Published online Mar 24, 2025. doi: 10.5306/wjco.v16.i3.102076
Revised: November 25, 2024
Accepted: December 12, 2024
Published online: March 24, 2025
Processing time: 105 Days and 13.2 Hours
The combination of anti-epidermal growth factor receptor (EGFR) therapy and chemotherapy is currently a preferred first-line treatment for patients with unre
To compare overall survival (OS) between frontline and subsequent anti-EGFR treatment in patients with unresectable, RAS and BRAF wild-type, left-sided mCRC.
We retrospectively reviewed the medical records of mCRC patients treated at The King Chulalongkorn Memorial Hospital and Songklanagarind Hospital, Thailand, between January 2013 and April 2023. Patients were classified into two groups based on the sequence of their anti-EGFR treatment. The primary endpoint was OS.
Among 222 patients with a median follow-up of 29 months, no significant difference in OS was observed between the frontline and subsequent-line groups (HR 1.03, 95%CI: 0.73-1.46, P = 0.878). The median OS was 35.53 months (95%CI: 26.59-44.47) for the frontline group and 31.60 months (95%CI: 27.83-35.37) for the subsequent-line group. In the subsequent-line group, 71 patients (32.4%) who ultimately never received anti-EGFR therapy had a significantly worse median OS of 19.70 months (95%CI: 12.87-26.53).
Frontline and subsequent-line anti-EGFR treatments provide comparable OS in unresectable, RAS/BRAF wild-type, left-sided mCRC patients, but early exposure is vital for those unlikely to receive subsequent therapy.
Core Tip: Anti-epidermal growth factor receptor (EGFR) therapy improves survival in RAS/BRAF wild-type metastatic colorectal cancer, whether used in frontline or subsequent settings. No direct evidence demonstrates frontline treatment’s superiority, particularly in unresectable, left-sided tumors. Our study found no significant difference in overall survival between these approaches, suggesting timing does not affect overall survival if anti-EGFR therapy is administered. However, omitting frontline anti-EGFR risks some patients losing the chance to benefit later. These findings highlight the importance of early exposure and offer valuable guidance for optimizing treatment sequencing in this patient population.
- Citation: Pakvisal N, Goldberg RM, Sathitruangsak C, Silaphong W, Faengmon S, Teeyapun N, Teerapakpinyo C, Tanasanvimon S. Overall survival with frontline vs subsequent anti-epidermal growth factor receptor therapies in unresectable, RAS/BRAF wild-type, left-sided metastatic colorectal cancer. World J Clin Oncol 2025; 16(3): 102076
- URL: https://www.wjgnet.com/2218-4333/full/v16/i3/102076.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i3.102076
Colorectal cancer (CRC) is the third most common malignancy and the second leading cause of cancer-related mortality worldwide[1]. In metastatic CRC (mCRC), the five-year survival rate is approximately 15%[2], with most long-term survivors having undergone surgical removal of their primary and metastatic lesions[3]. As a result, the longevity of mCRC patients with unresectable disease is mainly dependent on systemic treatments[4,5].
Chemotherapy, including fluoropyrimidines[6], oxaliplatin[7], and irinotecan[8,9], has long been the backbone of systemic treatment for mCRC[10,11]. Over the past two decades, two biological agents-monoclonal antibodies against the epidermal growth factor receptor (anti-EGFR: Cetuximab and panitumumab)[12] and against the vascular endothelial growth factor (anti-VEGF: Bevacizumab)[12] have been coupled with chemotherapy, improving survival outcomes[13-15]. Anti-EGFR benefits only patients with RAS wild-type tumors[14-18]. Several randomized phase 3 studies comparing the administration of anti-EGFR to anti-VEGF targeted antibodies coupled with chemotherapy for frontline treatment of mCRC treatment in patients with RAS wild-type tumors have yielded inconclusive results[19-21]. Nevertheless, meta-analyses suggest that patients with left-sided tumors derive greater benefit from initial therapy with an antibody targeting EGFR[22,23]. The PARADIGM trial, a phase 3 randomized controlled study, demonstrated superior overall survival (OS) with anti-EGFR compared to anti-VEGF in combination with chemotherapy for treatment-naive mCRC patients with left-sided and RAS wild-type tumors[24]. As a result, anti-EGFR plus doublet chemotherapy is currently considered to be the preferred first-line regimen in guidelines issued by both ESMO and NCCN for unresectable mCRC patients with left-sided and RAS wild-type tumors[10,25,26].
However, several studies still show the benefit of anti-EGFR in subsequent line settings[27-30]. There has been no direct evidence supporting the superiority of frontline over subsequent-line anti-EGFR therapy in these patients. In clinical practice, various clinicopathologic factors including tumor sidedness, patient characteristics (age, performance status, comorbidities and socioeconomic factors), tumor burden and symptoms, and treatment toxicity are considered to individualize systemic treatment options[10,25].
This study compared OS between frontline and subsequent-line anti-EGFR therapies in patients with unresectable, RAS and BRAF wild-type, left-sided mCRC in real-world clinical practice in two academic medical centers in Thailand.
We retrospectively extracted data from the Thai Society of Clinical Oncology CRC registry and reviewed medical records for additional information on patients with mCRC treated at The King Chulalongkorn Memorial Hospital and Songklanagarind Hospital between January 2013 and April 2023. The study population included patients with unresectable, RAS and BRAF wild-type, left-sided mCRC who underwent systemic therapy.
Patients with either synchronous or metachronous metastases were eligible for inclusion in the study. Metachronous mCRC was defined as metastatic tumors diagnosed more than 6 months after the diagnosis of the primary tumor. Patients with unresectable metastatic tumors were defined as those with extrahepatic metastasis or liver-limited me
In the frontline group, patients received at least one cycle of combination chemotherapy plus an EGFR targeted monoclonal antibody in the first-line setting. In the subsequent-line group, patients were treated with chemotherapy, with or without bevacizumab, in the first-line setting and received anti-EGFR therapy in subsequent lines upon tumor progression. We excluded the patients with any missing data.
The primary endpoint of the study was OS. We compared OS between frontline and subsequent anti-EGFR therapies in patients with unresectable, RAS and BRAF wild-type, left-sided mCRC. For the subsequent anti-EGFR therapy group, only patients who received anti-EGFR treatment upon tumor progression after first-line chemotherapy were included in the primary analysis. OS was defined as the time from the initiation of first-line systemic treatment to the date of death from any cause.
Secondary outcomes included identifying the percentage of patients who never received anti-EGFR therapy if it was not administered as first-line treatment and evaluating prognostic factors associated with poor survival outcomes in this study population.
Comparisons of demographic and clinical characteristics between the frontline and subsequent-line approach groups were analyzed using χ2 tests for categorical data and student’s t-tests for continuous data. To evaluate the association between OS, anti-EGFR therapy approach, and other factors, we applied a univariate Cox regression analysis to calculate the unadjusted HR. Significant factors identified in the univariate analysis were included in a multivariate Cox regression analysis to adjust for risks and determine the potential impact of the sequence of anti-EGFR therapy on OS.
The difference in OS between the two groups was analyzed using a two-sided log-rank test, and survival curves were generated using the Kaplan-Meier method. P-values of less than 0.05 were considered statistically significant. Statistical analyses were performed using SPSS version 28.0 (IBM Corp., Armonk, NY, United States).
This study was conducted in accordance with the Declaration of Helsinki and approved by the International Review Board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (No. 0375/66).
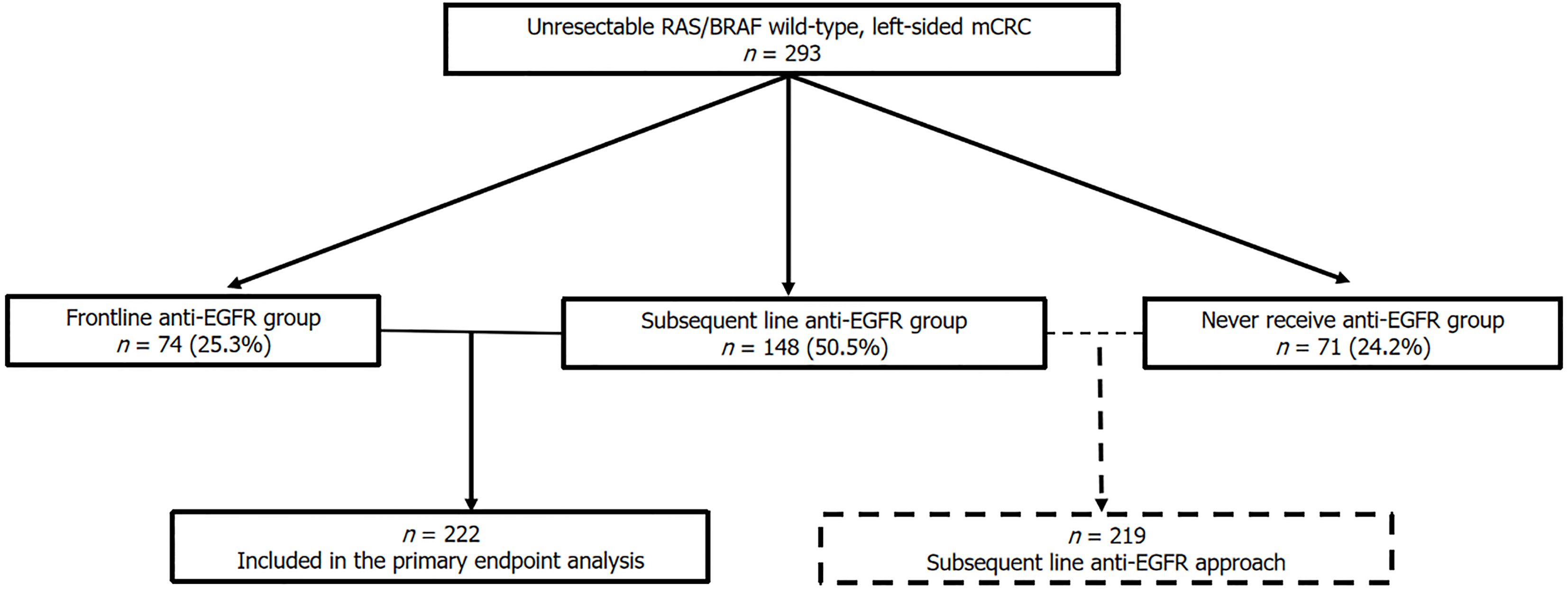
A total of 293 eligible patients were identified. Of these, 222 who received anti-EGFR therapies were included in the primary endpoint analysis. 74 patients (25.3%) received anti-EGFR as frontline treatment, while 148 (50.5%) received it as subsequent-line therapy. The higher proportion of subsequent anti-EGFR treatment is due to the lack of reimbursement for frontline anti-EGFR therapy in Thailand for unresectable mCRC. Additionally, 71 patients (24.2%) never received anti-EGFR in any lines of treatment, as shown in Figure 1.
Table 1 shows the demographic and clinical characteristics of all patients who received anti-EGFR targeted therapy. There were no statistically significant differences in age, sex, ECOG performance status, histologic subtype, tumor grading, metastatic type and number of metastatic sites, but significant differences in comorbidities, tumor location and site(s) of metastasis between frontline and subsequent-line groups. In the frontline anti-EGFR group, there was a higher proportion of patients with single-site metastasis, especially liver metastasis, but a lower proportion of patients with lung metastasis at presentation. Only half of patients underwent testing to determine the presence or absence of mismatch repair proteins or were evaluated for microsatellite instability.
| Characteristics | All (n = 222) | Frontline anti-EGFR (n = 74) | Subsequent-line anti-EGFR (n = 148) | P value |
| Age at diagnosis | ||||
| Median (range), years | 60 (26-92) | 61 (26-83) | 59.5 (30-92) | |
| ≥ 65 years | 65 (29.3) | 24 (32.4) | 41 (27.7) | 0.465 |
| Sex | ||||
| Male | 144 (64.9) | 49 (66.2) | 95 (64.2) | |
| Female | 78 (35.1) | 25 (33.8) | 53 (35.8) | 0.766 |
| ECOG | ||||
| 0 | 35 (15.8) | 9 (12.2) | 26 (17.6) | |
| 1 | 182 (82) | 64 (86.5) | 118 (79.7) | 0.452 |
| 2 | 5 (2.3) | 1 (1.4) | 4 (2.7) | |
| Underlying disease | ||||
| No | 107 (48.2) | 43 (58.1) | 64 (43.2) | 0.037 |
| Hypertension | 76 (34.2) | 17 (23) | 59 (39.9) | 0.012 |
| Diabetes | 44 (19.8) | 13 (17.6) | 31 (20.9) | 0.552 |
| Dyslipidemia | 48 (21.6) | 17 (23) | 31 (20.9) | 0.729 |
| Cerebrovascular disease | 8 (3.6) | 3 (4.1) | 5 (3.4) | 0.799 |
| Ischemic heart disease | 7 (3.2) | 3 (4.1) | 4 (2.7) | 0.587 |
| Other cancers | 6 (2.7) | 4 (5.4) | 2 (1.4) | 0.079 |
| Tumor location | ||||
| Splenic flexure | 7 (3.2) | 1 (1.4) | 6 (4.1) | 0.032 |
| Descending colon | 21 (9.5) | 9 (12.2) | 12 (8.1) | |
| Sigmoid colon | 118 (53.2) | 41 (55.4) | 77 (52) | |
| Rectosigmoid | 25 (11.3) | 11 (14.9) | 14 (9.5) | |
| Rectum | 51 (23) | 12 (16.2) | 39 (26.4) | |
| Histologic subtype | 0.409 | |||
| Mucinous adenocarcinoma | 3 (1.4) | 2 (2.7) | 1 (0.7) | |
| Signet ring cell carcinoma | 2 (0.9) | 1 (1.4) | 1 (0.7) | |
| Tumor grade | 0.596 | |||
| Well differentiated | 63 (33.7) | 22 (36.7) | 41 (32.3) | |
| Moderately differentiated | 109 (58.3) | 32 (53.3) | 77 (60.6) | |
| Poorly differentiated | 15 (8) | 6 (10) | 9 (7.1) | |
| Metastatic type | 0.109 | |||
| Synchronous | 189 (85.1) | 67 (90.5) | 122 (82.4) | |
| Metachronous | 33 (14.9) | 7 (9.5) | 26 (17.6) | |
| Number of metastatic sites | 0.053 | |||
| 1 | 133 (59.9) | 53 (71.6) | 80 (54.1) | |
| 2 | 67 (30.2) | 18 (24.3) | 49 (33.1) | |
| 3 | 13 (5.9) | 2 (2.7) | 11 (7.4) | |
| ≥ 4 | 9 (4.1) | 1 (1.4) | 8 (5.4) | |
| Site of metastasis | ||||
| Liver | 172 (77.5) | 63 (85.1) | 109 (73.6) | 0.053 |
| Liver-limited metastasis | 97 (43.7) | 46 (42.2) | 51 (34.5) | < 0.001 |
| Lung | 65 (29.3) | 13 (17.6) | 52 (35.1) | 0.007 |
| Peritoneum | 49 (22.1) | 13 (17.6) | 36 (24.3) | 0.252 |
| Distant lymph nodes | 30 (13.5) | 6 (8.1) | 24 (16.2) | 0.096 |
| MMR or MSI status | 0.265 | |||
| MMR deficient/high MSI | 3 (1.4) | 1 (1.4) | 2 (1.4) | |
| MMR proficient/stable MSI | 101 (45.5) | 28 (37.8) | 73 (49.3) | |
| Unknown | 118 (53.2) | 45 (60.8) | 73 (49.3) | |
| Line of anti-EGFR | < 0.001 | |||
| 1 | 74 (33.3) | 74 (100) | - | |
| 2 | 47 (21.2) | - | 47 (31.8) | |
| 3 | 75 (33.8) | - | 75 (50.7) | |
| 4 | 19 (8.6) | - | 19 (12.8) | |
| 5 | 7 (3.2) | - | 7 (4.7) | |
| Retreatment anti-EGFR | 53 (23.9) | 25 (33.8) | 28 (18.9) | 0.014 |
| Line of bevacizumab | ||||
| 1 | 51 (23) | - | 51 (34.5) | < 0.001 |
| 2 | 75 (33.8) | 25 (33.8) | 50 (33.8) | 1 |
| 3 | 22 (9.9) | 7 (9.5) | 15 (10.1) | 0.874 |
| 4 | 16 (7.2) | 5 (6.8) | 11 (7.4) | 0.854 |
| 5 | 6 (2.7) | 2 (2.7) | 4 (2.7) | 1 |
| Any lines of bevacizumab | 143 (64.4) | 33 (44.6) | 110 (74.3) | < 0.001 |
| Regimen chemotherapy at first-line | 0.166 | |||
| 5FU/Capecitabine | 5 (2.3) | - | 5 (3.4) | |
| FOLFOX/CAPEOX | 167 (75.2) | 55 (74.3) | 112 (75.7) | |
| FOLFIRI/CAPIRI | 47 (21.2) | 19 (25.7) | 28 (18.9) | |
| FOLFOXIRI/mFOLFORINOX | 3 (1.4) | - | 3 (2) | |
| Line number of systemic treatments | ||||
| Median (range) | 3 (1-8) | 2.5 (1-7) | 4 (2-8) | < 0.001 |
| 1 | 21 (9.5) | 21 (28.4) | - | |
| 2 | 34 (15.3) | 18 (21.6) | 18 (12.2) | |
| 3 | 63 (28.4) | 19 (25.7) | 44 (29.7) | |
| 4 | 56 (25.2) | 10 (13.5) | 46 (31.1) | |
| ≥ 5 | 48 (21.6) | 8 (10.8) | 40 (27) | |
| Local treatment at metastatic sites | 122 (55) | 51 (68.9) | 71 (48) | 0.003 |
| Metastectomy at liver | 73 (32.9) | 38 (51.4) | 35 (23.6) | < 0.001 |
| Patient status at last follow-up | ||||
| No evidence of disease | 13 (5.9) | 11 (14.9) | 2 (1.4) | < 0.001 |
| Alive with disease | 51 (23) | 18 (24.3) | 33 (22.3) | |
| Death | 157 (70.7) | 44 (59.5) | 113 (76.4) | |
| Unknown | 1 (0.5) | 1 (1.4) | - |
Most patients in the subsequent-line anti-EGFR group received anti-EGFR therapy in the second- or third-line settings. In this group, 34.5% received bevacizumab as part of their first-line treatment, and more patients received bevacizumab in any lines of treatment compared to the frontline anti-EGFR group. Despite this, there was no significant difference in the first-line chemotherapy regimen between the groups; most patients received oxaliplatin-based chemotherapy. Additionally, the frontline group had a significantly higher proportion of patients receiving local treatments at metastatic sites, including liver metastectomy, compared to the subsequent-line group.
Among patients not receiving frontline anti-EGFR therapy, 71 patients (32.4%) never received anti-EGFR targeted therapy in subsequent-line treatment. Their demographic and clinical characteristics are shown in Supplementary Table 1. All patients in this group experienced disease progression after first-line chemotherapy. However, only 47 patients (66.2%) and 20 patients (28.2%) in this group received second- and third-line therapy, respectively. The most common reason for not receiving subsequent-line treatments in this group after tumor progression was poor performance status, rendering the patients unfit for further treatment, as shown in Supplementary Figure 1. All systemic treatments administered during any lines of therapy for each group of patients are shown in Supplementary Table 2.
At the time of data cutoff, the median follow-up time was 29 months. The percentage of deaths was significantly higher in the subsequent line compared to the frontline anti-EGFR group (76.4% vs 59.5%, P < 0.001). The median OS for all patients who received anti-EGFR targeted therapy was 32.50 months (95%CI: 28.64-35.36).
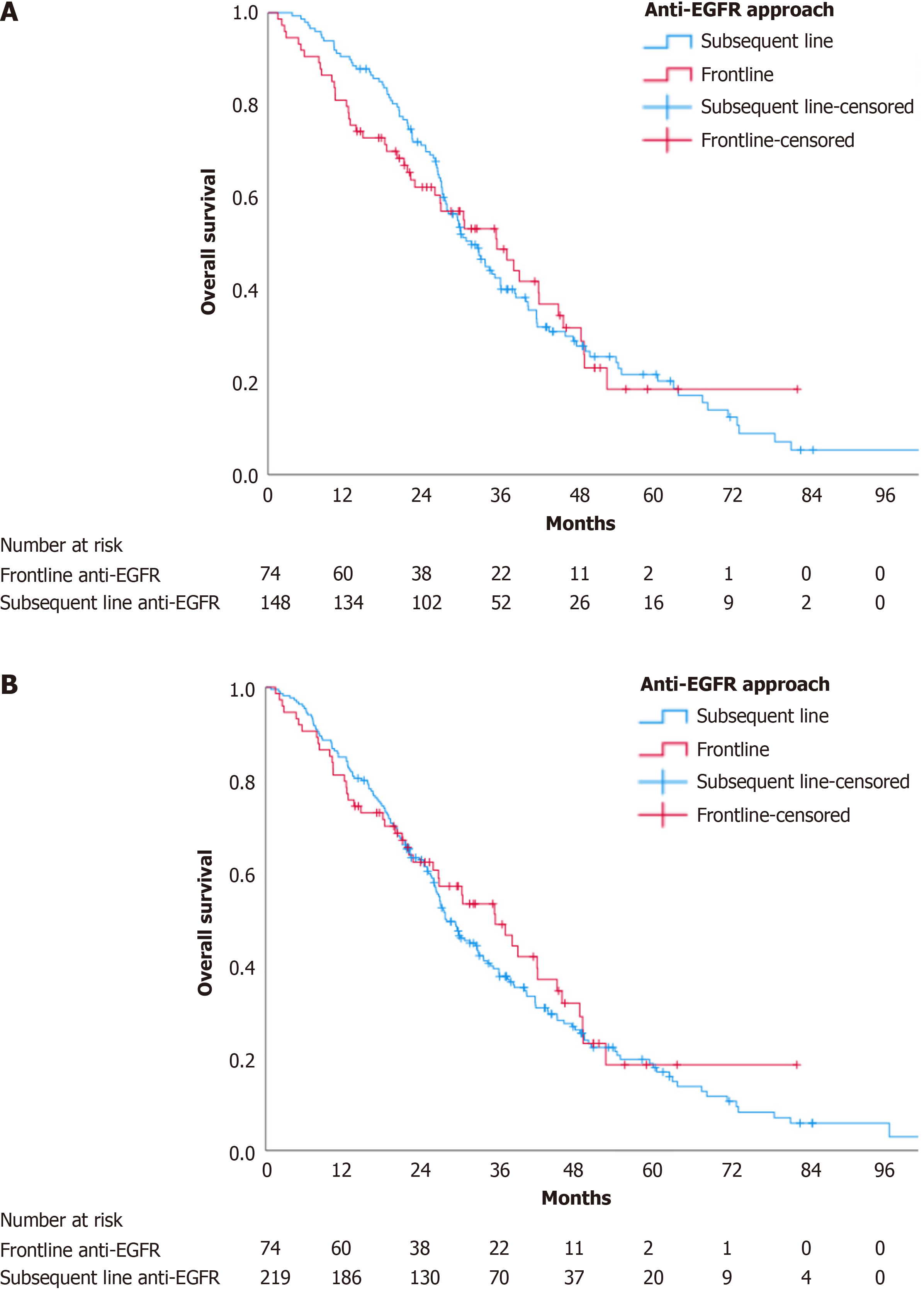
There was no statistically significant difference in OS between the two groups (HR 1.03, 95%CI: 0.73-1.46, P = 0.878). The median OS in the frontline group and subsequent-line groups was 35.53 months (95%CI: 26.59-44.47) and 31.60 months (95%CI: 27.83-35.37), respectively, as shown in Figure 2A.
The median OS in patients who never received anti-EGFR targeted therapy was 19.70 months (95%CI: 12.87-26.53). Including these patients into the subsequent anti-EGFR approach resulted in a median OS of 27.77 months (95%CI: 24.54-30.99). Despite the lower median OS, there remained no statistically significant difference between the subsequent line and the frontline anti-EGFR targeted therapy groups (HR 0.90, 95%CI: 0.65-1.25, P = 0.52), as shown in Figure 2B. When comparing the OS between patients who received anti-EGFR targeted therapy at any point and those who did not, the OS was significantly longer in the group that received anti-EGFR therapy (HR 0.65, 95%CI: 0.48-0.88, P = 0.005) as shown in Supplementary Figure 2.
The univariate and multivariate analyses of OS in the patients who received anti-EGFR targeted therapy are shown in Table 2. The timing of the anti-EGFR targeted therapy was not a statistically significant factor in either univariate or multivariate analyses. Univariate analysis identified signet ring cell features, poorly differentiated tumors, multiple metastasis sites, and peritoneal metastasis as significant poor prognostic factors, while liver-limited metastasis and liver resection were favorable prognostic factors. In multivariate analysis, the remaining significant factors were signet ring cell features, poorly differentiated tumors, and liver resection. Patients who underwent liver metastectomy had better survival outcomes than those who did not (HR 0.25, 95%CI: 0.15-0.42, P < 0.001).
| Covariate | Univariate | Multivariate | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age | ||||||
| ≥ 65 vs < 65 years | 0.83 | 0.56-1.16 | 0.242 | NA | NA | NA |
| Female vs male | 0.9 | 0.65-1.25 | 0.525 | NA | NA | NA |
| ECOG PS | ||||||
| 1 vs 0 | 1.06 | 0.70-1.62 | 0.78 | NA | NA | NA |
| 2 vs 0 | 1.19 | 0.41-3.42 | 0.748 | |||
| Underlying disease | ||||||
| Yes vs no | 0.91 | 0.66-1.24 | 0.54 | NA | NA | NA |
| Histologic subtype | ||||||
| Mucinous vs adenocarcinoma | 0.67 | 0.17-2.72 | 0.575 | 0.35 | 0.08-1.43 | 0.142 |
| Signet ring cell vs adenocarcinoma | 10.74 | 2.58-44.74 | 0.001 | 9.79 | 2.31-41.57 | 0.002 |
| Tumor grade | ||||||
| Moderately vs well differentiated | 1.12 | 0.76-1.65 | 0.557 | 0.98 | 0.64-1.50 | 0.92 |
| Poorly vs well differentiated | 2.38 | 1.24-4.58 | 0.009 | 2.18 | 1.07-4.43 | 0.032 |
| Metastatic type | ||||||
| Metachronous vs synchronous | 0.92 | 0.59-1.42 | 0.696 | NA | NA | NA |
| Number of metastases | ||||||
| ≥ 2 vs 1 site | 1.44 | 1.05-1.98 | 0.024 | 1.44 | 0.87-2.39 | 0.161 |
| Liver-limited metastasis | ||||||
| Yes vs no | 0.6 | 0.43-0.84 | 0.002 | 1.71 | 0.92-3.17 | 0.088 |
| Peritoneal metastasis | ||||||
| Yes vs no | 1.85 | 1.30-2.63 | < 0.001 | 1.31 | 0.84-2.03 | 0.233 |
| Anti-EGFR approach | ||||||
| Frontline vs subsequent-line | 1.03 | 0.73-1.46 | 0.878 | 1.12 | 0.79-1.82 | 0.393 |
| First-line bevacizumab | ||||||
| Yes vs no | 0.92 | 0.64-1.32 | 0.66 | NA | NA | NA |
| Metastectomy at liver | ||||||
| Yes vs no | 0.32 | 0.22-0.47 | < 0.001 | 0.25 | 0.15-0.42 | < 0.001 |
This study aimed to evaluate the impact of the administration of anti-EGFR targeted therapy either as first-line or in subsequent-line treatment in patients with unresectable RAS and BRAF wild-type, left-sided mCRC. We compared OS between patients who received frontline and subsequent-line anti-EGFR therapies in a real-world clinical practice in two Thai academic centers. Our primary analysis found no significant difference in OS between patients receiving frontline and subsequent-line anti-EGFR therapies. However, median OS was significantly shorter in patients who never received anti-EGFR therapy, as compared to patients who received anti-EGFR therapy at any treatment lines.
Anti-EGFR therapy is a standard treatment option for patients with unresectable RAS and BRAF wild-type, left-sided mCRC. Although the PARADIGM study supported the administration of frontline anti-EGFR therapy with chemothe
In this cohort, only 25% of patients received combination chemotherapy and anti-EGFR targeted therapy as first-line treatment. This was largely because of the anti-EGFR therapy reimbursement protocol in Thailand, where only third-line anti-EGFR is covered with the exception that first-line anti-EGFR therapy is covered in patients with potentially resectable mCRC[25]. This also led to the finding that there were more patients with liver-limited metastasis and liver resection groups in the frontline anti-EGFR therapy. In contrast, there were more patients receiving bevacizumab in the subsequent-line group. However, only liver resection was an independent favorable prognostic factor for OS in our cohort.
Thirty-two percent of patients experienced disease progression after first-line chemotherapy without anti-EGFR therapy and ultimately did not receive anti-EGFR therapy. This result was comparable to the findings reported in the PARADIGM study, where 29.5% of patients in the bevacizumab arm did not receive anti-EGFR therapy as subsequent treatment[24]. Notably, these patients in our cohort had the poorest survival outcomes, with a median OS of 19.7 months. The primary reason for not receiving subsequent therapy was the deterioration in performance status after tumor progression, which rendered these patients unfit for further treatment. Although both frontline and subsequent-line anti-EGFR therapies are reasonable for these patients based on our primary analysis, OS outcomes were worse in the cohort of patients that did not receive frontline anti-EGFR therapy who were deemed unfit to receive subsequent chemotherapy with anti-EGFR at the time of tumor progression.
There were some limitations in our study. Firstly, the sample size is limited because not all patients in our centers undergo RAS and BRAF testing, as testing is not reimbursable. It is only performed for patients that are considered to be candidates for anti-EGFR therapy. Secondly, as is common in a retrospective study, there was an imbalance in some baseline characteristics. We addressed this issue by using multivariate analysis to adjust for significant prognostic risks. Thirdly, this study was conducted in two centers in Thailand, where healthcare reimbursement regulations can limit options for patients, particularly in the systemic therapy of mCRC.
In conclusion, the timing of anti-EGFR targeted treatment does not impact OS in patients with unresectable RAS and BRAF wild-type, left-sided mCRC, providing that the patients receive anti-EGFR targeted therapy in at least one line of their treatment. These findings support flexibility in the strategy for deployment of anti-EGFR targeted treatments, allowing for personalized treatment plans based on patient and disease characteristics. In regions where upfront anti-EGFR targeted treatment use is financially constrained, these insights ensure patients can still benefit from anti-EGFR targeted therapy at some point in their treatments. However, if anti-EGFR targeted treatment is not given in the frontline setting, some patients may not receive it upon tumor progression, thereby missing the opportunity to benefit from the therapy. Clinicians might consider early use of anti-EGFR targeted therapy for patients at risk of rapid disease pro
This study is a result from Thai Society of Clinical Oncology (TSCO) CRC Registry. We would like to thank Chula Data Management Center, Faculty of Medicine, Chulalongkorn University, especially Ashaya Ingkasatien, for their support in data management.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 8170] [Article Influence: 8170.0] [Reference Citation Analysis (2)] |
| 2. | Morris VK, Kennedy EB, Baxter NN, Benson AB 3rd, Cercek A, Cho M, Ciombor KK, Cremolini C, Davis A, Deming DA, Fakih MG, Gholami S, Hong TS, Jaiyesimi I, Klute K, Lieu C, Sanoff H, Strickler JH, White S, Willis JA, Eng C. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. J Clin Oncol. 2023;41:678-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 314] [Article Influence: 157.0] [Reference Citation Analysis (0)] |
| 3. | Zeineddine FA, Zeineddine MA, Yousef A, Gu Y, Chowdhury S, Dasari A, Huey RW, Johnson B, Kee B, Lee MS, Morelli MP, Morris VK, Overman MJ, Parseghian C, Raghav K, Willis J, Wolff RA, Kawaguchi Y, Vauthey JN, Sun R, Kopetz S, Shen JP. Survival improvement for patients with metastatic colorectal cancer over twenty years. NPJ Precis Oncol. 2023;7:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 82] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 4. | Osterlund P, Salminen T, Soveri LM, Kallio R, Kellokumpu I, Lamminmäki A, Halonen P, Ristamäki R, Lantto E, Uutela A, Osterlund E, Ovissi A, Nordin A, Heervä E, Lehtomäki K, Räsänen J, Murashev M, Aroviita L, Jekunen A, Lindvall-Andersson R, Nyandoto P, Kononen J, Lepistö A, Poussa T, Muhonen T, Ålgars A, Isoniemi H; Members of The RAXO study group are collaborators of this study and are listed in Appendix Table 4B. Repeated centralized multidisciplinary team assessment of resectability, clinical behavior, and outcomes in 1086 Finnish metastatic colorectal cancer patients (RAXO): A nationwide prospective intervention study. Lancet Reg Health Eur. 2021;3:100049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 5. | Hernandez Dominguez O, Yilmaz S, Steele SR. Stage IV Colorectal Cancer Management and Treatment. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 6. | Van Cutsem E, Hoff PM, Harper P, Bukowski RM, Cunningham D, Dufour P, Graeven U, Lokich J, Madajewicz S, Maroun JA, Marshall JL, Mitchell EP, Perez-Manga G, Rougier P, Schmiegel W, Schoelmerich J, Sobrero A, Schilsky RL. Oral capecitabine vs intravenous 5-fluorouracil and leucovorin: integrated efficacy data and novel analyses from two large, randomised, phase III trials. Br J Cancer. 2004;90:1190-1197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 286] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and Fluorouracil With or Without Oxaliplatin as First-Line Treatment in Advanced Colorectal Cancer. J Clin Oncol. 2023;41:5080-5089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2407] [Cited by in RCA: 2381] [Article Influence: 95.2] [Reference Citation Analysis (1)] |
| 9. | Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2273] [Cited by in RCA: 2222] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 10. | Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, Seligmann J, De Baere T, Osterlund P, Yoshino T, Martinelli E; ESMO Guidelines Committee. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:10-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 817] [Article Influence: 408.5] [Reference Citation Analysis (34)] |
| 11. | Scheithauer W, Rosen H, Kornek GV, Sebesta C, Depisch D. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ. 1993;306:752-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 464] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 12. | Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 905] [Cited by in RCA: 1032] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 13. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7733] [Article Influence: 368.2] [Reference Citation Analysis (1)] |
| 14. | Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Oliner KS, Wolf M, Gansert J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697-4705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1296] [Cited by in RCA: 1394] [Article Influence: 92.9] [Reference Citation Analysis (0)] |
| 15. | Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2901] [Cited by in RCA: 3126] [Article Influence: 195.4] [Reference Citation Analysis (1)] |
| 16. | Bokemeyer C, Köhne CH, Ciardiello F, Lenz HJ, Heinemann V, Klinkhardt U, Beier F, Duecker K, van Krieken JH, Tejpar S. FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur J Cancer. 2015;51:1243-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 190] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 17. | Sorich MJ, Wiese MD, Rowland A, Kichenadasse G, McKinnon RA, Karapetis CS. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta-analysis of randomized, controlled trials. Ann Oncol. 2015;26:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 404] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 18. | Peeters M, Karthaus M, Rivera F, Terwey JH, Douillard JY. Panitumumab in Metastatic Colorectal Cancer: The Importance of Tumour RAS Status. Drugs. 2015;75:731-748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Hielscher J, Scholz M, Müller S, Link H, Niederle N, Rost A, Höffkes HG, Moehler M, Lindig RU, Modest DP, Rossius L, Kirchner T, Jung A, Stintzing S. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1331] [Article Influence: 121.0] [Reference Citation Analysis (0)] |
| 20. | Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, Schrag D, Greene C, O'Neil BH, Atkins JN, Berry S, Polite BN, O'Reilly EM, Goldberg RM, Hochster HS, Schilsky RL, Bertagnolli MM, El-Khoueiry AB, Watson P, Benson AB 3rd, Mulkerin DL, Mayer RJ, Blanke C. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA. 2017;317:2392-2401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 681] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 21. | Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon JL, Hecht JR, Yu H, Oliner KS, Go WY. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32:2240-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 495] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 22. | Yoshino T, Hooda N, Younan D, Muro K, Shitara K, Heinemann V, O'neil BH, Herrero FR, Peeters M, Soeda J, Suh M, Reichert H, Mezzi K, Fryzek J, Chia V, Rehn M, Stintzing S. A meta-analysis of efficacy and safety data from head-to-head first-line trials of epidermal growth factor receptor inhibitors versus bevacizumab in adult patients with RAS wild-type metastatic colorectal cancer by sidedness. Eur J Cancer. 2024;202:113975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 23. | Ciliberto D, Staropoli N, Caglioti F, Chiellino S, Ierardi A, Ingargiola R, Botta C, Arbitrio M, Correale P, Tassone P, Tagliaferri P. The best strategy for RAS wild-type metastatic colorectal cancer patients in first-line treatment: A classic and Bayesian meta-analysis. Crit Rev Oncol Hematol. 2018;125:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Watanabe J, Muro K, Shitara K, Yamazaki K, Shiozawa M, Ohori H, Takashima A, Yokota M, Makiyama A, Akazawa N, Ojima H, Yuasa Y, Miwa K, Yasui H, Oki E, Sato T, Naitoh T, Komatsu Y, Kato T, Hihara M, Soeda J, Misumi T, Yamamoto K, Akagi K, Ochiai A, Uetake H, Tsuchihara K, Yoshino T. Panitumumab vs Bevacizumab Added to Standard First-line Chemotherapy and Overall Survival Among Patients With RAS Wild-type, Left-Sided Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA. 2023;329:1271-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 135] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 25. | Yoshino T, Cervantes A, Bando H, Martinelli E, Oki E, Xu RH, Mulansari NA, Govind Babu K, Lee MA, Tan CK, Cornelio G, Chong DQ, Chen LT, Tanasanvimon S, Prasongsook N, Yeh KH, Chua C, Sacdalan MD, Sow Jenson WJ, Kim ST, Chacko RT, Syaiful RA, Zhang SZ, Curigliano G, Mishima S, Nakamura Y, Ebi H, Sunakawa Y, Takahashi M, Baba E, Peters S, Ishioka C, Pentheroudakis G. Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with metastatic colorectal cancer. ESMO Open. 2023;8:101558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 26. | National Comprehensive Cancer Network. Colon Cancer. 2024. Available from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. |
| 27. | Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, Tebbutt NC, van Hazel G, Wierzbicki R, Langer C, Moore MJ. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1468] [Cited by in RCA: 1488] [Article Influence: 82.7] [Reference Citation Analysis (1)] |
| 28. | Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3767] [Cited by in RCA: 3708] [Article Influence: 176.6] [Reference Citation Analysis (1)] |
| 29. | Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, André T, Chan E, Lordick F, Punt CJ, Strickland AH, Wilson G, Ciuleanu TE, Roman L, Van Cutsem E, Tzekova V, Collins S, Oliner KS, Rong A, Gansert J. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706-4713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 758] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 30. | Seymour MT, Brown SR, Middleton G, Maughan T, Richman S, Gwyther S, Lowe C, Seligmann JF, Wadsley J, Maisey N, Chau I, Hill M, Dawson L, Falk S, O'Callaghan A, Benstead K, Chambers P, Oliver A, Marshall H, Napp V, Quirke P. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. Lancet Oncol. 2013;14:749-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 311] [Article Influence: 25.9] [Reference Citation Analysis (0)] |