Published online Mar 24, 2025. doi: 10.5306/wjco.v16.i3.101236
Revised: December 7, 2024
Accepted: December 27, 2024
Published online: March 24, 2025
Processing time: 134 Days and 20.1 Hours
Primary hepatic neuroendocrine tumors (PHNETs) are extremely rare tumors originating from neuroendocrine cells. Due to lack of neuroendocrine symptoms and specific radiographic characteristics, PHNETs are challenging to differentiate from other liver tumors.
This case involved a 67-year-old male who was admitted with a discovered hepatic mass and a suspicious lung lesion. Primary hepatic carcinoma was initially speculated based on the characteristic magnetic resonance imaging findings. The patient underwent a laparoscopic right partial hepatectomy, and subsequent immunohistochemical examination revealed a HNET. To exclude other potential origins, a positron emission tomography-computed tomography scan and gastrointestinal endoscopy were performed, leading to a final diagnosis of PHNETs. Then we conducted a literature review using the PubMed database, identifying 99 articles and 317 cases related to PHNETs. The characteristics, diagnostic methods, and treatment of PHNETs have been described. Finally, we elaborate on the presumed origins, pathological grades, clinical features, diagnosed methods, and treatments associated with PHNETs.
The diagnosis of PHNETs was primarily an exclusionary process. A definitive diagnosis of PHNETs relied mainly on immunohistochemical markers (chromogranin A, synaptophysin, and cluster of differentiation 56) and exclusion of primary foci in other organs. Radical surgery was the preferred treatment for early-stage tumors.
Core Tip: This paper reports a case of a rare primary hepatic neuroendocrine tumor (PHNET). Subsequently, we conducted a literature review using the PubMed database to describe the characteristics, diagnostic methods, and treatment of PHNETs. The diagnosis of PHNETs relies on definitive pathological findings, with the primary objective of preoperative examinations being the exclusion of extrahepatic primary sources. Radical surgery is the preferred treatment for early-stage tumors, and overall outcomes of PHNETs are generally better than those of gastrointestinal NETs with liver metastasis.
- Citation: Lv HY, Liu MX, Hong WT, Li XW. Primary hepatic neuroendocrine tumor with a suspicious pulmonary nodule: A case report and literature review. World J Clin Oncol 2025; 16(3): 101236
- URL: https://www.wjgnet.com/2218-4333/full/v16/i3/101236.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i3.101236
Neuroendocrine tumors (NETs) are a rare type of tumor originating from neuroendocrine cells and distributing throughout the body, with the lung and gastrointestinal tract being the most common sites of origin[1]. Hepatic NETs (HNETs) are primarily caused by metastases from other organs, while primary HNETs (PHNETs) account for only 0.3% of all NETs[2]. The etiology and pathogenesis of PHNETs remain unclear. Diagnosing PHNET is challenging due to the absence of typical imaging or serological markers. Based on imaging characteristics, differentiating PHNETs from other hepatic malignancies, such as hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCC), is difficult. Most patients do not exhibit overt clinical symptoms, leading to incidental discovery during physical examinations or treatment for other conditions. As a result, the diagnosis and treatment of PHNETs are often delayed, posing significant risks to patient health.
This paper reported the case of a 67-year-old male who was admitted to our hospital because of a discovered hepatic mass. Hepatic carcinoma was initially suspected based on the magnetic resonance imaging (MRI) findings. Until a pathological examination after surgical resection, along with positron emission tomography-computed tomography (PET-CT) and gastrointestinal endoscopy to exclude other origins, the hepatic mass was ultimately diagnosed as a PHNET.
A 67-year-old male discovered a hepatic mass 6 days prior.
The patient’s routine examination revealed a hepatic mass, without abdominal pain, bloating, diarrhea, jaundice, or other symptoms.
The patient has had a nodule in the left lung for several years, with no significant changes noted during regular reexaminations.
The patient denied any family history of malignant tumors.
On physical examination, the vital signs were as follows: Body temperature, 36.5 °C; blood pressure, 135/86 mmHg; heart rate, 82 beats per minute; respiratory rate, 19 breaths per minute. Additionally, no mass was touched on the abdominal examination. There was no pressure, rebound pain, or muscle tension on palpation over the whole abdomen.
The liver function indicators were within the normal range. Among the tumor-related markers, the gastrin-releasing peptide precursor level increased to 93.87 pg/mL (> 63 pg/mL). Both hepatitis B surface antigen and hepatitis C antibody tests were negative.
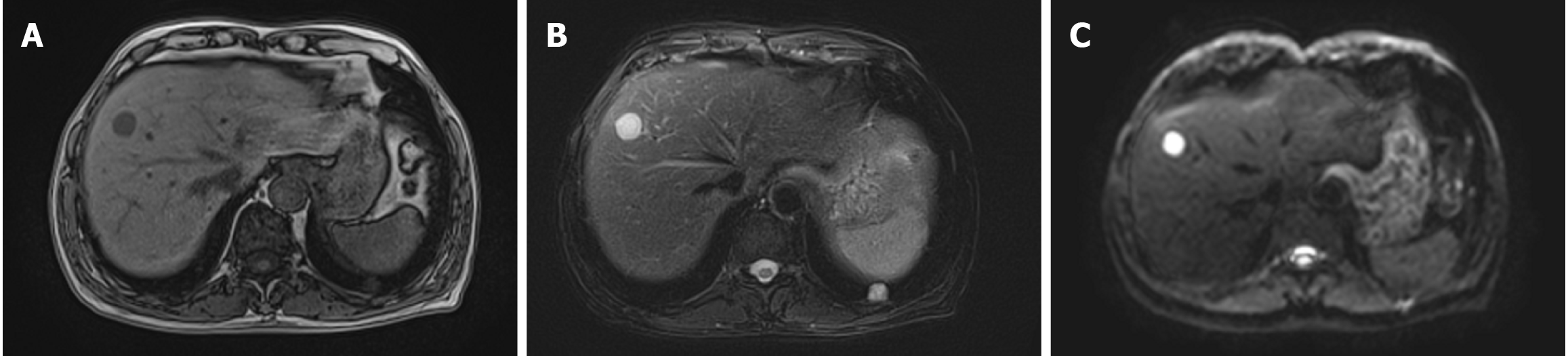
Hepatic MRI with contrast enhancement and diffusion-weighted imaging (DWI) indicated a nodule measuring 20 mm × 18 mm in hepatic segment VIII. The nodule exhibited long T1 and long T2 signals, along with hyperintensity on DWI. The enhanced scan demonstrated the circumferential enhancement, raising concerns for potential malignancy and metastasis (Figure 1). A high-resolution chest CT (HRCT) scan showed a solid nodule in the basal segment of the left lung’s lower lobe (Im163), measuring approximately 15 mm × 13 mm, and CT enhancement was recommended. The enhanced chest CT scan indicated that the nodule displayed mild to moderate inhomogeneous enhancement.
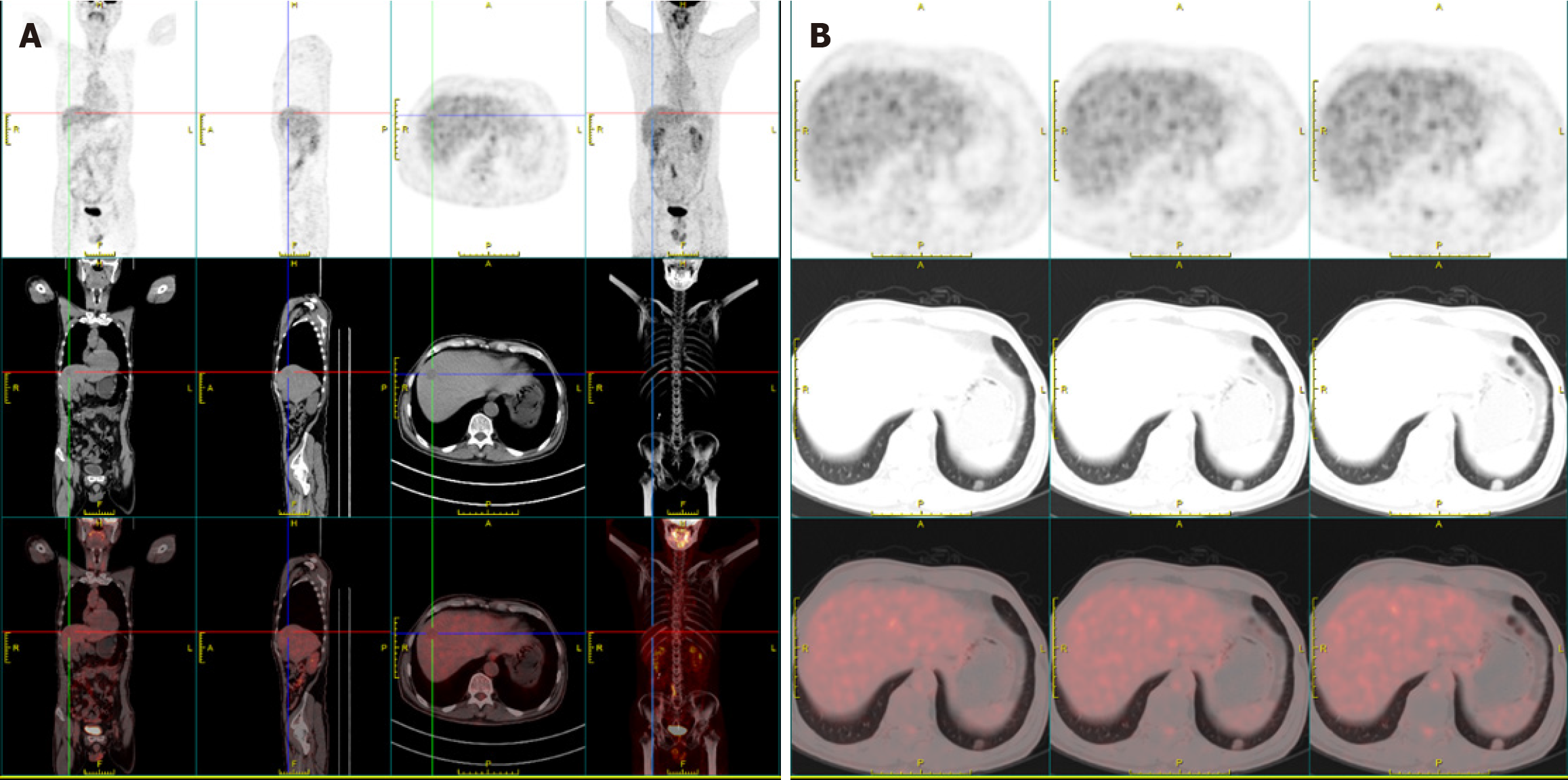
After consulting with the multidisciplinary treatment team, primary hepatic tumor was considered as a temporary diagnosis. To determine whether the pulmonary nodule was benign or malignant and to exclude the possibility of metastasis, we carried out PET-CT and gastrointestinal endoscopy. The PET-CT demonstrated a slightly hypodense nodule in the hepatic segment VIII with low glucose metabolism, which was considered malignancy (Figure 2). By contrast, the pulmonary nodule exhibited slightly elevated glucose metabolism, suggesting it was a benign lesion. The gastrointestinal endoscopy showed no significant abnormalities.
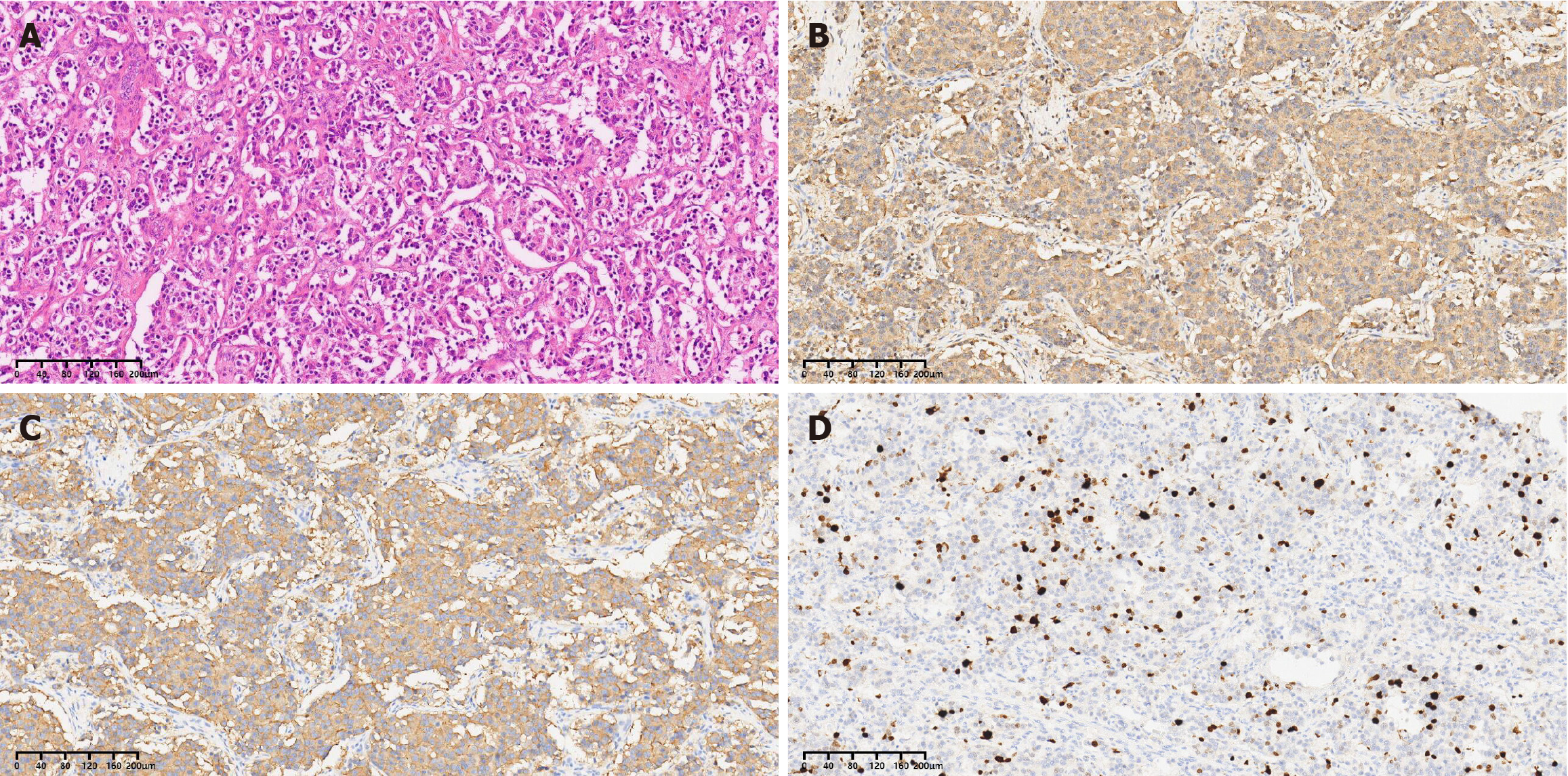
Based on the combined pathological and immunohistochemical results, the tumor was consistent with a PHNET and was classified as small cell carcinoma.
Based on the examinations conducted, primary hepatic cancer was diagnosed, and a laparoscopic right partial he
After the operation, the lung mass underwent a puncture biopsy, which revealed no tumor cells. Additionally, there was no signs of liver recurrence or emergence of another PHNET at the 1-year follow-up.
We have summarized cases reported in the English literature over the past 15 years. A systematic search was conducted in the PubMed database using the term ‘primary hepatic neuroendocrine tumor’. The search produced 99 publications and reported 317 cases. The mean age of patients was 52 years, with the oldest patient being 87 years old[3] and the youngest 17 years[4]. The literature review showed that the age at diagnosis of PHNETs was generally older. One patient had a preoperative course lasting 26 years. She was initially diagnosed with a benign hepatic tumor, and was later found to have PHNETs after tumor recurrence following resection[5]. The male-to-female ratio for PHNET was 1.01:1 (148 males to 146 females), indicating no significant sex difference. Approximately 34.3% of the tumors were multiple, while 65.7% were single, with tumors in the right liver being more common. There were seven cases of giant tumors, with a maximum diameter of 35 cm[6-12]. PHNET metastasis primarily involved multiple intrahepatic sites[13,14], with some cases presenting in the lungs, mesentery, spleen, adrenal glands, bone marrow, and brain[11,15-17]. Rasmussen et al[6] reported a unique case of PHNET with orbital metastasis.
In most cases, patients underwent hepatitis viruses test, tumor marker test [carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), carbohydrate antigen 19-9 (CA19-9)], chest and upper abdominal CT scans, and hepatic MRI. A total of 50.2% of patients received 18F-fludeoxyglucose PET/CT scans to exclude extrahepatic lesions and to assist in diagnosing whether the lesions were benign or malignant[18,19]. Twenty-five percent of patients underwent somatostatin receptor scintigraphy (SRS), some of which were performed postoperatively. This may be associated with preoperative susceptibility to the diagnosis of HCC or hepatic hemangioma[20-22]. To clarify the nature of the lesions preoperatively, 62.8% of patients underwent ultrasound (US)- or CT-guided liver puncture[23,24]. A total of 48.6% of patients underwent gas
Regarding treatment strategies, 170 patients underwent surgery, predominantly radical resection[27,28]. Twenty-seven patients received transcatheter arterial chemoembolization (TACE)[24,29], and six patients underwent liver transplants due to giant tumors or liver failure[30,31]. A study by Qiu et al[32] study demonstrated that radical surgery was sig
NENs are a heterogeneous group of tumors originating from diffuse NETs. NENs are mostly found in the gastrointestinal tract, pancreas, and lungs, with a lower incidence in the liver, accounting for only 0.3% of all NEN cases and 0.28%-0.46% of all hepatic cancers[2].
The origin of PHNETs remains unclear, with three hypotheses proposed in the literature. First, PHNETs may originate from ectopic tissues with endocrine functions in the liver, such as the pancreas and adrenal glands[41,42]. Second, they may arise from the neuroendocrine differentiation of malignant stem cells within the liver. Third, PHNETs could originate from neuroendocrine cells located in the epithelium of the intrahepatic capillary bile ducts[2,43]. The third hypothesis is the most widely accepted. Nakano described a case of HCC combined with the neuroendocrine carcinoma (NEC). Microscopic examination revealed that HCC and NEC are closely intermingled, with a transitional area evident between them. Therefore, the author proposed that moderately or poorly differentiated HCC transdifferentiate into a neuroendocrine phenotype, which aligns with third hypothesis[44].
NENs are classified into well-differentiated (NETs) and poorly differentiated (NECs). NETs are further categorized into three grades based on mitotic count and/or the Ki67 proliferation index: Low-grade (G1), intermediate-grade (G2), and high-grade (G3). By definition, NECs are considered high-grade, characterized by early dissemination and a poor prognosis[45]. The latest World Health Organization 2022 classification of endocrine and NETs, distinguished NECs and subclasses them into small cell NEC (SCNEC) and large cell NEC (LCNEC), each defined by cytological features (Table 1)[46,47]. SCNECs exhibit highly atypical with small cell morphology, forming solid proliferations with prominent necrosis. LCNECs display large cell morphology with vesicular chromatin or prominent nucleoli, consisting of large nodules arranged in an organoid pattern with focal necrosis[48].
| Neuroendocrine neoplasm | Classification | Diagnostic criteria |
| Well-differentiated neuroendocrine neoplasm | Grade 1 (G1) | < 2 mitoses/2 mm2 and/or Ki67 < 3% |
| Grade 2 (G2) | 2-20 mitoses/2 mm2 and/or Ki67 3%-20% | |
| Grade 3 (G3) | > 20 mitoses/2 mm2 and/or Ki67 > 20% | |
| Poorly differentiated neuroendocrine neoplasm | Small cell NEC | > 20 mitoses/2 mm2 and/or Ki67 > 20% (often > 70%), and small cell cytomorphology |
| Large cell NEC | > 20 mitoses/2 mm2 and/or Ki67 > 20% (often > 70%), and large cell cytomorphology |
PHNETs grow slowly, and most patients are clinically asymptomatic. These tumors are often discovered incidentally, with common symptoms including abdominal pain, jaundice, and a palpable mass in the right upper quadrant, which may result from mass effects on the liver and adjacent organs[49]. Metastatic HNETs (MHNETs) present with flushing, diarrhea, and abdominal pain, which are associated with the typical carcinoid syndrome due to the secretion of vasoa
PHNETs lack of definitive imaging and characteristic serological markers to facilitate the diagnosis. Conventional tumor markers, such as AFP, CEA, and CA19-9, are generally within normal ranges or mildly elevated in patients with PHNETs. For functional PHNETs, the metabolic products of biogenic amines and peptides, such as serotonin, insulin, and gastrin, are useful to disease diagnosis. However, most PHNETs are non-functional and do not have specific serological markers. In our case, elevated level of gastrin-releasing peptide precursor was associated with NETs. CT imaging reveals single or multiple low-density nodules or heterogeneous masses in the liver, often accompanied by cystic degeneration or liquefied necrosis. MRI imaging demonstrates slightly long T1 and T2 signals, as well as hyperintensity on DWI. Following a dynamic enhanced scan, the lesions exhibit heterogeneous enhancement with irregular internal margins in the arterial phase. In the portal venous phase, the lesions show continuous enhancement with irregular, non-enhanced low-density foci at the center[52]. Solid PHNETs are frequently misdiagnosed preoperatively as the HCC or cholangiocarcinoma (CCC) due to imaging similarities. HCC typically displays a ‘fast-in-fast-out’ enhancement pattern on CT/MRI, characterized by marked enhancement in the arterial phase followed by rapid clearance in the portal venous phase. CCC is radiographically characterized by bile duct dilation, thickening of the bile duct wall, or periductal infiltration, and usually demonstrates a slowly progressive enhancement on enhanced imaging (Table 2). Cystic PHNETs easily confused with hepatic mucinous cystadenoma, cystadenocarcinoma, and Echinococcus cysts on CT and MRI[50,53,54]. Wang et al[55] compared pathological grading with CT and MRI features, revealing that G1 tumors were singular solid nodules, while G2 and G3 tumors exhibit multiple diffuse lesions or a large tumor with multiple satellite lesions characterized by focal hemorrhage, necrosis, and portal vein thrombosis. PHNETs show significant enhancement in the arterial phase, indicating that they are tumors with a rich blood supply. On US, PHNET lesions are predominantly hyperechoic, exhibiting strong arterial hyperenhancement on contrast-enhanced US (CEUS). By contrast, CEUS in patients with MHNETs showed arterial hypo-enhancement or iso-enhancement[56]. The somatostatin analogue octreotide receptor scintigraphy (SRS) can also be used in the diagnosis of NENs, with a sensitivity of 90% and specificity of 80%[57]. PET/CT offers superior specificity and sensitivity compared to SRS for the diagnosis of NENs. Gallium-dotatate PET/CT not only clarifies the location of the primary tumor, but it also highly sensitive to microscopic metastatic lesions, which is essential for the staging and prognosis of patients with PHNETs[58,59].
| PHNENs | HCC | CCC | |
| Source | Neuroendocrine cells | Liver cells | Bile duct cells |
| Incidence rate (of all hepatic cancers) | 0.28%-0.46% | 80% | 15% |
| Risk factors | Un clear | Viral hepatitis, alcoholic liver disease, non-alcoholic fatty liver disease, aflatoxin | Cholangitis, bile duct stones, bile duct cysts, hepatic schistosome infection |
| Clinical manifestations | No manifestations in the early stage. Abdominal pain, jaundice and an epigastric mass in the late stage. Functional PHNENs may be associated with carcinoid syndrome | No manifestations in the early stage. The first symptom is abdominal pain, followed by an epigastric mass, fatigue, lethargy and abdominal distension | No manifestations in the early stage. Abdominal discomfort, abdominal pain, fatigue, nausea, an epigastric mass, jaundice and fever in the late stage |
| Imaging manifestations | Single or multiple low-density nodules or inhomogeneous masses in the liver, often with cystic degeneration or liquefied necrosis | ‘Fast-in-fast-out’ enhancement pattern on CT/MRI | Bile duct dilatation, thickening of the bile duct wall or periductal infiltration on CT/MRI, and a slowly progressive enhancement on enhanced imaging |
| Serum tumor markers | No specific tumor markers. Serotonin, insulin and gastrin elevated in the functional PHNENs | AFP, PIVKA II, AFP-L3, microRNA elevated | No specific tumor markers. CA19-9, CA125 and CEA elevated |
| Immunohistochemical markers | CgA, Syn, NSE, CD56 | HepPar1, CD34, pCEA, COX-2, arginase-1 | MUC-1, CK19, AQP-1 |
| Treatment | Preferred surgery | Preferred surgery | Preferred surgery |
The diagnosis of PHNETs relies on definitive pathological findings, with the primary objective of preoperative examinations being the exclusion of extrahepatic primary sources. Gastroscopy and colonoscopy are performed to exclude gastrointestinal NETs. Lung evaluations, including (HRCT and bronchoscopy, are conducted to identify any primary pulmonary lesions. PET/CT has gained popularity prior to surgery because of its comprehensive whole-body imaging findings. While assessing the hepatic lesion through abdominal MRI and CT scans, it is crucial to consider the possibility of a primary pancreatic mass. Consequently, the diagnosis of PHNETs can be regarded as an exclusionary diagnosis.
Preoperative puncture biopsy is not recommended, as several literatures indicate that it has a low diagnostic accuracy, with only 11.3% reported[2,60]. Postoperative histological and immunohistochemical evaluations are the most essential method for definitive diagnosis. Mitoses and the Ki67 proliferation index serve as the fundamental criteria for tumor staging and grading. CgA, Syn, and CD56 are widely recognized as the most specific immunohistochemical markers. CgA is predominantly found in the neuroendocrine cells and is considered a highly specific neuroendocrine marker[61]. It exhibits strong and diffuse immunostaining in NETs, but may be weak or even absent in NECs[62]. CgA also correlates with tumor burden and patient prognosis, making it a valuable indicator for postoperative follow-up observations[63]. Neuron-specific enolase (NSE) is a glycolytic enzyme involved in neurons and neuroendocrine cells. However, the sensitivity of NSE, which ranges from 39%-43%, and its specificity, which ranges from 65% to 73%, limit its clinical application in distinguishing NENs from non-NENs[64]. Syn is a primary component of synaptic vesicles in neurons and is expressed in both NETs and NECs. It is the most sensitive neuroendocrine marker rather than the specific marker[65,66]. The CD56, a neural cell adhesion molecule, exhibits high sensitivity in identifying the neuroendocrine phenotype of tumors, but it is also not a specific neuroendocrine marker[61,67]. Additional neuroendocrine markers are summarized in the Table 3[68-71]. In conclusion, a variety of immunological markers are necessary for the accurate diagnosis of NETs.
| Immunohistological markers | Interpretation |
| Chromogranin A | The major constituents of neuroendocrine secretory granules, the first-choice marker to confirm nets, responding prognosis |
| Synaptophysin | The main constituent of synaptic vesicles of neurons, most sensitive but no specific nets marker |
| Neuron-specific enolase | A glycolytic enzyme produced in neurons and neuroendocrine cells, with low sensitivity and specificity |
| CD56 | The neural cell adhesion molecule, a sensitive but no specific neuroendocrine marker |
| ASH1 | The key transcription factor in neuroendocrine cell differentiation, used as a differential diagnosis for high-grade extra-pulmonary NECs[68,69] |
| CK AE1/AE3 | Marking the epithelial and epithelial-derived tumors[61,70] |
| SSTR 2/5 | The most frequently expressed SSTR subtypes in NENs, with type-specific differences[71] |
Although there is no treatment guidelines of PHNETs, surgical resection is the main treatment for both primary and secondary HNETs[72,73]. Achieving an R0 resection to ensure negative margins and appropriate lymph node dissection is considered the optimal surgical approach. Overall survival outcomes of PHNETs were generally better than those of HCC (mean survival time: 30.64 vs 25.11 months) and gastrointestinal NETs with liver metastasis (5-year survival rate: 18.57% vs 8.63%). However, the mean survival time of PHNETs was inferior to gastrointestinal NETs without metastasis (30.64 months vs 41.62 months)[74]. Knox reviewed the long-term survival following PHNET resection, and the results showed that the 10-year survival rate was 68%. There was no significant influence on survival associated with preo
As shown in our case, PHNETs are extremely rare tumors for which early detection and diagnosis are particularly important. Due to the absence of neuroendocrine symptoms and their nonspecific radiographic appearance, PHNETs are challenging to differentiate from other liver tumors. The diagnosis of PHNETs is a continuum that spans from the preoperative to the postoperative stage, necessitating long-term follow-up to exclude a primary extrahepatic source. Immunohistochemical markers of the postoperative mass, such as CgA, Syn, and CD56, represent the most accurate method for diagnosing the PHNETs. Surgical resection is the preferred treatment, and most patients tend to recover well after surgery.
We are grateful for the support from the participant and his family.
| 1. | Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology. 2005;128:1717-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 524] [Article Influence: 26.2] [Reference Citation Analysis (1)] |
| 2. | Quartey B. Primary Hepatic Neuroendocrine Tumor: What Do We Know Now? World J Oncol. 2011;2:209-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Morishita A, Yoneyama H, Nomura T, Sakamoto T, Fujita K, Tani J, Miyoshi H, Haba R, Masaki T. Primary hepatic neuroendocrine tumor: A case report. Mol Clin Oncol. 2016;4:954-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Gorla AK, Basher RK, Kaman L, Bal A, Bhattacharya A, Mittal BR. 68Ga-DOTATATE PET/CT in Primary Hepatic Neuroendocrine Tumor. Clin Nucl Med. 2017;42:118-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Meng XF, Pan YW, Wang ZB, Duan WD. Primary hepatic neuroendocrine tumor case with a preoperative course of 26 years: A case report and literature review. World J Gastroenterol. 2018;24:2640-2646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Rasmussen JØ, von Holstein SL, Prause JU, Vainer B, Hansen AB, Fehr A, Stenman G, Heegaard S. Genetic analysis of an orbital metastasis from a primary hepatic neuroendocrine carcinoma. Oncol Rep. 2014;32:1447-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Almas T, Inayat F, Ehtesham M, Khan MK. Primary hepatic neuroendocrine tumour masquerading as a giant haemangioma: an unusual presentation of a rare disease. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Pastrián LG, Ruz-Caracuel I, Gonzalez RS. Giant Primary Neuroendocrine Neoplasms of the Liver: Report of 2 Cases With Molecular Characterization. Int J Surg Pathol. 2019;27:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Rocca A, Calise F, Marino G, Montagnani S, Cinelli M, Amato B, Guerra G. Primary giant hepatic neuroendocrine carcinoma: a case report. Int J Surg. 2014;12 Suppl 1:S218-S221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Sotiropoulos GC, Charalampoudis P, Delladetsima I, Stamopoulos P, Dourakis S, Kouraklis G. Surgery for giant primary neuroendocrine carcinoma of the liver. J Gastrointest Surg. 2014;18:839-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Lee MH, Hsu YH. Primary hepatic neuroendocrine carcinoma with nonbacterial thrombotic endocarditis: a case report. Kaohsiung J Med Sci. 2011;27:36-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Mima K, Beppu T, Murata A, Otao R, Miyake K, Okabe H, Masuda T, Okabe K, Sugiyama S, Chikamoto A, Ishiko T, Takamori H, Baba H. Primary neuroendocrine tumor in the liver treated by hepatectomy: report of a case. Surg Today. 2011;41:1655-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Yu WM, Li R, Sun BL, Du JK, Tuo HF. Primary hepatic neuroendocrine tumour with multiple liver metastases: A case report with literature review. Int J Surg Case Rep. 2021;89:106590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Nikeghbalian S, Eshraghian A, Kazemi K, Shamsaeefar A, Hosseinzadeh M, Geramizadeh B, Malek-Hosseini SA. Liver Transplantation for High-Grade Primary Hepatic Neuroendocrine Tumor with Diffuse Liver Metastasis. J Gastrointest Cancer. 2020;51:304-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Tang Y, Chen X, Lu X, Yuan Z, Yang Y, Qiu C, Li H. Case Report: Primary hepatic neuroendocrine tumor: two cases report with literature review. Front Oncol. 2023;13:1225583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Ogasawara Y, Ito N, Kogiso T, Yoshizawa S, Nagashima Y, Tokushige K. A case of neuroendocrine carcinoma with massive invasion to the liver and multiorgan causing acute liver failure. Clin J Gastroenterol. 2023;16:580-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Nakano S, Minaga K, Tani Y, Tonomura K, Hanawa Y, Morimura H, Terashita T, Matsumoto H, Iwagami H, Nakatani Y, Akamatsu T, Uenoyama Y, Maeda C, Ono K, Watanabe T, Yamashita Y. Primary Hepatic Neuroendocrine Carcinoma with Thrombocytopenia Due to Diffuse Bone Marrow and Splenic Infiltration: An Autopsy Case. Intern Med. 2022;61:3361-3368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Mitamura K, Yamamoto Y, Tanaka K, Sanomura T, Murota M, Nishiyama Y. (18)F-FDG PET/CT Imaging of Primary Hepatic Neuroendocrine Tumor. Asia Ocean J Nucl Med Biol. 2015;3:58-60. [PubMed] |
| 19. | Zhang Z, Li H. Surgical treatment of primary hepatic neuroendocrine tumor diagnosed by Al(18)F-NOTA-Octreotide PET/CT: a case report. Front Med (Lausanne). 2023;10:1256176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | DeLuzio MR, Barbieri AL, Israel G, Emre S. Two Cases of Primary Hepatic Neuroendocrine Tumors and a Review of the Current Literature. Ann Hepatol. 2017;16:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Bouzayan L, Madani A, Malki S, Abbou W, Skiker I, Benani A, Jabi R, Bouziane M. Primary hepatic origin of a neuroendocrine tumor: A rare case report. Ann Med Surg (Lond). 2022;84:104937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Baek SH, Yoon JH, Kim KW. Primary hepatic neuroendocrine tumor: gadoxetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging. Acta Radiol Short Rep. 2013;2:2047981613482897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Zhao B, Mao J, Li Y. Primary hepatic neuroendocrine tumor associated with hypertension: A case report. Front Surg. 2022;9:1021806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Zhang X, Yang H, Xin Y, Yang Y, Lu H, Zhou X. Complete Response After Pre-Operative Transcatheter Arterial Chemoembolization for Unresectable Primary Hepatic Neuroendocrine Tumour: A Case Report and Literature Review. Front Oncol. 2022;12:893403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Ghattas S, Al Bitar J, Chahine G, Kamar F, Haddad M, Wakim R. Primary Hepatic Neuroendocrine Tumor: A Case Report and Literature Review. Case Reports Hepatol. 2024;2024:9181560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Shi L, Sun L. Colorectal adenocarcinoma with hepatic neuroendocrine carcinoma: A case report. Medicine (Baltimore). 2023;102:e35428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Shah D, Mandot A, Cerejo C, Amarapurkar D, Pal A. The Outcome of Primary Hepatic Neuroendocrine Tumors: A Single-Center Experience. J Clin Exp Hepatol. 2019;9:710-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Parray A, Patkar S, Goel M. Primary hepatic neuroendocrine tumours of liver- a rarity: Single centre analysis of 13 patients. Ann Hepatobiliary Pancreat Surg. 2020;24:17-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Rao YY, Zhang HJ, Wang XJ, Li MF. Primary hepatic neuroendocrine tumor - (18)F-fluorodeoxyglucose positron emission tomography/computed tomography findings: A case report. World J Clin Cases. 2021;9:6450-6456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Khan J, Jingmei L. Primary Neuroendocrine Tumor of the Liver With Papillary Features in a Multivisceral Transplant Patient. Cureus. 2021;13:e17394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Alekseev D, Goralczyk A, Lorf T, Ramadori G, Obed A. Ten years survival with excellent outcome after living donor liver transplantation from 70 years old donor for primary hepatic neuroendocrine carcinoma: Case report. Int J Surg Case Rep. 2012;3:34-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Qiu MJ, Chen YB, Bi NR, Yang SL, He XX, Xiong ZF. Comparative Clinical Analysis of Gastroenteropancreatic Neuroendocrine Carcinomas with Liver Metastasis and Primary Hepatic Neuroendocrine Carcinomas. Dis Markers. 2018;2018:9191639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Han Y, Li L, Sun H. Computed Tomography and Magnetic Resonance Imaging in the Diagnosis of Primary Neuroendocrine Tumors of the Liver. World Neurosurg. 2020;138:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Chen RW, Qiu MJ, Chen Y, Zhang T, He XX, Li Y, Sun WJ, Xie T, Yang SL, Hu JL. Analysis of the clinicopathological features and prognostic factors of primary hepatic neuroendocrine tumors. Oncol Lett. 2018;15:8604-8610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Li MX, Li QY, Xiao M, Wan DL, Chen XH, Zhou L, Xie HY, Zheng SS. Survival comparison between primary hepatic neuroendocrine neoplasms and primary pancreatic neuroendocrine neoplasms and the analysis on prognosis-related factors. Hepatobiliary Pancreat Dis Int. 2019;18:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Wang H, Yang D, Wu Z, Luo Y, Ling W. Contrast-Enhanced Ultrasound Findings of Hepatocellular Carcinoma With Neuroendocrine Carcinoma: A Case Report. Front Med (Lausanne). 2021;8:602346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Kano Y, Kakinuma S, Goto F, Azuma S, Nishimura-Sakurai Y, Itsui Y, Nakagawa M, Kudo A, Tanabe M, Kirimura S, Amano T, Ito T, Akashi T, Asahina Y, Watanabe M. Primary hepatic neuroendocrine carcinoma with a cholangiocellular carcinoma component in one nodule. Clin J Gastroenterol. 2014;7:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Grenn EE, Shannon Orr W 3rd, Mark Earl T. Primary Hepatic Neuroendocrine Carcinoma : Amphicrine Type. Am Surg. 2022;88:149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 39. | Lu J, Xiong XZ, Cheng NS. Hepatobiliary and pancreatic: coexisting cancers: Hepatic neuroendocrine carcinomas with gall bladder adenocarcinoma. J Gastroenterol Hepatol. 2014;29:1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Kaneko R, Kimura Y, Sakata H, Ikehara T, Mitomi H, Uekusa T, Ohbu M, Kubo S. A case of primary hepatic mixed neuroendocrine-non-neuroendocrine tumor (MiNEN) associated with gallbladder carcinosarcoma. Clin J Gastroenterol. 2020;13:1280-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Andreola S, Lombardi L, Audisio RA, Mazzaferro V, Koukouras D, Doci R, Gennari L, Makowka L, Starzl TE, van Thiel DH. A clinicopathologic study of primary hepatic carcinoid tumors. Cancer. 1990;65:1211-1218. [PubMed] [DOI] [Full Text] |
| 42. | Sioutos N, Virta S, Kessimian N. Primary hepatic carcinoid tumor. An electron microscopic and immunohistochemical study. Am J Clin Pathol. 1991;95:172-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Gravante G, De Liguori Carino N, Overton J, Manzia TM, Orlando G. Primary carcinoids of the liver: a review of symptoms, diagnosis and treatments. Dig Surg. 2008;25:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 44. | Nakano A, Hirabayashi K, Yamamuro H, Mashiko T, Masuoka Y, Yamamoto S, Ozawa S, Nakagohri T. Combined primary hepatic neuroendocrine carcinoma and hepatocellular carcinoma: case report and literature review. World J Surg Oncol. 2021;19:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 45. | Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, Busam KJ, de Krijger RR, Dietel M, El-Naggar AK, Fernandez-Cuesta L, Klöppel G, McCluggage WG, Moch H, Ohgaki H, Rakha EA, Reed NS, Rous BA, Sasano H, Scarpa A, Scoazec JY, Travis WD, Tallini G, Trouillas J, van Krieken JH, Cree IA. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31:1770-1786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 713] [Article Influence: 101.9] [Reference Citation Analysis (0)] |
| 46. | Rindi G, Mete O, Uccella S, Basturk O, La Rosa S, Brosens LAA, Ezzat S, de Herder WW, Klimstra DS, Papotti M, Asa SL. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr Pathol. 2022;33:115-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 481] [Article Influence: 160.3] [Reference Citation Analysis (2)] |
| 47. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2441] [Article Influence: 488.2] [Reference Citation Analysis (3)] |
| 48. | Pilichowska M, Kimura N, Ouchi A, Lin H, Mizuno Y, Nagura H. Primary hepatic carcinoid and neuroendocrine carcinoma: clinicopathological and immunohistochemical study of five cases. Pathol Int. 1999;49:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Song JE, Kim BS, Lee CH. Primary hepatic neuroendocrine tumor: A case report and literature review. World J Clin Cases. 2016;4:243-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 50. | Shetty PK, Baliga SV, Balaiah K, Gnana PS. Primary hepatic neuroendocrine tumor: an unusual cystic presentation. Indian J Pathol Microbiol. 2010;53:760-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Tohyama T, Matsui K, Kitagawa K. Primary hepatic carcinoid tumor with carcinoid syndrome and carcinoid heart disease: a case report of a patient on long-term follow-up. Intern Med. 2005;44:958-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Kellock T, Tuong B, Harris AC, Yoshida E. Diagnostic imaging of primary hepatic neuroendocrine tumors: a case and discussion of the literature. Case Rep Radiol. 2014;2014:156491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 53. | Kim JM, Lee WA, Shin HD, Song IH, Kim SB. Cystic Primary Hepatic Neuroendocrine Tumor. Korean J Gastroenterol. 2021;78:300-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Krohn M, Grieser C, Weichert W, Pascher A, Denecke T. Well-differentiated neuroendocrine carcinoma mimicking an echinococcus cyst of the liver in CT-MRI findings with hepatocyte specific contrast material. J Gastrointestin Liver Dis. 2011;20:439-442. [PubMed] |
| 55. | Wang LX, Liu K, Lin GW, Jiang T. Primary hepatic neuroendocrine tumors: comparing CT and MRI features with pathology. Cancer Imaging. 2015;15:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 56. | Li R, Tang CL, Yang D, Zhang XH, Cai P, Ma KS, Guo DY, Ding SY. Primary hepatic neuroendocrine tumors: clinical characteristics and imaging features on contrast-enhanced ultrasound and computed tomography. Abdom Radiol (NY). 2016;41:1767-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Critchley M. Octreotide scanning for carcinoid tumours. Postgrad Med J. 1997;73:399-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Sadowski SM, Neychev V, Millo C, Shih J, Nilubol N, Herscovitch P, Pacak K, Marx SJ, Kebebew E. Prospective Study of 68Ga-DOTATATE Positron Emission Tomography/Computed Tomography for Detecting Gastro-Entero-Pancreatic Neuroendocrine Tumors and Unknown Primary Sites. J Clin Oncol. 2016;34:588-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 266] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 59. | Yang J, Kan Y, Ge BH, Yuan L, Li C, Zhao W. Diagnostic role of Gallium-68 DOTATOC and Gallium-68 DOTATATE PET in patients with neuroendocrine tumors: a meta-analysis. Acta Radiol. 2014;55:389-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 60. | Hwang S, Lee YJ, Lee SG, Kim CW, Kim KH, Ahn CS, Moon KM, Ko KH, Kim KW, Choi NK, Ha TY. Surgical treatment of primary neuroendocrine tumors of the liver. J Gastrointest Surg. 2008;12:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | Uccella S, La Rosa S, Volante M, Papotti M. Immunohistochemical Biomarkers of Gastrointestinal, Pancreatic, Pulmonary, and Thymic Neuroendocrine Neoplasms. Endocr Pathol. 2018;29:150-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 62. | Weiler R, Feichtinger H, Schmid KW, Fischer-Colbrie R, Grimelius L, Cedermark B, Papotti M, Bussolati G, Winkler H. Chromogranin A and B and secretogranin II in bronchial and intestinal carcinoids. Virchows Arch A Pathol Anat Histopathol. 1987;412:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 63. | Arnold R, Wilke A, Rinke A, Mayer C, Kann PH, Klose KJ, Scherag A, Hahmann M, Müller HH, Barth P. Plasma chromogranin A as marker for survival in patients with metastatic endocrine gastroenteropancreatic tumors. Clin Gastroenterol Hepatol. 2008;6:820-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 64. | Mariën L, Islam O, Chhajlani S, Lybaert W, Peeters M, Van Camp G, Op de Beeck K, Vandamme T. The Quest for Circulating Biomarkers in Neuroendocrine Neoplasms: a Clinical Perspective. Curr Treat Options Oncol. 2023;24:1833-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Gould VE, Lee I, Wiedenmann B, Moll R, Chejfec G, Franke WW. Synaptophysin: a novel marker for neurons, certain neuroendocrine cells, and their neoplasms. Hum Pathol. 1986;17:979-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 134] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 66. | Gould VE, Wiedenmann B, Lee I, Schwechheimer K, Dockhorn-Dworniczak B, Radosevich JA, Moll R, Franke WW. Synaptophysin expression in neuroendocrine neoplasms as determined by immunocytochemistry. Am J Pathol. 1987;126:243-257. [PubMed] |
| 67. | Jin L, Hemperly JJ, Lloyd RV. Expression of neural cell adhesion molecule in normal and neoplastic human neuroendocrine tissues. Am J Pathol. 1991;138:961-969. [PubMed] |
| 68. | La Rosa S, Marando A, Gatti G, Rapa I, Volante M, Papotti M, Sessa F, Capella C. Achaete-scute homolog 1 as a marker of poorly differentiated neuroendocrine carcinomas of different sites: a validation study using immunohistochemistry and quantitative real-time polymerase chain reaction on 335 cases. Hum Pathol. 2013;44:1391-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Altree-Tacha D, Tyrrell J, Li F. mASH1 is Highly Specific for Neuroendocrine Carcinomas: An Immunohistochemical Evaluation on Normal and Various Neoplastic Tissues. Arch Pathol Lab Med. 2017;141:288-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 70. | Bahrami A, Gown AM, Baird GS, Hicks MJ, Folpe AL. Aberrant expression of epithelial and neuroendocrine markers in alveolar rhabdomyosarcoma: a potentially serious diagnostic pitfall. Mod Pathol. 2008;21:795-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 71. | Hofland J, Kaltsas G, de Herder WW. Advances in the Diagnosis and Management of Well-Differentiated Neuroendocrine Neoplasms. Endocr Rev. 2020;41:371-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 72. | Park CH, Chung JW, Jang SJ, Chung MJ, Bang S, Park SW, Song SY, Chung JB, Park JY. Clinical features and outcomes of primary hepatic neuroendocrine carcinomas. J Gastroenterol Hepatol. 2012;27:1306-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 73. | Frilling A, Clift AK. Surgical Approaches to the Management of Neuroendocrine Liver Metastases. Endocrinol Metab Clin North Am. 2018;47:627-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 74. | Zhang Z, Zhao X, Li Z, Wu Y, Liu Y, Li Z, Li G. Development of a nomogram model to predict survival outcomes in patients with primary hepatic neuroendocrine tumors based on SEER database. BMC Cancer. 2021;21:567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Knox CD, Anderson CD, Lamps LW, Adkins RB, Pinson CW. Long-term survival after resection for primary hepatic carcinoid tumor. Ann Surg Oncol. 2003;10:1171-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 76. | Qu C, Qu LL, Zhu CZ, Wang ZS, Cao J. Treatment of primary hepatic neuroendocrine tumors with associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): A case report and literature review. Medicine (Baltimore). 2018;97:e12408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 77. | Iwao M, Nakamuta M, Enjoji M, Kubo H, Fukutomi T, Tanabe Y, Nishi H, Taguchi KI, Kotoh K, Nawata H. Primary hepatic carcinoid tumor: case report and review of 53 cases. Med Sci Monit. 2001;7:746-750. [PubMed] |
| 78. | Gurung A, Yoshida EM, Scudamore CH, Hashim A, Erb SR, Webber DL. Primary hepatic neuroendocrine tumour requiring live donor liver transplantation: case report and concise review. Ann Hepatol. 2012;11:715-720. [PubMed] |
| 79. | Chatziioannou A, Georgopoulou V, Katsiki E, Sinakos E. Primary hepatic neuroendocrine tumor treated with liver transplantation. Dig Liver Dis. 2022;54:1441-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Moris D, Tsilimigras DI, Ntanasis-Stathopoulos I, Beal EW, Felekouras E, Vernadakis S, Fung JJ, Pawlik TM. Liver transplantation in patients with liver metastases from neuroendocrine tumors: A systematic review. Surgery. 2017;162:525-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |