Published online Apr 24, 2025. doi: 10.5306/wjco.v16.i4.100729
Revised: November 24, 2024
Accepted: January 2, 2025
Published online: April 24, 2025
Processing time: 213 Days and 20.3 Hours
Hepatocellular carcinoma (HCC) ranks as the sixth most common cancer and the third- leading cause of cancer-related deaths worldwide. The multidisciplinary tumor board (MDTB) has been recognized for improving outcomes in cancer management, but its role in patients with HCC undergoing liver transplantation (LT) remains underexplored.
To evaluate the impact of an MDTB on survival outcomes in patients with HCC undergoing LT.
We retrospectively analyzed 393 patients with HCC who underwent LT at our institution from October 2015 to October 2021. Patients were categorized into the MDTB and non-MDTB groups. We compared preoperative and postoperative characteristics, overall survival (OS), and disease-free survival (DFS) between the two groups.
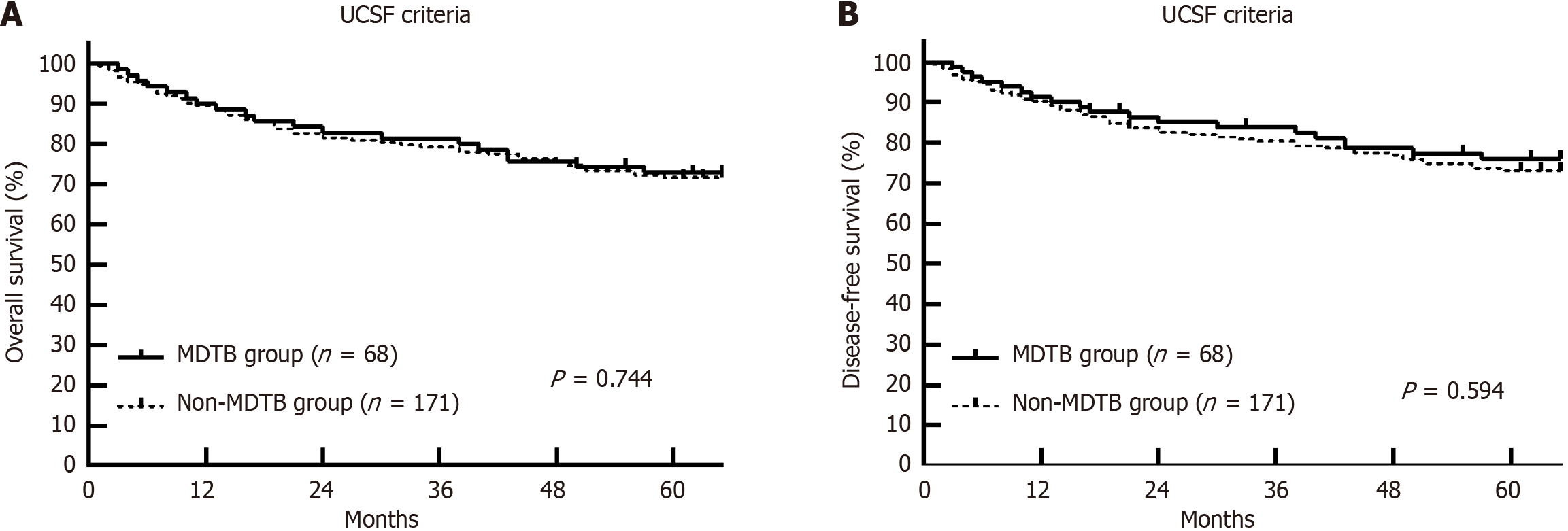
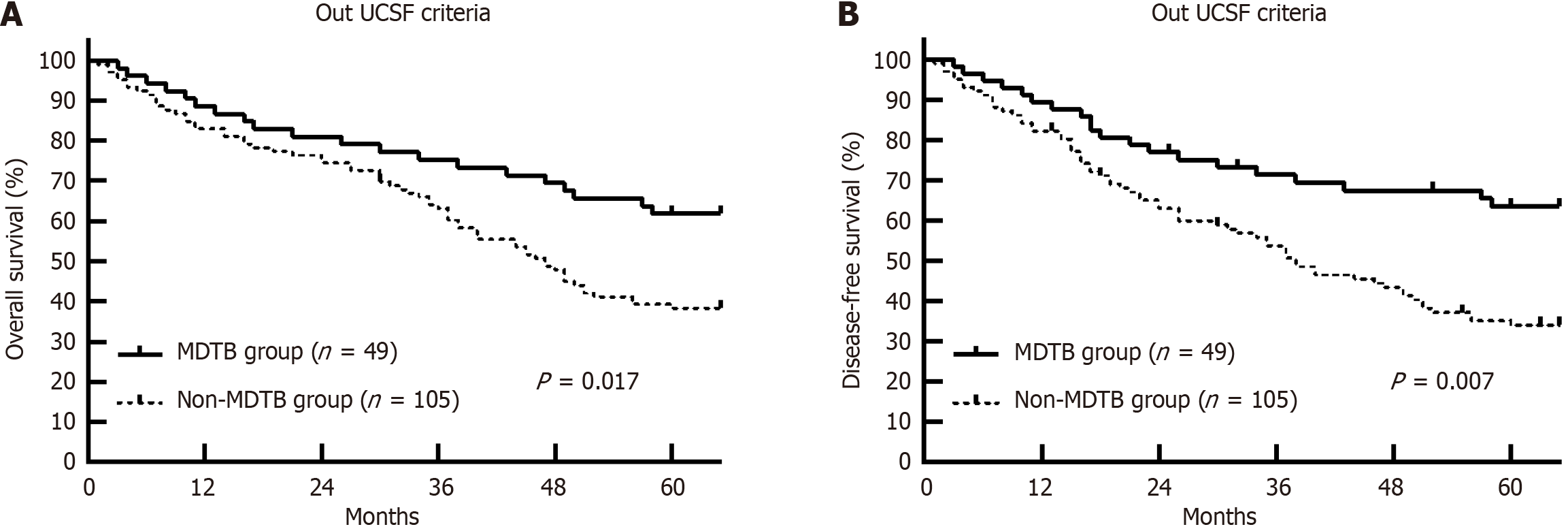
Within the University of California, San Francisco (UCSF) criteria, no significant differences in OS and DFS were noted between the MDTB and non-MDTB groups. However, for patients who exceeded the UCSF criteria, the MDTB group ex
The MDTB approach was particularly beneficial for patients with HCC exceeding the UCSF criteria, significantly improving OS and DFS. These findings advocate for integrating MDTB into clinical practice for optimizing the management of high-risk patients with HCC undergoing LT.
Core Tip: This study evaluated the impact of a multidisciplinary tumor board (MDTB) on the survival outcomes of patients with hepatocellular carcinoma undergoing liver transplantation. While MDTB and non-MDTB groups showed similar outcomes within the University of California, San Francisco criteria, MDTB involvement significantly improved overall survival and disease-free survival in patients exceeding University of California, San Francisco criteria. By integrating expertise from various specialties, MDTB enhanced bridging therapies, perioperative management, and individualized treatment strategies, advocating for its adoption to optimize outcomes for high-risk patients with hepatocellular carcinoma undergoing liver transplantation.
- Citation: Zhang L, Yang J, Li JJ, Chen CY, Wang XD, Xie Y, Jiang WT. Multidisciplinary tumor board is associated with improved survival in patients with hepatocellular carcinoma after liver transplantation. World J Clin Oncol 2025; 16(4): 100729
- URL: https://www.wjgnet.com/2218-4333/full/v16/i4/100729.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i4.100729
Hepatocellular carcinoma (HCC) ranks as the sixth most common cancer and the third-leading cause of cancer-related deaths worldwide, with mortality rates rising in many regions[1]. The majority of patients with HCC present with severe cirrhosis or liver dysfunction, complicating their management[2]. Liver transplantation (LT) is widely regarded as the optimal treatment for patients with cirrhosis and small HCC or for those with unresectable HCC and partial liver dysfunction[3]. However, LT for HCC faces challenges such as organ shortages, leading to prolonged waiting times, tumor progression, and missed transplant opportunities[4]. HCC presents a unique clinical challenge due to its complex nature characterized by diverse etiologies, atypical clinical presentations, aggressive malignancy, and chronic liver damage. These factors frequently render traditional, single-specialty therapeutic approaches inadequate[5]. To address these challenges, the multidisciplinary tumor board (MDTB) approach has emerged as a critical strategy in managing patients with HCC undergoing LT[6].
MDTB involves a team of multidisciplinary experts, including hepatologists, transplant surgeons, radiologists, onco
We retrospectively analyzed 393 patients who underwent orthotopic LT for HCC at our institution between October 2015 and October 2021. The patients were categorized into two groups: 117 patients (29.8%) received LT with MDTB involvement (MDTB group), and 276 patients (70.2%) underwent LT without MDTB participation (non-MDTB group). Inclusion criteria for both groups included: (1) Indication for LT; (2) Age ≥ 18 years; (3) Primary diagnosis of HCC without extrahepatic or distant metastasis; (4) A Model for End-Stage Liver Disease score < 25 with a stable clinical condition; and (5) Diagnosis based on imaging criteria [dynamic CT/magnetic resonance imaging (MRI)] and/or histopathological confirmation. All grafts used for LT were obtained from deceased donors. No organs from executed prisoners were used. All deceased donations complied with the China Organ Donation Program. This study was app
Preoperative and postoperative characteristics, including demographics, tumor characteristics, and donor information, were collected and compared between the MDTB and non-MDTB groups. Imaging data (CT and MRI) of the chest, abdomen, and pelvis were analyzed to assess the number, size, and metastatic potential of hepatic tumors. Preoperative assessments were validated against postoperative pathological findings. Follow-up continued until the last census date on July 31, 2023.
HCC was diagnosed based on dynamic imaging criteria and/or histopathological findings. Specifically, imaging modalities, such as dynamic contrast-enhanced CT or MRI, were used to identify arterial phase hyperenhancement and venous phase washout, characteristic features of HCC. Histopathological confirmation was obtained when imaging findings were inconclusive or required further validation.
Initiated in March 2018, MDTB meetings were held weekly and comprised hepatologists, transplant surgeons, diagnostic radiologists, sonographers, interventional radiologists, medical oncologists, pathologists, and coordinators. Cases were presented by the treating physician, with imaging reviewed by radiologists, followed by open discussions. For patients exceeding the UCSF criteria, MDTB recommendations often included bridging treatments such as transhepatic arterial chemoembolization (TACE) or radiofrequency ablation based on tumor characteristics and expected wait times for transplantation. Controversial cases were revisited in subsequent MDTB meetings. The MDTB guidelines considered for the effective management of HCC included international recommendations such as the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases guidelines.
Preoperative and postoperative characteristics were compared between the MDTB and non-MDTB groups using χ2 tests or Fisher’s exact tests for categorical variables and t tests for continuous variables. OS and DFS were analyzed using Kaplan-Meier curves, with comparisons made using the log-rank test. Subgroup analyses were conducted for patients within and exceeding the UCSF criteria. A P value < 0.05 was considered statistically significant.
Table 1 and Table 2 summarize the demographic and clinical characteristics of the study population. Most patients in both groups had hepatitis B cirrhosis as the underlying liver disease, with similar Model for End-Stage Liver Disease scores and waiting times for LT. The UCSF criteria were met by 58.1% of patients in the MDTB group and 61.9% in the non-MDTB group (P = 0.4989). A higher percentage of patients in the MDTB group received bridging therapy (74.4%) compared to the non-MDTB group (50.4%, P = 0.021), with a significant difference in the use of TACE (55.6% vs 31.5%, P < 0.001). Additionally, 12% of patients had multifocal disease, and 8% had extrahepatic disease. No significant differences were observed in the remaining preoperative characteristics between the MDTB and non-MDTB groups.
| Characteristic | MDTB group (n = 117) | non-MDTB group (n = 276) | P value |
| Gender, male/female | 73/44 | 189/87 | 0.651 |
| Age, years | 52.7 ± 17.1 | 49.2 ± 15.8 | 0.219 |
| Indication for transplantation | |||
| Hepatitis B cirrhosis | 84 (71.8) | 208 (75.4) | 0.662 |
| Alcoholic cirrhosis | 18 (15.4) | 39 (14.1) | 0.772 |
| Other | 15 (12.8) | 29 (10.5) | > 0.990 |
| MELD score at transplantation | 17.1 ± 4.7 | 16.5 ± 4.9 | 0.215 |
| PreOP biology | |||
| Total bilirubin, μmol/L | 79.5 ± 76.9 | 74.7 ± 68.2 | 0.841 |
| Serum creatinine, μmol/L | 86.1 ± 72.6 | 82.6 ± 66.3 | 0.377 |
| INR | 1.4 ± 0.6 | 1.5 ± 0.6 | 0.447 |
| Plasma ammonia, μmol/L | 39.7 ± 21.5 | 42.6 ± 23.4 | 0.669 |
| AFP, ng/mL1 | 23.4 (7-194) | 30.1 (7-388) | 0.165 |
| PIVKA-II, mAU/mL1 | 39.5 (19-372) | 47.3 (21-463) | 0.312 |
| Waiting time for LT | |||
| From HCC diagnosis, months | 3.3 ± 2.6 | 2.9 ± 2.5 | 0.153 |
| From listing, days | 47.8 ± 51.4 | 44.7 ± 48.8 | 0.571 |
| Radiological characteristics | |||
| Number of nodules | 2.4 ± 1.3 | 2.3 ± 1.3 | 0.486 |
| Size of the largest nodule, mm | 47.9 ± 18.3 | 45.6 ± 17.9 | 0.248 |
| Multifocal tumors | 41 (35) | 88 (31.9) | 0.652 |
| UCSF criteria | 0.499 | ||
| Within UCSF criteria | 68 (58.1) | 171 (61.9) | |
| Exceeding UCSF criteria | 49 (41.9) | 105 (38.1) | |
| Bridging therapy | 87 (74.4) | 139 (50.4) | 0.021 |
| TACE | 65 (55.6) | 86 (31.5) | < 0.001 |
| RFA | 11 (9.4) | 28 (10.1) | > 0.990 |
| SBRT | 2 (1.7) | 3 (1.1) | 0.637 |
| Resection | 4 (3.4) | 8 (2.9) | 0.756 |
| Other | 5 (4.3) | 14 (5.1) | > 0.990 |
| Pathological characteristics | |||
| Poorly (Edmonson grade III/IV) | 56 (47.8) | 129 (46.7) | 0.912 |
| (Micro-) vascular invasion | 37 (31.6) | 82 (29.7) | 0.719 |
| Capsular invasion | 33 (28.2) | 77 (27.9) | > 0.990 |
| Characteristics | MDTB group (n = 117) | non-MDTB group (n = 276) | P value |
| Gender, male/female | 84/33 | 179/97 | > 0.990 |
| Age, years | 41.5 ± 16.9 | 43.3 ± 17.2 | 0.665 |
| Cause of death | |||
| Cerebral trauma | 65 (55.6) | 148 (53.6) | > 0.990 |
| Cerebrovascular accident | 27 (23.1) | 53 (19.2) | 0.792 |
| Brain tumor (glioma) | 11 (9.4) | 33 (11.9) | 0.735 |
| Hypoxic brain injury | 5 (4.3) | 20 (7.2) | 0.672 |
| Other | 9 (7.7) | 22 (7.9) | > 0.990 |
| ICU stay, days | 2.85 ± 2.05 | 2.93 ± 2.24 | 0.798 |
Table 3 presents the postoperative outcomes. The incidence of grade III/IV complications did not differ significantly between the MDTB group (20.5%) and the non-MDTB group (25.7%, P = 0.304). Postoperative mortality rates were 7.7% in the MDTB group and 10.1% in the non-MDTB group (P = 0.572), with no significant difference in re-transplantation rates between the groups (10.3% vs 8.3%, P = 0.563).
| Outcomes | MDTB group (n = 117) | non-MDTB group (n = 276) | P value |
| Complications grade III/IV | 24 (20.5) | 71 (25.7) | 0.304 |
| Post-operative deaths | 9 (7.7) | 28 (10.1) | 0.572 |
| Tumor progression/MOF | 2 (1.7) | 10 (3.6) | |
| Sepsis | 3 (2.6) | 9 (3.2) | |
| ARDS | 2 (1.7) | 4 (1.4) | |
| Sudden cardiac arrest | 1 (0.8) | 3 (1.1) | |
| GVHD | 1 (0.8) | 2 (0.7) | |
| Re-transplantation | 12 (10.3) | 23 (8.3) | 0.563 |
| Hepatic graft dysfunction | 6 (5.1) | 10 (3.6) | |
| Cholestatic graft dysfunction | 4 (3.4) | 9 (3.3) | |
| Hepatic artery thrombosis | 2 (1.7) | 4 (1.4) | |
| Tumor recurrence | 24 (20.8) | 87 (31.5) | 0.104 |
| Liver only | 8 (6.8) | 26 (9.4) | |
| Liver and other | 7 (5.9) | 24 (8.7) | |
| Other | 14 (11.9) | 37 (13.4) | |
| Recurrence time, months | 28.7 ± 14.9 | 21.5 ± 10.3 | < 0.001 |
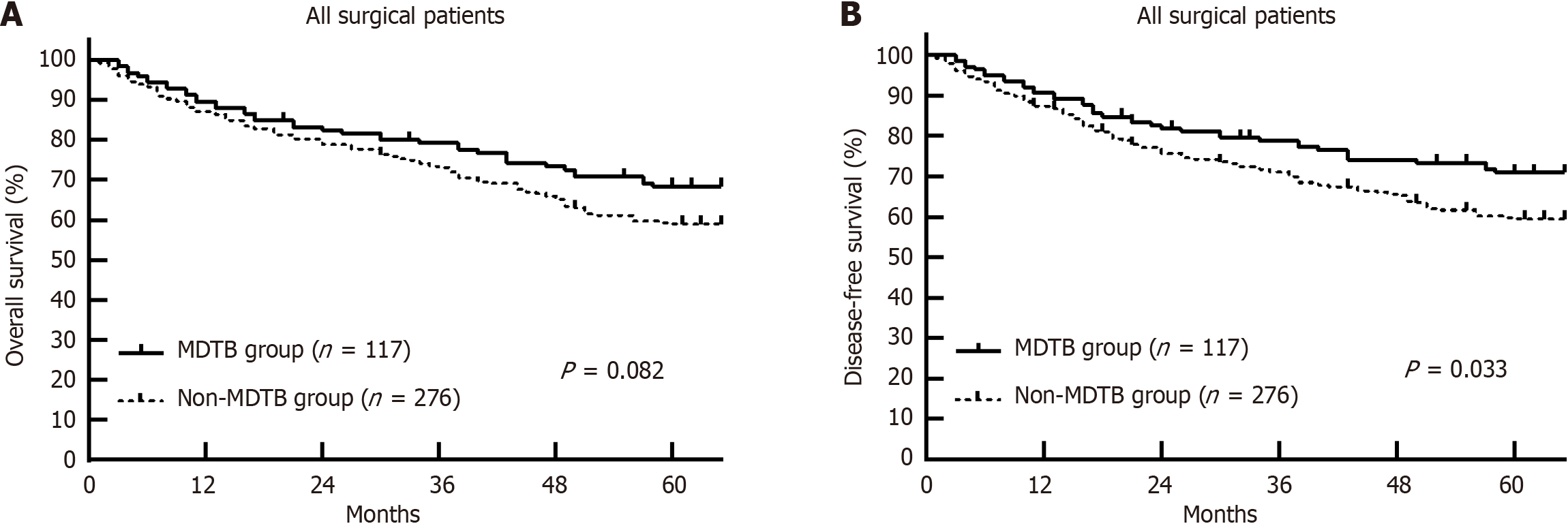
Tumor recurrence occurred in 20.8% of patients in the MDTB group and 31.5% in the non-MDTB group (P = 0.104), with the MDTB group showing a significantly longer mean recurrence time (28.7 ± 14.9 months vs 21.5 ± 10.3 months, P < 0.001). No significant differences in OS and DFS were found between the MDTB and non-MDTB groups (Figure 1), and differences between the two groups were even smaller within the UCSF criteria (Figure 2). However, for patients ex
This study highlighted that an MDTB approach significantly improved OS and DFS in patients with HCC undergoing LT, particularly in those exceeding the UCSF criteria. The holistic approach of the MDTB, integrating expertise from hepatologists, transplant surgeons, radiologists, oncologists, and pathologists, allows for comprehensive treatment planning, enhancing both preoperative and postoperative care. The MDTB approach proved to be especially effective for patients exceeding UCSF criteria, as demonstrated by the improved 1-year, 3-year, and 5-year OS rates in this subgroup compared to the non-MDTB group (Figure 2). Similarly, the 1-year, 3-year, and 5-year DFS rates were significantly higher in the MDTB group. This improvement is primarily attributed to the multidisciplinary evaluation of the MDTB, which fa
The role of the MDTB in individualized decision-making is crucial, especially for patients with more advanced disease or complex tumor characteristics. This indicates that comprehensive assessment and tailored treatment strategies of the MDTB play a significant role in improving outcomes for high-risk patients. Additionally, the mean recurrence time was significantly longer in the MDTB group (28.7 ± 14.9 months) compared to the non-MDTB group (21.5 ± 10.3 months, P < 0.001), emphasizing the effectiveness of the MDTB in managing recurrence risk through coordinated perioperative care. MDTB involvement also ensures optimal timing for transplantation and donor selection. The multidisciplinary nature of MDTB allows for thorough evaluation of each patient’s condition, enabling informed decisions that contribute to improved OS and DFS rates. The recurrence-free survival benefit observed in the MDTB group further supports the value of coordinated, multidisciplinary care in minimizing recurrence and improving long-term outcomes (Figure 3). This benefit is consistent with findings from other cancer types, such as colorectal cancer with liver metastases and lung cancer, where MDTB interventions have led to improved survival and reduced recurrence rates.
Finally, while MDTB implementation is resource-intensive, its long-term cost-effectiveness cannot be overlooked. Clinical pharmacists’ involvement in MDTB has been found to reduce medication-related issues, thereby optimizing treatment and improving cost-effectiveness. This study supports the integration of MDTB into clinical practice, particularly for high-risk patients with HCC who benefit significantly from comprehensive, multidisciplinary management that extends beyond conventional single-specialty care.
The integration of a MDTB into the treatment protocol for patients with HCC undergoing LT markedly improved OS and DFS, especially in patients who exceeded the UCSF criteria. This study strongly advocates for the broader adoption of MDTB in clinical practice, not only as a means to optimize the management of high-risk patients with HCC but also as a model for enhancing interdisciplinary collaboration in complex oncological care.
We thank the staff of the Department of Liver Transplantation, Tianjin First Center Hospital, for their invaluable contributions to this study.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55814] [Article Influence: 7973.4] [Reference Citation Analysis (132)] |
| 2. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6052] [Article Influence: 864.6] [Reference Citation Analysis (3)] |
| 3. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Cheng AL, Vogel A, Tovoli F, Ueshima K, Aikata H, López CL, Pracht M, Meng Z, Daniele B, Park JW, Palmer D, Tamai T, Saito K, Dutcus CE, Lencioni R. Overall survival and objective response in advanced unresectable hepatocellular carcinoma: A subanalysis of the REFLECT study. J Hepatol. 2023;78:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1458] [Cited by in RCA: 1381] [Article Influence: 197.3] [Reference Citation Analysis (0)] |
| 5. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2595] [Article Influence: 865.0] [Reference Citation Analysis (59)] |
| 6. | Kudo M. Systemic Therapy for Hepatocellular Carcinoma: Latest Advances. Cancers (Basel). 2018;10:412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 7. | Gadsden MM, Kaplan DE. Multidisciplinary Approach to HCC Management: How Can This Be Done? Dig Dis Sci. 2019;64:968-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Ducreux M, Abou-Alfa GK, Bekaii-Saab T, Berlin J, Cervantes A, de Baere T, Eng C, Galle P, Gill S, Gruenberger T, Haustermans K, Lamarca A, Laurent-Puig P, Llovet JM, Lordick F, Macarulla T, Mukherji D, Muro K, Obermannova R, O'Connor JM, O'Reilly EM, Osterlund P, Philip P, Prager G, Ruiz-Garcia E, Sangro B, Seufferlein T, Tabernero J, Verslype C, Wasan H, Van Cutsem E. The management of hepatocellular carcinoma. Current expert opinion and recommendations derived from the 24th ESMO/World Congress on Gastrointestinal Cancer, Barcelona, 2022. ESMO Open. 2023;8:101567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 84] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 9. | Baker T, Tabrizian P, Zendejas I, Gamblin TC, Kazimi M, Boudjema K, Geller D, Salem R. Conversion to resection post radioembolization in patients with HCC: recommendations from a multidisciplinary working group. HPB (Oxford). 2022;24:1007-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Liu X, Lu Y, Zhou W, Peng T, Zhou J, Bi H, Xia F, Chen X. Chinese Multidisciplinary Expert Consensus on Immune Checkpoint Inhibitor-Based Combination Therapy for Hepatocellular Carcinoma (2023 Edition). Liver Cancer. 2024;13:355-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |