Published online Mar 24, 2025. doi: 10.5306/wjco.v16.i3.101681
Revised: December 1, 2024
Accepted: December 27, 2024
Published online: March 24, 2025
Processing time: 120 Days and 0.9 Hours
Flat bone metastases are common in patients with advanced cancers, often resulting in severe pain, limited mobility, and reduced quality of life (QOL). Traditional treatment options, such as radiotherapy or systemic therapies, often fail to provide sufficient pain relief or improve functional outcomes in these patients. Microwave ablation (MWA) offers advantages, such as shorter pro
To evaluate the efficacy and safety of C-arm computed tomography (CT)-guided MWA combined with PO for managing painful flat bone metastases, focusing on pain relief, functional improvement, and QOL enhancement.
A total of 45 patients with refractory moderate-to-severe pain resulting from flat bone metastases who underwent C-arm CT-guided MWA combined with PO between January 2015 and January 2021 were included. The efficacy of the pro
No serious complications were observed in any of the patients. A significant reduction in VAS and ODI was noted at 1 week, 1 month, and 3 months post-procedure. A marked improvement in QOL was observed at all follow-up points. Bone cement extravasation was observed in 10 patients; however, none exhibited significant clinical symptoms. Based on RECIST v1.1, the ORR was 26.7% and the DCR was 88.9%. The mRECIST evaluation revealed a higher ORR of 51.1% and DCR of 88.9%.
C-arm CT-guided MWA with PO provides a dependable and effective strategy for managing flat bone metastases. It demonstrates significant pain relief, improved functional outcomes, and enhanced QOL. This treatment combination also shows promising tumor response rates with a low complication profile.
Core Tip: This study retrospectively evaluated the effectiveness and safety of using C-arm computed tomography-guided microwave ablation in conjunction with percutaneous osteoplasty to manage painful flat bone metastases. The key outcomes included postoperative pain relief, functional improvement, quality of life (QOL) enhancement, and occurrence of serious complications. The results indicate that this combined therapy is effective and safe for managing painful flat bone metastases. It offers significant benefits for pain control, functionality, and QOL.
- Citation: Lin ZP, Zou XG, Huang DB, Chen Y, Lin JW, Li XQ, Zhang J. Efficacy and safety of C-arm computed tomography-guided microwave ablation with percutaneous osteoplasty for flat bone metastases. World J Clin Oncol 2025; 16(3): 101681
- URL: https://www.wjgnet.com/2218-4333/full/v16/i3/101681.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i3.101681
Bone metastases occur in 50%-70% of patients with advanced cancer[1]. The spine is the most common site, followed by the pelvis, ribs, and proximal femur[2]. Patients with bone metastases often experience debilitating pain, an increased likelihood of pathological fractures, and mobility restrictions, leading to a significant decline in quality of life (QOL)[3]. Severe pain occurs in nearly 75% of patients with bone metastases, whereas 56%-82.3% of these cases are undertreated[4]. Pain relief and the prevention of complications are the primary goals when managing patients with advanced bone metastases, particularly those who have a limited life expectancy and poor overall health.
Percutaneous thermal ablation has become widely adopted for treating bone metastases. Compared with radiofrequency ablation (RFA), microwave ablation (MWA) has several advantages, such as faster heating, shorter procedure time, larger ablation zones, and no requirement for grounding electrodes[5]. In addition, percutaneous osteoplasty (PO) enhances bone stability, which effectively prevents and ameliorates pathological fractures[6].
There are few studies describing the use of MWA combined with PO for flat bone metastases, such as those occurring in the ribs, ilium, and scapula. Flat bones primarily consist of cancellous bone with a thin cortical bone and poor mechanical strength. They are frequently associated with osteolysis and osteoporosis, which increases the difficulty of needle stabilization during puncture. In addition, the limited thickness of the cortical bone restricts the volume of the bone cement injected and increases the risk of leakage. Specific challenges involve the ribs, which are close to the pleura and lungs and pose a risk of mis-puncture. Moreover, the scapula, surrounded by muscles and ligaments, requires precise planning to avoid tissue injury, and the ilium, with its proximity to neurovascular structures, necessitates careful navigation. During the procedure, needle trajectory planning and depth measurements are dependent on C-arm com
Because of the anatomical and clinical complexities of flat bones as well as the limited research available, a detailed examination of interventional treatments for flat bone metastases is necessary and valuable. This retrospective analysis determined the therapeutic effects of C-arm CT-guided MWA combined with PO for treating flat bone metastases. We focused on postoperative outcomes, including pain relief, functional improvement, QOL, and complications, to de
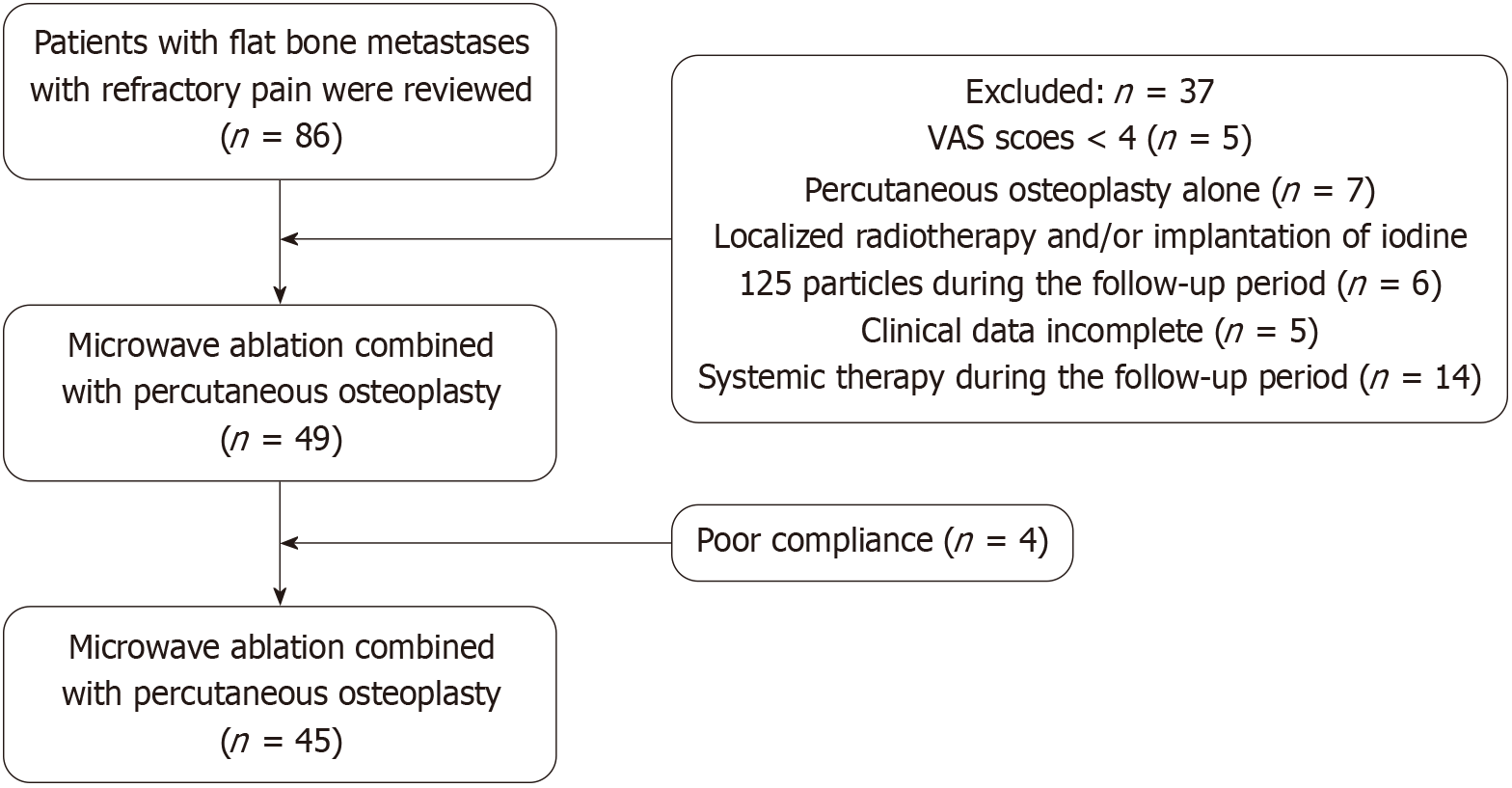
The ethics committee of our institution reviewed and approved this study (approval number: 2024-043) and determined that individual informed consent was not required due to the retrospective design. Patients who underwent MWA combined with PO for flat bone metastases at our hospital between January 2015 and January 2021 were included. The inclusion criteria were: (1) Focal pain limited to 1-2 areas with bone metastases confirmed by imaging; and (2) Intractable pain [visual analog scale (VAS) ≥ 4] unresponsive to conventional treatment (e.g., opioids, chemotherapy, or radiation). The exclusion criteria included: (1) Unmanageable coagulation abnormalities; (2) Active infections near the targeted lesion; (3) Significant organ dysfunction involving the liver, kidneys, heart, or lungs; (4) Proximity to critical nerves or blood vessels (< 1 cm from the tumor edge); (5) Incomplete data; (6) Radiotherapy targeting a specific area, cryoablation, or iodine-125 seed implantation performed during the follow-up period; and (7) Administration of systemic treatments during the follow-up phase. Figure 1 provides an overview of the study workflow. This research was carried out fo
The interventional plan was formulated based on the lesion site and scope observed in the CT or magnetic resonance imaging (MRI) images while avoiding critical nerves and blood vessels. When possible, the shortest and most convenient approach to the area of greatest bone destruction was selected. To determine the appropriate needle lengths, the distance from the entry point to the lesion was carefully measured.
To optimize the operation time, ensure patient comfort, and maintain positional stability, most patients were placed in either a supine or prone position. Following local anesthesia administration, a 13G bone puncture needle was guided into the lesion center using digital subtraction angiography (DSA). Two- and three-dimensional reconstruction was performed using C-arm CT imaging technology. Reconstruction techniques such as maximum intensity projection (MIP), mul
Tomographic images reconstructed by C-arm CT were used to confirm that the puncture needle had reached the target lesion. After confirming the accurate placement of the puncture needle, the stylet (inner needle core) was withdrawn, and a standard 16G ablation needle was introduced into the lesion. The puncture needle was retracted slightly to ensure that the working section of the ablation needle was fully exposed. The power and ablation time were adjusted based on the size and extent of the target lesion.
After completing thermal ablation, the ablation needle was removed and the bone puncture needle was repositioned into the target lesion. Under continuous DSA fluoroscopic monitoring, mixed bone cement was injected into the target lesion via the puncture needle to initially evaluate the risk of cement leakage. Postoperatively, C-arm CT imaging was conducted for two- and three-dimensional reconstruction to evaluate the distribution of the bone cement and determine the extent of leakage.
The efficacy of the procedure was determined by evaluating the VAS, Oswestry disability index (ODI), and QOL scores at baseline (before the procedure) and at 1 day, 1 week, 1 month, and 3 months following the procedure. The ODI evaluates a wide range of functional domains, such as pain intensity, lifting, walking, sitting, and daily activities that are relevant to patients with flat bone metastases. Although originally developed for lower back pain, its components overlap with the functional impairments caused by metastases in flat bones, such as the scapula, ribs, and ileum. These afflictions can similarly restrict mobility and daily functioning. For example, rib metastases can restrict breathing and movement; scapular metastases may impair upper limb mobility to affect daily activities such as dressing or reaching; and ilium metastases can limit walking and standing because of pelvic instability and pain. Although not region-specific, the ODI provides a standardized tool to capture these functional limitations. There are currently limited validated tools spe
Tumor response in the target lesion was assessed using RECIST v1.1 and mRECIST criteria, which were tailored specifically for local target lesions to assess the efficacy of MWA combined with PO.
RECIST v1.1 is a global tumor assessment tool that evaluates the sum of the longest diameters of all target lesions. In this study, it was applied to local target lesions to assess the changes in the entire lesion, including both necrotic and viable components. This method is particularly relevant for assessing structural changes following treatment. Conversely, mRECIST focuses solely on the viable (enhancing) portion of the tumor as identified by arterial-phase imaging. By applying mRECIST to local target lesions, we evaluated the reduction in viable tumor burden, which is a key indicator of locoregional treatment efficacy.
According to Local Target Lesion RECIST v1.1, complete response (CR) was defined as the disappearance of all measurable components of the target lesion upon imaging; partial response (PR) as a ≥ 30% reduction in the longest diameter of the target lesion; stable disease (SD) as neither sufficient shrinkage to qualify for a PR, nor a sufficient in
According to Local Target Lesion mRECIST, CR was defined as the complete disappearance of the viable (enhancing) portion of the target lesion; PR as a ≥ 30% reduction in the viable tumor size of the target lesion; SD as a reduction of < 30% or increase < 20% in viable tumor size, and PD as a ≥ 20% increase in viable tumor size or the appearance of new lesions.
The tumor size and viable tumor size were measured using CT or MRI before and at 1- and 3-month follow-ups. Two radiologists, each with over 10 years of expertise in oncologic imaging, independently conducted the measurements, resolving discrepancies through discussion. Overall response rate (ORR) was defined as the sum of PR and CR, while disease control rate (DCR) was calculated as PR, CR, and SD combined.
Data analyses were conducted using SPSS 20.0 software. Normally distributed measurements are represented as the mean ± SD. A paired t-test was used to analyze changes in VAS, ODI, and QOL scores before and after the procedure. P < 0.05 was considered statistically significant.
The cohort included 35 male patients and 10 female patients aged between 23 and 76 years, with a mean age of 62.3 ± 10.4 years. The largest lesion in the target area was 8.2 cm × 2.5 cm. There were 20 cases of rib metastases, 16 cases of scapular metastases, and 9 cases of ilium metastases. All patients had bone metastases from malignant tumors as confirmed by pathological or imaging data. The primary malignancies included 19 cases of primary liver cancer, 15 cases of lung cancer, 3 cases of cholangiocarcinoma, 2 cases of esophageal cancer, 1 case of pheochromocytoma, 1 case of multiple myeloma, 1 case of colon cancer, and 3 cases of nasopharyngeal carcinoma (Table 1). All patients underwent MWA combined with PO. Before the procedure, the patients had poor responses to traditional treatment, such as analgesics and radiotherapy, and had received imaging evaluations such as CT, MRI, or positron emission tomography/CT.
| Classification | Number | % |
| Age | 62.3 ± 10.4 | - |
| Sex | ||
| Male | 35 | 77.8 |
| Female | 10 | 22.2 |
| Primary tumor | ||
| Primary liver cancer | 19 | 42.3 |
| Lung cancer | 15 | 33.3 |
| Cholangiocarcinoma | 3 | 6.7 |
| Esophageal cancer | 2 | 4.4 |
| Pheochromocytoma | 1 | 2.2 |
| Multiple myeloma | 1 | 2.2 |
| Colon cancer | 1 | 2.2 |
| Nasopharyngeal carcinoma | 3 | 6.7 |
| Site of bone metastases | ||
| Rib | 20 | 44.4 |
| Shoulder blade | 16 | 35.6 |
| Ilium | 9 | 20 |
| Lesion size | ||
| < 3 cm | 13 | 28.9 |
| ≥ 3 cm | 32 | 71.1 |
| Nature of the lesion | ||
| Osteolytic | 40 | 88.9 |
| Osteogenic | 0 | 0 |
| Hybrid | 5 | 11.1 |
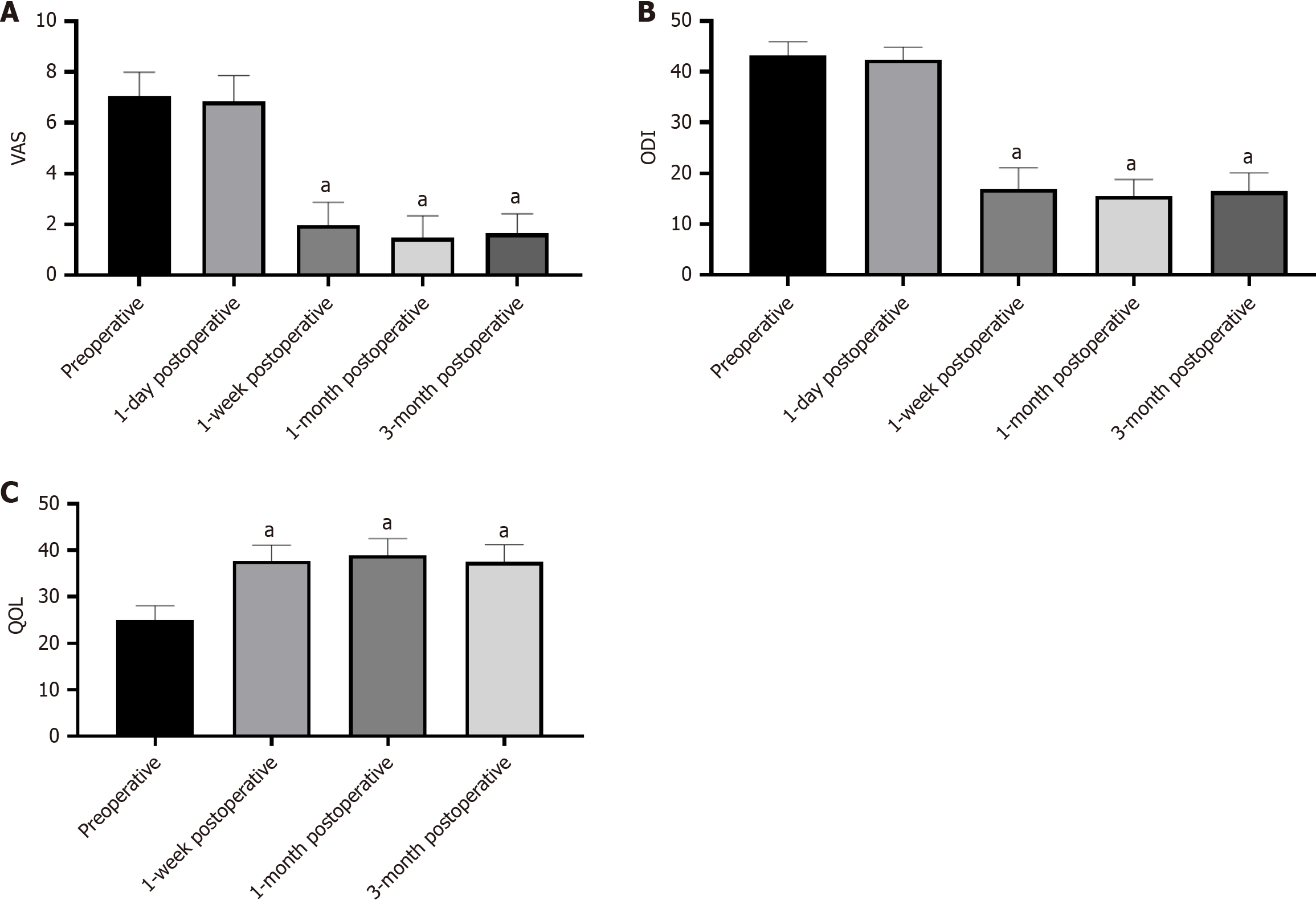
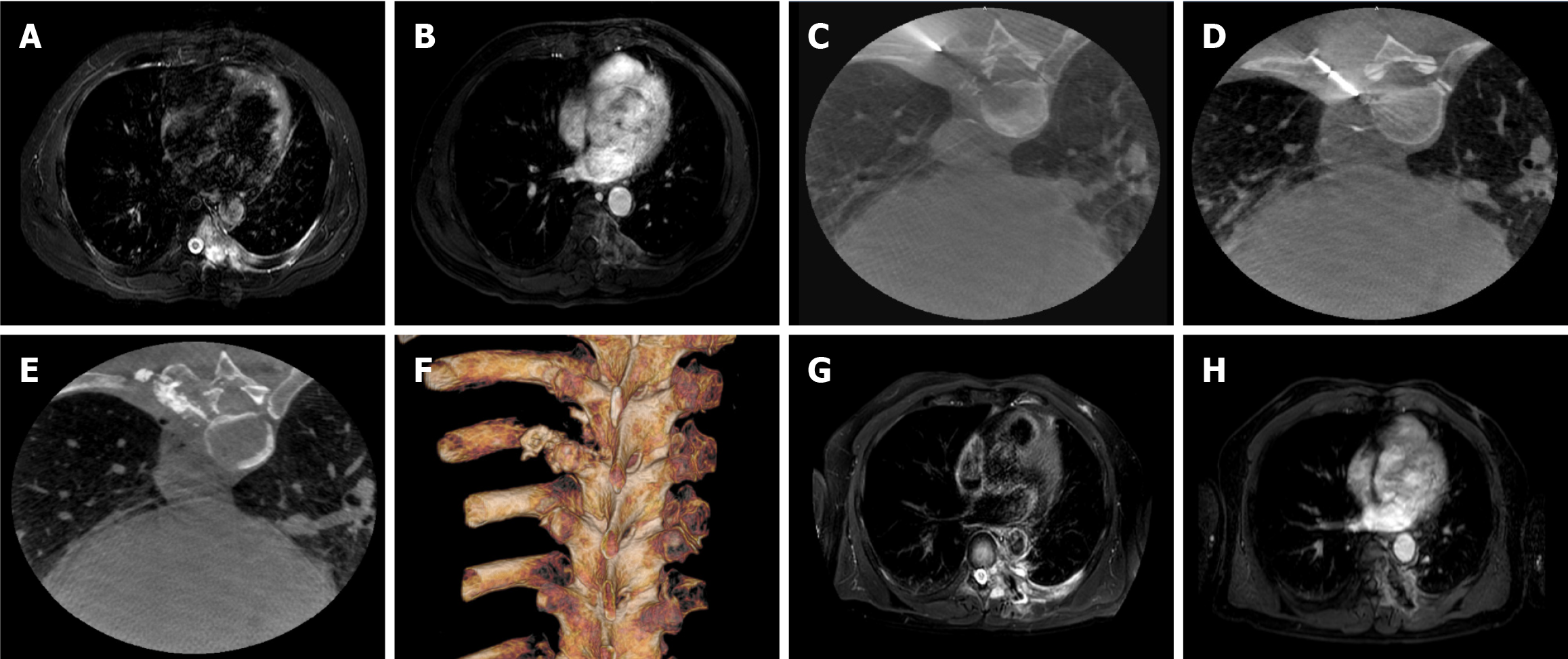
The recorded VAS scores were 7.04 ± 0.95 before procedure, 6.84 ± 1.02 at 1 day post-procedure, 1.98 ± 0.89 at 1 week, 1.47 ± 0.87 at 1 month, and 1.64 ± 0.77 at 3 months post-procedure. Compared with preoperative VAS, the scores at 1 week (t = 29.4, P < 0.001), 1 month (t = 31.3, P < 0.001), and 3 months (t = 34.4, P < 0.001) after the procedure decreased significantly, whereas the VAS score at 1 day after the procedure was not significantly lower compared with that before the procedure (t = 1.59, P = 0.12; Figure 2A). The ODI scores were recorded as 43.20 ± 0.68 pre-procedure, 42.36 ± 2.52 at 1 day post-procedure, 16.96 ± 4.11 at 1 week, 15.56 ± 3.27 at 1 month, and 16.49 ± 3.61 at 3 months after procedure. The ODI scores at 1 week (t = 38.5, P < 0.001), 1 month (t = 42.9, P < 0.001), and 3 months (t = 41.1, P < 0.001) after the procedure were significantly lower compared with that before the procedure (Figure 2B). The QOL scores were recorded as 24.89 ± 3.22 pre-procedure, 37.78 ± 3.38 at 1 week post-procedure, 38.91 ± 3.62 at 1 month, and 37.49 ± 3.75 at 3 months after procedure. The QOL increased significantly at 1 week (t = -20.9, P < 0.001), 1 month (t = -22.6, P < 0.001), and 3 months (t = -20.9, P < 0.001) after the procedure compared with the preoperative value (Figure 2C). All patients were followed by imaging examinations at 1 and 3 months following the procedure. The treatment process for a patient with lung cancer and left 7th rib metastasis is shown in Figure 3.
Target lesion tumor treatment responses were assessed using mRECIST and RECIST v1.1. The number of cases of CR, PR, SD, and PD as assessed by mRECIST was 0, 23 (51.1%), 17 (37.8%), and 5 (11.1%), respectively. The ORR was 51.1% and the DCR was 88.9%. The number of cases of CR, PR, SD, and PD as assessed by RECIST v1.1 was 0, 12 (26.7%), 28 (62.2%), and 5 (11.1%), respectively. The ORR was 26.7% and the DCR was 88.9% (Table 2).
| Variable | CR | PR | SD | PD | ORR | DCR |
| mRECIST | 0 (0) | 23 (51.1) | 17 (37.8) | 5 (11.1) | 23 (51.1) | 40 (88.9) |
| RECIST v1.1 | 0 (0) | 12 (26.7) | 28 (62.2) | 5 (11.1) | 12 (26.7) | 40 (88.9) |
A small amount of bone cement extravasation was observed in 10 patients, but none of the patients developed significant clinical symptoms. One patient (3.3%) developed a localized skin infection along the puncture tract, which improved after symptomatic treatment. No other complications, such as skin burns, nerve injury, bone cement embolism, or perioperative death, were observed.
Bone metastases are common in advanced cancer patients, where the main objectives of treatment are pain relief, complication prevention, and enhancing QOL. For advanced cancer patients, surgery is rarely the optimal choice. While radiotherapy can help reduce cancer-associated pain, it typically takes 5 to 20 weeks to produce palliative effects and has a pain relief success rate of only 60% to 70%[7]. For treating bone metastases, percutaneous thermal ablation provides benefits such as affordability, real-time imaging, integration with other treatment modalities, reliability, and quick procedural times. RFA generates heat through high-frequency currents to produce a high local temperature (70 °C-100 °C) within the tumor tissue to induce coagulative necrosis[8]. Similarly, MWA induces coagulative necrosis by heating tissues, a process driven by the agitation of water molecules through electromagnetic microwave energy[9]. Both thermal ablation methods effectively cause coagulative necrosis of the tumor cells. Compared with RFA, MWA is more effective for high-impedance tissues. Bone tissue, characterized by its relative permeability and low conductivity, enables deeper microwave penetration[10]. Thermal ablation alleviates cancer-related pain in bone metastases through several mechanisms: (1) Disrupting pain fibers in the periosteum and bone cortex, thereby reducing pain signaling; (2) Decreasing tumor load, which minimizes nerve-ending stimulation; (3) Suppressing osteoclast activity around tumor cells; and (4) Inducing local coagulative necrosis, which lowers the production of pain-inducing cytokines, such as interleukins and tumor necrosis factor-α[11].
Osteonecrosis and osteoporosis may occur following bone ablation in weight-bearing areas. Bone cement has an important role in increasing bone stability, reducing the incidence of pathological fractures, alleviating pain, and controlling tumor progression. The analgesic mechanism of PO may involve the following factors: (1) Thermal effect: Heat released as bone cement hardens destroys tumor cells and pain-transmitting nerve endings, providing lasting ablation; (2) Mechanical effect: By reinforcing bone structure, cement injection stabilizes micro-fractures, limits small-scale movement at fracture ends, and increases vertebral support, alleviating tissue compression and reducing friction; (3) Vascular effect: Bone cement blocks the blood supply to local tissues, damaging tumor cells and nociceptive peripheral nerves; and (4) Chemical toxicity: The toxic effects of bone cement are detrimental to tumor cells and nerve cells[12,13]. Masala et al[14] demonstrated that the combined effects of ablation and PO are synergistic, thus enhancing overall treatment efficacy. Similarly, Halpin et al[15] reported that thermal ablation combined with PO reduces the spread of tumor cells, induces venous plexus thrombosis, and decreases the risk of bone cement extravasation.
Flat bones include the ribs, scapula, and ilium. Rib metastases cause localized pain and in severe cases, impair breathing. The scapula connects the upper extremity to the spine and facilitates six types of movements: Lifting, lowering, external rotation, internal rotation, abduction, and adduction. Metastases involving the scapula can restrict upper limb mobility, which significantly affects daily activities. As part of the pelvis, the ilium supports the trunk and connects the lower limbs. It is traversed by major blood vessels and nerves, thus pelvic bone metastases are particularly impactful by limiting lower limb movement. Overall, flat bone metastases result in severe localized pain and restricted limb mobility, profoundly affecting patient QOL.
There are no specific reports focused on the treatment of flat bone metastases using percutaneous MWA combined with PO. Limited studies have examined the safety and efficacy of MWA combined with PO for the treatment of extraspinal bone metastases. Pusceddu et al[16] reported on 35 patients with 37 bone metastases, including 25 extraspinal metastases, who underwent MWA combined with PO. The results indicated a reduction in VAS scores by 84%, 90%, and 90% at 1 week, 1 month, and 6 months post-procedure, respectively, with no severe complications. Similarly, Wei et al[17] reported 33 extraspinal bone metastases in 26 Lung cancer patients treated by MWA combined with PO. All procedures were successful, with significant pain relief; however, complications occurred in eight patients (28%).
In the present retrospective analysis, a large number of extra-vertebral metastases were treated by CT-guided MWA combined with PO. VAS scores were significantly reduced during follow-up, consistent with the findings from the aforementioned studies. For functional assessment, the ODI scores for the patients showed significant improvement, QOL notably increased, and mobility and overall QOL were markedly enhanced.
All of the patients in the present study successfully completed the procedure without serious complications. One case of skin infection was observed, which is consistent with a previous report[18]. To minimize the risk of local infections, we recommend the following: (1) Strict adherence to aseptic techniques throughout the procedure; (2) Prophylactic use of antibiotics in cases involving large lesions or prolonged ablation times. If necessary, antibiotics may be incorporated into the bone cement to further reduce the risk of infection; and (3) Determining the skin needle insertion point based on imaging exams before the procedure to avoid further adjustments during the procedure. Bone cement leakage has been described in the literature[18]. The incidence after PO varies across studies and may be influenced by factors, such as cortical defects and the sensitivity of imaging modalities used for procedure guidance and detection of extravasation[19]. In the present study, bone cement leakage was observed in 10 cases (22.2%) during the procedure; however, no significant clinical symptoms were observed, such as neurological impairment or damage to adjacent structures. To mitigate this risk, we recommend the following: (1) Completing the injection of bone cement under continuous fluoroscopic mo
In this retrospective analysis, treatments were performed using C-arm CT, an imaging device that combines a C-arm digital flat-panel detector and upgraded CT reconstruction technology. After the information is collected by the C-arm CT vascular tablet machine and transmitted to the imaging workstation, MPR, MIP, and VR post-processing techniques are used to generate CT-like soft tissue tomographic images within 5 s. This enables the immediate visualization of the positions of the bone cement needle, ablation needle, and the distribution of the bone cement, thus enhancing the ef
This retrospective study had several limitations. First, the heterogeneity of the different primary tumors and different pathological types cannot be excluded. Second, it was a single-center study. Third, the follow-up time was short as long-term follow-up data was not obtained.
In conclusion, C-arm CT-guided MWA combined with PO is an effective, safe, and clinically viable treatment option for flat bone metastases.
| 1. | Cetin K, Christiansen CF, Jacobsen JB, Nørgaard M, Sørensen HT. Bone metastasis, skeletal-related events, and mortality in lung cancer patients: a Danish population-based cohort study. Lung Cancer. 2014;86:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 2. | Kakhki VR, Anvari K, Sadeghi R, Mahmoudian AS, Torabian-Kakhki M. Pattern and distribution of bone metastases in common malignant tumors. Nucl Med Rev Cent East Eur. 2013;16:66-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Soeharno H, Povegliano L, Choong PF. Multimodal Treatment of Bone Metastasis-A Surgical Perspective. Front Endocrinol (Lausanne). 2018;9:518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J; ESMO Guidelines Working Group. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2014;25 Suppl 3:iii124-iii137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 369] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 5. | Kurup AN, Callstrom MR. Ablation of skeletal metastases: current status. J Vasc Interv Radiol. 2010;21:S242-S250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Clark W, Bird P, Gonski P, Diamond TH, Smerdely P, McNeil HP, Schlaphoff G, Bryant C, Barnes E, Gebski V. Safety and efficacy of vertebroplasty for acute painful osteoporotic fractures (VAPOUR): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2016;388:1408-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 284] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 7. | Zeng L, Chow E, Bedard G, Zhang L, Fairchild A, Vassiliou V, Alm El-Din MA, Jesus-Garcia R, Kumar A, Forges F, Tseng LM, Hou MF, Chie WC, Bottomley A. Quality of life after palliative radiation therapy for patients with painful bone metastases: results of an international study validating the EORTC QLQ-BM22. Int J Radiat Oncol Biol Phys. 2012;84:e337-e342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Qiu YY, Wu QS, Zhang XS, Yang S, Zhang KX. Progress of image-guided physical ablation and percutaneous cementoplasty for bone metastatic pain. Zhongguo Jieruyingxiang Yu Zhiliaoxue. 2019;16:121-124. [DOI] [Full Text] |
| 9. | Lubner MG, Brace CL, Hinshaw JL, Lee FT Jr. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21:S192-S203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 582] [Cited by in RCA: 491] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 10. | Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics. 2005;25 Suppl 1:S69-S83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 592] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 11. | Botsa E, Mylona S, Koutsogiannis I, Koundouraki A, Thanos L. CT image guided thermal ablation techniques for palliation of painful bone metastases. Ann Palliat Med. 2014;3:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 12. | Anselmetti GC, Manca A, Kanika K, Murphy K, Eminefendic H, Masala S, Regge D. Temperature measurement during polymerization of bone cement in percutaneous vertebroplasty: an in vivo study in humans. Intervent Radiol. 2009;32:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Yang HL, Sun ZY, Wu GZ, Chen KW, Gu Y, Qian ZL. Do vertebroplasty and kyphoplasty have an antitumoral effect? Med Hypotheses. 2011;76:145-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Masala S, Lunardi P, Fiori R, Liccardo G, Massari F, Ursone A, Simonetti G. Vertebroplasty and kyphoplasty in the treatment of malignant vertebral fractures. J Chemother. 2004;16 Suppl 5:30-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Halpin RJ, Bendok BR, Sato KT, Liu JC, Patel JD, Rosen ST. Combination treatment of vertebral metastases using image-guided percutaneous radiofrequency ablation and vertebroplasty: a case report. Surg Neurol. 2005;63:469-74; discussion 474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Pusceddu C, Sotgia B, Fele RM, Ballicu N, Melis L. Combined Microwave Ablation and Cementoplasty in Patients with Painful Bone Metastases at High Risk of Fracture. Cardiovasc Intervent Radiol. 2016;39:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Wei Z, Zhang K, Ye X, Yang X, Zheng A, Huang G, Wang J. Computed tomography-guided percutaneous microwave ablation combined with osteoplasty for palliative treatment of painful extraspinal bone metastases from lung cancer. Skeletal Radiol. 2015;44:1485-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Qiu YY, Zhang KX, Ye X, Zhang XS, Xing C, Wu QS, Hu MM, Li PX, Wang JJ. Combination of Microwave Ablation and Percutaneous Osteoplasty for Treatment of Painful Extraspinal Bone Metastasis. J Vasc Interv Radiol. 2019;30:1934-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Kurup AN, Morris JM, Schmit GD, Atwell TD, Schmitz JJ, Rose PS, Callstrom MR. Balloon-assisted osteoplasty of periacetabular tumors following percutaneous cryoablation. J Vasc Interv Radiol. 2015;26:588-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |