Published online Mar 24, 2025. doi: 10.5306/wjco.v16.i3.101098
Revised: November 30, 2024
Accepted: December 17, 2024
Published online: March 24, 2025
Processing time: 139 Days and 1.6 Hours
Based on the discovery that human β-defensin-1 (hBD-1) triggers autophagy in colon cancer cells and inhibits proliferation, we proposed the consideration of its druggability. As a protein, its stability, targetability and bioavailability must be improved. Compared with the traditional medicinal chemistry technology, nano
Core Tip: Nanotechnology is a cost-effective strategy for addressing the druggable properties of proteins. We propose an immunoliposome system that improves the sta
- Citation: Huang Y, Wang XY, Huang JY, Huang ZW. Incorporation of human β-defensin-1 into immunoliposomes to facilitate targeted autophagy therapy of colon carcinoma. World J Clin Oncol 2025; 16(3): 101098
- URL: https://www.wjgnet.com/2218-4333/full/v16/i3/101098.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i3.101098
In the World Journal of Gastrointestinal Oncology, Zhao et al[1] published an important work titled, ‘Human β-defensin-1 affects the mammalian target of rapamycin pathway and autophagy in colon cancer cells through long non-coding RNA TCONS_00014506’. Cancer is one of the most common causes of death worldwide[2], with colon cancer as the third most common type[3]. New anticancer drugs are urgently needed to prevent the proliferation of colon cancer cells. Several drugs have been developed to treat colon cancer, including sildenafil, exisulind, tadalafil, vardenafil and panafil as phosphodiesterase type 5 inhibitors that target colorectal cancer cells[4], doxorubicin and etoposide, which are topoi
| Section | Summary |
| Background | Colorectal cancer has a low 5-year survival rate and a high mortality rate. The hBD-1 may function in the innate immune system, which recognises and destroys cancer cells. LncRNAs are involved in cell differentiation and growth |
| Methods | Cell proliferation was detected using the cell counting kit 8 method to determine the optimal drug concentration. The effect of hBD-1 on the SW620 cell proliferation was evaluated by a colony formation assay. Bioinformatics was used to screen LncRNAs for potential biological significance associated with the mTOR pathway. In addition, the expression of p-mTOR, beclin1 and LC3I/II in the SW620 cells was detected by western blot analysis |
| Results | The hBD-1 inhibited the SW620 cell proliferation, as shown by the reduced colony-forming ability of SW620 cells following the hBD-1 exposure. The hBD-1 decreased the expression of the p-mTOR (Ser2448) protein and increased the expression of the Beclin1 and LC3II/I proteins. In addition, a bioinformatics analysis identified seven lncRNAs associated with the mTOR pathway (two up-regulated and five down-regulated). Finally, lncRNA TCONS_00014506 was selected. Following the lncRNA TCONS_00014506 inhibition, exposure to hBD-1 inhibited p-mTOR (Ser2448) and promoted beclin1 and LC3II/I protein expression |
| Conclusion | In the SW620 colon cancer cells, hBD-1 increased the lncRNA TCONS_00014506 expression to suppress the mTOR pathway and promote autophagy |
The study of Zhao et al[1] preliminarily revealed the anticancer potential of hBD-1. We should bear in mind that there is still an ‘enduring war’ to fight, and the clinical translation of hBD-1 as an antitumor remedy is warranted. The drug
One strategy for enhancing druggability is to perform structural modification through medicinal chemistry to enhance stability, targetability and bioavailability[15]. Nevertheless, this strategy is considered time-consuming and expensive. Moreover, clinical translation is often challenging, particularly for protein-based drugs[16]. In comparison, nanotechnology is a cost-effective strategy that can address the druggability issues of proteins. Specifically, with the application of easy-to-produce nanocarriers, the stability, targetability and bioavailability may be increased without sophisticated chemical reactions for structural modification[17]. Many commercialised anticancer products based on nanotechnology are available in the global market, such as Doxil®[18], Abraxane®[19] and Onivyde®[20], indicating the potential clinical translation. Thus, we proposed the design of a nanocarrier-based formulation for hBD-1.
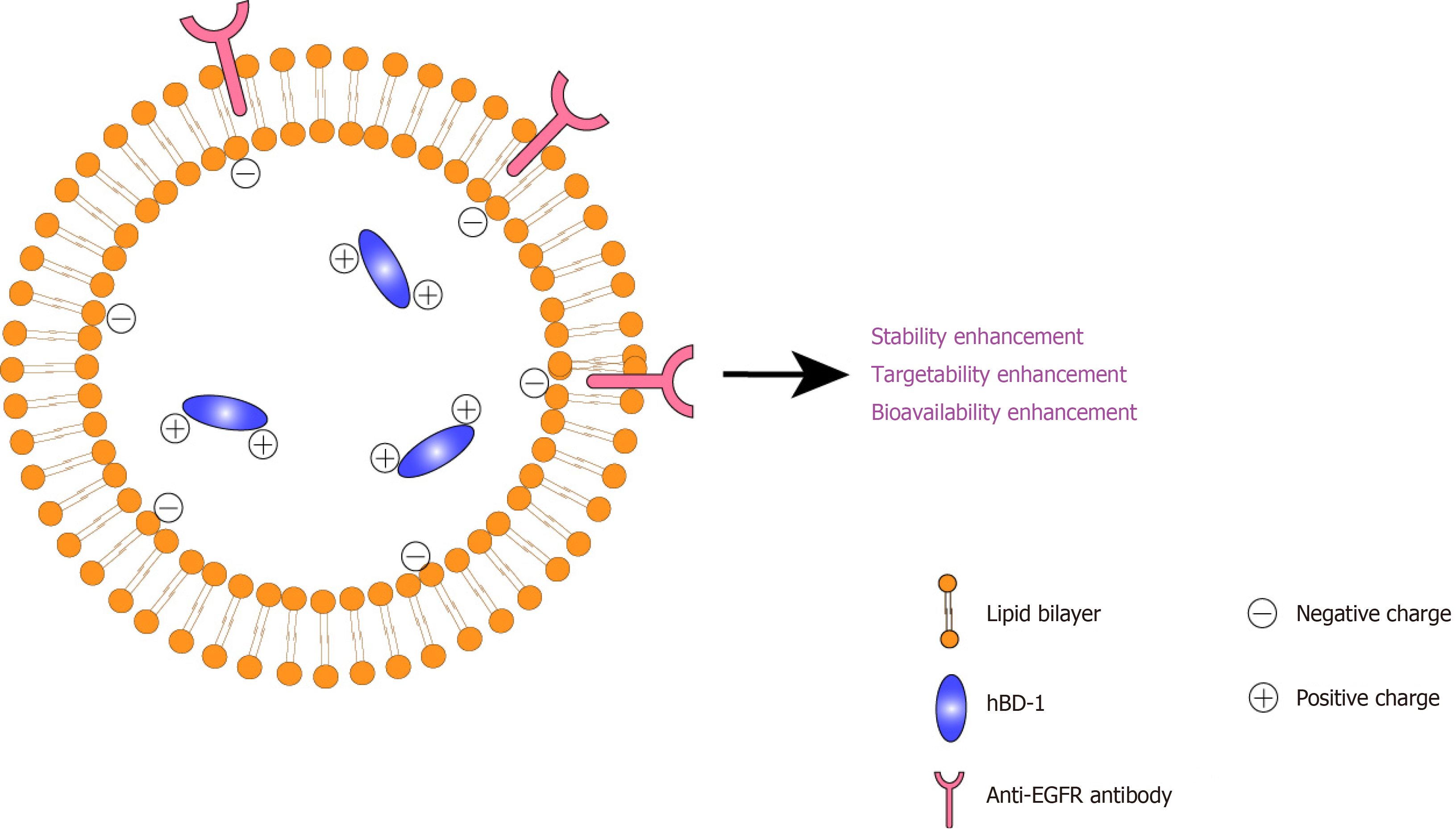
An immunoliposome system was proposed to achieve high stability, targetability and bioavailability. The design details are as follows: (1) To improve stability and bioavailability, hBD-1 should be accommodated in a liposomal system. The liposomal system serves as a confinement framework for hBD-1 to mitigate contact with water and oxygen, thereby reducing hydrolysis and oxidative stress[21]. This mechanism stabilises hBD-1. Furthermore, this system comprises a biocompatible, lecithin-based lipid bilayer, which is favourable for fusion with cell membranes[22], facilitates trans
The abovementioned design is illustrated in Figure 1. The proposed immunoliposomes are anticipated to enhance the stability, targetability and bioavailability of hBD-1 to facilitate clinical translation. For clinical application, intravenous injection may be a preferred route of administration for the system because the anti-EGFR structure may be destroyed by gastric potential of hydrogen or digestive enzymes associated with oral administration[34].
Clinical trials and large-scale manufacture of the proposed immunoliposomes require further study. On the one hand, nanotoxicology in humans must be considered during clinical trials[35]. On the other hand, strong standardised methodologies must be developed and implemented to support research practices involving the engineering and manipulation of nanomaterials[36]. Safety and reproducibility will be necessary for hBD-1 immunoliposomes to be commercially su
Overall, immunoliposomes are suitable nanovesicles for improving the druggability of hBD-1; however, further basic and translational studies on this system are warranted. It is suggested that journals, such as the World Journal of Gastrointestinal Oncology, should increase awareness of this topic.
| 1. | Zhao YX, Cui Y, Li XH, Yang WH, An SX, Cui JX, Zhang MY, Lu JK, Zhang X, Wang XM, Bao LL, Zhao PW. Human β-defensin-1 affects the mammalian target of rapamycin pathway and autophagy in colon cancer cells through long non-coding RNA TCONS_00014506. World J Gastrointest Oncol. 2024;16:1465-1478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 2. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11913] [Article Influence: 2978.3] [Reference Citation Analysis (4)] |
| 3. | Benarba B, Pandiella A. Colorectal cancer and medicinal plants: Principle findings from recent studies. Biomed Pharmacother. 2018;107:408-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Nemr MT, Teleb M, Aboulmagd AM, El-naggar ME, Gouda N, Abdel-ghany A, Elshaier YA. Design, synthesis and chemoinformatic studies of new thiazolopyrimidine derivatives as potent anticancer agents via phosphodiesterase-5 inhibition and apoptotic inducing activity. J Mol Struct. 2023;1272:134216. [RCA] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 5. | Sengupta S, Tyagi P, Chandra S, Kochupillai V, Gupta SK. Encapsulation in cationic liposomes enhances antitumour efficacy and reduces the toxicity of etoposide, a topo-isomerase II inhibitor. Pharmacology. 2001;62:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Fadaly WAA, Mohamed FEA, Nemr MTM, Sayed AM, Khalil RG, Zidan TH. Novel benzenesulfonamide derivatives as potential selective carbonic anhydrase IX, XII inhibitors with anti-proliferative activity: Design, synthesis and in silico studies. Bioorg Chem. 2024;153:107881. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Abd El-Mawgoud HK, AboulMagd AM, Nemr MTM, Hemdan MM, Hassaballah AI, Farag PS. Design, synthesis and cytotoxic evaluation of new thieno[2,3-d]pyrimidine analogues as VEGFR-2/AKT dual inhibitors, apoptosis and autophagy inducers. Bioorg Chem. 2024;150:107622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 8. | Nemr MTM, Yousif MNM, Barciszewski J. Interaction of small molecules with polynucleotide repeats and frameshift site RNA. Arch Pharm (Weinheim). 2019;352:e1900062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Xie H, Zhang C. Potential of the nanoplatform and PROTAC interface to achieve targeted protein degradation through the Ubiquitin-Proteasome system. Eur J Med Chem. 2024;267:116168. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Pazgier M, Prahl A, Hoover DM, Lubkowski J. Studies of the biological properties of human beta-defensin 1. J Biol Chem. 2007;282:1819-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Zhang T, Li L, Chunta S, Wu W, Chen Z, Lu Y. Enhanced oral bioavailability from food protein nanoparticles: A mini review. J Control Release. 2023;354:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 12. | Jia HP, Starner T, Ackermann M, Kirby P, Tack BF, McCray PB Jr. Abundant human beta-defensin-1 expression in milk and mammary gland epithelium. J Pediatr. 2001;138:109-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Vardar-Sengul S, Demirci T, Sen BH, Erkizan V, Kurulgan E, Baylas H. Human beta defensin-1 and -2 expression in the gingiva of patients with specific periodontal diseases. J Periodontal Res. 2007;42:429-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Singh PK, Jia HP, Wiles K, Hesselberth J, Liu L, Conway BA, Greenberg EP, Valore EV, Welsh MJ, Ganz T, Tack BF, McCray PB Jr. Production of beta-defensins by human airway epithelia. Proc Natl Acad Sci U S A. 1998;95:14961-14966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 443] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 15. | Alam Khan S, Jawaid Akhtar M. Structural modification and strategies for the enhanced doxorubicin drug delivery. Bioorg Chem. 2022;120:105599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Nestor JJ Jr. The medicinal chemistry of peptides. Curr Med Chem. 2009;16:4399-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Farouk F, Shamma R. Chemical structure modifications and nano-technology applications for improving ADME-Tox properties, a review. Arch Pharm (Weinheim). 2019;352:e1800213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Redruello-Guerrero P, Córdoba-Peláez P, Láinez-Ramos-Bossini AJ, Rivera-Izquierdo M, Mesas C, Ortiz R, Prados J, Perazzoli G. Liposomal Doxorubicin In vitro and In vivo Assays in Non-small Cell Lung Cancer: A Systematic Review. Curr Drug Deliv. 2024;21:1346-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Zhao M, Lei C, Yang Y, Bu X, Ma H, Gong H, Liu J, Fang X, Hu Z, Fang Q. Abraxane, the Nanoparticle Formulation of Paclitaxel Can Induce Drug Resistance by Up-Regulation of P-gp. PLoS One. 2015;10:e0131429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 20. | Milano G, Innocenti F, Minami H. Liposomal irinotecan (Onivyde): Exemplifying the benefits of nanotherapeutic drugs. Cancer Sci. 2022;113:2224-2231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 21. | Chang Z, Wang W, Huang Z, Huang Y, Wu C, Pan X. Lecithin Reverse Micelle System is Promising in Constructing Carrier Particles for Protein Drugs Encapsulated Pressurized Metered-Dose Inhalers. Adv Ther (Weinh). 2023;6. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 22. | Guo M, Peng T, Wu C, Pan X, Huang Z. Engineering Ferroptosis Inhibitors as Inhalable Nanomedicines for the Highly Efficient Treatment of Idiopathic Pulmonary Fibrosis. Bioengineering. 10:727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Zalba S, Contreras AM, Haeri A, Ten Hagen TL, Navarro I, Koning G, Garrido MJ. Cetuximab-oxaliplatin-liposomes for epidermal growth factor receptor targeted chemotherapy of colorectal cancer. J Control Release. 2015;210:26-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Antonello A, Tarozzi A, Morroni F, Cavalli A, Rosini M, Hrelia P, Bolognesi ML, Melchiorre C. Multitarget-directed drug design strategy: a novel molecule designed to block epidermal growth factor receptor (EGFR) and to exert proapoptotic effects. J Med Chem. 2006;49:6642-6645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Fadaly WAA, Nemr MTM, Kahk NM. Discovery of novel pyrazole based Urea/Thiourea derivatives as multiple targeting VEGFR-2, EGFR(WT), EGFR(T790M) tyrosine kinases and COX-2 Inhibitors, with anti-cancer and anti-inflammatory activities. Bioorg Chem. 2024;147:107403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Reference Citation Analysis (0)] |
| 26. | Wu P, Zhou Q, Zhu H, Zhuang Y, Bao J. Enhanced antitumor efficacy in colon cancer using EGF functionalized PLGA nanoparticles loaded with 5-Fluorouracil and perfluorocarbon. BMC Cancer. 2020;20:354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 27. | Karpov OA, Fearnley GW, Smith GA, Kankanala J, Mcpherson MJ, Tomlinson DC, Harrison MA, Ponnambalam S. Receptor tyrosine kinase structure and function in health and disease. AIMS Biophys. 2015;2:476-502. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Celik I, Ayhan-kılcıgil G, Karayel A, Guven B, Onay-besikci A. Synthesis, molecular docking, in silico ADME, and EGFR kinase inhibitor activity studies of some new benzimidazole derivatives bearing thiosemicarbazide, triazole, and thiadiazole. J Heterocyclic Chem. 2022;59:371-387. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Maruyama IN. Mechanisms of activation of receptor tyrosine kinases: monomers or dimers. Cells. 2014;3:304-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 30. | Butti R, Das S, Gunasekaran VP, Yadav AS, Kumar D, Kundu GC. Receptor tyrosine kinases (RTKs) in breast cancer: signaling, therapeutic implications and challenges. Mol Cancer. 2018;17:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 249] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 31. | Naishima NL, Faizan S, Raju RM, Sruthi ASVL, Ng V, Sharma GK, Vasanth KS, Shivaraju VK, Ramu R, Kumar BP. Design, Synthesis, Analysis, Evaluation of Cytotoxicity Against MCF-7 Breast Cancer Cells, 3D QSAR Studies and EGFR, HER2 Inhibition Studies on Novel Biginelli 1,4-Dihydropyrimidines. J Mol Struct. 2023;1277:134848. [RCA] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 32. | Fadaly WAA, Nemr MTM, Zidan TH, Mohamed FEA, Abdelhakeem MM, Abu Jayab NN, Omar HA, Abdellatif KRA. New 1,2,3-triazole/1,2,4-triazole hybrids linked to oxime moiety as nitric oxide donor selective COX-2, aromatase, B-RAFV600E and EGFR inhibitors celecoxib analogs: design, synthesis, anti-inflammatory/anti-proliferative activities, apoptosis and molecular modeling study. J Enzyme Inhib Med Chem. 2023;38:2290461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Liu Y, Li X, Pen R, Zuo W, Chen Y, Sun X, Gou J, Guo Q, Wen M, Li W, Yu S, Liu H, Huang M. Targeted delivery of irinotecan to colon cancer cells using epidermal growth factor receptor-conjugated liposomes. Biomed Eng Online. 2022;21:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 34. | Cao Y, Xiong EM, True AD, Xiong YL. The pH-dependent protection of α-galactosidase activity by proteins against degradative enzymes during soymilk in vitro digestion. LWT-Food Sci Technol. 2016;69:244-250. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Domingues C, Santos A, Alvarez-Lorenzo C, Concheiro A, Jarak I, Veiga F, Barbosa I, Dourado M, Figueiras A. Where Is Nano Today and Where Is It Headed? A Review of Nanomedicine and the Dilemma of Nanotoxicology. ACS Nano. 2022;16:9994-10041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 96] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 36. | Roubert F, Beuzelin-ollivier M, Hofmann-amtenbrink M, Hofmann H, Hool A. “Nanostandardization” in Action: Implementing Standardization Processes in a Multidisciplinary Nanoparticle-Based Research and Development Project. Nanoethics. 2016;10:41-62. [DOI] [Full Text] |