Published online Apr 24, 2025. doi: 10.5306/wjco.v16.i4.100957
Revised: January 13, 2025
Accepted: March 8, 2025
Published online: April 24, 2025
Processing time: 205 Days and 18.3 Hours
Splenic artery aneurysm (SAA) rupture is a rare, life-threatening condition characterized by acute intra-abdominal hemorrhage and hemodynamic instability. Ruptured SAAs may exhibit a biphasic and relatively slow clinical progression, commonly referred to as the “double-rupture phenomenon”. The reported in
A 61-year-old female presented to the Department of Emergency with upper abdominal pain, abdominal distension, dizziness, and vomiting. Her vital signs were initially stable. Physical examination revealed abdominal tenderness and positive-shifting dullness. Abdominal contrast-enhanced computed tomography revealed cirrhosis, severe portal hypertension, and splenomegaly. Acute rupture was suggested by a hematoma on the upper left side outside the SAA. Surgeons deemed intravascular intervention challenging and open splenectomy inevitable. Circulatory collapse occurred after anesthesia induction, likely due to a double rupture of the SAA. This double-rupture phenomenon may have resulted from an initial rupture of the SAA into the omental bursa, forming a hematoma that exerted a tamponade effect. A second rupture into the peritoneal cavity may have been triggered by decreased intra-abdominal pressure following anesthesia in
Anesthesia-induced pressure reduction may trigger a second SAA rupture, causing collapse. Early diagnosis and multidisciplinary teamwork improve outcomes. This is a rare and life-threatening case of SAA rupture, which is of great significance to the medical community for understanding and handling such emergencies.
Core Tip: Ruptured splenic artery aneurysm (SAA) exhibits a biphasic and relatively slow clinical progression, referred to as a “double-rupture phenomenon”. Anesthesia induction can decrease the intra-abdominal pressure and lead to a second rupture of the SAA, resulting in circulatory collapse and life-threatening conditions. This report presents a rare case of SAA with a double-rupture phenomenon and circulatory collapse following anesthesia induction. The patient was successfully discharged after cardiopulmonary resuscitation and emergency splenectomy. Managing patients with ruptured SAA poses significant challenges. Early diagnosis and timely multidisciplinary team coordination and collaboration are critical for saving lives in such cases.
- Citation: Xu GY, Gong YH, Wang Y, Han XL, Hao C, Xu L. Splenic artery aneurysm with double-rupture phenomenon and circulatory collapse following anesthesia induction: A case report. World J Clin Oncol 2025; 16(4): 100957
- URL: https://www.wjgnet.com/2218-4333/full/v16/i4/100957.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i4.100957
Rupture of a splenic artery aneurysm (SAA) is an uncommon but potentially fatal condition, typically occurring in patients with vasculitis or portal hypertension. The mortality rate among patients with ruptured SAAs and portal hypertension is as high as 65%-75%[1,2]. In cases of ruptured SAA, delayed intraperitoneal bleeding may occur 6-96 hours later, with blood initially contained within the lesser omental sac[3-5]. Ruptured SAAs probably exhibit a biphasic and relatively slow clinical progression referred to as the “double-rupture phenomenon”[6]. The reported incidence of this phenomenon ranges 12%-21% among patients with ruptured SAAs, possibly due to variations in intra-abdominal pressure[6,7]. Following anesthesia induction, muscle relaxation can decrease intra-abdominal pressure in patients with ruptured SAAs, potentially triggering a double-rupture phenomenon and resulting in hemodynamic instability. Ma
A 61-year-old female was admitted to the Department of Emergency of our hospital with upper abdominal pain and distension, accompanied by dizziness and vomiting.
Upon admission, the patient exhibited the following normal vital signs: Her blood pressure (BP) was 104/70 mmHg and her heart rate was 103 bpm. Nonclotting blood was aspirated from the abdomen, raising suspicion of intra-abdominal hemorrhage.
The patient had been diagnosed with liver cirrhosis 6 months earlier and was currently in the decompensated stage. Additionally, she had renal insufficiency.
Both her personal and familial medical history were free of significant findings.
Physical examination revealed tenderness on abdominal palpation and positive shifting dullness.
Laboratory tests demonstrated a hemoglobin level of 54 g/L, prothrombin time of 16.5 seconds, activated partial thromboplastin time of 30.1 seconds, D-Dimer of 11.49 mg/L, and fibrinogen 1.07 g/L.
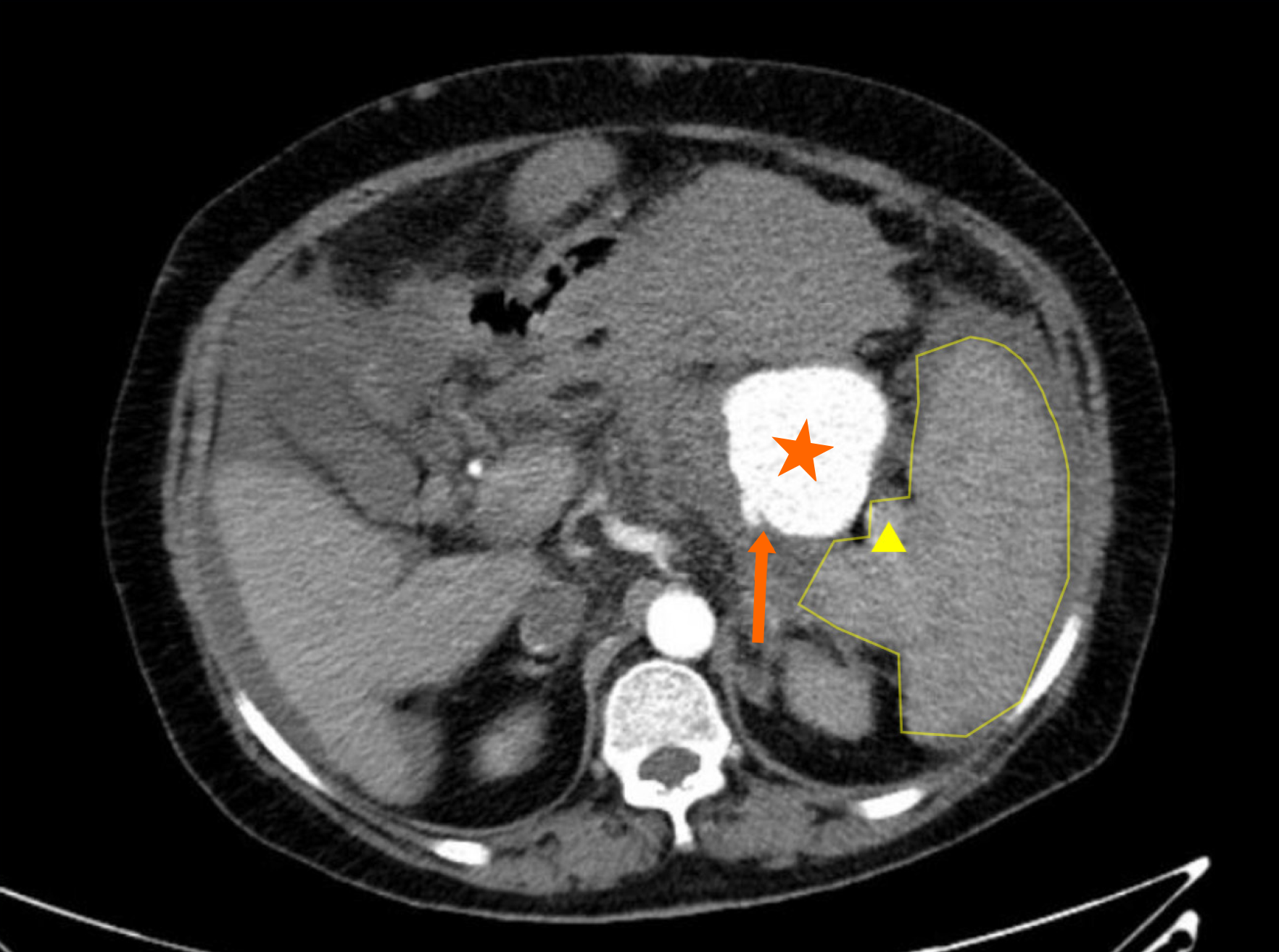
Abdominal contrast-enhanced computed tomography (CT) revealed cirrhosis, and severe portal hypertension with splenomegaly, esophageal varices, and extensive portosystemic collaterals. Multiple filling defects were detected in the portal, splenic, and superior mesenteric veins, leading to a diagnosis of venous thrombosis. Several tumor-like expansions were observed in the distal and middle segments of the splenic artery. The largest tumor, which had a rough border, measured 53 mm × 53 mm, and acute rupture was suggested by a hematoma located on the upper left side outside the SAA (Figure 1).
Rupture and hemorrhage of SAA.
In this case, contrast-enhanced abdominal CT revealed cirrhosis, severe portal hypertension, and splenomegaly. The large hematoma located on the upper left side outside the SAA suggested that an acute rupture had occurred. Surgeons and interventional radiologists determined that intravascular intervention would be difficult due to the large, multiple SAAs being too close to the splenic hilum. Open surgical options include splenectomy with excision of the aneurysm, distal and proximal splenic artery ligation with or without aneurysm resection, and transaneurysmal arterial ligation, with the specific approach determined based on the patient’s clinical profile. Given the patient’s preoperative condition, we opted for surgical intervention, as splenectomy was deemed inevitable.
Emergency exploratory laparotomy was performed while the patient received blood transfusion and fluid re
During CPR, central venous access was urgently established, and the patient’s BP was maintained at 60-70/25-30 mmHg using intravenous norepinephrine at 2-3 μg/kg per minute. Four pressurized venous access points were used for blood transfusion and fluid resuscitation. A second rupture of the SAAs was suspected, prompting emergency laparotomy.
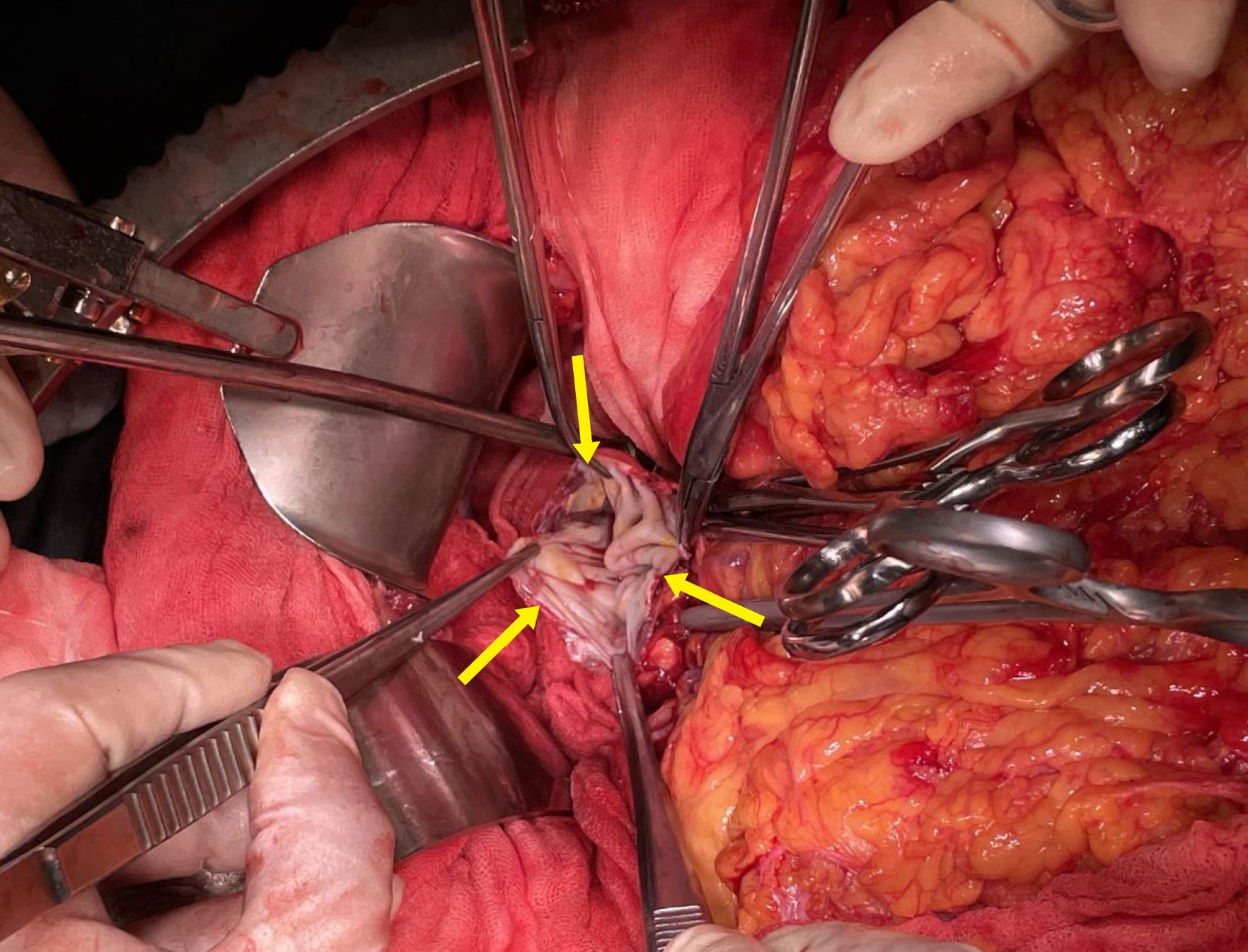
Massive amounts of nonclotting blood and blood clots were removed, and a second set of cell savers was immediately assembled. 25 minutes after CPR was initiated, surgeons identified a ruptured SAA, measuring 6 cm in diameter, with a 4 cm laceration (Figure 2). Upon ligation of the SAA, the patient’s BP immediately improved to 98/45 mmHg. CPR was discontinued, and the norepinephrine infusion was quickly reduced to 0.8 μg/kg per minute. Stable circulation was restored, and BP was maintained at 110-120/50-60 mmHg with ongoing fluid and blood transfusion. Norepinephrine was gradually discontinued. The surgery lasted for 2.5 hours, during which the spleen was resected. The total estimated blood loss was 6500 mL. The patient received 3300 mL of crystalloids, 2000 mL of colloids, 8 units of allogeneic red blood cells, and 1367 mL of recovered autologous red blood cells. Plasma (1000 mL), 1 unit of platelets, 2 g of fibrinogen, and 400 IU of prothrombin complex were administered to correct coagulopathy. Fluids and blood were heated using fluid warmers during the massive transfusion. The patient’s temperature was continuously monitored and maintained between 35.3 °C and 35.8 °C throughout the operation. No forced air-warming devices were used for rewarming. After the procedure, the patient was transferred to the intensive care unit for further management.
The patient recovered without any significant postoperative bleeding. Both pupils were equal in size, with a diameter of approximately 3.0 mm. On postoperative day 2, increased muscle tone was suspected in the upper limbs; however, head CT imaging revealed no clear abnormal high- or low-density shadows. Considering the possibility of hypoxic encephalopathy, sedation was continued and body temperature was maintained below 37 °C. On postoperative day 4, sedation was discontinued, and the patient regained consciousness. No neurological abnormalities were detected during physical examination.
SAA rupture is a rare but life-threatening condition, typically occurring in pregnant women or patients with vasculitis or portal hypertension, resulting in hemodynamic instability[11,12]. Ruptured SAAs may exhibit a biphasic and relatively slow clinical progression, known as the “double-rupture phenomenon”[5,6]. This phenomenon occurs when an initial rupture of the SAA into the omental bursa forms a hematoma that exerts a tamponade effect on bleeding. A second rupture in the peritoneal cavity may occur 6-96 hours later, likely triggered by changes in intra-abdominal pressure[5,13]. Treatment options for ruptured SAAs include open surgical approaches, laparoscopic aneurysmectomy with or without splenectomy, and endovascular interventions. Open surgical procedures may involve splenectomy, proximal and distal splenic artery ligation with or without aneurysm resection, and transaneurysmal arterial ligation[12]. Open surgery remains the gold standard for patients with a large SAA or significant hemodynamic instability. However, the open surgical approach is very challenging, with a perioperative mortality rate of approximately 25%-36%[10]. In patients with portal hypertension, the mortality rate can reach as high as 65%-75%[1,2]. Preoperative embolization of the splenic artery is essential for successful resection of a giant SAA, as it shortens the duration of surgery and reduces intraoperative blood loss[14].
In this case, the patient presented with a ruptured SAA due to portal hypertension before admission, manifesting as severe anemia and dizziness. Contrast-enhanced CT confirmed the diagnosis. Surgeons and interventional radiologists considered splenic artery embolization unfeasible due to the large size, numerous SAAs, and their proximity to the splenic hilum. Consequently, an emergency open splenectomy was performed. The most significant challenge during the procedure was refractory hypotension following anesthesia induction. This hypotension was exacerbated by muscle relaxation and decreased intra-abdominal pressure, which likely triggered a second rupture of the SAA. Additionally, general anesthesia can suppress the sympathetic nervous system, reduce vascular tone, and aggravate refractory hypotension. A meta-analysis revealed that the perioperative mortality rate for endovascular repair of abdominal aortic aneurysms under local anesthesia is significantly lower than that for open surgery under general anesthesia[7,15]. Furthermore, Chen et al[16] found that in a cohort of patients who had already developed hypotension before surgery, the odds ratio for general anesthesia compared to local anesthesia was 4.64, significantly higher than across the entire cohort (2.08). We propose that open splenectomy under general anesthesia may have two potential adverse effects on patients. First, anesthesia induction and abdominal decompression may cause re-rupture of the SAA and refractory hypotension. Second, the surgery itself may induce traumatic stress. Therefore, exploratory laparotomy may pose an extremely high risk of mortality in patients with preoperative decompensated hemorrhagic shock.
With advances in interventional techniques, endovascular intervention of SAA has demonstrated a success rate of 85%-92%[17]. Additionally, postoperative complications and mortality rates associated with endovascular treatment are lower than those associated with open resection[18,19]. Recent guidelines recommend endovascular intervention as the standard treatment for SAA[19,20]. Furthermore, some experts suggest that even in hemodynamically unstable patients, ruptured SAA could be managed using vascular embolization, stents, or balloon tamponade to achieve hemostasis[21-23]. However, exploratory laparotomy remains necessary in certain cases[24,25].
The patient in this case suffered from portal hypertension, which led to thrombosis in the portal and splenic veins, resulting in extremely high pressure within the splenic circulation. Although the preoperative hemodynamics appeared stable, the patient was actually in a precarious compensated state. During anesthesia induction and laparotomy, this fragile compensation could be disrupted at any moment, leading to circulatory collapse and life-threatening conditions. Although endovascular intervention for large ruptured SAAs is challenging, and the reoperation rate is high, performing endovascular techniques under local anesthesia before laparotomy can help control major active bleeding and provide a clearer surgical field. Additionally, the presence of coils within the aneurysm enables surgeons to locate the SAA more quickly and accurately. However, in patients with unstable hemodynamics, there is a potential risk of delayed surgical intervention if endovascular techniques fail, highlighting the importance of experienced surgeons with sound decision-making abilities.
For patients whose circulation has already collapsed, emergency laparotomy must be performed to ligate the ruptured SAA. Every effort must be made to maintain BP and buy time for surgery. When the surgeons opened the abdomen, CPR was performed, and four venous access points were established for rapid fluid resuscitation. A central venous line was inserted for vasopressor infusion, and an extremely high dose of noradrenaline was administered to maintain BP. Throughout the procedure, five anesthesiologists and five circulating nurses were called upon. One anesthesiologist led the entire rescue operation and managed the adjustment of vasoactive drugs. Another anesthesiologist performed internal jugular vein catheterization, while one anesthesiologist monitored two cell savers. Another was responsible for pressurized blood transfusion and fluid infusion, and the final anesthesiologist handled anesthesia documentation and regular blood gas monitoring. The circulating nurses collaborated with the surgeons to establish peripheral venous access, prepare vasoactive drugs, and initiate rapid blood transfusion. One nurse focused on brain protection using ice packs and ice cap, while two others assisted the surgeons with chest compressions. Thanks to regular crisis resource management training in high-fidelity simulation centers, the medical team performed their respective duties methodically, demonstrating seamless coordination during the procedure.
Before the ruptured SAA was ligated, BP could not be maintained despite continued CPR and the administration of an extremely large dose of vasopressors. However, systolic BP immediately rose to over 100 mmHg upon ligation of the SAA. The dose of norepinephrine was promptly reduced to maintain a satisfactory BP. This observation suggests that vasoactive drugs can be significantly lost due to rapid arterial bleeding. Therefore, in cases of major bleeding, the potential loss of vasoactive drugs should be considered.
In terms of body temperature management, significant blood loss and rapid fluid resuscitation can cause severe hypothermia, which further worsens coagulopathy. However, while severe hypotension may result in ischemic-hypoxic encephalopathy, for which targeted hypothermia could be beneficial, a recent study demonstrated that targeted hypothermia did not reduce the incidence of death at 6 months compared to targeted normothermia in comatose patients following cardiac arrest. Consequently, while localized head cooling was applied to prevent cerebral injury, rewarming methods were implemented to prevent severe hypothermia (body temperature below 35 °C). Coagulopathy was ma
Nevertheless, this study has certain limitations. As an individual case report, the findings may not be broadly generalizable. However, it presents a rare and life-threatening case of SAA re-rupture, which is of considerable significance to the medical community for improving the understanding and management of such emergencies. Overall, this study provides a detailed report emphasizing the critical importance of rapid diagnosis, prompt intervention, timely decision-making, and effective multidisciplinary team collaboration in managing SAA ruptures, making it a valuable learning resource for medical professionals.
A ruptured SAA is a rare but potentially fatal condition, typically occurring in patients with vasculitis or portal hy
| 1. | Jackson HT, Diaconu SC, Maluso PJ, Abell B, Lee J. Ruptured splenic artery aneurysms and the use of an adapted fast protocol in reproductive age women with hemodynamic collapse: case series. Case Rep Emerg Med. 2014;2014:454923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Moon DB, Lee SG, Hwang S, Kim KH, Ahn CS, Ha TY, Song GW, Jung DH, Ko GY, Sung KB. Characteristics and management of splenic artery aneurysms in adult living donor liver transplant recipients. Liver Transpl. 2009;15:1535-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Davis T, Minardi J, Knight J, Larrabee H, Schaefer G. Ruptured Splenic Artery Aneurysm: Rare Cause of Shock Diagnosed with Bedside Ultrasound. West J Emerg Med. 2015;16:762-765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Al-Habbal Y, Christophi C, Muralidharan V. Aneurysms of the splenic artery - a review. Surgeon. 2010;8:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Kim JH, Chung HS, Kim JH, Park SY, Lee SB, Do BS. Splenic artery aneurysm with the double-rupture phenomenon. Clin Exp Emerg Med. 2017;4:113-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Stanley JC, Thompson NW, Fry WJ. Splanchnic artery aneurysms. Arch Surg. 1970;101:689-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 237] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Deng J, Liu J, Rong D, Ge Y, Zhang H, Liu X, Guo W. A meta-analysis of locoregional anesthesia versus general anesthesia in endovascular repair of ruptured abdominal aortic aneurysm. J Vasc Surg. 2021;73:700-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Lin JL, Lin C, Wang HL, Wu SJ, Tang Y, Yang CS, Luo JW, Chi W, Fang ZT. Splenic Artery Embolization and Splenectomy for Spontaneous Rupture of Splenic Hemangioma and Its Imaging Features. Front Cardiovasc Med. 2022;9:925711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 9. | Lim HJ. A review of management options for splenic artery aneurysms and pseudoaneurysms. Ann Med Surg (Lond). 2020;59:48-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Akbulut S, Otan E. Management of Giant Splenic Artery Aneurysm: Comprehensive Literature Review. Medicine (Baltimore). 2015;94:e1016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Luo R, Gao J, Gan W, Xie WB. Clinical-radiomics nomogram for predicting esophagogastric variceal bleeding risk noninvasively in patients with cirrhosis. World J Gastroenterol. 2023;29:1076-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (5)] |
| 12. | Yuan F, He L, Yao Z, Long Y, Xu S. Spontaneous rupturing of splenic artery aneurysm: Another reason for fatal syncope and shock (Case report and literature review). Open Med (Wars). 2022;17:601-605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 13. | Kohara Y, Fujimoto K, Katsura H, Komatsubara T, Ichikawa K, Higashiyama H. Biphasic clinical course of a ruptured right gastric artery aneurysm caused by segmental arterial mediolysis: a case report. BMC Surg. 2020;20:191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Huang L, Yu Q, Peng H, Zhen Z. Postoperative Outcomes Following a Modified Method of Surgical Division of the Splenic Pedicle in 719 Patients During Splenectomy for Portal Hypertension: A 12-Year, Retrospective, Single-Center Study. Med Sci Monit. 2022;28:e937763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 15. | Kontopodis N, Galanakis N, Antoniou SA, Tsetis D, Ioannou CV, Veith FJ, Powell JT, Antoniou GA. Meta-Analysis and Meta-Regression Analysis of Outcomes of Endovascular and Open Repair for Ruptured Abdominal Aortic Aneurysm. Eur J Vasc Endovasc Surg. 2020;59:399-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Chen SL, Kabutey NK, Whealon MD, Kuo IJ, Donayre CE, Fujitani RM. Locoregional Anesthesia Offers Improved Outcomes after Endovascular Repair of Ruptured Abdominal Aortic Aneurysms. Ann Vasc Surg. 2019;59:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Jesinger RA, Thoreson AA, Lamba R. Abdominal and pelvic aneurysms and pseudoaneurysms: imaging review with clinical, radiologic, and treatment correlation. Radiographics. 2013;33:E71-E96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Hogendoorn W, Lavida A, Hunink MG, Moll FL, Geroulakos G, Muhs BE, Sumpio BE. Open repair, endovascular repair, and conservative management of true splenic artery aneurysms. J Vasc Surg. 2014;60:1667-76.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Lakin RO, Bena JF, Sarac TP, Shah S, Krajewski LP, Srivastava SD, Clair DG, Kashyap VS. The contemporary management of splenic artery aneurysms. J Vasc Surg. 2011;53:958-64; discussion 965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Mariúba JVO. Splenic aneurysms: natural history and treatment techniques. J Vasc Bras. 2019;19:e20190058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Huang YK, Hsieh HC, Tsai FC, Chang SH, Lu MS, Ko PJ. Visceral artery aneurysm: risk factor analysis and therapeutic opinion. Eur J Vasc Endovasc Surg. 2007;33:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Lee SH, Yang S, Park I, Im YC, Kim GY. Ruptured splenic artery aneurysms in pregnancy and usefulness of endovascular treatment in selective patients: A case report and review of literature. World J Clin Cases. 2022;10:9057-9063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (1)] |
| 23. | Kalipatnapu S, Kota AA, Agarwal S. Giant splenic artery aneurysm. J Vasc Surg. 2019;69:1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Ologun G, Sharpton K, Granet P. Successful use of resuscitative endovascular balloon occlusion of the aorta in the treatment of ruptured 8.5-cm splenic artery aneurysm. J Vasc Surg. 2017;66:1873-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Wernheden E, Brenøe AS, Shahidi S. Emergency endovascular coiling of a ruptured giant splenic artery aneurysm. J Vasc Surg Cases Innov Tech. 2017;3:240-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Sandroni C, Natalini D, Nolan JP. Temperature control after cardiac arrest. Crit Care. 2022;26:361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |