Published online Mar 24, 2025. doi: 10.5306/wjco.v16.i3.101705
Revised: November 23, 2024
Accepted: January 7, 2025
Published online: March 24, 2025
Processing time: 110 Days and 6.6 Hours
Older patients are more likely to have a poor performance status and comor
To investigated the role of palliative radiotherapy in older patients and patients who were expected to demonstrate a therapeutic effect.
From February 2019 to February 2022, 33 patients aged ≥ 80 years underwent palliative radiotherapy. The prognosis in palliative care study predictor (PiPS), palliative prognostic index (PPI), and delirium-palliative prognostic score (D-PaP) models were used for prognosis prediction. D-PaP scores calculated according to the doctor's prediction of clinical prediction of survival (CPS) were excluded and then analyzed for comparison. Radiation was prescribed at a dose of 2.5-7 Gy per fraction, up to a median of 39 Gy10 (range, 28-75 Gy10).
The median follow-up was 2.4 months (range, 0.2-27.5 months), and 28 patients (84.8%) showed subjective symptom improvements following treatment. The 2- and 6-month survival rates of all patients were 91.5% and 91.5%, respectively. According to regression analysis, the performance status index, symptom type, and radiation dose all showed no significant correlation with the treatment re
This study shows that the prognosis prediction model used in palliative care can be used to identify patients suitable for treatment.
Core Tip: This is a retrospective study to investigate the role of palliative radiotherapy in older patients and patients who were expected to demonstrate a great therapeutic effect. The prognosis in palliative care study predictor, palliative prognostic index, and delirium-palliative prognostic score models were used for prognosis prediction. Most of patients showed subjective symptom improvements following treatment. The prognosis prediction model showed good correlation with survival. In order to increase the therapeutic effectiveness in palliative radiotherapy, it is necessary to assess a patient's exact prognosis and select appropriate patients accordingly.
- Citation: Park H. Validation of the prognostic model for palliative radiotherapy in older patients with cancer. World J Clin Oncol 2025; 16(3): 101705
- URL: https://www.wjgnet.com/2218-4333/full/v16/i3/101705.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i3.101705
The incidence of cancer is high among individuals 60-69 years old and is 11 times greater among those ≥ 65-years-old compared to those < 65-years-old. For this reason, about half of all cancer cases are diagnosed in individuals aged ≥ 70 years, and older patients account for a large portion of the total population regarding the prevalence of cancer[1]. Cancer is one of the most significant diseases in older patients. About 60% of all cancer-related deaths occur in older patients aged 70 years[1,2]. Moreover, cancer accounts for about one-third of the causes of death in the elderly population[1,2]. When choosing a cancer treatment, both the characteristics of the cancer and the overall health status of the patient, such as their general condition and any underlying diseases, should be considered[2]. Older patients have a shorter life expectancy than younger patients; moreover, they typically have many accompanying underlying diseases and have a poorer general condition. For this reason, older patients are often rejected from receiving active testing and treatment services. Therefore, even if other factors, such as the underlying disease, are the same in young and old patients, older patients typically receive less treatment due to the simple fact that they are older[3].
Palliative treatment is a treatment approach that improves the pain and symptoms of a patient and their quality of life. Although palliative treatment is applicable regardless of patient age and the type and severity of their disease, most patients requiring palliative treatment are cancer patients. Palliative radiotherapy is relatively effective for cancer patients and tends to be a well-tolerated treatment. Although some studies have reported the usefulness of palliative radiotherapy in elderly patients, a large number of patients and caregivers are not receiving treatment due to fears of treatment, the risks of side effects, and doubts about treatment effectiveness[1]. Since actual age is not always associated with physical ability, the determination of treatment based solely on age can be an obstacle preventing appropriate treatment opportunities. The importance of palliative care is increasing due to the recent growth of the elderly population, as well as, the increase in cancer incidence, and the changes in traditional views or perceptions, such as a growing acceptance of the pursuit of a dignified death[4]. Therefore, in this study, we investigated the role of palliative radiotherapy in older patients and in patients who are expected to show a great therapeutic effect.
This study was approved by the Institutional Review Board (DKUH 2024-09-006). From February 2019 to February 2022, a total of 353 patients received palliative radiotherapy. Palliative radiotherapy was recommended: (1) When symptoms developed due to advanced cancer; and (2) When other treatment options, such as surgery or chemotherapy, were unavailable due to general conditions and underlying diseases. Among the 353 total patients, 39 were ≥ 80 years old, and 33 received planned palliative radiotherapy. Four of the six patients who did not receive the planned treatment did not complete treatment for reasons such as deterioration of their general condition. Patients who underwent same-site re-irradiation were excluded from the analysis. Prognosis in palliative care study predictor (PiPS), palliative prognostic index (PPI), and delirium-palliative prognostic score (D-PaP) models were used to predict patient prognosis[5]. For a more objective comparison, scores based on the physician's prediction of survival (CPS) were excluded from the D-PaP model and then compared and analyzed. The following data were collected for the PiPS model: Cognitive function, pulse, anorexia, dyspnea, dysphagia, fatigue, performance status index Eastern Cooperative Oncology Group (ECOG) score, global health status, leukocyte count, platelet count, urea, alanine aminotransferase, albumin, and C-reactive protein. Total scores were calculated using a computer-based interface, and the estimated survival time was expressed as days (0-13 days), weeks (14-55 days), or months (> 55 days). For the D-PaP model, the following data were collected: Dyspnea, anorexia, Karnofsky performance status (KPS), leukocyte count, lymphocyte percentage, and delirium. The total D-PaP score ranged from 0-19.5 points, of which the CPS contributed 0-8.5 points; however, these points were excluded at the time of score calculation. For the PPI model, the degree of dietary intake, swelling, the presence or absence of breathing difficulties at rest, delirium, and the palliative performance scale scores were collected. The total PPI score ranged from 0-15 points.
Simulation computed tomography imaging was performed for treatment planning in all patients. Radiotherapy was prescribed at a dose of 2.5-7 Gy per fraction and up to the median biologically effective dose of 39 Gy10 (range, 28-75 Gy10). In general, for symptom relief, 30 Gy and 10 fractions, 20 Gy and 5 fractions, or 7 Gy and 1 fraction were prescribed. There is no known difference in treatment effect between treatment schedules[6]. Based on these results, the treatment dose and number of fractions were prescribed in consideration of the treatment site and the patient's general condition, as well as the patient's residence, treatment cost, and treatment preference. Primarily, if the patient’s general condition was good and there was no discomfort during movement, a long-term treatment regimen was prescribed, such as 30 Gy and 10 fractions. For patients with discomfort during movement or who lived at a far distance from the hospital, a short-term treatment regimen, such as 20 Gy and 5 fractions or 7 Gy and 1 fraction, was prescribed.
Patient symptom evaluation and physical examinations were performed during and at the end of radiotherapy. Given the patient's general condition and life expectancy, imaging tests at the treatment site were not performed at the time of follow-up. The treatment-response evaluation results were recorded according to the patient's subjective evaluation. Each patient was evaluated according to their main symptoms. The pain level was evaluated by the change in the numerical rating scale (NRS), whereas bleeding was evaluated via the presence or absence of gross bleeding and changes in the amount of bleeding. Neurological symptoms, dyspnea, and obstructive symptoms were evaluated based on improve
Patient characteristics are described in Table 1. The median age of all patients was 82 years (range, 80-92 years), and more than half of the patients were male (69.7%). The ECOG score was 1 point for 112 patients (36.4%), 2 points for 13 patients (36.4%), and 3 points for seven patients (29.4%). Lung cancer was the most common primary tumor. Most patients (72.7%) had metastatic tumors at the time of radiotherapy. Seventeen patients (51.5%) received palliative radiotherapy as the first treatment after tumor diagnosis, and the remaining patients had received other treatments, such as surgery or che
| Characteristics | Total (n = 33) |
| Age (years) | |
| Median | 82 |
| Range | 80-92 |
| Gender | |
| Male | 23 (69.7) |
| Female | 10 (30.3) |
| ECOG | |
| 0 | 1 (3.0) |
| 1 | 12 (36.4) |
| 2 | 13 (39.4) |
| 3 | 7 (21.2) |
| 4 | 0 |
| Primary site of neoplasm | |
| Lung | 13 (39.4) |
| Gastrointestinal | 7 (21.2) |
| Genitourinary | 5 (15.2) |
| Others | 11 (24.2) |
| Distant metastasis | |
| No | 9 (27.3) |
| Yes | 24 (72.7) |
| Previous treatment | |
| No | 17 (51.5) |
| Surgery | 6 (18.2) |
| Chemotherapy | 6 (18.2) |
| Radiotherapy | 1 (3.0) |
| Others | 3 (9.1) |
The distribution of patients according to the prognostic prediction model is described in Table 2. According to the PiPS model, most patients (81.8%) were expected to survive for > 55 days. When considering the D-PaP model, which excludes the PPI and CPS values, about one-third of the patients scored ≥ 4 points.
| Variable | Total (n = 33) |
| PiPS | |
| Months+ (> 55 days) | 27 (81.8) |
| Weeks (14-55 days) | 6 (18.2) |
| Days (0-13 days) | 0 |
| PPI | |
| < 4 | 28 (84.8) |
| ≥ 4 | 5 (15.2) |
| D-PaP without CPS | |
| < 4 | 28 (84.8) |
| ≥ 4 | 5 (15.2) |
There were no patients who demonstrated changes in ECOG scores before and after treatment. The median follow-up duration was 2.4 months (range, 0.2-27.5 months), and five patients (15.2%) died during follow-up. A total of 31 patients had symptoms before treatment. Two patients who had no symptoms were treated with palliative radiotherapy for metastatic brain tumors. Subjective symptom improvement was confirmed in 26 patients (78.8%), whereas the remaining five patients (15.2%) experienced no symptom improvement. Among the patients who were treated for pain, 89.5% (17/19) experienced improvements in their NRS scores. Moreover, all three patients who were treated for bleeding ex
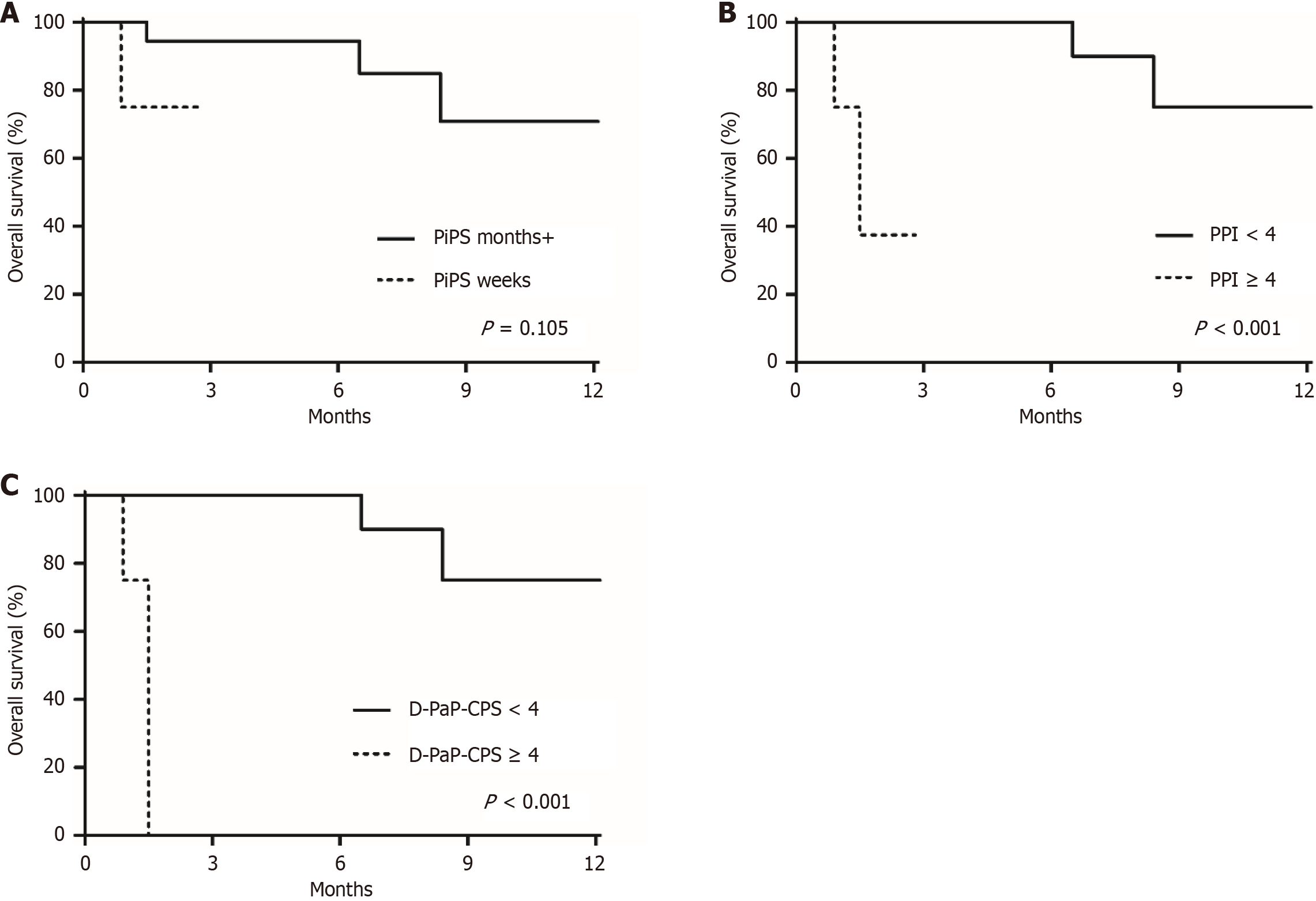
Logistic regression analysis showed that the age, ECOG score, type of symptoms, treatment location, and palliative radiotherapy dose were not significantly associated with treatment response (Table 3). The prognostic prediction model revealed no significant association with treatment response but did reveal a significant association with survival (Figure 1). The 2-month survival rate was 94.4% among the 27 patients who were expected to survive for > 55 days in the PiPS model. Among the six patients who were expected to survive ≤ 55 days in the PiPS model, one patient died at about one month after the end of treatment, whereas the remaining five patients were lost to follow-up. The 2-month survival rate of 28 patients with a PPI score of < 4 points was 100%. Of the remaining five patients who scored ≥ 4 points in this model, three were lost to follow-up, and two died at 1 month and 1.5 months after the end of treatment (P < 0.001). After excluding the CPS, the 2-month survival rates in the D-PaP model were 100% in the < 4 points group and 0% in the ≥ 4 points group (P < 0.001).
| Characteristics | OR (95%CI) | P value |
| Age | 0.807 (0.579-1.126) | 0.207 |
| ECOG | 0.956 (0.303-3.022) | 0.940 |
| Symptoms (pain vs others) | 2.833 (0.389-20.179) | 0.298 |
| Treatment site (bone vs brain vs others) | 0.834 (0.299-2.325) | 0.728 |
| Radiation dose, BED | 1.021 (0.942-1.106) | 0.610 |
| PiPS (Months vs Weeks) | 1.375 (0.120-15.721) | 0.798 |
| PPI (< 4 vs ≥ 4) | 5.111 (0.592-44.146) | 0.138 |
| D-PaP without CPS (< 4 vs ≥ 4) | 1.375 (0.120-15.721) | 0.798 |
Palliative treatment aims to prevent and alleviate pain. It is a treatment approach that improves the quality of life of a patient by diagnosing and evaluating their pain, as well as other physical and psychosocial problems[7]. Cancer patients complain of symptoms due to various causes[8]. Palliative radiotherapy has been used for symptom relief in all types of tumors that cause symptoms since the early 1900s, and its effectiveness has been proven through a number of studies[9]. Approximately half of advanced cancer patients receive radiotherapy, and 30%-40% of radiotherapy are used to relieve symptoms in cancer patients[10]. However, in older patients, the rate of cancer treatment, including palliative radio
Similarly, age was not a prognostic factor for survival in older patients treated with palliative radiotherapy[14]. Nieder et al[14] reported that one-year survival rates after palliative radiotherapy were 35% and 33% in patients who were 80 years or older and who were younger than 80 years, respectively. Brain metastasis and poor performance status were significant prognostic factors for survival. These results have also been confirmed in other studies[12,15]. Katano et al[15] analyzed the clinical outcomes of palliative radiotherapy in patients with head and neck squamous cell carcinoma. The results showed that the one-year survival rate was 35.6%, and the KPS was the only significant prognostic factor for survival. A study published by Hickish et al[12] analyzed 290 patients who received palliative chemotherapy for lung cancer and reported that performance status was a significant prognostic factor for survival, regardless of patient age.
Although there is a beneficial effect of palliative radiotherapy, if immediate death is expected, the expected treatment side effects outweigh symptom relief, or treatment compliance is low, the treatment effect will not be significant[16]. Therefore, it is important to select appropriate patients who can be expected to experience treatment effects while minimizing side effects via prognosis prediction[17]. However, it is not easy to predict a patient's prognosis and deter
These prognostic prediction models have several limitations. There is controversy with respect to which model most accurately predicts prognosis; however, it can be appropriate to apply different models depending on the clinical situation and available variables[5]. A prognostic prediction model can be applied to patients who receive palliative radiotherapy, as performed in this study. With proper prognosis prediction, unnecessary treatment can be avoided in patients with a short life expectancy, and appropriate patient selection for palliative radiotherapy is possible. Another limitation is that the models use patient symptoms such as dyspnea, dysphagia, and edema. These symptoms can be relieved after treatment. Therefore, physician can still be important, and caution is required when these prediction models are used.
This study has several limitations. First, the number of patients was low, and the number of patients who were lost to follow-up loss was high. Therefore, the statistical robustness of the study is weak. In addition, it was difficult to evaluate treatment toxicity and treatment responses through long-term follow-up. Second, due to the inherent nature of re
This study shows that palliative radiotherapy can be used as an effective palliative treatment in older patients. In order to increase the therapeutic effectiveness of palliative radiotherapy, it is necessary to assess a patient's exact prognosis and select appropriate patients accordingly. Although no factors were significantly correlated with symptom improvement, this study showed that good performance status and dietary intake were correlated with improved prognosis. Therefore, it is important to consider these factors in determining the use of palliative radiotherapy in such patients. This study confirmed the possibility that the existing prognostic prediction model can be applied to patients who receive palliative radiotherapy and that more appropriate treatment can consequently be provided to patients through prognostic prediction.
| 1. | Campos S, Presutti R, Zhang L, Salvo N, Hird A, Tsao M, Barnes EA, Danjoux C, Sahgal A, Mitera G, Sinclair E, DeAngelis C, Nguyen J, Napolskikh J, Chow E. Elderly patients with painful bone metastases should be offered palliative radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1500-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Kim ES, Lee JK, Kim MH, Noh HM, Jin YH. Validation of the prognosis in palliative care study predictor models in terminal cancer patients. Korean J Fam Med. 2014;35:283-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Townsley C, Pond GR, Peloza B, Kok J, Naidoo K, Dale D, Herbert C, Holowaty E, Straus S, Siu LL. Analysis of treatment practices for elderly cancer patients in Ontario, Canada. J Clin Oncol. 2005;23:3802-3810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Jones JA, Simone CB 2nd. Palliative radiotherapy for advanced malignancies in a changing oncologic landscape: guiding principles and practice implementation. Ann Palliat Med. 2014;3:192-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 5. | Baba M, Maeda I, Morita T, Inoue S, Ikenaga M, Matsumoto Y, Sekine R, Yamaguchi T, Hirohashi T, Tajima T, Tatara R, Watanabe H, Otani H, Takigawa C, Matsuda Y, Nagaoka H, Mori M, Tei Y, Hiramoto S, Suga A, Kinoshita H. Survival prediction for advanced cancer patients in the real world: A comparison of the Palliative Prognostic Score, Delirium-Palliative Prognostic Score, Palliative Prognostic Index and modified Prognosis in Palliative Care Study predictor model. Eur J Cancer. 2015;51:1618-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Chow R, Hoskin P, Chan S, Mesci A, Hollenberg D, Lam H, DeAngelis C, Chow E. Efficacy of multiple fraction conventional radiation therapy for painful uncomplicated bone metastases: A systematic review. Radiother Oncol. 2017;122:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Meyers DS. Palliative Radiation Therapy for the Hospice Patient: Nuances of Care and Reimbursement. J Oncol Pract. 2015;11:e87-e88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 8. | Enck RE. Palliative radiation therapy in hospice care. Am J Hosp Palliat Care. 2002;19:151-152. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Lutz S, Spence C, Chow E, Janjan N, Connor S. Survey on use of palliative radiotherapy in hospice care. J Clin Oncol. 2004;22:3581-3586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Shi DD, DiGiovanni J, Skamene S, Noveroske Philbrick S, Wang Y, Barnes EA, Chow E, Sullivan A, Balboni TA. Patterns of symptom control and palliative care-focused original research articles in the International Journal of Radiation Oncology *Biology* Physics and the Radiotherapy and Oncology Journal, 2005-2014. Ann Palliat Med. 2018;7:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Zachariah B, Balducci L, Venkattaramanabalaji GV, Casey L, Greenberg HM, DelRegato JA. Radiotherapy for cancer patients aged 80 and older: a study of effectiveness and side effects. Int J Radiat Oncol Biol Phys. 1997;39:1125-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 109] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 12. | Hickish TF, Smith IE, O'Brien ME, Ashley S, Middleton G. Clinical benefit from palliative chemotherapy in non-small-cell lung cancer extends to the elderly and those with poor prognostic factors. Br J Cancer. 1998;78:28-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Turner NJ, Muers MF, Haward RA, Mulley GP. Do elderly people with lung cancer benefit from palliative radiotherapy? Lung Cancer. 2005;49:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Nieder C, Angelo K, Haukland E, Pawinski A. Survival after palliative radiotherapy in geriatric cancer patients. Anticancer Res. 2014;34:6641-6645. [PubMed] |
| 15. | Katano A, Minamitani M, Tongyu G, Ohira S, Yamashita H. Survival Following Palliative Radiotherapy for Head and Neck Squamous Cell Carcinoma: Examining Treatment Indications in Elderly Patients. Cancer Diagn Progn. 2024;4:46-50. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Aggarwal A, Hughes S. Palliative radiotherapy: Evolving role and policy challenges. J Cancer Policy. 2016;10:21-29. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Jones JA, Lutz ST, Chow E, Johnstone PA. Palliative radiotherapy at the end of life: a critical review. CA Cancer J Clin. 2014;64:296-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Lutz ST, Jones J, Chow E. Role of radiation therapy in palliative care of the patient with cancer. J Clin Oncol. 2014;32:2913-2919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 19. | Gripp S, Mjartan S, Boelke E, Willers R. Palliative radiotherapy tailored to life expectancy in end-stage cancer patients: reality or myth? Cancer. 2010;116:3251-3256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Chen ATC, Mauro GP, Gabrielli F, Chaves CLG, Castro I, Vasconcelos KM, Reis M, Saraiva T, Carvalho HA. PROGRAD - An observational study of the prognosis of inpatients evaluated for palliative radiotherapy. Radiother Oncol. 2018;127:299-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Uneno Y, Kanai M. Prognosis Prediction Models and their Clinical Utility in Palliative Care. Highlights on Several Underestimated Topics in Palliative Care. 2017. [DOI] [Full Text] |
| 22. | Niki K, Okamoto Y, Yasui M, Omae T, Kohno M, Matsuda Y. Is a Combination of Six Clinical Tests Useful as a Measure to Predict Short-Term Prognosis in Terminal Cancer Patients? A Prospective Observational Study in a Japanese Palliative Care Unit. Palliat Med Rep. 2024;5:430-437. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |