Published online Feb 24, 2025. doi: 10.5306/wjco.v16.i2.95642
Revised: September 28, 2024
Accepted: October 22, 2024
Published online: February 24, 2025
Processing time: 240 Days and 1.6 Hours
Bone is a major site of metastasis in nasopharyngeal carcinoma (NPC). Recently, nuclear factor kappa-beta ligand (RANKL) inhibitors have garnered attention for their ability to inhibit osteoclast formation and bone resorption, as well as their potential to modulate immune functions and thereby enhance the efficacy of programmed cell death protein 1 (PD-1) inhibitor therapy.
We present a case of a patient with NPC who developed sternal stalk metastasis and multiple bone metastases with soft tissue invasion following radical chemoradiotherapy and targeted therapy. Prior to chemotherapy, the patient experienced severe bone marrow suppression and opted out of further chemotherapy sessions. However, the patient received combination therapy, including RANKL inhibitors (denosumab) alongside PD-1, radiotherapy, and granulocyte-macrophage colony-stimulating factor (PRaG) therapy (NCT05435768), and achieved 16 months of progression-free survival and more than 35 months of overall survival, without encountering any grade 2 or higher treatment-related adverse events.
Denosumab combined with PRaG therapy could be a new therapeutic approach for the second-line treatment in patients with bone metastases.
Core Tip: Bone is a common metastatic site in nasopharyngeal carcinoma (NPC). We report a patient with NPC who developed multiple bone metastases and soft tissue invasion after 1 year of curative chemoradiotherapy and targeted therapy. Due to severe bone marrow suppression from prior chemotherapy, the patient declined further treatment and received denosumab (receptor activating factor ligand inhibitor) combined with PRaG therapy. This regimen resulted in progression-free survival of 16 months and overall survival of more than 35 months, without any grade 2 or higher treatment-related adverse reactions, suggesting a novel therapeutic strategy for patients with refractory NPC.
- Citation: Chen WW, Kong YH, Zhang LY. Denosumab combined with immunotherapy, radiotherapy, and granulocyte-macrophage colony-stimulating factor for the treatment of metastatic nasopharyngeal carcinoma: A case report. World J Clin Oncol 2025; 16(2): 95642
- URL: https://www.wjgnet.com/2218-4333/full/v16/i2/95642.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i2.95642
Despite simultaneous chemoradiotherapy, approximately 30% of patients with locally advanced cancer experience distant or recurring metastasis[1]. Bone is one of the most common metastatic sites of nasopharyngeal carcinoma (NPC), accounting for more than 50% of all metastatic occurrences[2].
Systemic treatments for metastatic NPC primarily include platinum-based chemotherapy, such as cisplatin. Recent studies have reported a 5-year survival rate of 19.2% with the first-line gemcitabine and cisplatin (GP) regimen[3]. The advent of immunotherapy in combination with platinum-based chemotherapy has significantly improved the survival outcomes of patients with metastatic NPC, increasing the median progression-free survival (PFS) from 6.9 months to 10.8 months[4]. This approach has become the standard treatment for metastatic NPC[5]. However, current treatment options remain limited for metastatic NPC due to failure in the first-line treatment or refractory to chemotherapy.
In this case report, we aimed to present a patient with NPC who developed multiple bone metastases following standard chemoradiotherapy and targeted therapy, which led to therapy-induced severe bone marrow suppression, resulting in patient noncompliance. Consequently, the patient opted for an innovative regimen combining receptor of nuclear factor kappa-beta ligand (RANKL) inhibitors with PRaG therapy.
A 63-year-old male Chinese patient was diagnosed with advanced NPC with multiple bone metastases 1 year after completing curative chemoradiotherapy.
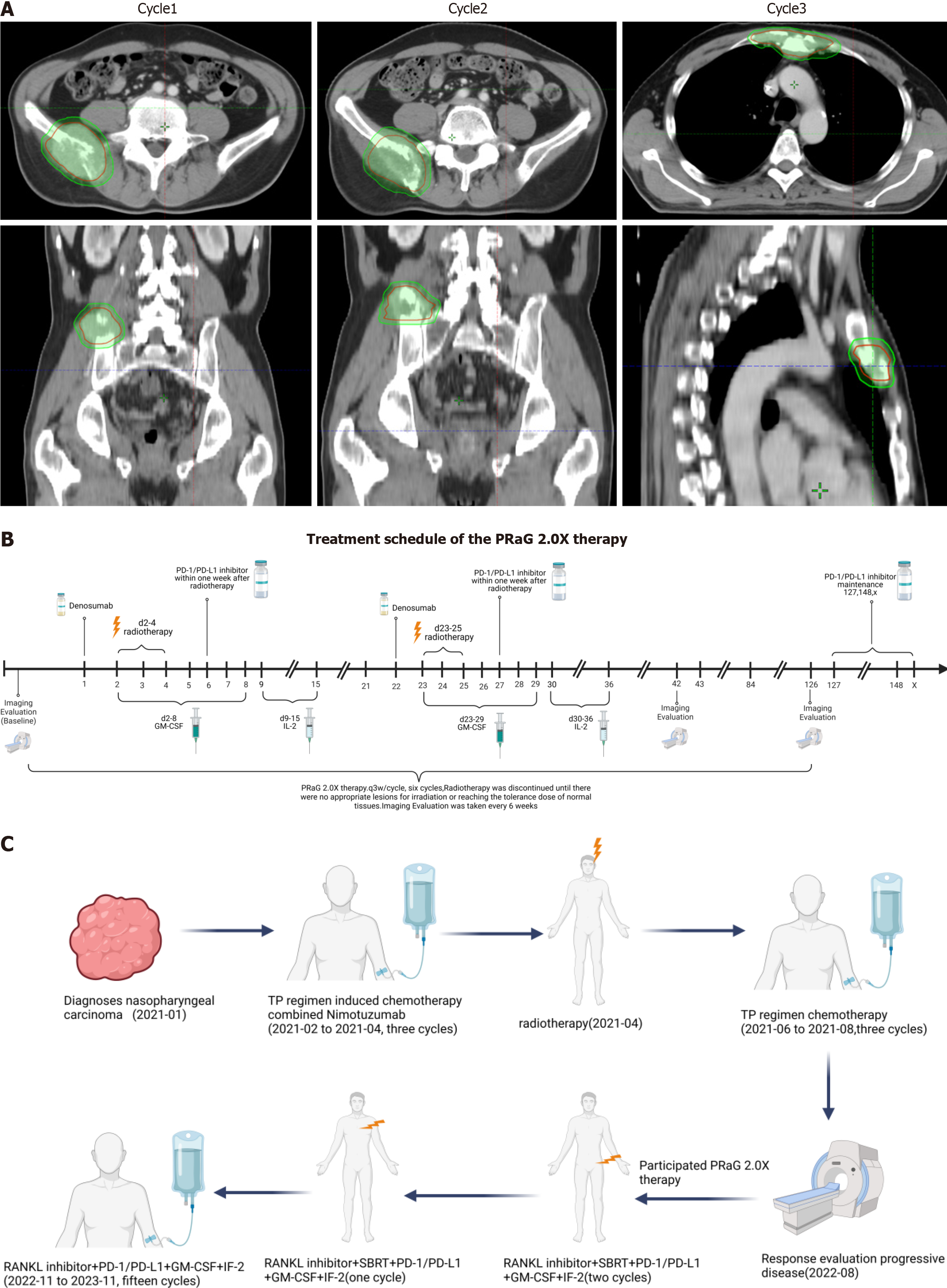
The patient was diagnosed with stage IVA NPC with poorly differentiated squamous cell carcinoma in January 2021. He underwent three cycles of induction chemotherapy with docetaxel plus cisplatin (TP) regimen, consisting of paclitaxel (240 mg on day 1) + cisplatin (45 mg for 3 consecutive days from day 1 to day 3), in combination with nimotuzumab (400 mg on day 1) as targeted therapy. Radiation therapy was administered from April 1, 2021, to May 17, 2021. The radiation therapy consists of primary tumor lesion and metastatic retropharyngeal lymph node (PGTVnx) 71.28 Gy/2.16 Gy/33 Fx; PGTVnd (metastatic lymph nodes in bilateral II region) 66 Gy/2.0 Gy/33 Fx; planning target volume (PTV) 1 (includes high-risk areas around the primary focus, pterygoid sinus, skull base, sieve sinus, right soft palate, slope, pterygopalatine fossa, parotid gland, parapharyngeal space, and oropharynx) 66 Gy/2 Gy/33 Fx; PTV2 (right Ib, bilateral II, III, and Va regions) 61.05 Gy/1.85 Gy/33 Fx; PTV3 (bilateral IVa and Vb regions) 50.4 Gy/1.8 Gy/28 Fx.
Three cycles of TP regimen chemotherapy were administered from June to August 2021, with cycle one consisting of paclitaxel (240 mg on day 1) and cisplatin (45 mg from day 1 to day 3). Due to the development of severe bone marrow suppression (leukopenia), the dose was reduced by 75% (i.e., paclitaxel 180 mg on day one and cisplatin 30 mg from day 1 to day 3) in the subsequent second and third cycles.
After chemoradiotherapy, the follow-up magnetic resonance imaging indicated size reduction in the nasopharyngeal primary and cervical lymph nodes, with the tumor lesion remaining stable in several subsequent reviews. The partial response and progressive disease were evaluated per the RECIST 1.1 criteria. In August 2022, a follow-up examination revealed metastasis in the sternal stem and right iliac bone surrounded with soft tissue shadows.
The patient had no history of chronic diseases, including hypertension, diabetes, heart disease, or infectious diseases such as hepatitis B and tuberculosis.
There was no personal or familial history of relevant tumor diseases.
The patient had pressure pain in the sternal and right iliac areas.
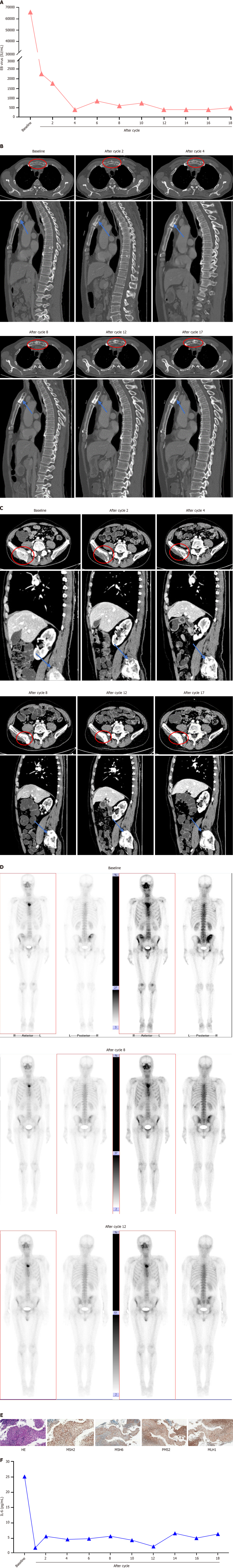
The Epstein–Barr-DNA copy count was 6.62 × 104 IU/mL (Figure 1A).
Based on the patient’s medical history and relevant examinations, the ultimate diagnosis was multiple bone metastatic NPC.
After being diagnosed with multiple bone metastases and declining chemotherapy, the patient was enrolled in a clinical trial (NCT05435768) and began treatment with RANKL inhibitors combined with PRaG therapy on September 1, 2022. The treatment protocol included a subcutaneous injection of denosumab (XGEVA; 120 mg, day 1), followed by ste
In early November 2022, after three cycles of treatment, the patient began consolidation therapy. Each cycle of consolidation therapy consisted of a subcutaneous injection of denosumab (XGEVA) (120 mg, day 1), followed by GM-CSF (200 µg, days 2-8) for 7 consecutive days, and sequential IL-2 injections (2 million IU, days 9-15) for 7 consecutive days. A PD-L1 inhibitor (Envolizumab 400 mg) was administered subcutaneously every 21 days (the patient’s course of treatment is shown in Figure 2C).
During the combination therapy (RANKL inhibitors combined with PRaG therapy), follow-up examinations revealed a significant reduction in size in both sternal and right iliac metastases, and a significant decrease in Epstein–Barr virus (EBV) copy number. In December 2023, the PFS of the patient was 16 months, and the overall survival (OS) exceeded 35 months, with no grade 2 or higher treatment-related adverse reactions. The patient is currently undergoing treatment with Envolizumab immunotherapy as monotherapy.
According to the phase 3 clinical trial, GP is the preferred first-line chemotherapy regimen for recurrent or metastatic NPC[3]. With the advent of immunotherapy, the CAPTAIN-1 clinical trial introduced camrelizumab into the GP regimen, which markedly increased the median PFS of patients with local recurrence and metastatic NPC to 10.8 months, co
The “PRaG therapy” designed by our research group and registered under ChiCTR1900026175, combines programmed cell death protein 1 (PD-1)/PD-L1 inhibitors, radiotherapy, and GM-CSF to treat patients with advanced posterior line tumor. The PRaG therapy targets three stages of the tumor-immunity cycle: (1) Radiotherapy to expose tumor antigens; (2) GM-CSF to activate antigen-presenting cells; and (3) PD-1/PD-L1 inhibitors to enhance the tumor-killing ability of specific CD8+T lymphocytes, thereby fostering a synergistic and effective tumor immune response[6]. The therapy aims to establish immune memory and addresses the tumor heterogeneity through multiple radiation therapy cycles, targeting different tumor parts. RANKL is a cytokine involved in osteoclast differentiation and plays a key role in pathologic bone resorption in patients with bone metastasis[7]. RANKL inhibitors are known for treating bone metastases or multiple myelomas, exhibiting antitumor immune effects when combined with immune checkpoint inhibitors (ICIs)[8]. A pre
Based on our study results, combining RANKL inhibitors with PRaG therapy may further improve anticancer efficacy in patients with bone metastases. In this case, the patient was already in an advanced stage at the initial diagnosis. Given the high malignancy and the critical condition of the patient, induction of chemotherapy and targeted therapy were administered, which resulted in severe bone marrow suppression during adjuvant chemotherapy. Furthermore, after developing multiple bone metastases and considering the significant toxic side effects, the patient was noncompliant with undergoing chemotherapy, and opted for experimental treatment with RANKL inhibitors combined with PRaG therapy. After treatment, a significant reduction in bone metastases and copy numbers of EBV were observed.
In addition, the concentration of IL-6 significantly decreased during the treatment (Figure 1F). IL-6 is secreted by various cells, including dendritic cells, macrophages, B lymphocytes, T lymphocytes, and tumor cells[11]. It activates the IL-6/signal transduction and transcription activation factor 3-signaling pathway in cancer cells, supporting tumorigenesis by inhibiting apoptosis and promoting survival[12]. Notably, IL-6 was upregulated in different types of cancer. High IL-6 levels in the tumors were also associated with poor prognosis[13].
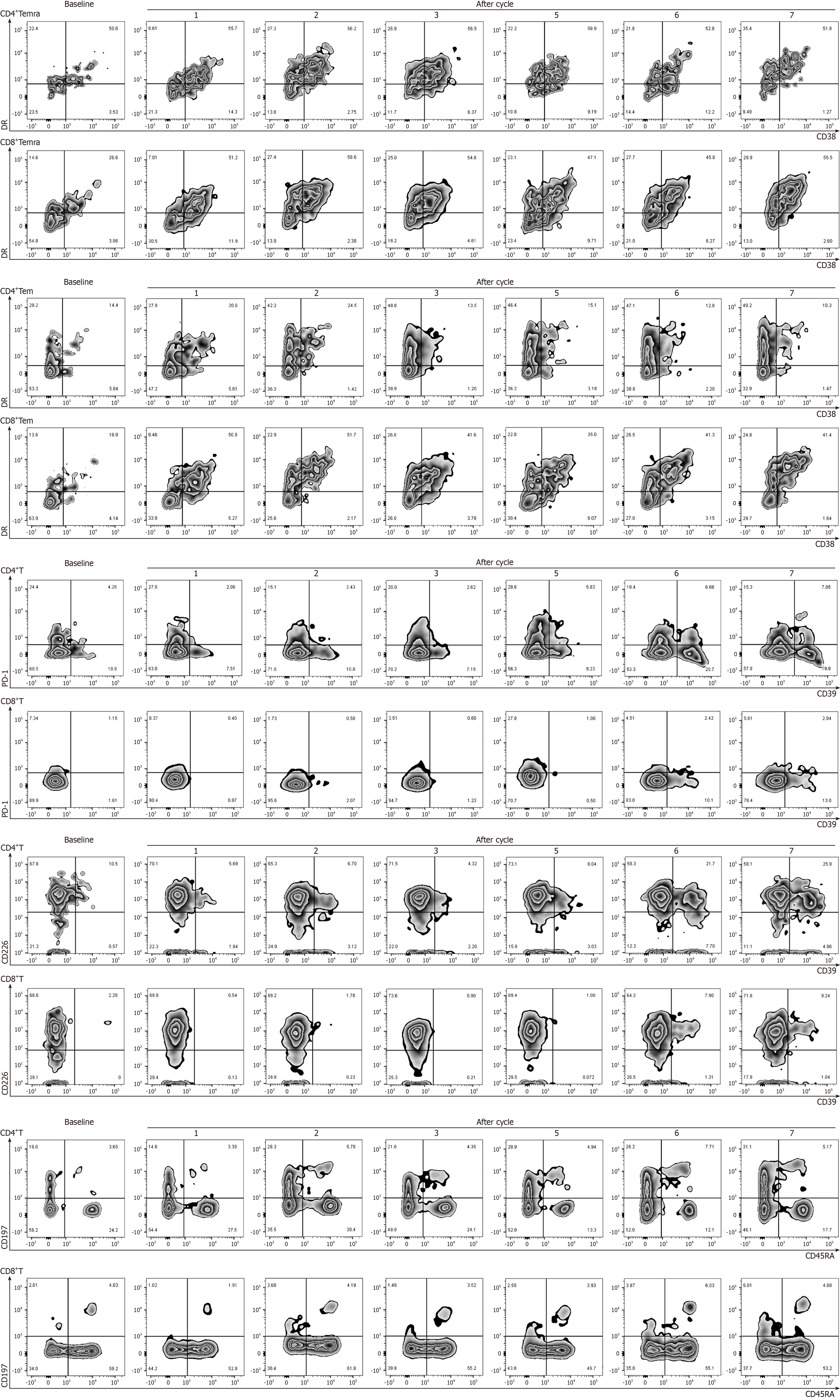
Flow cytometric analysis of immune function activation during the PRaG treatment in this patient revealed a slight increase in several immune cell populations, including HLA-DR+ CD38+CD8+Temra cells, CD4+CD39+T cells, CD4+CD39+PD-1+T cells, CD8+CD39+T cells, CD4+CD39+CD226+T cells, and CD8+CD39 +CD226+T cells of the patient (Figure 3). CD226 is an adhesion molecule that stimulates natural killer cells and CD8+T cell-mediated cytotoxicity[14]. Recent research suggests that the absence of CD226 in CD8+T cells indicates a correlation with tumor progression, whereas an increase in CD226+CD8+T cells may correlate with better antitumor efficacy[15]. Moreover, CD39, an enzyme that works in concert with CD73, initiates the conversion of adenosine triphosphate to adenosine diphosphate and cyclic adenosine mono
The patient’s PFS reached 16 months, and OS exceeded 35 months, and was continuing with the single-agent immune maintenance therapy, with a survival period higher than the median OS of first-line GP combined with immunotherapy (PFS, 95%CI: 8.5-13.6 months). Importantly, no grade 2 or higher adverse reactions were observed during the treatment. The results of the short-term efficacy evaluation of this case were significantly better than those previously reported in the literature, providing a new reference for the treatment options for patients with NPC and bone metastases and who have failed the first-line treatment or are unable to tolerate chemotherapy.
After treatment with RANKL inhibitors coupled with the PRaG therapy regimen, the patient achieved prolonged survival with a PFS of 16 months and an OS exceeding 35 months, along with a high safety profile characterized by only grade 1 treatment-related adverse events. Bone is the predominant site of metastasis in advanced NPCs. The treatment regimen used in this case provides valuable insights for patients with bone metastases who either fail in first-line treatment or have difficulty tolerating chemotherapy. To the best of our knowledge, this is the first prospective report on the safety and efficacy of RANKL inhibitors combined with PD-1 inhibitors. The efficacy and safety of this treatment paradigm need to be further analyzed and validated in open-label prospective studies.
We are grateful to the patient and her family.
| 1. | Lee AW, Ma BB, Ng WT, Chan AT. Management of Nasopharyngeal Carcinoma: Current Practice and Future Perspective. J Clin Oncol. 2015;33:3356-3364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 577] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 2. | Zou X, You R, Liu H, He YX, Xie GF, Xie ZH, Li JB, Jiang R, Liu LZ, Li L, Zhang MX, Liu YP, Hua YJ, Guo L, Qian CN, Mai HQ, Chen DP, Luo Y, Shen LF, Hong MH, Chen MY. Establishment and validation of M1 stage subdivisions for de novo metastatic nasopharyngeal carcinoma to better predict prognosis and guide treatment. Eur J Cancer. 2017;77:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 3. | Zhang L, Huang Y, Hong S, Yang Y, Yu G, Jia J, Peng P, Wu X, Lin Q, Xi X, Peng J, Xu M, Chen D, Lu X, Wang R, Cao X, Chen X, Lin Z, Xiong J, Lin Q, Xie C, Li Z, Pan J, Li J, Wu S, Lian Y, Yang Q, Zhao C. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet. 2016;388:1883-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 385] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 4. | Yang Y, Qu S, Li J, Hu C, Xu M, Li W, Zhou T, Shen L, Wu H, Lang J, Hu G, Luo Z, Fu Z, Qu S, Feng W, Chen X, Lin S, Zhang W, Li X, Sun Y, Lin Z, Lin Q, Lei F, Long J, Hong J, Huang X, Zeng L, Wang P, He X, Zhang B, Yang Q, Zhang X, Zou J, Fang W, Zhang L. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2021;22:1162-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 272] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 5. | Tang LL, Chen YP, Chen CB, Chen MY, Chen NY, Chen XZ, Du XJ, Fang WF, Feng M, Gao J, Han F, He X, Hu CS, Hu DS, Hu GY, Jiang H, Jiang W, Jin F, Lang JY, Li JG, Lin SJ, Liu X, Liu QF, Ma L, Mai HQ, Qin JY, Shen LF, Sun Y, Wang PG, Wang RS, Wang RZ, Wang XS, Wang Y, Wu H, Xia YF, Xiao SW, Yang KY, Yi JL, Zhu XD, Ma J. The Chinese Society of Clinical Oncology (CSCO) clinical guidelines for the diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun (Lond). 2021;41:1195-1227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 237] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 6. | Kong Y, Ma Y, Zhao X, Pan J, Xu Z, Zhang L. Optimizing the Treatment Schedule of Radiotherapy Combined With Anti-PD-1/PD-L1 Immunotherapy in Metastatic Cancers. Front Oncol. 2021;11:638873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Wilkinson AN, Viola R, Brundage MD. Managing skeletal related events resulting from bone metastases. BMJ. 2008;337:a2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Liede A, Hernandez RK, Wade SW, Bo R, Nussbaum NC, Ahern E, Dougall WC, Smyth MJ. An observational study of concomitant immunotherapies and denosumab in patients with advanced melanoma or lung cancer. Oncoimmunology. 2018;7:e1480301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Ahern E, Harjunpää H, Barkauskas D, Allen S, Takeda K, Yagita H, Wyld D, Dougall WC, Teng MWL, Smyth MJ. Co-administration of RANKL and CTLA4 Antibodies Enhances Lymphocyte-Mediated Antitumor Immunity in Mice. Clin Cancer Res. 2017;23:5789-5801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | van Dam PA, Verhoeven Y, Trinh XB, Wouters A, Lardon F, Prenen H, Smits E, Baldewijns M, Lammens M. RANK/RANKL signaling inhibition may improve the effectiveness of checkpoint blockade in cancer treatment. Crit Rev Oncol Hematol. 2019;133:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 11. | Lotz M, Jirik F, Kabouridis P, Tsoukas C, Hirano T, Kishimoto T, Carson DA. B cell stimulating factor 2/interleukin 6 is a costimulant for human thymocytes and T lymphocytes. J Exp Med. 1988;167:1253-1258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 398] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Kumari N, Dwarakanath BS, Das A, Bhatt AN. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016;37:11553-11572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 735] [Article Influence: 81.7] [Reference Citation Analysis (0)] |
| 13. | Tsai MS, Chen WC, Lu CH, Chen MF. The prognosis of head and neck squamous cell carcinoma related to immunosuppressive tumor microenvironment regulated by IL-6 signaling. Oral Oncol. 2019;91:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Martinet L, Smyth MJ. Balancing natural killer cell activation through paired receptors. Nat Rev Immunol. 2015;15:243-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 369] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 15. | Weulersse M, Asrir A, Pichler AC, Lemaitre L, Braun M, Carrié N, Joubert MV, Le Moine M, Do Souto L, Gaud G, Das I, Brauns E, Scarlata CM, Morandi E, Sundarrajan A, Cuisinier M, Buisson L, Maheo S, Kassem S, Agesta A, Pérès M, Verhoeyen E, Martinez A, Mazieres J, Dupré L, Gossye T, Pancaldi V, Guillerey C, Ayyoub M, Dejean AS, Saoudi A, Goriely S, Avet-Loiseau H, Bald T, Smyth MJ, Martinet L. Eomes-Dependent Loss of the Co-activating Receptor CD226 Restrains CD8(+) T Cell Anti-tumor Functions and Limits the Efficacy of Cancer Immunotherapy. Immunity. 2020;53:824-839.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 16. | Timperi E, Barnaba V. CD39 Regulation and Functions in T Cells. Int J Mol Sci. 2021;22:8068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 17. | Koh J, Kim Y, Lee KY, Hur JY, Kim MS, Kim B, Cho HJ, Lee YC, Bae YH, Ku BM, Sun JM, Lee SH, Ahn JS, Park K, Ahn MJ. MDSC subtypes and CD39 expression on CD8(+) T cells predict the efficacy of anti-PD-1 immunotherapy in patients with advanced NSCLC. Eur J Immunol. 2020;50:1810-1819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |