Published online Mar 24, 2025. doi: 10.5306/wjco.v16.i3.101240
Revised: December 19, 2024
Accepted: January 2, 2025
Published online: March 24, 2025
Processing time: 134 Days and 17.2 Hours
Paraganglioma (PGL) is a neuroendocrine tumor originating from paraganglia that can occur in various locations, such as the head, neck, chest, abdomen, and pelvis. Retroperitoneal PGLs are rare, and recurrent cases in this area are particularly uncommon, posing considerable surgical complexities. Owing to their neu
We report a 64-year-old man with a recurrent retroperitoneal PGL. The patient underwent retroperitoneal mass resection in 2013, with postoperative pathology revealing a PGL. Regular follow-up was not conducted until April 2024, when a computed tomography scan revealed a huge mass in the retroperitoneum, closely adjacent to the abdominal aorta. Laboratory examinations revealed elevated levels of catecholamines in the patient's blood serum. Upon admission, volume expan
PGL recurrence is rare but non-negligible. PGLs adjacent to major arteries com
Core Tip: Recurrent retroperitoneal paragangliomas are infrequent but pose substantial surgical challenges, particularly when located adjacent to critical vascular structures such as the abdominal aorta. Effective perioperative management of he
- Citation: Feng YF, Pan YF, Zhou HL, Hu ZH, Wang JJ, Chen B. Surgical resection of a recurrent retroperitoneal paraganglioma: A case report. World J Clin Oncol 2025; 16(3): 101240
- URL: https://www.wjgnet.com/2218-4333/full/v16/i3/101240.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i3.101240
Paraganglioma (PGL) is a neuroendocrine tumor originating from paraganglia, which are associated with the autonomic nervous system[1]. These tumors arise from chromaffin cells or similar cells capable of secreting catecholamines, such as adrenaline and noradrenaline. PGLs manifest in various anatomical locations, including the head, neck, chest, abdomen, and pelvis, and are characterized by neurosecretory and chief cells surrounded by prominent vascular stroma. Although typically benign, malignant forms of PGL are also recognized, and they may exhibit a tendency for recurrence or me
In May 2024, a patient with recurrent retroperitoneal PGL was admitted to our hospital, and the details are reported below.
A 64-year-old male patient was admitted on May 2, 2024, due to the discovery of a retroperitoneal mass 1 month prior.
The patient reported no dizziness, headache, chest tightness, shortness of breath, abdominal pain, bloating, or discomfort in the lumbar or sacral regions.
The patient underwent left retroperitoneal mass resection at a local hospital 11 years prior, and postoperative pathology revealed a PGL. Immunohistochemistry revealed positive staining for synaptophysin, chromogranin A, neuron-specific enolase (NSE), glial fibrillary acidic protein, S-100, and cytokeratin as well as a Ki-67 index of approximately 5%. Intraoperative findings revealed that the tumor was in close proximity to the abdominal aorta, celiac trunk, and mesentery, with clear margins and a size of approximately 4 cm × 4 cm. The tumor was completely excised with preservation of the renal hilum, and no lymph nodes were identified at the hilum. The patient experienced significant intraoperative blood pressure (BP) fluctuations, peaking at 280 mmHg. After discharge, the patient did not undergo regular follow-up visits.
The patient denied any family history of tumors.
The vital signs were as follows: (1) Body temperature: 36.3 °C; (2) BP: 102 mmHg/67 mmHg; (3) Heart rate (HR): 85 beats per minute; and (4) Respiratory rate: 20 breaths per minute. Physical examination revealed an old scar of 10 cm on the left abdomen with no tenderness on palpation and normal bowel sounds.
Laboratory tests during hospitalization revealed normal plasma cortisol levels and rhythm, as well as normal plasma renin-angiotensin-aldosterone levels. Serum catecholamine hormones were significantly elevated, including: (1) Adrenaline 449.00 pg/mL (normal reference range < 141.00 pg/mL); (2) Methoxyepinephrine 329.70 pg/mL (normal reference range < 98.60 pg/mL); and (3) Methoxynorepinephrine 930.80 pg/mL (normal reference range < 164.90 pg/mL).
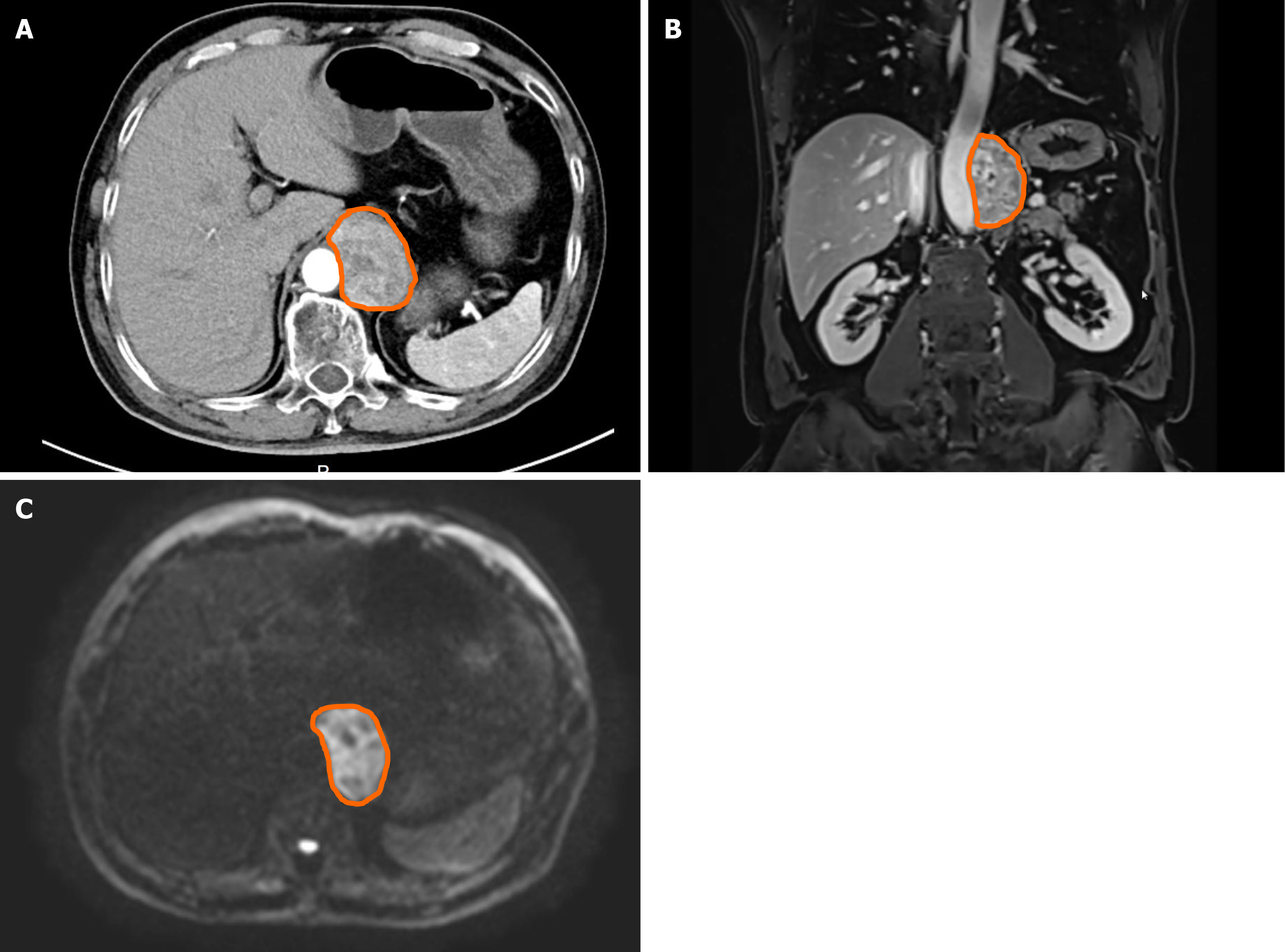
An emergency computed tomography (CT) scan at a local hospital revealed a mass approximately 35 mm × 40 mm in size. Imaging studies at our hospital included a contrast-enhanced CT scan of the abdomen, which revealed a well-defined, heterogeneous retroperitoneal mass measuring approximately 5.5 cm × 4.0 cm × 6.1 cm with cystic changes and heterogeneous enhancement (Figure 1A and B). Abdominal magnetic resonance imaging with diffusion-weighted imaging (DWI) revealed a similar abnormal signal mass in the left retroperitoneal space, with restricted diffusion on DWI and progressive heterogeneous enhancement, indicating a cystic component within the mass (Figure 1C).
The diagnosis was determined to be recurrent PGL.
The patient underwent preoperative expansion fluid replacement at a rate of 1000 mL per day with a crystalloid-colloid ratio of 1:1 for one week before the surgery. Upon admission, BP was maintained at 100−120 mmHg/70−90 mmHg, and HR was 60-90 beats per minute. Serum catecholamine hormones were re-evaluated and normalized one week post-admission. The day before the surgery, the patient underwent a cleansing enema, and a gastric tube was inserted. Central venous catheterization was also performed before the surgery.
The patient underwent surgery on May 15, 2024. Once general anesthesia was successfully induced, the patient was positioned supine, a urinary catheter was inserted, and invasive arterial BP monitoring was established.
Following the administration of prophylactic antibiotics, a combined thoracoabdominal incision (a 15 cm oblique incision along the 6th–7th left rib interspace below the left nipple) was made. The surgery involved layer-by-layer entry into the chest, cutting the left rib arch, pulling the chest with a soft tissue retractor to expose the left pleural cavity, suspending the pericardium with 2–0 silk sutures, and exposing the diaphragm. The procedure continued with a longitudinal incision along the left side of the mediastinum through the diaphragm muscle to the left diaphragmatic crus, revealing the abdominal cavity. Based on imaging data, the tumor was located at the thoracoabdominal junction, po
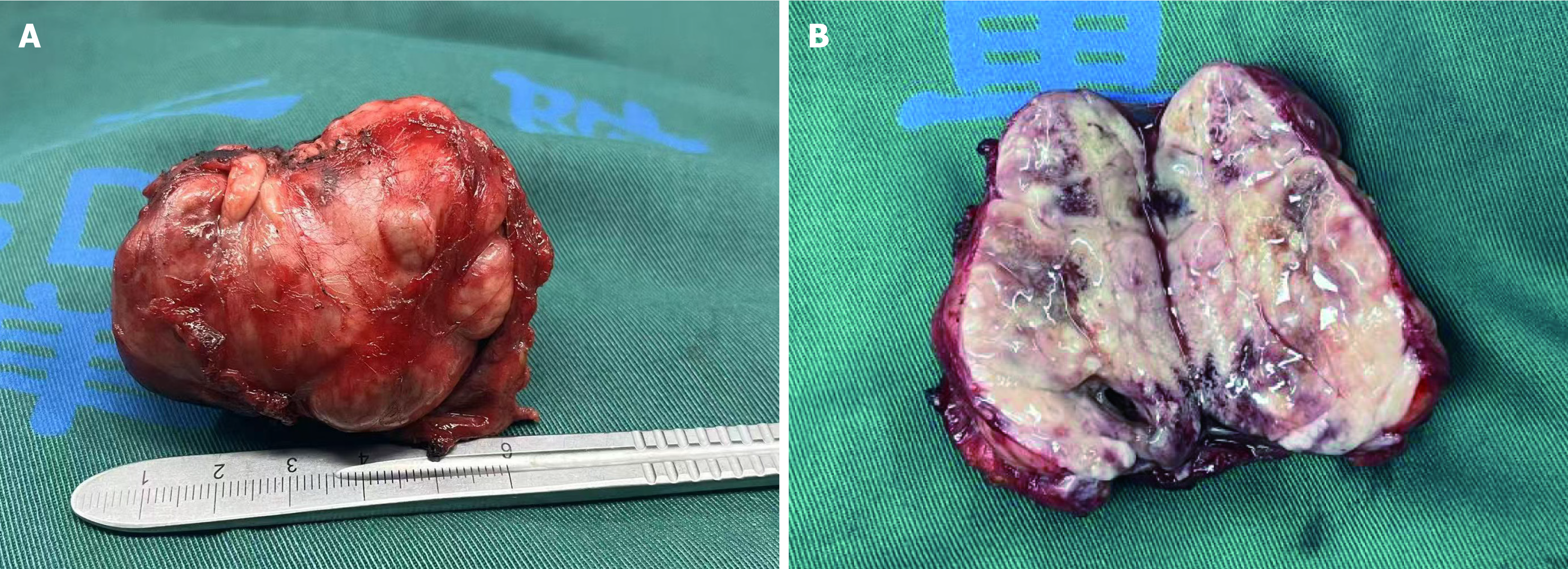
During tumor resection, the surgical and anesthesiology teams maintained close coordination. Upon a rapid rise in systolic BP to 180-200 mmHg, the procedure was promptly halted. Intravenous nitroprusside was administered, along with rapid phentolamine infusion to restore hemodynamic stability. Once systolic BP was reduced to below 140 mmHg, the resection was resumed. The procedure lasted for approximately one and a half hours, during which the surgical team carefully dissected the tumor from the abdominal aorta and surrounding tissues along its outer capsule, achieving complete resection of a tumor measuring approximately 5.0 cm × 5.0 cm (Figure 2).
Although a decrease in systolic BP was anticipated following tumor resection, the rate of reduction was unexpectedly rapid, with a precipitous drop from 240 mmHg to 30 mmHg. The anesthesiology team swiftly implemented volume resuscitation and vasopressor therapy, administering norepinephrine and intravenous epinephrine. Despite three doses of 1 mg epinephrine over 10 minutes, the systolic BP increased only marginally. It was not until the fourth dose of epinephrine that systolic pressure gradually rose to 80 mmHg.
Once hemodynamic stability was achieved, the surgical team proceeded with the operation. The damaged abdominal aortic adventitia was repaired with 3–0 sutures, and aortoplasty was performed. The abdominal and thoracic cavities were washed, and hemostasis was carefully achieved. The posterior thoracoabdominal fascia was closed. A drain tube was placed in the posterior peritoneum, and the left diaphragmatic crus was sutured. Two bovine pericardial patches (6 cm × 8 cm and 4 cm × 6 cm) were used to repair the diaphragm and close the abdominal cavity. The left pleural cavity was washed. The anastomosis site was free of bleeding, and the left chest wall was closed. Then, the lung was inflated, and the incision was closed layer by layer. A drainage tube was placed in the left chest wall. Intraoperative blood loss was approximately 400 mL.
The patient was transferred into the intensive care unit for surveillance. Upon stabilization of hemodynamic status, the patient was transferred to the general ward on the following day for continued postoperative recovery and supportive management. The patient was discharged on the ninth postoperative day.
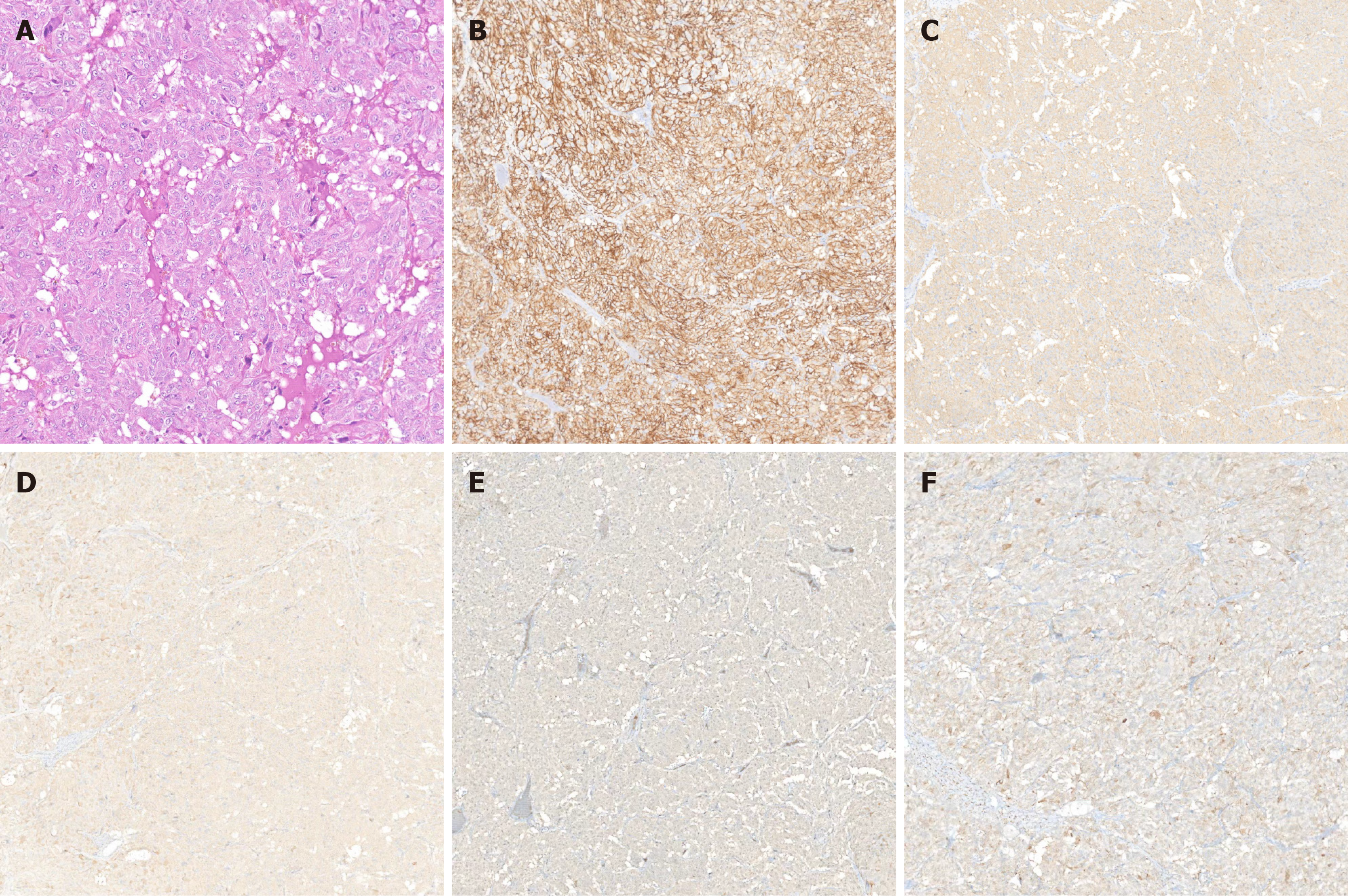
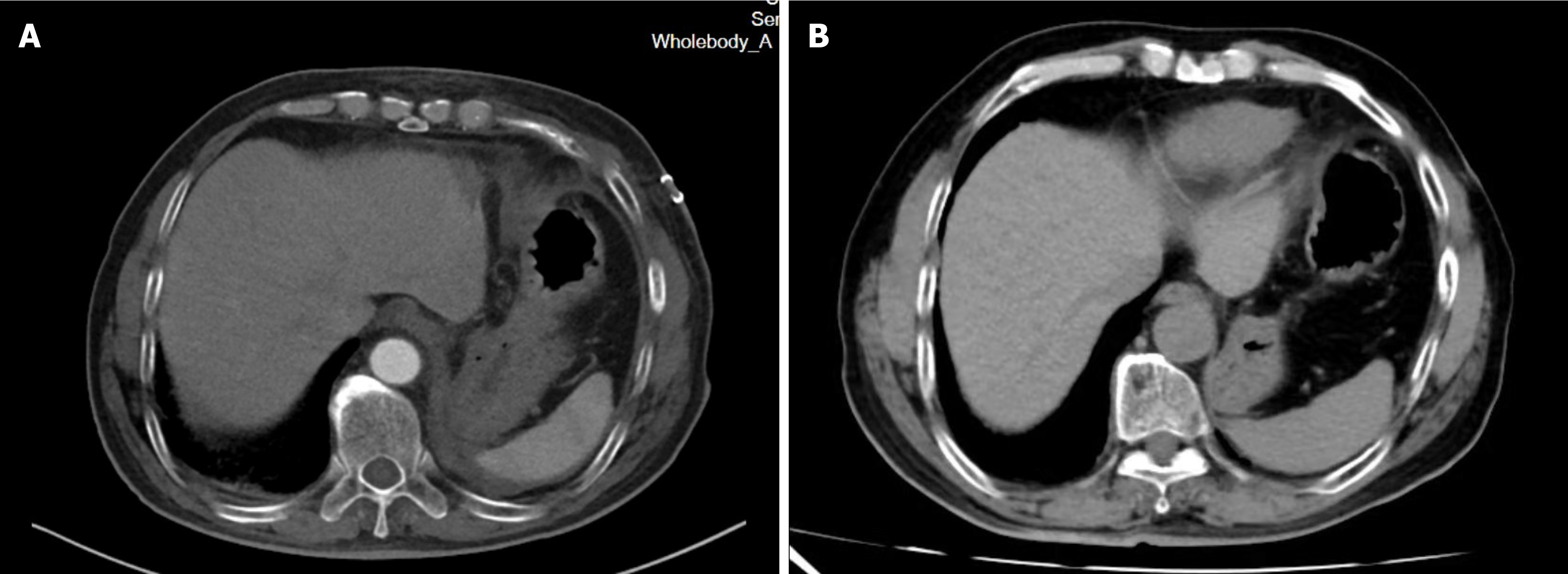
The pathological examination of the resected specimen revealed an epithelioid cell tumor in the retroperitoneum, showing characteristics of an organoid arrangement of medium to large polygonal cells with eosinophilic to amphophilic cytoplasm. The tumor measured 6.5 cm × 4.5 cm and invaded the surrounding skeletal muscle and adipose tissue, with vascular invasion. Immunohistochemical analysis revealed positive staining for synaptophysin, chromogranin A, CD56, S100, and NSE (Figure 3). The diagnosis was suggestive of a recurrent PGL. A follow-up CT scan of the abdomen two week postoperatively revealed no residual tumor in the surgical area and no significant effusion (Figure 4A). Six months postoperatively, a follow-up CT scan revealed no evidence of tumor residue or recurrence (Figure 4B). Serum adrenaline, methoxyepinephrine, and methoxynorepinephrine levels all returned to normal, measuring 92.89 pg/mL, 87.25 pg/mL, and 105.61 pg/mL, respectively. BP remained stable, and the patient reported no notable symptoms or discomfort.
Pheochromocytoma (PHEO) and PGL are uncommon neuroendocrine tumors[3,4]. The former originates from chromaffin cells of the adrenal medulla, whereas the latter develops from the extra-adrenal paraganglia of the autonomous sympathetic nervous system[5]. Although these two tumors are histologically difficult to distinguish, they have distinct anatomical features[5]. The specific incidence of PGL is unclear, as it is often described alongside PHEO, with the literature reporting an approximate annual incidence of 0.8 cases per 100000 person-years for such diseases[6]. PGLs can originate from the parasympathetic or sympathetic ganglia[5,6]. Although they cannot be differentiated on the basis of histological examinations, parasympathetic and sympathetic PGLs differ in their anatomical distributions, clinical characteristics, and likelihood of associated genetic syndromes[5,6]. Parasympathetic PGLs are commonly found in the neck and skull base, specifically along the glossopharyngeal and vagus nerve branches. Carotid body tumors are the most prevalent, followed by jugulotympanic and vagal PGLs[7]. In more than 80% of cases, the tumors are non-secreting, and symptoms are typically determined by the endocrine nature of the mass[6,7]. Sympathetic PGL occurs in approximately 75% of cases in the abdomen, most commonly at the confluence of the inferior vena cava and left renal vein or at the organ of Zuckerkandl (located at the aortic bifurcation or origin of the inferior mesenteric artery), approximately 10% of which occurs in the chest, and 10% of which occurs in the bladder and prostate[6,7]. Most sympathetic PGLs are functional, with symptoms determined by high secretion of catecholamines, mostly norepinephrine[7].
Approximately 33%-50% of PGLs may be associated with hereditary syndromes. Most PGLs are benign. Currently, there are no prognostic markers to accurately predict the malignant behavior of these tumors, with metastasis being evidence of malignant PGL[5]. The 2017 update of the World Health Organization classification replaced the terms "malignant" and "benign" with "metastatic" and "nonmetastatic", respectively[5].
Currently, the main treatment method for PGL is surgical resection[1]. In this case, the patient underwent tumor resection 13 years ago due to the discovery of an "adrenal mass", with significant BP fluctuations during surgery. Pa
Preoperative preparations included plasma expansion, BP monitoring, HR monitoring, and anesthetic planning to reduce perioperative mortality. The preparation aimed to mitigate the risks associated with sudden BP fluctuations and perioperative hypotension and shock in patients with PHEO[8]. Prior to surgery, an anesthesiologist performed central venous catheterization, monitored arterial BP, and prepared vasoactive drugs (preoperative pressor preparation: Ad
For complex PGLs that are not amenable to curative surgery or have confirmed metastatic disease, treatment options may include radioisotope therapy and/or palliative chemotherapy. Cyclophosphamide, vincristine, dacarbazine has been reported as a successful chemotherapy regimen, with 50% of patients experiencing relief of tumor symptoms, albeit with a short remission period[9]. In this case, as no metastasis or residual tumor was detected postoperatively, adjuvant therapy was not administered. Long-term follow-up is recommended for monitoring potential invasion into lymphatics, blood vessels, and muscles[10]. Based on a review of the literature on recurrent PGLs reported over the past decade, we revealed significant variation in recurrence time, ranging from as short as a few months to as long as 22 years. Fur
| Number | Gender | Age at diagnosis (years) | Time to recurrence | Primary site | Site of recurrence/metastasis | Gene mutation |
| 1 | Female | 17 | 7 years | Vulva | Vulva | SDHB |
| 2 | Male | 14 | 7 years | Right para-ureteral | Para-aortic | |
| 4 | Female | 19 | 9 months | Thyroid | Left paravertebral | SDHB |
| 5 | Female | 40 | 11 years | Right adrenal gland | Right adrenal gland | |
| 6 | Female | 35 | 5 months | Left mastoid | Left jugular vein | |
| 7 | Male | 79 | 2 years | Pituitary tumor | Sella turcica | |
| 8 | Male | 5 | 4 years | Right renal hilum | Left border of the abdominal aorta | |
| 9 | Female | 27 | 16 years | Right adrenal gland | Right adrenal gland | |
| 10 | Female | 46 | 2 years | Right thyroid | Laryngeal nerve | |
| 11 | Male | 33 | 2 years | Right neck | Right neck | |
| 12 | Female | 38 | 1 years | Bilateral cervical area | Cervical recurrence and liver metastasis | |
| 13 | Female | 60 | 10 years | Neck | Neck and vertebral column | |
| 14 | Female | 18 | 18 years | Left adrenal | Left adrenal recurrence with pulmonary metastases | |
| 15 | Male | 60 | 10 years | Right adrenal | Right adrenal | |
| 16 | Male | 32 | 6 years | Superior to the renal artery | Superior to aortic bifurcation | |
| 17 | Male | 18 | 10 years | Right carotid body | Left carotid body and right hip | |
| 18 | Male | 9 | 22 years | Left carotid body | Aortic root and the right ventricular outflow tract | SDHB |
| 29 | Male | 30 | 6 years | Carotid body | Carotid body and T7 vertebral metastases | |
| 20 | Female | 26 | 3 years | Left carotid body | Bilateral pulmonary, liver, and peripancreatic lymph nodes | |
| 21 | Male | 53 | 8 years | Bladder | Bladder and pelvic | |
| 22 | Male | 40 | 4 years | Bladder | Bladder and renal pelvis |
Distinguishing between benign and malignant forms of PHEO/PGL based exclusively on pathological morphology is challenging, necessitating evidence of invasiveness or metastasis to confirm malignancy[11]. At present, no reliable prognostic markers exist to accurately predict the metastatic potential of PGLs. However, histological scoring systems such as the Pheochromocytoma of the Adrenal Gland Scaled Score and Grading of Adrenal Pheochromocytoma and Paraganglioma Score are valuable tools for evaluation. It is crucial to acknowledge the risk of recurrence and metastasis in all patients with a history of PHEO/PGL[11]. Therefore, long-term follow-up should not be solely based on patho
This case we presented offers valuable insights into the diagnosis and management of recurrent PGLs. The recurrence was detected 11 years after the initial surgery, underscoring the importance of regular surveillance. Recurrent PGLs in proximity to major vascular structures, such as the abdominal aorta in this instance, are seldom reported by vascular surgeons, rendering this case particularly pertinent for their clinical practice. Furthermore, despite meticulous perioperative planning to mitigate hemodynamic instability, unanticipated fluctuations in BP occurred, emphasizing the critical need for robust hemodynamic management to ensure surgical safety.
Surgical resection is a critical therapeutic approach for PGL. PGLs located in the retroperitoneum are particularly rare and present complex anatomical challenges given their proximity to vital organs and major blood vessels, contributing to increased surgical difficulty. Moreover, their secretion of catecholamines underscores the importance of thorough preoperative preparation and meticulous intraoperative coordination between surgical and anesthesia teams to manage potential perioperative hemodynamic instability. Postoperative surveillance is essential given the risk of PGL recurrence or metastasis.
We thank Dr. Zhao J (Department of Anesthesiology, Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China) for her support to this paper.
| 1. | Iacobone M, Belluzzi A, Torresan F. Surgical approaches and results of treatment for hereditary paragangliomas. Best Pract Res Clin Endocrinol Metab. 2019;33:101298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Buffet A, Burnichon N, Favier J, Gimenez-Roqueplo AP. An overview of 20 years of genetic studies in pheochromocytoma and paraganglioma. Best Pract Res Clin Endocrinol Metab. 2020;34:101416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 3. | Farrugia FA, Martikos G, Tzanetis P, Charalampopoulos A, Misiakos E, Zavras N, Sotiropoulos D. Pheochromocytoma, diagnosis and treatment: Review of the literature. Endocr Regul. 2017;51:168-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 4. | Hodin R, Lubitz C, Phitayakorn R, Stephen A. Diagnosis and management of pheochromocytoma. Curr Probl Surg. 2014;51:151-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and Paraganglioma. N Engl J Med. 2019;381:552-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 391] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 6. | Calissendorff J, Juhlin CC, Bancos I, Falhammar H. Pheochromocytomas and Abdominal Paragangliomas: A Practical Guidance. Cancers (Basel). 2022;14:917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Lee JA, Duh QY. Sporadic paraganglioma. World J Surg. 2008;32:683-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Naranjo J, Dodd S, Martin YN. Perioperative Management of Pheochromocytoma. J Cardiothorac Vasc Anesth. 2017;31:1427-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 9. | Granberg D, Juhlin CC, Falhammar H. Metastatic Pheochromocytomas and Abdominal Paragangliomas. J Clin Endocrinol Metab. 2021;106:e1937-e1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 10. | Plouin PF, Amar L, Dekkers OM, Fassnacht M, Gimenez-Roqueplo AP, Lenders JW, Lussey-Lepoutre C, Steichen O; Guideline Working Group. European Society of Endocrinology Clinical Practice Guideline for long-term follow-up of patients operated on for a phaeochromocytoma or a paraganglioma. Eur J Endocrinol. 2016;174:G1-G10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 330] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 11. | Lam AK. Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours. Endocr Pathol. 2017;28:213-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 298] [Article Influence: 37.3] [Reference Citation Analysis (0)] |