Published online Jan 24, 2025. doi: 10.5306/wjco.v16.i1.96131
Revised: September 3, 2024
Accepted: September 23, 2024
Published online: January 24, 2025
Processing time: 185 Days and 18.1 Hours
Extramedullary plasmacytoma (EMP) represents one of the rarer forms of plasma cell malignancies, capable of impacting a variety of tissues and organs throughout the body. The majority of EMP cases are predominantly found in the head and neck region, especially within the laryngopharynx, as well as in the gastroin
A 51-year-old man was admitted to our hospital because of a slowly enlarging neck mass. A physical examination revealed a palpable left lymph node, and ma
We have reported a rare case of extramedullary plasmacytoma with the uvula as the first affected site and the relevant literature is reviewed to improve clinicians' awareness of such rare comorbidities.
Core Tip: The high-risk characteristics of extramedullary plasmacytoma in clinical manifestations stem from its pathogenesis and the high-risk trait of cytogenetics. There are limited recommendations and guidelines for this type of tumor owing to the rarity of extramedullary plasmacytoma (EMP) of the uvula. Radiotherapy is the preferred treatment after diagnosis. Che
- Citation: Yang J, Peng H, Tu SK, Li M, Song K. Extramedullary plasmacytoma with the uvula as first affected site: A case report. World J Clin Oncol 2025; 16(1): 96131
- URL: https://www.wjgnet.com/2218-4333/full/v16/i1/96131.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i1.96131
Extramedullary plasmacytoma (EMP) is an exceptionally rare form of plasma cell malignancy that can impact various tissues and organs throughout the body. The majority of EMP cases are found in the head and neck region, especially within the laryngopharynx, as well as in the gastrointestinal (GI) tract. Although instances of oropharyngeal involvement have been documented in the medical literature, EMP specifically affecting the uvula is an extremely uncommon oc
A 51-year-old man was admitted to our hospital because of a slowly enlarging mass of his neck.
On physical examination, the patient was a well-nourished man with a palpable, immovable nontender left lymph node measuring 2.5 cm × 2.5 cm. Oropharyngeal examination showed a neoformation of the left uvula. The rest of the physical examination was unremarkable. It was not clear whether the mass was an oropharyngeal tumor with cervical lymph node metastasis, or a lymphoma. However, this supported the clinical diagnosis of a primary lesion of the uvula with regional nodal involvement. The pathological diagnosis of the soft tissue mass and the cervical lymph node was pla
No special notes.
No special notes.
Upon conducting a physical examination, the patient presented as a well-nourished individual. Notably, a lymph node on the left side was identified; it was palpable, immovable, and nontender, measuring approximately 2.5 cm × 2.5 cm. Additionally, the examination of the oropharynx revealed the presence of a neoformation located on the left uvula. Aside from these findings, the remainder of the physical examination did not reveal any significant abnormalities.
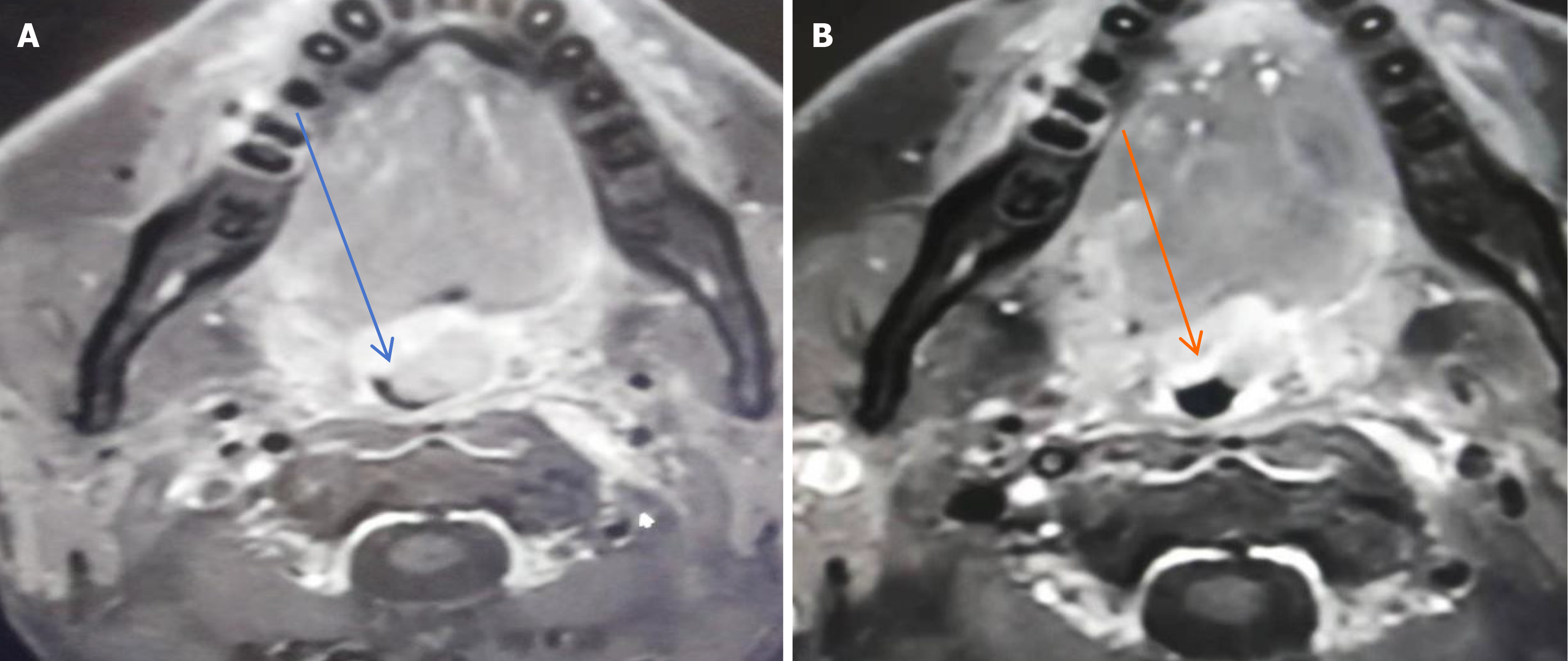
Magnetic resonance imaging (MRI) of the oropharynx showed an irregular, sharply defined soft tissue mass measuring 1.8 cm × 1.9 cm × 2.2 cm with heterogeneous enhancement (Figure 2A). The mass was closely related to the uvula and grew into the oropharyngeal cavity, which led to the narrowing of the corresponding oropharyngeal cavity. An enhanced scanning scan showed similar signals and enhancement signals. Therefore, we suspected that the mass was a primary tumor. Moreover, MRI of the neck showed nodular and massive signals, with the left larger one being about 3.1 cm × 3.6 cm and the right larger one being about 2.0 cm × 2.9 cm.
EMP of the uvula.
The patient eventually received a three-agent chemotherapy regimen [bortezomib, epirubicin, and dexamethasone (PAD)]. After four cycles, repeat MRI showed a significant reduction of both the mass in the left wall of the oropharynx and the multiple lymph nodes of different sizes in the neck (Figure 2B). It indicated that the chemotherapy was effective, so the patient was advised to intensify his chemotherapy regimen by using the VDECP chemotherapy regimen [cisplatin, etoposide, dexamethasone, and bortezomib (VDECP)]. After 6-mo chemotherapy, repeat immunoglobulin assay showed that the λ light chain returned to normal levels and the monoclonal immunoglobulins were normal. Finally, there were no evidence of MM on bone marrow biopsy and no lytic lesions on skeletal survey.
Plasmacytomas represent a form of malignant hyperplasia involving plasma cells, and they can manifest in two primary locations: Within the bone, referred to as medullary plasmacytomas, or in soft tissue, known as EMPs. These lesions can appear in varying quantities, meaning that a patient may exhibit either a solitary lesion or multiple lesions, depending on the extent and progression of the disease[1].
Plasma cell tumors are classified into four distinct categories: MM, plasma cell leukemia, solitary bone plasmacytoma (SBP), and EMP. Among these categories, EMP is notable for representing about 3% of all plasma cell malignancies. A significant proportion of EMP cases, approximately 80%, are localized to anatomical regions such as the head, neck, and gastrointestinal tract. Additionally, EMP can manifest in various other locations, including the urinary bladder, central nervous system, thyroid, breast, testes, parotid gland, lymph nodes, and skin. Nevertheless, the occurrence of EMP in the uvula is quite rare, underscoring its atypical presentation in this particular site. Demographic trends indicate that men have a threefold increased risk of developing EMP compared to women. The condition predominantly affects adults, especially those in their 50s and 60s, with a median age at diagnosis being 55 years. This age distribution emphasizes the importance of monitoring this demographic for potential signs of extramedullary plasmacytoma[2].
Solitary plasmacytoma can manifest in two distinct forms: Bone and extramedullary types. Both of these types have the potential to advance to MM, a more advanced and systemic form of the disease. Specifically, SBP is noted to progress to MM in approximately 50% to 60% of affected individuals. In terms of prognosis, the overall survival rate for these pa
The high-risk characteristics of EMP in clinical manifestations stem from its pathogenesis and the high-risk trait of cytogenetics[4]. Although it is currently unclear how plasma cells break through the bone marrow stromal cells and the bone marrow microenvironment, myeloma cells have been found to express adhesion factors such as very late appearing antigen-4 (VLA-4), CD56, and CD44[5,6]. The interaction between bone marrow plasma cells and vascular cell adhesion molecule-1 (VCAM-1) promotes the homing of myeloma cells[7,8]. However, for patients with EMP, the expression levels of these adhesion factors and endothelial cell receptors are decreased, which makes the adhesion ability of plasma cells to stromal cells decrease. Subsequently, plasma cells are likely to escape from the bone marrow microenvironment, thus developing EMP. Stromal cell-derived factor-1 (SDF-1) is one of the members of the CXC subfamily of chemokines, named CXCL12, also known as B cell stimulating factor[9]. SDF-1 induces cytoskeleton remodeling, pseudopod for
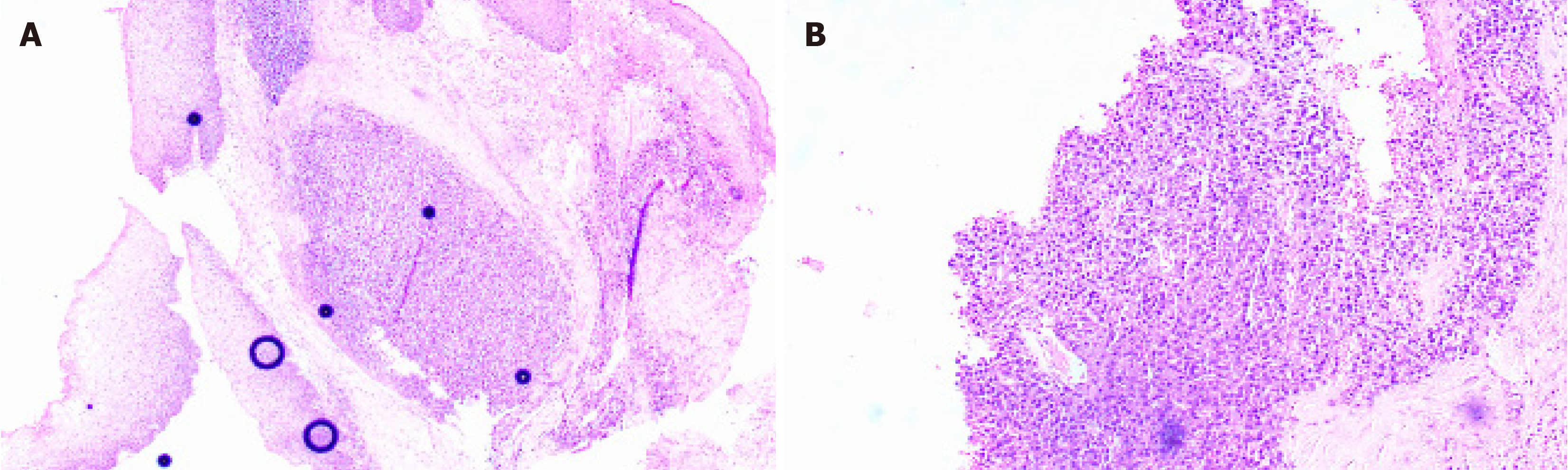
The International Myeloma Working Group has established specific criteria for the definition of EMP. First, EMP is characterized by the presence of a tumor that consists of monoclonal plasma cells localized in a single extramedullary site. Importantly, the diagnosis of EMP requires the absence of any lesions throughout the entire skeletal system. Furthermore, there must be no detectable lesions within the bone marrow itself. In addition, it is crucial to ensure that there is no involvement of any organs, confirming that the disease has not spread to other areas. Lastly, the assessment must reveal an absence of monoclonal immunoglobulin in either serum or urine, which further supports the diagnosis of EMP[11]. The diagnostic methods for EMP mainly include imaging and histological examination. EMP has a low incidence, is difficult to be diagnose, and lacks specificity on early imaging, but it still has certain features. Computed tomographic (CT) imaging characteristics of EMP can be summarized as local lesions, small necrosis, moderate enhancement, fusion tendency, and encapsulation of adjacent blood vessels to form a "sandwich" sign. In addition, positron emission tomography (PET)-CT can clarify the regional activity of the tumor and can be used to distinguish EMP from MM[12]. EMP histology shows proliferation of plasmacytoid tumor cells, with immunohistochemical markers CD38, CD138, and CD79a, Kappa, or Lambda. Differentiation from MM and extramedullary lesions of MM must be made before a diagnosis of EMP or taking any therapeutic measure. Multiple bone marrow biopsies are required to exclude MM and look for evidence of clonal plasma cell expansion. In this case, the uvula showed an irregular mass without infiltrative growth, and the immunohistochemical results were positive for CD79a, D38, CD138, and Kappa. Meanwhile, bone marrow aspiration examinations ruled out the possibility of advanced extramedullary manifestations of MM. As our patient fulfilled the above criteria, the tumor was diagnosed as primary EMP of the uvula.
Plasmacytomas are difficult to cure, and there are limited recommendations and guidelines for this type of tumor because of the rarity of EMP. Due to the high rate of control, radiotherapy is the treatment of choice after diagnosis. Either tumors that can be resected as a whole or lesions that are insensitive to radiotherapy can be treated with surgical re
EMP is mostly a low-grade malignancy, and even though responding well to treatment, it remains an aggressive tumor with a poor prognosis, prone to local recurrence and distant metastasis. Common metastatic sites include cervical lymph nodes, the lung, liver, spinal cord, and bone marrow. Because of the high evolution rate to MM, it leads to poor overall survival in such patients. Due to the rarity of the disease, there is no uniform consensus on prognostic factors and treatment. Radiotherapy remains the mainstay of treatment, but there are variations in the sensitivity of treatment. According to statistics, after treatment, about 20% of EMP patients have local recurrence within 2-3 years, and 15% convert to highly malignant MM[14]. The International Guidelines for Radiotherapy of Lymphoma clearly state that surgical treatment is still necessary for solitary plasmacytoma. Therefore, the establishment of perfect classification and recovery assessment criteria is crucial for the selection of treatment methods.
We propose that patients undergo follow-up reviews at 3-mo intervals during the first year. Following that initial period, reviews should shift to 6-mo intervals for the subsequent year, and then transition to an annual review schedule for the rest of the patient's life. During each of these visits, it is critical to conduct a comprehensive history and physical examination of the patient. Additionally, a variety of laboratory tests should be performed, which include a complete blood count, measurements of serum calcium levels, as well as urea, creatinine, and electrolyte testing. It is also important to incorporate serum and immunofixation protein electrophoresis, urine testing for Bence-Jones proteins, free light chains analysis, and a skeletal survey into the routine testing protocol. Furthermore, patients should undergo yearly monitoring with MRI to track any changes over time. Importantly, if there are any indications of disease progression, a complete reevaluation is warranted. This reevaluation should entail performing a bone marrow biopsy along with an integrated CT/PET scan to comprehensively assess the situation. This structured approach is essential to ensure that any changes in the patient's condition are monitored closely and addressed promptly[1]. It is believed that with the further research of large samples, the treatment plan for EMP will gradually be individualized, standardized, and scientific.
We have reported a rare case of extramedullary plasmacytoma with the uvula as the first affected site and the relevant literature is reviewed to improve clinicians' awareness of such rare comorbidities.
| 1. | Bazaadut S, Soodin D, Singh P, Khalafallah A, Withers S, Taylor S, Fernando R. Extramedullary plasmacytoma of the tonsil with nodal involvement. Int J Otolaryngol. 2010;2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Townend PJ, Kraus G, Coyle L, Nevell D, Engelsman A, Sidhu SB. Bilateral extramedullary adrenal plasmacytoma: case report and review of the literature. Int J Endocr Oncol. 2017;4:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Soutar R, Lucraft H, Jackson G, Reece A, Bird J, Low E, Samson D; Working Group of the UK Myeloma Forum; British Committee for Standards in Haematology; British Society for Haematology. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Clin Oncol (R Coll Radiol). 2004;16:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | de Haart SJ, Willems SM, Mutis T, Koudijs MJ, van Blokland MT, Lokhorst HM, de Weger RA, Minnema MC. Comparison of intramedullary myeloma and corresponding extramedullary soft tissue plasmacytomas using genetic mutational panel analyses. Blood Cancer J. 2016;6:e426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Dahl IM, Rasmussen T, Kauric G, Husebekk A. Differential expression of CD56 and CD44 in the evolution of extramedullary myeloma. Br J Haematol. 2002;116:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Ryu D, Kim SJ, Hong Y, Jo A, Kim N, Kim HJ, Lee HO, Kim K, Park WY. Alterations in the Transcriptional Programs of Myeloma Cells and the Microenvironment during Extramedullary Progression Affect Proliferation and Immune Evasion. Clin Cancer Res. 2020;26:935-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Ullah TR. The role of CXCR4 in multiple myeloma: Cells' journey from bone marrow to beyond. J Bone Oncol. 2019;17:100253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | Hao P, Zhang C, Wang R, Yan P, Peng R. Expression and pathogenesis of VCAM-1 and VLA-4 cytokines in multiple myeloma. Saudi J Biol Sci. 2020;27:1674-1678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | Weinstock M, Aljawai Y, Morgan EA, Laubach J, Gannon M, Roccaro AM, Varga C, Mitsiades CS, Paba-Prada C, Schlossman R, Munshi N, Anderson KC, Richardson PP, Weller E, Ghobrial IM. Incidence and clinical features of extramedullary multiple myeloma in patients who underwent stem cell transplantation. Br J Haematol. 2015;169:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Handa H, Kuroda Y, Kimura K, Masuda Y, Hattori H, Alkebsi L, Matsumoto M, Kasamatsu T, Kobayashi N, Tahara KI, Takizawa M, Koiso H, Ishizaki T, Shimizu H, Yokohama A, Tsukamoto N, Saito T, Murakami H. Long non-coding RNA MALAT1 is an inducible stress response gene associated with extramedullary spread and poor prognosis of multiple myeloma. Br J Haematol. 2017;179:449-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749-757. [PubMed] |
| 12. | Caers J, Withofs N, Hillengass J, Simoni P, Zamagni E, Hustinx R, Beguin Y. The role of positron emission tomography-computed tomography and magnetic resonance imaging in diagnosis and follow up of multiple myeloma. Haematologica. 2014;99:629-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Wirk B, Wingard JR, Moreb JS. Extramedullary disease in plasma cell myeloma: the iceberg phenomenon. Bone Marrow Transplant. 2013;48:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Sato K, Fumimoto S, Fukada T, Ichihashi Y, Ochi K, Satomi H, Morita T, Hanaoka N, Okada Y, Katsumata T. Extramedullary Plasmacytoma Arising From the Anterior Mediastinum. Ann Thorac Surg. 2017;103:e393-e395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |