Published online Mar 24, 2025. doi: 10.5306/wjco.v16.i3.100030
Revised: October 23, 2024
Accepted: December 20, 2024
Published online: March 24, 2025
Processing time: 168 Days and 5 Hours
In recent years, endoscopic resection (ER) has been employed for the excision of submucosal tumors (SMTs). Nonetheless, ER in the duodenum is linked to ele
To establish a clinical score model for supporting suture decision-making of duodenal SMTs.
This study included 137 individuals diagnosed with duodenal SMTs who under
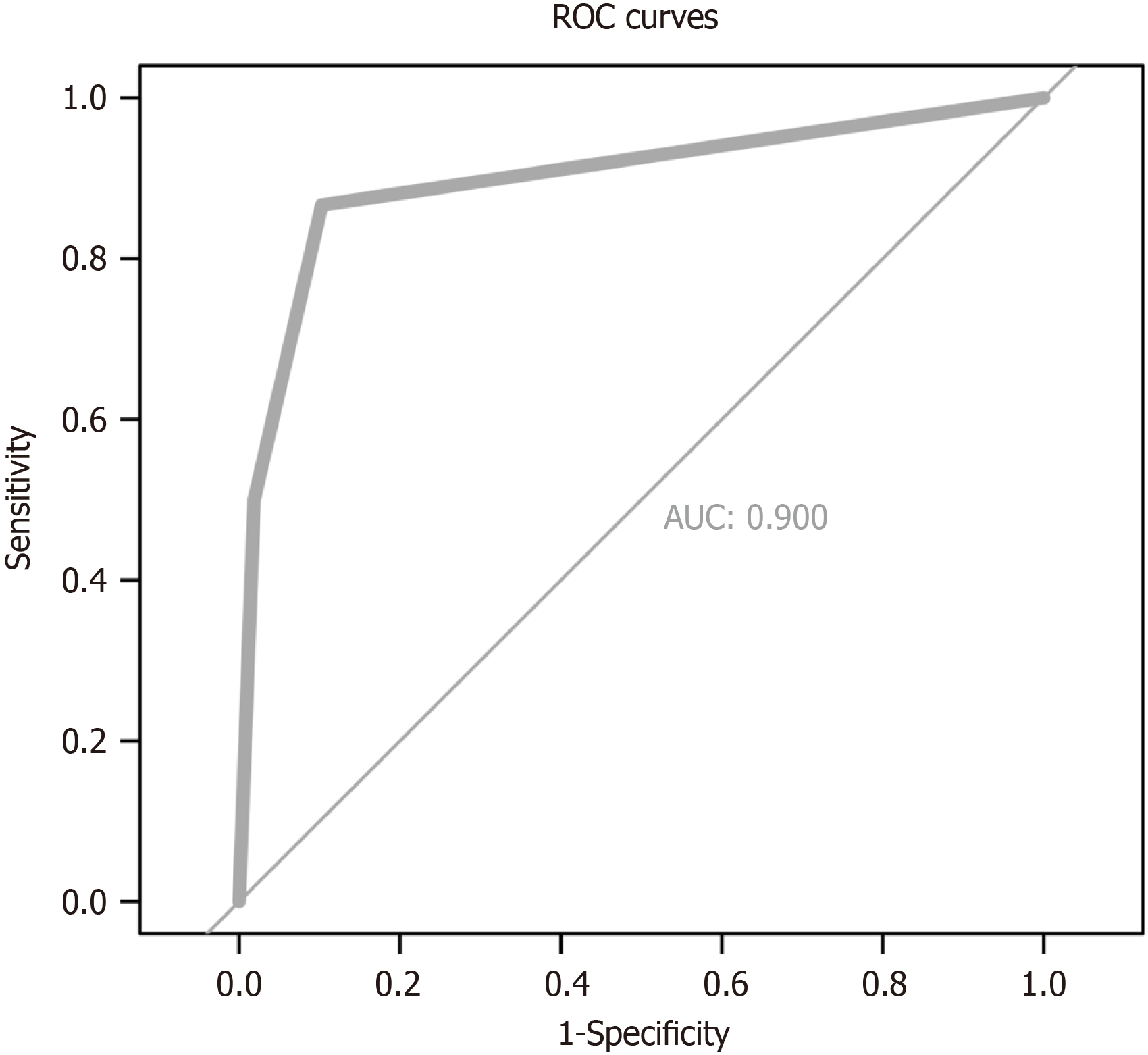
The clinical scoring system incorporated two key factors: Extraluminal growth, which was assigned 2 points, and endoscopic full-thickness resection, which was given 3 points. This model demonstrated strong predictive accuracy, as evidenced by the area under the receiver operating characteristic curve of 0.900 (95% confidence interval: 0.823-0.976). Additionally, the model’s goodness-of-fit was validated by the Hosmer-Lemeshow test (P = 0.404). The probability of purse-string suturing in low (score 0-2) and high (score > 3) categories were 3.0% and 64.3% in the TC, and 6.1% and 88.9% in the VC, respectively.
This scoring system may function as a beneficial instrumentality for medical practitioners, facilitating the decision-making process concerning suture techniques in the context of duodenal SMTs.
Core Tip: Endoscopic resection of duodenal submucosal tumors carries high risks of hemorrhage and perforation, hi
- Citation: Geng ZH, Qu YF, Zhu Y, Fu PY, Chen WF, Li QL, Zhou PH. Scoring system supporting suture decision-making for duodenal submucosal tumors. World J Clin Oncol 2025; 16(3): 100030
- URL: https://www.wjgnet.com/2218-4333/full/v16/i3/100030.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i3.100030
Surgical interventions, specifically open or laparoscopic wedge resection, have traditionally been the treatment of choice for the majority of patients presenting with submucosal tumors (SMTs) within the gastrointestinal tract. Yet, such surgical interventions could potentially impact the patient’s quality of life. Endoscopic approaches have demonstrated their viability, safety, and efficacy in managing these digestive SMTs. However, the duodenum poses unique challenges for endoscopic resection (ER) due to its thin wall, limited luminal space, abundant blood supply, and its adjacency to critical organs, as well as its exposure to harsh fluids like gastric acid, bile, and pancreatic enzymes. Consequently, ER within the duodenum is associated with an increased propensity for significant adverse outcomes, with a particular emphasis on the risks of perforation and delayed bleeding[1,2].
Several studies have indicated that ER can be a minimally invasive approach for duodenal SMTs, maintaining curative potential when performed by skilled endoscopists[3,4]. Throughout the excision procedure, proper suturing is essential for minimizing complications. However, there is a scarcity of research on suturing techniques specifically for duodenal SMTs. We retrospectively conducted an in-depth analysis of the medical records pertaining to a sizable case series of individuals afflicted with duodenal SMTs who had undergone ER. Subsequently, we formulated a clinical scoring model designed to guide clinical decision-making with respect to the selection of suture techniques for these neoplasms. The objective was to guide less experienced endoscopists in making tailored suturing decisions, thereby enhancing clinical outcomes.
We conducted a single-institution retrospective analysis involving 137 successive patients with a diagnosis of duodenal SMTs who received ER at Zhongshan Hospital, Fudan University in Shanghai, China, from December 2016 to August 2022. The study included only those patients for whom comprehensive demographic and clinical data, as well as follow-up information, were accessible. Prior to surgery, patients underwent assessments using endoscopy, computed to
For the dissection of the tumor, a standard endoscope equipped with a transparent cap, along with specialized instru
The determination of ER approach for duodenal SMTs was predicated upon the morphological characteristics of the tumor as ascertained through endoscopic examination. For polypoid or superficial SMTs in the duodenum, endoscopic mucosal resection or electrosurgical cutting is typically employed. In cases where the SMT is deeply situated, endoscopic submucosal dissection or endoscopic full-thickness resection (EFTR) is chosen, depending on the lesion’s depth. For le
The key outcomes measured in this study encompassed: (1) The length of time for the surgical procedure and the pa
Continuous variables were expressed as the average values plus or minus the standard deviation (SD), while categorical variables were depicted as counts (with their respective percentages). Baseline patient characteristics were compared utilizing Student’s t-tests or χ2-tests. Univariate analysis was conducted to identify potential predictive factors. Su
The beta coefficients derived from the logistic regression analysis were employed to devise a scoring system for the prediction of suturing techniques, with the scores being rounded to the nearest whole number corresponding to the absolute value of the beta coefficient. Only beta coefficients associated with P values less than 0.05 were incorporated. Ultimately, the scoring system was utilized to forecast suturing techniques, allocating two points for extraluminal growth and three points for EFTR. Each subject’s total score was determined by summing the individual variable scores, and then categorizing them into low and high risk groups. The rates of purse-string suturing were also computed for each ca
The discriminatory power of the model was assessed utilizing receiver operating characteristic curves. The model’s calibration was evaluated using the Hosmer-Lemeshow goodness-of-fit test, which is a statistical method employed to determine the fit of the model to the observed data. The logistic regression analysis was conducted employing the ‘caret’ R package, with the refinement of model performance parameters accomplished through a ten-fold cross-validation process. All statistical analyses were carried out utilizing SPSS 26.0 and R programming language 4.0.2.
Patients with duodenal SMTs (n = 137), consisting of 67 females and 70 males with a mean age of 53.1 years (SD: 11.6 years), were consecutively included and underwent ER at Zhongshan Hospital, Fudan University, Shanghai, China. It is noteworthy that none of these subjects reported a history of endoscopic/surgical procedures on the duodenum. The predominant locations affected were the duodenal bulb, accounting for 68 cases (49.6%), and the descending portion, with 19 cases (13.9%) and 49 cases (35.8%) for SMTs in proximity to and away from the papilla, respectively. An ex
The 137 patients who participated all underwent ER, which included electric cutting for 3 patients (2.2%), endoscopic mucosal resection for 28 patients (20.4%), endoscopic submucosal dissection for 65 patients (47.4%), EFTR for 37 patients (27.0%), and endoscopic piecemeal mucosal resection for 4 patients (2.9%). The array of endoscopic tools utilized during the excision encompassed the hook knife for 39 patients (28.5%), dual knife for 10 patients (7.3%), IT knife for 49 patients (35.8%), and a snare for 61 patients (44.5%). For suturing, metal clips were applied in 107 cases (78.1%), and purse-string suturing was used in 30 cases (21.9%). The average procedure time was 36.7 minutes with a SD of 29.8 minutes. Su
The mean length of hospitalization for the patients was 6.0 days, with a SD of 9.7 days. The pathological analysis revealed the following diagnoses: 29 cases (21.2%) of ectopic pancreas, 14 cases (10.2%) of lipoma, 17 cases (12.4%) of neuroendocrine tumors, 29 cases (21.2%) of gastrointestinal stromal tumors (GISTs), and 37 cases (27.0%) of Brunner’s gland adenomas. Complications arose in 8 patients (5.8%): three had delayed bleeding which was managed effectively with endoscopic hemostasis. Three experienced delayed perforations, with two recovering with conservative treatment and one requiring laparoscopic repair. One patient experienced a perforation accompanied by superficial ulcer bleeding in proximity to the surgical site, which was subsequently resolved following further surgical intervention. Additionally, one patient developed severe acute pancreatitis, which showed improvement with symptomatic management. No re
We further compared two different suturing techniques and the univariate analysis showed that the duodenal SMTs in the group of purse-string suturing tended to exhibit extraluminal growth (50% vs 1.9%, P < 0.001), often located within the muscularis propria (83.3% vs 24.3%, P < 0.001), predominantly diagnosed as GIST (56.7% vs 11.2%, P < 0.001). EFTR (86.7% vs 10.3%, P < 0.001) was commonly employed, accompanied by gastric tube insertion (96.7% vs 59.8%, P < 0.001). Moreover, procedures within this group typically had a longer duration (60.9 ± 32.3 vs 30.2 ± 25.5, P < 0.001) (Table 1).
| Metal clip (n = 107) | Purse-string suturing (n = 30) | P value | |
| Demographic information | |||
| Male | 55 (51.4) | 15 (50.0) | 0.892 |
| Age (years), mean ± SD | 53.8 ± 11.8 | 50.8 ± 10.8 | 0.215 |
| Hypertension | 29 (27.1) | 5 (16.7) | 0.242 |
| Diabetes mellitus | 5 (4.7) | 0 (0.0) | 0.512 |
| Lesion characteristics | |||
| Growth pattern | < 0.001 | ||
| Intraluminal growth | 105 (98.1) | 15 (50.0) | |
| Extraluminal growth | 2 (1.9) | 15 (50.0) | |
| Morphology | 0.490 | ||
| Regular | 97 (90.7) | 29 (96.7) | |
| Irregular | 10 (9.3) | 1 (3.3) | |
| Mucosa | 0.321 | ||
| Smooth | 90 (84.1) | 28 (93.3) | |
| Anabrotic or ulcerative | 17 (15.9) | 2 (6.7) | |
| Location | 0.269 | ||
| Duodenal bulb | 49 (45.8) | 19 (63.3) | |
| Descending part (near the papilla) | 17 (15.9) | 2 (6.7) | |
| Descending part (not near the papilla) | 40 (37.4) | 9 (30.0) | |
| Horizontal part | 1 (0.9) | 0 (0.0) | |
| Infiltration depth | < 0.001 | ||
| Submucosa | 81 (75.7) | 5 (16.7) | |
| Muscularis propria | 26 (24.3) | 25 (83.3) | |
| Max diameter (mm), mean ± SD | 12.9 ± 7.0 | 14.6 ± 6.8 | 0.227 |
| Procedural outcomes | |||
| Endoscopic methods | |||
| Electric cutting | 3 (2.8) | 0 (0.0) | 0.220 |
| EMR | 28 (26.2) | 0 (0.0) | 0.002 |
| ESD | 62 (57.9) | 3 (10.0) | < 0.001 |
| EFTR | 11 (10.3) | 26 (86.7) | < 0.001 |
| EPMR | 3 (2.8) | 1 (3.3) | 0.881 |
| Intraoperative endoscopic instruments | |||
| Hook knife | 30 (28.0) | 9 (30.0) | 0.833 |
| Dual knife | 8 (7.5) | 2 (6.7) | 1.000 |
| IT knife | 40 (37.4) | 9 (30.0) | 0.456 |
| Snare | 45 (42.1) | 16 (53.3) | 0.272 |
| Histopathologic evaluation | |||
| Ectopic pancreas | 24 (22.4) | 5 (16.7) | 0.495 |
| Lipoma | 14 (13.1) | 0 (0.0) | 0.080 |
| NET | 16 (15.0) | 1 (3.3) | 0.164 |
| GIST | 12 (11.2) | 17 (56.7) | < 0.001 |
| Brunner’s gland adenoma | 33 (30.8) | 4 (13.3) | 0.056 |
| Others | 8 (7.5) | 3 (10.0) | 0.945 |
| Submucosal fibrosis | 10 (9.3) | 2 (6.7) | 0.926 |
| En-bloc resection | 84 (78.5) | 23 (76.7) | 0.830 |
| Complete resection | 84 (78.5) | 23 (76.7) | 0.830 |
| Stomach tube | 64 (59.8) | 29 (96.7) | < 0.001 |
| Surgery time (minute), mean ± SD | 30.2 ± 25.5 | 60.9 ± 32.3 | < 0.001 |
| Hospital stay (day), mean ± SD | 5.1 ± 9.4 | 8.9 ± 10.5 | 0.059 |
| Complications | 6 (5.6) | 2 (6.7) | 1.000 |
The demographic and clinical features, as well as the outcomes of the procedures, for both the training cohort (TC) and the validation cohort (VC) are detailed in Supplementary Table 2. Encompassing a total of 137 patients, the study comprised 95 participants in the TC and 42 in the VC. It was observed that the clinical profiles and procedural results were well-matched between the two cohorts.
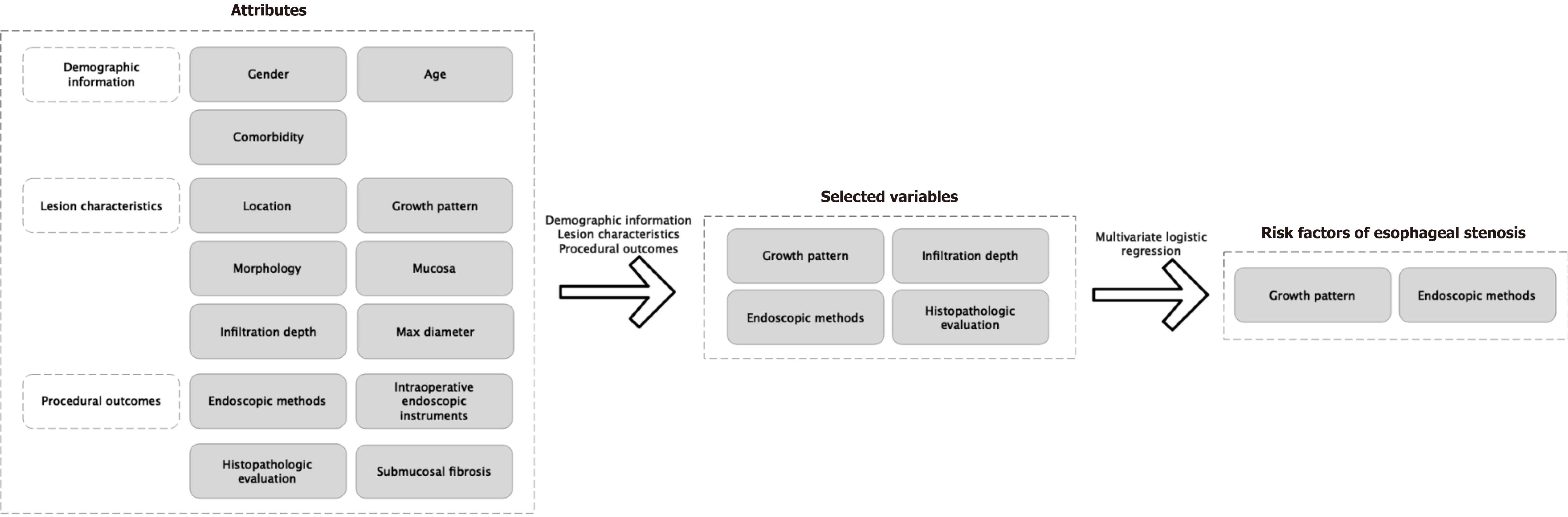
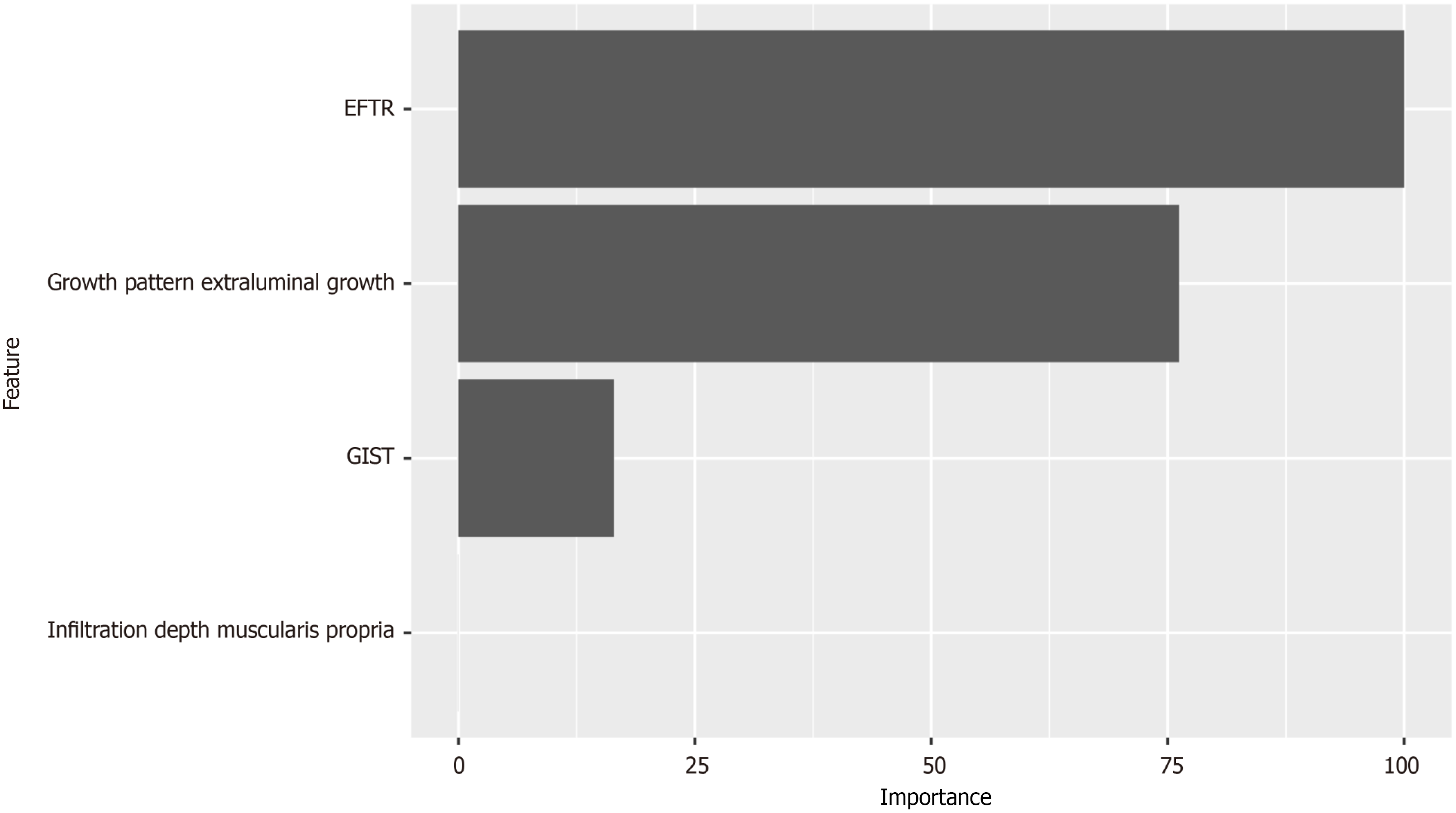
Four key variables demonstrated significant differences based on the two distinct suturing techniques: These were growth pattern, infiltration depth, endoscopic methods, and histopathologic evaluation (Figure 1). These variables were consequently incorporated into the MLRA. Upon conducting the multivariate analysis with 10-fold cross-validation, it was determined that growth pattern and endoscopic methods were the model’s predictors with statistical significance (Table 2). Figure 2 illustrates the relative importance of each variable as determined by the sensitivity analysis.
| Factors | OR (95%CI) | β coefficient | P value | Point assigned |
| Growth pattern | ||||
| Intraluminal growth | 1 | |||
| Extraluminal growth | 6.694 (1.212-55.684) | 1.901 | 0.044 | 2 |
| Infiltration depth | ||||
| Submucosa | 1 | |||
| Muscularis propria | 1.237 (0.049-13.673) | 0.213 | 0.873 | NA |
| Endoscopic methods | ||||
| Non-full-thickness resection | 1 | |||
| EFTR | 30.518 (3.251-788.983) | 3.418 | 0.009 | 3 |
| Histopathologic evaluation | ||||
| GIST | 0.606 (0.095-3.371) | -0.502 | 0.575 | NA |
| Non-GIST | 1 |
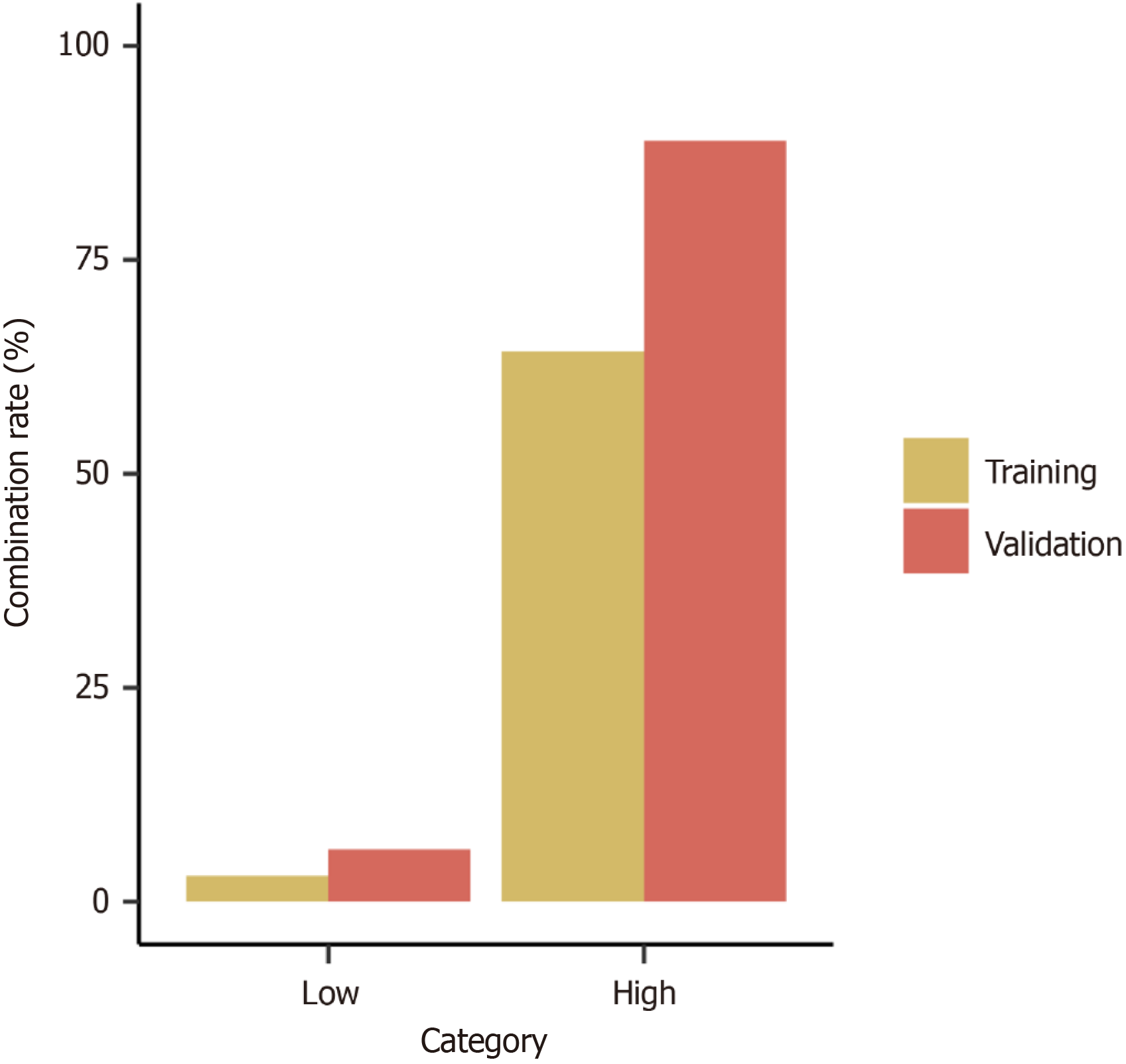
A predictive scoring model for suturing techniques was developed utilizing variables that exhibited significant statistical disparities within the demographic and lesion feature analysis. This scoring model was formulated utilizing MLRA within the TC and subsequently validated within the VC. We applied a scoring system for predicting suturing technique by assigning two points to extraluminal growth and three points to EFTR (Table 2). For each case within the TC, the cumulative score was determined by summing up the points allocated to the respective variables (Table 3). Higher scores indicated a greater probability of utilizing purse-string suturing for wound management. The probability of purse-string suturing in low (score = 0-2) and high (score > 3) categories were 3.0% and 64.3% in the TC, and 6.1% and 88.9% in the VC, respectively (Table 4, Figure 3). The area under the receiver operating characteristic curve for the TC and VC were 0.908 and 0.891, respectively (Supplementary Figure 1). The scoring model presented good discriminatory power (area under the curve: 0.900, 95%CI: 0.823-0.976) (Figure 4) as well as goodness-of-fit using the Hosmer-Lemeshow test (P = 0.404).
| Total points | TC, patients | TC, combination | TC, combination rate (%) | VC, patients | VC, combination | VC, combination rate (%) |
| 0 | 67 | 2 | 3.0 | 33 | 2 | 6.1 |
| 3 | 15 | 7 | 46.7 | 5 | 4 | 80.0 |
| 5 | 13 | 11 | 84.6 | 4 | 4 | 100.0 |
| Category | Total points | TC, patients | TC, purse-string suturing | TC, rate (%) | VC, patients | VC, purse-string suturing | VC, pate (%) |
| Low | 67 | 2 | 3.0 | 33 | 2 | 6.1 | |
| High | ≥ 3 | 28 | 18 | 64.3 | 9 | 8 | 88.9 |
Previous studies has indicated that ER for duodenal SMTs is linked to a significant incidence of complications, as re
In our center, purse-string suture closure was predominantly utilized for tumors that exhibited extraluminal growth, infiltrated the muscularis propria, or were diagnosed as GIST, as well as for those patients who had undergone EFTR. The sensitivity analysis highlighted that the tumor’s growth pattern and the endoscopic techniques employed were significant determinants in the selection of suturing approaches. Compared to lesions growing intraluminally, those growing extraluminally were situated deeper, necessitating a more secure purse-string suture for post-resection closure. Besides, the wounds following EFTR were often deeper compared with non-full-thickness resection, necessitating purse-string suturing for secure closure.
Based on the analyses, our research has developed and confirmed a new, straightforward scoring system designed to forecast the suturing technique for duodenal SMTs. This scoring system had two factors: Growth pattern and endoscopic methods. When the score ranged from zero to two, metal clips were indicated for suturing the wound. Conversely, a score exceeding three suggested that purse-string suturing was more appropriate. Our scoring system had a number of advantages. Firstly, it was founded on readily accessible clinical characteristics, making it practical for use in clinical practice and potentially aiding in the prediction of suturing methods. Moreover, the incorporation of ten-fold cross-validation in our model parameter tuning resulted in a scoring system with strong discriminative power. This system was capable of classifying duodenal SMTs into various risk groups and identifying appropriate suturing techniques, thus acting as a valuable guide for novice endoscopists.
Our study, while informative, was not without its constraints. Firstly, we conducted only internal validation for our scoring system, suggesting that its robustness needs to be further tested with cases from external sources to enhance the system’s applicability across different settings. Secondly, given that this was a retrospective analysis, there was a possibility of inherent bias. Therefore, it is imperative for future prospective studies to address these limitations and to strengthen the validity of our findings.
To sum up, the scoring system we developed might offer clinicians a valuable tool for predicting the appropriate suturing techniques for duodenal SMTs.
| 1. | Geng ZH, Zhu Y, Qu YF, Fu PY, Chen WF, Zhou PH, Li QL. Risk factors for complications and incomplete resection after endoscopic resection for duodenal submucosal tumors. Surg Endosc. 2023;37:9183-9189. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Ochiai Y, Kato M, Kiguchi Y, Akimoto T, Nakayama A, Sasaki M, Fujimoto A, Maehata T, Goto O, Yahagi N. Current Status and Challenges of Endoscopic Treatments for Duodenal Tumors. Digestion. 2019;99:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Li C, Liang C, Wang X, Le M, Liu D, Tan Y. Safety and efficacy of surgical and endoscopic resection in the treatment of duodenal subepithelial lesions. Surg Endosc. 2022;36:4145-4153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Kantsevoy SV. Duodenal endoscopic submucosal dissection: Is it ready for primetime? (with video). Gastrointest Endosc. 2020;91:1138-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Fukuhara S, Kato M, Iwasaki E, Sasaki M, Tsutsumi K, Kiguchi Y, Akimoto T, Takatori Y, Nakayama A, Maehata T, Minami K, Ogata H, Kanai T, Yahagi N. Management of perforation related to endoscopic submucosal dissection for superficial duodenal epithelial tumors. Gastrointest Endosc. 2020;91:1129-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Vanbiervliet G, Strijker M, Arvanitakis M, Aelvoet A, Arnelo U, Beyna T, Busch O, Deprez PH, Kunovsky L, Larghi A, Manes G, Moss A, Napoleon B, Nayar M, Pérez-Cuadrado-Robles E, Seewald S, Barthet M, van Hooft JE. Endoscopic management of ampullary tumors: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021;53:429-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (1)] |
| 7. | Qiao Z, Ling X, Zhu J, Ying G, Xu L, Zhu H, Tang J. Therapeutic application of purse-string sutures with nylon loops and metal clips under single-channel endoscopy for repair of gastrointestinal wall defects. Exp Ther Med. 2018;15:4356-4360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Luo XB, Huang JS, Liu M, Li Y, Li AM, Zhang Q, Wang Z, Xing TY, Huang Y, Huang R, Bai Y, Liu SD, Han ZL. Endoscopic resection via antral submucosal tunneling for en bloc removal of tumors in the duodenal bulb. Dig Endosc. 2022;34:1063-1068. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |