectively. According to the Lauren classification, the proportions of patients with intestinal, diffuse, mixed, and unknown types were 44.44%, 27.78%, 5.56%, and 19.44%, respectively. In terms of the primary site of occurrence, the proportions of gastric cancer and gastroesophageal junction cancer were 86.11% and 13.89%, respectively. Regarding metastatic sites, the proportions of patients with metastasis to lymph nodes, peritoneum, liver, lungs, and other sites were 58.33%, 38.89%, 33.33%, 25.00%, and 27.78%, respectively. Among those with metastasis, the proportions of patients with fewer than three metastatic sites (< 3) and those with three or more (≥ 3) were 69.44% and 30.56%. With regard to prior treatments, the proportions of patients who had received the Triplet regimen (platinum and fluoropyrimidine with anthracycline) and the Double regimen (platinum and fluoropyrimidine) were 22.22% and 75.00%, respectively. In terms of treatment lines, the proportions of patients receiving first-line and second-line treatments were 72.22% and 27.78%, respectively (Table 1).
| Characteristics | No. of patients (%) |
| Age | |
| Years | 58 |
| Range | 47-71 |
| Gender | |
| Male | 28 (77.78) |
| Female | 8 (22.22) |
| Number of metastasis sites | |
| 2 | 25 (69.44) |
| 3 | 11 (30.56) |
| Metastatic site | |
| Lymph node | 21 (58.33) |
| Peritoneum | 14 (38.89) |
| Liver | 12 (33.33) |
| Lung | 9 (25.00) |
| Others | 10 (27.78) |
| Primary tumor site | |
| Gastric | 31 (86.11) |
| GEJ | 5 (13.89) |
| ECOG score | |
| 0-1 | 30 (83.33) |
| 2 | 6 (16.67) |
| Tumor grade | |
| Well, differentiated | 2 (5.56) |
| Moderately diff erentiated | 9 (25.00) |
| Poorly differentiated | 20 (55.56) |
| Unknown or missing | 5 (13.89) |
| Histological subtype (Lauren classification) | |
| Intestinal | 16 (44.44) |
| Diffuse | 10 (27.78) |
| Mixed | 2 (5.56) |
| Unknown or not available | 8 (22.22) |
| Previous treatment | |
| Triplet: Platinum and fl uoropyrimidine with anthracycline | 8 (22.22) |
| Doublet: Platinum and fl uoropyrimidine | 27 (75.00) |
| HER2, EGFR, or other | 1 (2.78) |
| Treatment line | |
| 1 | 26 (72.22) |
| 2 | 10 (27.78) |
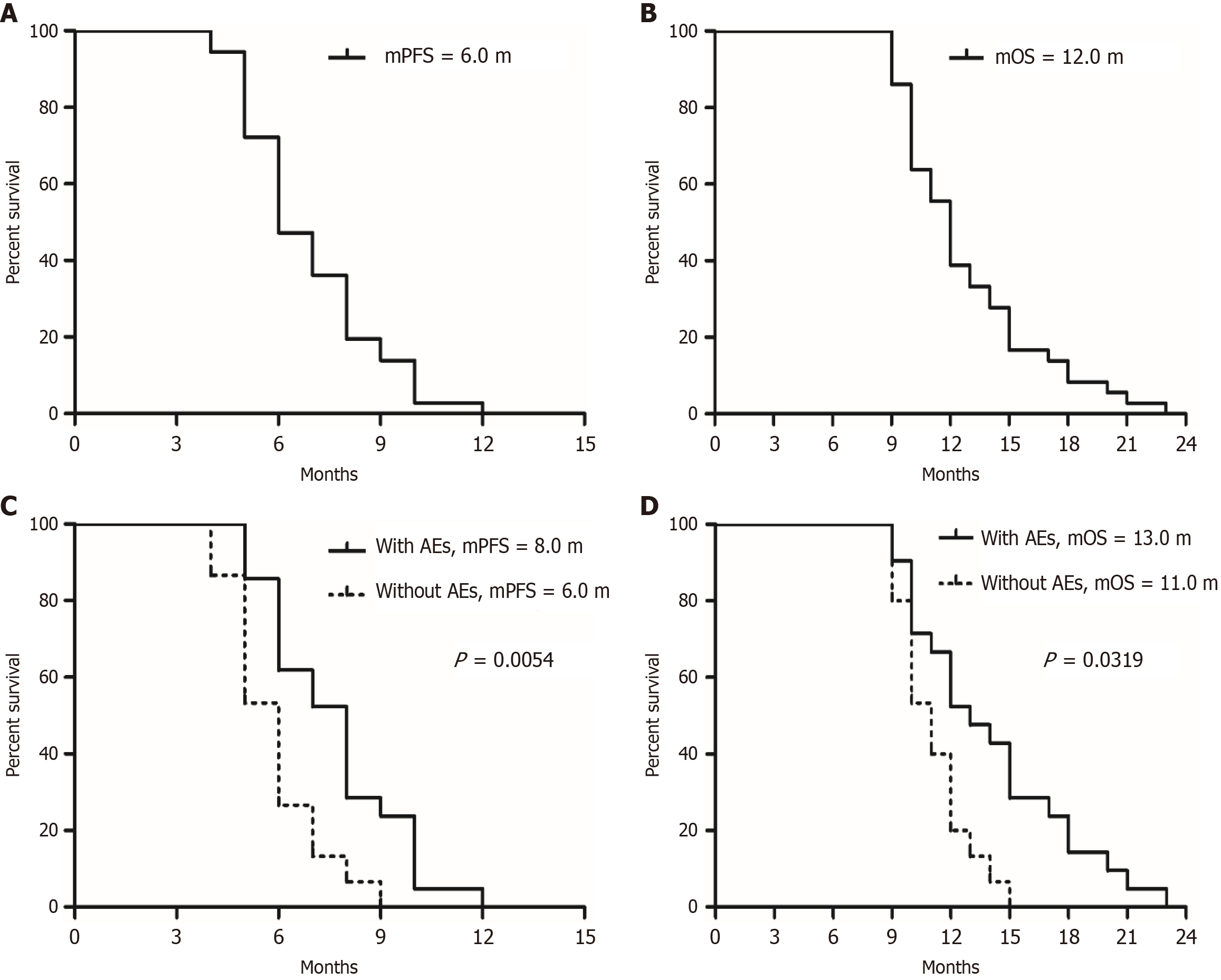
All patients received at least two cycles of treatment, and preliminary data were obtained after a median follow-up of 24 months. The ORR in this patient population was 44.44%, with 0 patients achieving CR and 16 patients achieving PR. Additionally, the DCR reached 77.78%, indicating that 12 patients maintained SD (Table 2). The median PFS was 6.0 months (95%CI: 3.51-10.27), while the median OS was 12.0 months (95%CI: 7.50-19.00). Subgroup analysis showed that patients who experienced hypertension, proteinuria, and hand-foot syndrome during treatment had better efficacy compared to those who did not experience these AEs [median PFS (mPFS) 8.0 months vs 6.0 months, P = 0.0054, HR = 0.25, 95%CI: 0.10-0.64; median OS (mOS) 13.0 months vs 11.0 months, P = 0.0319, HR = 0.30, 95%CI: 0.13-0.74] (Figure 1), indicating that the identification of certain AEs during treatment can serve as a basis for screening priority populations.
| Clinical efficacy | Patient No. | Proportion (%) |
| Complete response | 0 | 0 |
| Partial response | 16 | 44.44 (16/36) |
| Stable response | 12 | 33.33 (12/36) |
| Progressive disease | 8 | 22.23 (8/36) |
| Objective response | 44.44 | |
| Median PFS | 6.0 months | |
| Disease control rate | 77.78 | |
| Median OS | 12.0 months |
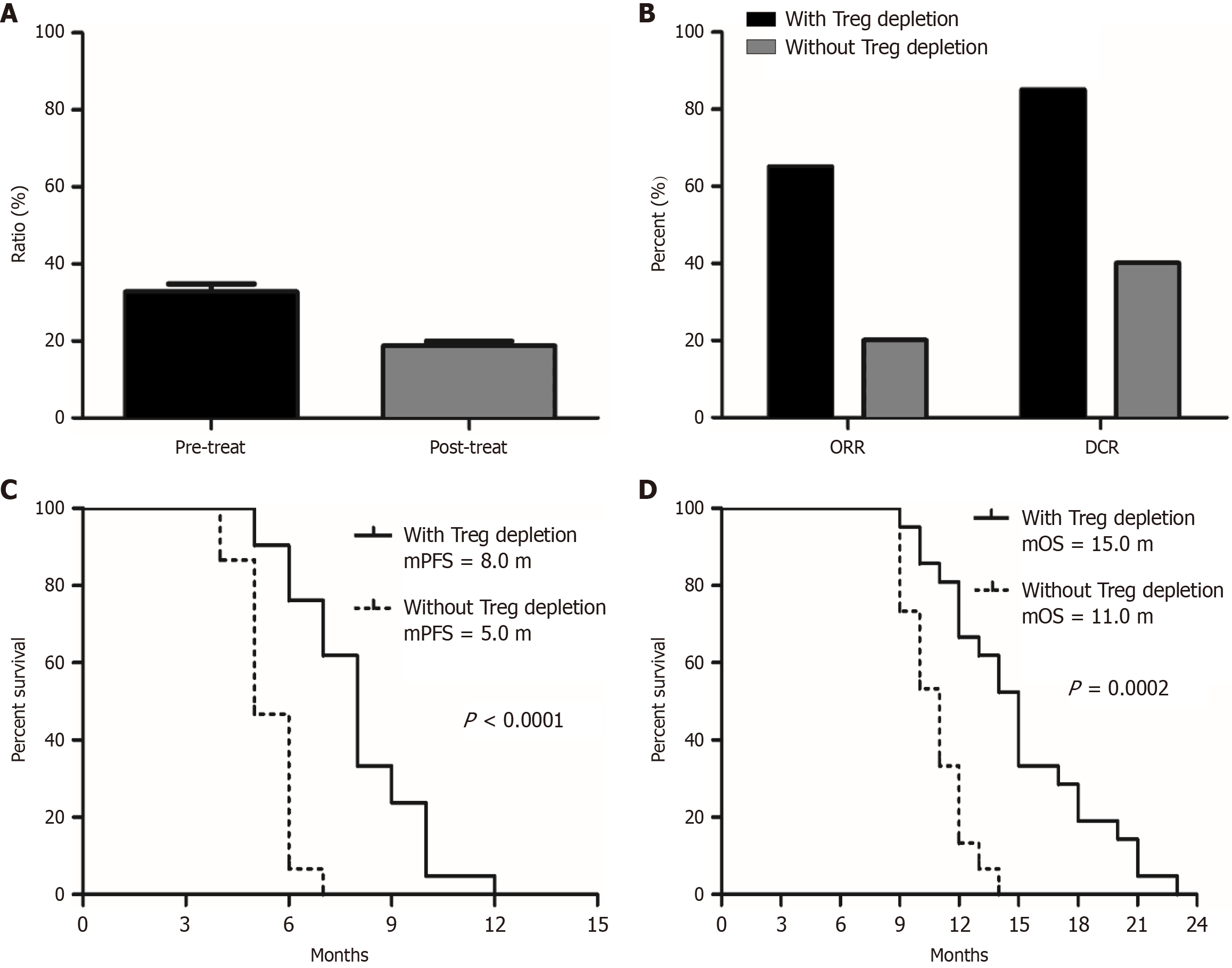
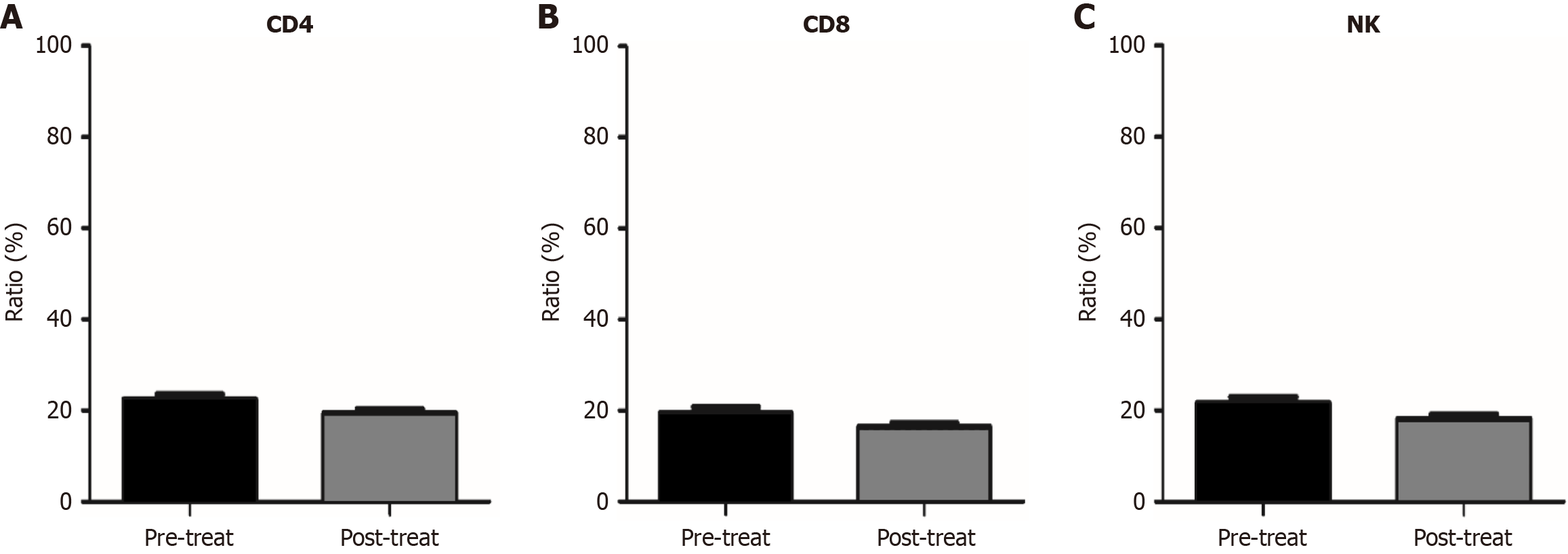
Given that this regimen may be related to modulation of the microenvironment, we also compared changes in the proportions of immune cells in peripheral blood before and after treatment. Preliminary results showed that the baseline proportion of regulatory T (Treg) cells in patients’ peripheral blood was generally elevated compared to the normal range, while the proportion of Treg cells significantly decreased after six weeks of treatment (Figure 2). However, there were no significant changes in the proportion of other immune cells (CD4+, CD8+, NK+) in the peripheral blood of patients before and after treatment (Figure 3). Patients who experienced Treg cell depletion after treatment had higher ORR and DCR, as well as longer PFS and OS, compared to those who did not show Treg cell depletion (patients with Treg cell depletion vs patients without Treg cell depletion, mPFS 8.0 months vs 5.0 months, P < 0.0001, HR = 0.08, 95%CI: 0.03-0.23; mOS 15.0 months vs 11.0 months, P = 0.0002, HR = 0.13, 95%CI: 0.05-0.35) (Figure 2). This indicates that the regimen likely exerts synergistic anti-tumor effects by depleting immunosuppression and activating the immune response. Additionally, the downregulation of Treg cell proportions may serve as a potential molecular biomarker for efficacy prediction.
Regarding safety, AEs can be broadly categorized into hematologic and non-hematologic reactions, indicating that the combination of nab-ptx and anlotinib did not result in additive toxicity; the combination therapy was generally well-tolerated. Common hematologic AEs included leukopenia (36.11%), neutropenia (33.33%), thrombocytopenia (30.56%), and anemia (27.78%). However, these were primarily grade 1-2 and resolved with appropriate management. The most common non-hematologic AEs were grade 1-2, including decreased appetite (52.78%), fatigue (44.44%), hand-foot syndrome (33.33%), hypertension (30.56%), proteinuria (25.00%), and peripheral neuropathy (19.44%). Serious AEs occurred in 22% of the patients, with only three cases leading to treatment discontinuation. Notably, no treatment-related deaths were reported. Management strategies for AEs included dose adjustments of anlotinib in 15% of patients and supportive care measures for symptomatic relief (Table 3).
| Adverse events | Any grade | Grade 3 or 4 |
| Hematological | ||
| Leukopenia | 13 (36.11) | 2 (5.56) |
| Neutropenia | 12 (33.33) | 2 (5.56) |
| Thrombocytopenia | 11 (30.56) | 2 (5.56) |
| To anemia | 10 (27.78) | 0 |
| Non-hematologic | ||
| Decreased appetite | 19 (52.78) | 0 |
| Fatigue | 16 (44.44) | 0 |
| Hypertension | 11 (30.56) | 2 (5.56) |
| Hand-foot syndrome | 12 (33.33) | 2 (5.56) |
| Proteinuria | 9 (25.00) | 2 (5.56) |
| Peripheral neuropathy | 7 (19.44) | 1 (2.78) |
| Elevated transaminase | 7 (19.44) | 1 (2.78) |
| Oral ulcer | 6 (16.67) | 0 |
| Stomatitis | 5 (13.89) | 0 |
| Abdominal pain | 5 (13.89) | 0 |
| Diarrhea | 5 (13.89) | 0 |
| Hyperbilirubinemia | 3 (8.33) | 0 |
| Elevated LDH | 3 (8.33) | 0 |
| ALP increased | 3 (8.33) | 0 |
| Elevated GGT | 3 (8.33) | 0 |
| Hypoproteinemia | 4 (11.11) | 0 |
| Dysphagia | 3 (8.33) | 0 |
| Dysphonia | 3 (8.33) | 0 |
| Bleeding | 0 | 0 |
The treatment landscape for AGC has evolved significantly over the past decade, with novel therapeutic approaches gaining traction[2]. Our study provides comprehensive insights into the efficacy and safety of anlotinib[14], a multi-targeted tyrosine kinase inhibitor, combined with nab-ptx in patients who had previously undergone second-line therapy or beyond[9]. The positive outcomes observed in this cohort offer important implications for the management of AGC.
The ORR of 44% is noteworthy, especially considering that these patients had already received multiple lines of therapy. The results indicated that the combination of anlotinib and nab-ptx yielded a significant clinical benefit, with a median PFS of 6.0 months and median OS of 12.0 months. These findings align with previous studies examining the efficacy of anlotinib in various malignancies, reinforcing its role as an effective agent in AGC treatment[7,13,15]. Our study also found that patients who experienced hypertension, proteinuria, and hand-foot syndrome during treatment had better efficacy, indicating that the identification of AEs plays an important role and holds value in distinguishing advantageous patient populations.
The mechanisms underlying the enhanced efficacy of this combination therapy can be attributed to the complementary action of anlotinib and nab-ptx[16,17]. Anlotinib targets multiple pathways involved in tumor angiogenesis and growth, including VEGFR, PDGFR, and FGFR[18], while nab-ptx facilitates improved delivery of paclitaxel to the tumor site through its unique formulation[8]. This dual approach not only enhances anti-tumor activity but also potentially mitigates some of the resistance mechanisms that commonly develop after several lines of chemotherapy[19-21]. Interestingly, our study found that the proportion of Treg cells in the peripheral blood of patients with AGC was ge
The safety profile observed in our study demonstrated that the combination therapy was generally well-tolerated. Common AEs associated with anlotinib include hypertension, proteinuria, and hand-foot syndrome. While serious AEs were reported in a small percentage of patients (16.67%), the absence of treatment-related deaths underscores the acceptability of this regimen in a heavily pretreated population[12]. The occurrence of grade 1-2 hematological toxicities such as leukopenia, neutropenia, anemia, and thrombocytopenia align with the known side effects of nab-ptx[11,22]. However, effective management strategies, including dose modifications and supportive care, contributed to maintaining patient quality of life and treatment adherence. The incidence of peripheral neuropathy, although noted in 20% of patients (primarily grade 1-2, with grade 3 or higher occurring in less than 3%), was manageable and did not necessitate discontinuation of therapy in the majority of cases. It is also important to highlight that the safety profile may vary based on individual patient characteristics. Factors such as age, comorbidities, and prior treatment history can influence tolerability. Consequently, careful patient selection and monitoring are paramount when implementing this combination therapy in clinical practice[3].
Our study has the following clinical significance: First, this is the first published research on the efficacy and safety of anlotinib combined with nab-ptx in second-line treatment for AGC, showing higher efficacy than previous control data[13,23]. In addition to the general population, the study also included a subset of patients with liver and peritoneal metastases, which broadens its clinical application[14,18]. Furthermore, subgroup analyses indicated that patients who experienced hypertension, proteinuria, and hand-foot syndrome during treatment had better outcomes, suggesting that the identification of AEs during treatment could serve as potential biomarkers for predicting efficacy[5,10]. This provides a basis for screening favorable populations when applying this treatment regimen clinically. In addition to its significant efficacy, our study also found that the mechanism of this regimen may involve reducing immunosuppression to activate the immune response, thereby exerting synergistic anti-tumor effects. This provides a direction and insights for future research. Aside from its considerable efficacy, this regimen also exhibits few and tolerable adverse reactions, which not only ensures treatment adherence but also further improves the quality of life for patients[24,25].
While our findings are encouraging, this study has inherent limitations characteristic of retrospective analyses, including potential selection bias and the inability to control for all confounding variables. Additionally, the relatively small sample size may limit the generalizability of the results. Future prospective, randomized controlled trials are necessary to validate our findings and to establish standard treatment protocols incorporating anlotinib and nab-ptx for AGC. Furthermore, the exploration of biomarkers predictive of response to this combination therapy could further refine patient selection, enabling a more personalized approach to treatment[6,26]. Understanding the pharmacogenomics of anlotinib and nab-ptx, as well as exploring combination strategies with other emerging therapies, may also enhance the overall efficacy and safety of AGC treatments[27,28].
This study presents compelling evidence supporting the use of anlotinib combined with nab-ptx as an effective and safe treatment strategy for patients with AGC who have received previous lines of therapy. As research in this area progresses, we anticipate that such combination therapies will play a pivotal role in improving patient outcomes in this challenging disease landscape.
We would like to thank Xichen Wang (MSD China, Shanghai, China) for providing academic information consulting support.
| 1. | López MJ, Carbajal J, Alfaro AL, Saravia LG, Zanabria D, Araujo JM, Quispe L, Zevallos A, Buleje JL, Cho CE, Sarmiento M, Pinto JA, Fajardo W. Characteristics of gastric cancer around the world. Crit Rev Oncol Hematol. 2023;181:103841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 154] [Reference Citation Analysis (3)] |
| 2. | Yang WJ, Zhao HP, Yu Y, Wang JH, Guo L, Liu JY, Pu J, Lv J. Updates on global epidemiology, risk and prognostic factors of gastric cancer. World J Gastroenterol. 2023;29:2452-2468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 181] [Article Influence: 90.5] [Reference Citation Analysis (12)] |
| 3. | Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, Vogel A, Smyth EC; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1005-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 654] [Article Influence: 218.0] [Reference Citation Analysis (0)] |
| 4. | Gao Y, Liu P, Shi R. Anlotinib as a molecular targeted therapy for tumors. Oncol Lett. 2020;20:1001-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 5. | Su Y, Luo B, Lu Y, Wang D, Yan J, Zheng J, Xiao J, Wang Y, Xue Z, Yin J, Chen P, Li L, Zhao Q. Anlotinib Induces a T Cell-Inflamed Tumor Microenvironment by Facilitating Vessel Normalization and Enhances the Efficacy of PD-1 Checkpoint Blockade in Neuroblastoma. Clin Cancer Res. 2022;28:793-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 83] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 6. | Yuan M, Guo XL, Chen JH, He Y, Liu ZQ, Zhang HP, Ren J, Xu Q. Anlotinib suppresses proliferation, migration, and immune escape of gastric cancer cells by activating the cGAS-STING/IFN-β pathway. Neoplasma. 2022;69:807-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 7. | Gangannapalle M, Shahnoor H, Sattar L, Nagi TK, Al-Tekreeti M, Khan MW, Haseeb MD, Khan A. Nanoparticle AlbuminBound Paclitaxel and Solvent-Based Paclitaxel as Chemotherapy Options for Patients With Advanced Gastric Cancer: A Systematic Review and Meta-Analysis. Cureus. 2023;15:e41711. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Roviello G, Conter FU, Mini E, Generali D, Traversini M, Lavacchi D, Nobili S, Sobhani N. Nanoparticle albumin-bound paclitaxel: a big nano for the treatment of gastric cancer. Cancer Chemother Pharmacol. 2019;84:669-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Ahmad S, Lambuk L, Ahmed N, Mussa A, Tee V, Mohd Idris RA, Sahran NF, Chan YY, Hassan R, Lee YY, Mohamud R. Efficacy and safety of nab-paclitaxel in metastatic gastric cancer: a meta-analysis. Nanomedicine (Lond). 2023;18:1733-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 10. | Li X, Wu D, Tang J, Wu Y. The Efficiency and Safety of Triple-Drug Combination of Albumin-Bound Paclitaxel, Anlotinib and PD-1/L1 Inhibitors in the 2(nd) or Above Line of Advanced NSCLC: A Retrospective Cohort Study. Cancer Manag Res. 2024;16:1003-1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 11. | Ismail U, Killeen RB. Taxane Toxicity. 2023 Mar 12. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 12. | Li S, Wang H. Research Progress on Mechanism and Management of Adverse Drug Reactions of Anlotinib. Drug Des Devel Ther. 2023;17:3429-3437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 13. | Nakasya A, Hagiwara Y, Ikoma T, Kurioka Y, Matsumoto T, Yamamoto Y, Tsuduki T, Kajiwara T, Moriwaki T, Nishina T, Yamashita N, Hyodo I. Nanoparticle albumin-bound paclitaxel and ramucirumab versus paclitaxel and ramucirumab as second-line chemotherapy for unresectable advanced or recurrent gastric cancer: a multicenter, propensity score-matched analysis (CROSS SELL study). Int J Clin Oncol. 2022;27:684-694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 14. | Nie C, He Y, Lv H, Gao M, Gao X, Chen B, Xu W, Wang J, Liu Y, Zhao J, Chen X. Clinical Study of Anlotinib as Third-Line or Above Therapy in Patients With Advanced or Metastatic Gastric Cancer: A Multicenter Retrospective Study. Front Oncol. 2022;12:885350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Jiang M, Zhang C, Hu Y, Li T, Yang G, Wang G, Zhu J, Shao C, Hou H, Zhou N, Liu K, Zhang X. Anlotinib Combined with Toripalimab as Second-Line Therapy for Advanced, Relapsed Gastric or Gastroesophageal Junction Carcinoma. Oncologist. 2022;27:e856-e869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 16. | Sha H, Tong F, Ni J, Sun Y, Zhu Y, Qi L, Li X, Li W, Yang Y, Gu Q, Zhang X, Wang X, Zhu C, Chen D, Liu B, Du J. First-line penpulimab (an anti-PD1 antibody) and anlotinib (an angiogenesis inhibitor) with nab-paclitaxel/gemcitabine (PAAG) in metastatic pancreatic cancer: a prospective, multicentre, biomolecular exploratory, phase II trial. Signal Transduct Target Ther. 2024;9:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Reference Citation Analysis (0)] |
| 17. | Liu H, Pan D, Yao Z, Wang H, Li Y, Qin X, Qu P, Tang J, Han Z. Efficacy and safety of gemcitabine/nab-paclitaxel combined with anlotinib and PD-1 inhibitors as a first-line treatment for advanced pancreatic cancer. Int Immunopharmacol. 2024;139:112635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Luo Q, Zhang S, Zhang D, Feng R, Li N, Chen W, Chen X, Yang S. Effects and Mechanisms of Anlotinib and Dihydroartemisinin Combination Therapy in Ameliorating Malignant Biological Behavior of Gastric Cancer Cells. Curr Pharm Biotechnol. 2021;22:523-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Gu W, Yang J, Wang Y, Xu J, Wang X, Du F, Hu X, Guo H, Song C, Tao R, Zhang X. Comprehensive identification of FGFR1-4 alterations in 5 557 Chinese patients with solid tumors by next-generation sequencing. Am J Cancer Res. 2021;11:3893-3906. [PubMed] |
| 20. | Jin Z, Lu Y, Wu X, Pan T, Yu Z, Hou J, Wu A, Li J, Yang Z, Li C, Yan M, Yan C, Zhu Z, Liu B, Qiu W, Su L. The cross-talk between tumor cells and activated fibroblasts mediated by lactate/BDNF/TrkB signaling promotes acquired resistance to anlotinib in human gastric cancer. Redox Biol. 2021;46:102076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 21. | Glorieux C, Xia X, You X, Wang Z, Han Y, Yang J, Noppe G, Meester C, Ling J, Robert A, Zhang H, Li SP, Wang H, Chiao PJ, Zhang L, Li X, Huang P. Cisplatin and gemcitabine exert opposite effects on immunotherapy with PD-1 antibody in K-ras-driven cancer. J Adv Res. 2022;40:109-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 22. | Hagiwara Y, Nakasya A, Matsumoto T, Ikoma T, Yamamoto Y, Kurioka Y, Tsuduki T, Kajiwara T, Nishina T, Yamashita N, Moriwaki T, Hyodo I. Risk factors and efficacy outcomes of early-onset severe neutropenia due to paclitaxel or nanoparticle albumin-bound paclitaxel combined with ramucirumab in advanced gastric cancer: a multicenter retrospective cohort study. J Gastrointest Oncol. 2022;13:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 23. | Zheng W, Sun G, Li Z, Wu F, Sun G, Cao H, Zhou J, Ma Y. The Effect of Anlotinib Combined with anti-PD-1 in the Treatment of Gastric Cancer. Front Surg. 2022;9:895982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 24. | Si X, Zhang L, Wang H, Zhang X, Wang M, Han B, Li K, Wang Q, Shi J, Wang Z, Cheng Y, He J, Shi Y, Chen W, Wang X, Luo Y, Nan K, Jin F, Li B, Chen Y, Zhou J, Wang D. Quality of life results from a randomized, double-blinded, placebo-controlled, multi-center phase III trial of anlotinib in patients with advanced non-small cell lung cancer. Lung Cancer. 2018;122:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Zhang D, Zhang Z, Luo J, Zheng J, Mao X, Tsilimigras DI, Chun HJ, Zeng H. Efficacy and safety of transarterial chemoembolization alone compared to its combination with anlotinib among patients with intermediate or advanced stage hepatocellular carcinoma: a phase II randomized controlled trial. J Gastrointest Oncol. 2024;15:1627-1635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 26. | Zhu M, Zhang P, Yu S, Tang C, Wang Y, Shen Z, Chen W, Liu T, Cui Y. Targeting ZFP64/GAL-1 axis promotes therapeutic effect of nab-paclitaxel and reverses immunosuppressive microenvironment in gastric cancer. J Exp Clin Cancer Res. 2022;41:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 27. | Wang J, Wu DX, Meng L, Ji G. Anlotinib combined with SOX regimen (S1 (tegafur, gimeracil and oteracil porassium capsules) + oxaliplatin) in treating stage IV gastric cancer: study protocol for a single-armed and single-centred clinical trial. BMJ Open. 2020;10:e034685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Ma J, Xiao M, Li X, Zhao Q, Ji W, Ling Y, Yang Q. Analysis of the efficacy and safety of paclitaxel (albumin-bound) combined with S-1 and oxaliplatin combined with S-1 in the first-line treatment of advanced gastric cancer: a cohort study. J Gastrointest Oncol. 2022;13:630-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |