Published online Jan 24, 2025. doi: 10.5306/wjco.v16.i1.93670
Revised: September 4, 2024
Accepted: October 11, 2024
Published online: January 24, 2025
Processing time: 240 Days and 20 Hours
Patients with BRAF V600E mutant metastatic colorectal cancer (mCRC) have a low incidence rate, poor biological activity, suboptimal response to conventional treatments, and a poor prognosis. In the previous cohort study on mCRC con
To evaluate the efficacy of integrated Chinese and Western medicine in the treatment of BRAF V600E mutant metastatic colorectal cancer.
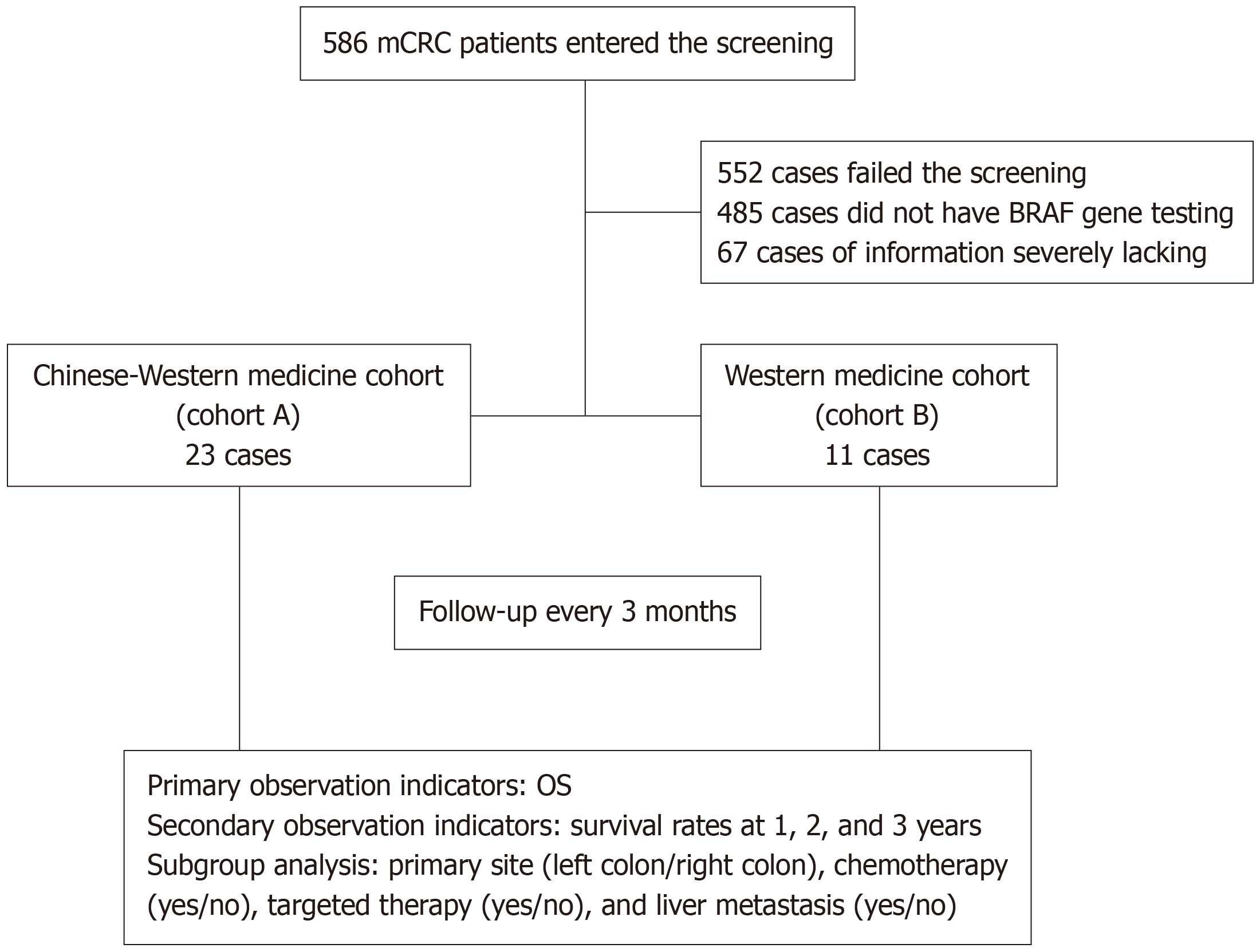
A cohort study was conducted on patients with BRAF V600E mutant metastatic colorectal cancer admitted to Xiyuan Hospital of China Academy of Chinese Medical Sciences and Traditional Chinese Medicine Hospital of Xinjiang Uygur Autonomous Region from January 2016 to December 2022. The patients were divided into two cohorts.
A total of 34 cases were included, with 23 in Chinese-Western medicine cohort (cohort A) and 11 in Western medicine cohort (cohort B). The median overall survival was 19.9 months in cohort A and 14.2 months in cohort B, with a statistically significant difference (P = 0.038, hazard ratio = 0.46). The 1-3-year survival rates were 95.65% (22/23), 39.13% (9/23), and 26.09% (6/23) in cohort A, and 63.64% (7/11), 18.18% (2/11), and 9.09% (1/11) in cohort B, respectively. Subgroup analysis showed statistically significant differences in median OS between the two cohorts in the right colon, liver metastasis, chemotherapy, and first-line treatment subgroups (P < 0.05).
Integrated Chinese and Western medicine can prolong the survival and reduce the risk of death in patients with BRAF V600E mutant metastatic colorectal cancer, with more pronounced benefits observed in patients with right colon involvement, liver metastasis, combined chemotherapy, and first-line treatment.
Core Tip: Patients with BRAF V600E mutant metastatic colorectal cancer (mCRC) have a much lower median overall survival than patients without BRAF V600E mutations. This study employed a retrospective cohort design and confirmed that in the real world, compared to chemotherapy and/or targeted therapy, combined treatment with integrated Chinese and Western medicine significantly extended overall survival and reduced the risk of death in BRAF V600E mutated mCRC patients, while being more effective in patients involving right colon, liver metastases, combined chemotherapy, and first-line therapy.
- Citation: Bian JY, Feng YF, He WT, Zhang T. Cohort study on the treatment of BRAF V600E mutant metastatic colorectal cancer with integrated Chinese and western medicine. World J Clin Oncol 2025; 16(1): 93670
- URL: https://www.wjgnet.com/2218-4333/full/v16/i1/93670.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i1.93670
In patients with metastatic colorectal cancer (mCRC), BRAF mutation are found in approximately 5%-12% of cases, with the majority (about 80%) being BRAF V600E mutation. Research has demonstrated that BRAF V600E mutant mCRC exhibits unique biological activity and clinical characteristics when compared to non-BRAF V600E mutant mCRC[1].
BRAF V600E mutant mCRC have been associated with older age, right-sided colon involvement, and female gender. These mutations are also correlated with reduced chemotherapy response and poor prognosis, making them a poor prognostic biomarker. The median overall survival (mOS) for patients with BRAF V600E mutant mCRC is typically only 9-14 months[2,3], which is significantly lower than the mOS of approximately 40 months for non-BRAF V600E mutant mCRC patients[4]. To address this challenge in clinical treatment, previous studies have explored the use of integrated Chinese and Western medicine in prolonging the overall survival (OS) of mCRC patients[5-7]. However, there is cur
This study collected data from patients with BRAF V600E mutant mCRC who were treated at Xiyuan Hospital of China Academy of Chinese Medical Sciences and Xinjiang Uyghur Autonomous Region Traditional Chinese Medicine Hospital from January 1, 2016 to December 31, 2022.
The study included patients who met the following: (1) Confirmed diagnosis of colorectal cancer through pathology; (2) Detection of BRAF V600E mutation using second-generation gene sequencing technology; (3) Presence of distant me
The study excludes patients who meet any of the following criteria: (1) Severe organ dysfunction in the heart, liver, lungs, kidneys, etc.; (2) Patients with complete intestinal obstruction or unable to take oral medications for various reasons; (3) History of previous or concurrent malignant tumors, excluding cured basal cell carcinoma of the skin and cervical car
If a patient cannot be followed up in an outpatient setting and three consecutive telephone follow-ups cannot be co
A retrospective cohort study was conducted, with "whether receiving ≥ 3 months of traditional Chinese medicine 'staged treatment'" as the exposure factor. Participants who meet this exposure factor are assigned to the Chinese-Western medicine cohort (cohort A) and receive integrated Chinese and Western medicine. Participants who do not meet this exposure factor are assigned to the Western medicine cohort (cohort B) and receive standard Western medicine treatment.
According to the “Clinical Practice Guidelines for Colorectal Cancer” (2021 edition) recommended by the National Comprehensive Cancer Network[8], the treatment regimen for colorectal cancer encompasses chemotherapy drugs, including fluorouracil. Additionally, targeted drugs like cetuximab, bevacizumab, regorafenib, and vemurafenib are also recommended. The specific implementation of these treatment drugs and dosages is determined by the clinical doctor based on the guidelines. It is important for the doctor to assess the individual patient’s condition and make personalized treatment decisions accordingly.
The Chinese medicine treatment for colorectal cancer can be divided into three stages as follows.
Stage 1: From the day before chemotherapy to the 6th day. During this stage, it is recommended to take Liu Jun An Wei Fang orally. This herbal formula consists of ingredients such as Taizishen, fried Baizhu, Fuling, and Jiang Banxia. Take one dose daily, decocted in water, and consume it warm twice in the morning and evening.
Stage 2: From the 7th day to the 20th day of chemotherapy. During this stage, it is advised to take Qi Tu Er Zhi Fang orally with modifications. This formula includes ingredients like raw Huangqi, Tusizi, Nüzhenzi, and Mò Hànlián. Take one dose daily, decocted in water, and consume it warm twice in the morning and evening.
Stage 3: Maintenance treatment stage of first-line or second-line Western medicine treatment. In this stage, which involves single-drug chemotherapy ± targeted therapy, the overall treatment principle is to “strengthen the spleen, nourish the kidneys, and detoxify”. The prescription is primarily based on Si Jun Zi Tang, with flexible modifications using Chinese herbs that have functions such as clearing heat and detoxification, resolving phlegm and dispersing nodules, and promoting blood circulation and resolving stasis. Some examples of these herbs include Baihua She
In addition to regular follow-up visits during hospitalization and outpatient visits, a telephone follow-up is also required every 3 months. The key points of the follow-up include the patient's survival status, whether they have received Chinese medicine treatment for more than 3 months, and any major adverse reactions during the treatment period.
Overall survival: To calculate the OS for mCRC patients, we need to consider the time from diagnosis to death. For patients who are still alive at the last follow-up, their OS is calculated based on the time from diagnosis to the last follow-up, considering them as censored. For patients lost to follow-up, their OS is calculated based on the time from diagnosis to the last follow-up before the loss, also considering them as censored.
Cumulative survival rates at 1, 2, and 3 years for two cohorts: The number of surviving patients at 1, 2, and 3 years is divided by the total number of cases in each group.
Using SAS JMP (Pro 14.0) software for data processing. Baseline analysis: t-test was used for continuous variables, rank sum test was used for non-conforming variables, and χ2 test was used for categorical variables. If the sample size does not meet the requirements for the χ2 test, Fisher's exact test will be used. OS analysis will be conducted using the Kaplan-Meier method to plot survival curves, and group comparisons will be performed using the log-rank test. Cumulative survival rates at 1, 2, and 3 years will be calculated. The significance level is set at α = 0.05. A P value less than 0.05 in
This study included a total of 586 participants, with 34 participants meeting the inclusion criteria. Among them, 23 participants were in the Chinese-Western medicine treatment group (cohort A), and 11 participants were in the Western medicine treatment group (cohort B; Figure 1). The last follow-up was conducted on June 20, 2023. 31 participants died by the last follow-up, and there were no cases lost to follow-up. The median follow-up time was 32.5 months. Baseline information is shown in Table 1.
| Project | Cohort A (n = 23) | Cohort B (n = 11) | Rank sum /χ2 /Fisher’s test | P value |
| Age (year) | 54 (51.64) | 53 (44.70) | -0.387 | 0.698 |
| Gender | ||||
| Male | 12 (52.17) | 4 (36.36) | - | 0.477 |
| Female | 11 (47.83) | 7 (63.63) | ||
| Primary site | ||||
| Right-side colon | 12 (52.17) | 6 (54.55) | 0.017 | 0.896 |
| Left-side colon | 11 (47.83) | 5 (45.45) | ||
| Liver metastasis | ||||
| Yes | 11 (47.83) | 5 (45.45) | 0.017 | 0.896 |
| No | 12 (52.17) | 6 (54.55) | ||
| Peritoneal metastasis | ||||
| Yes | 5 (21.74) | 3 (27.27) | - | 1.000 |
| No | 18 (78.26) | 8 (72.73) | ||
| Chemotherapy | ||||
| Yes | 16 (69.57) | 11 (100.00) | - | 0.069 |
| No | 7 (30.43) | 0 (0.00) | ||
| Targeted therapy | ||||
| Yes | 14 (60.87) | 6 (54.55) | 0.122 | 0.726 |
| No | 9 (39.13) | 5 (45.45) |
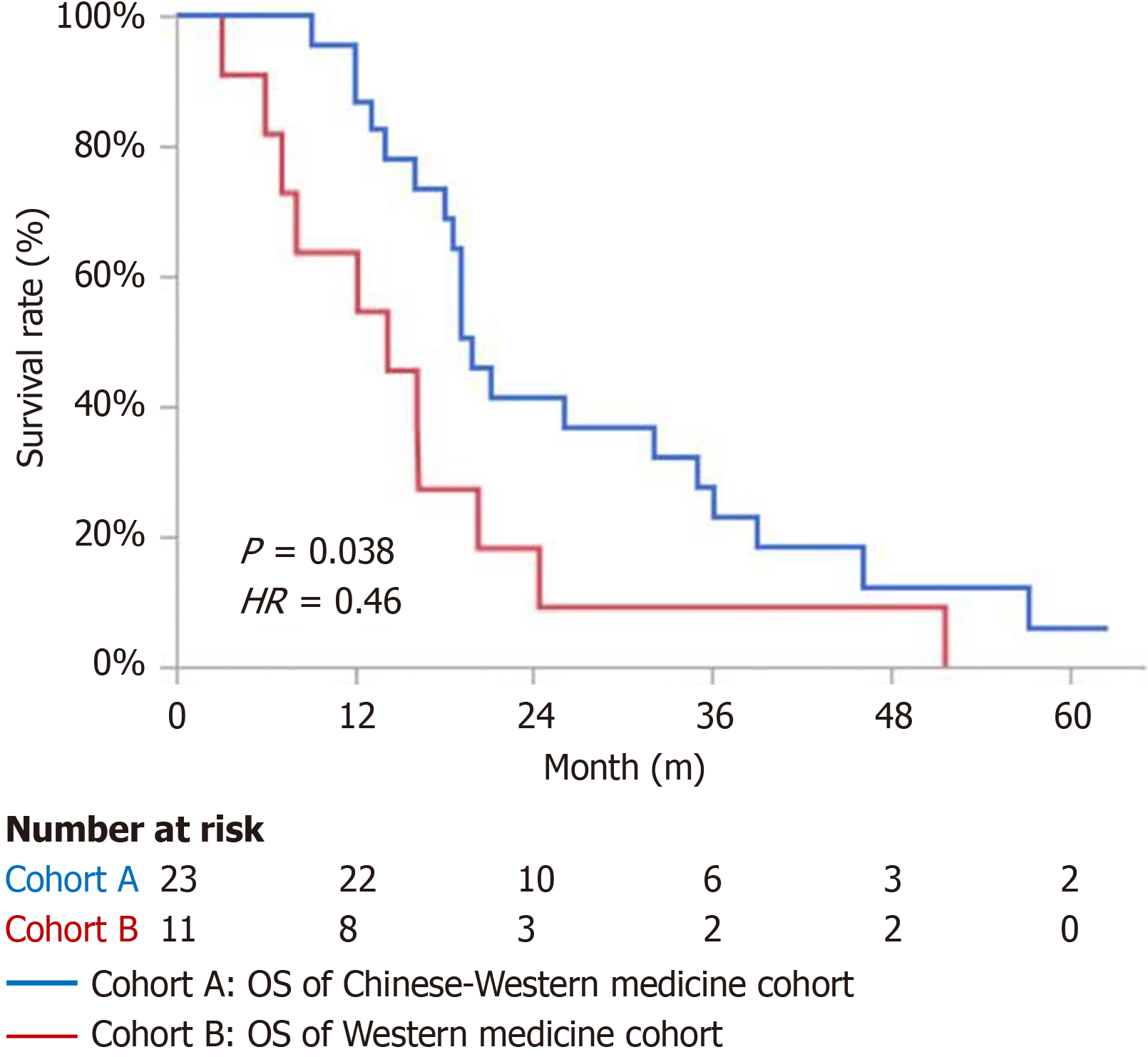
The mOS for the cohort A was 19.9 months, while the mOS for the cohort B was 14.2 months. The difference was statistically significant [P = 0.038, hazard ratio (HR) = 0.46], as shown in Figure 2.
The cumulative survival rates for the first, second, and third years in cohort A were 95.65% (22/23), 39.13% (9/23), and 26.09% (6/23) respectively. In cohort B, the survival rates for the first, second, and third years were 63.64% (7/11), 18.18% (2/11), and 9.09% (1/11) respectively.
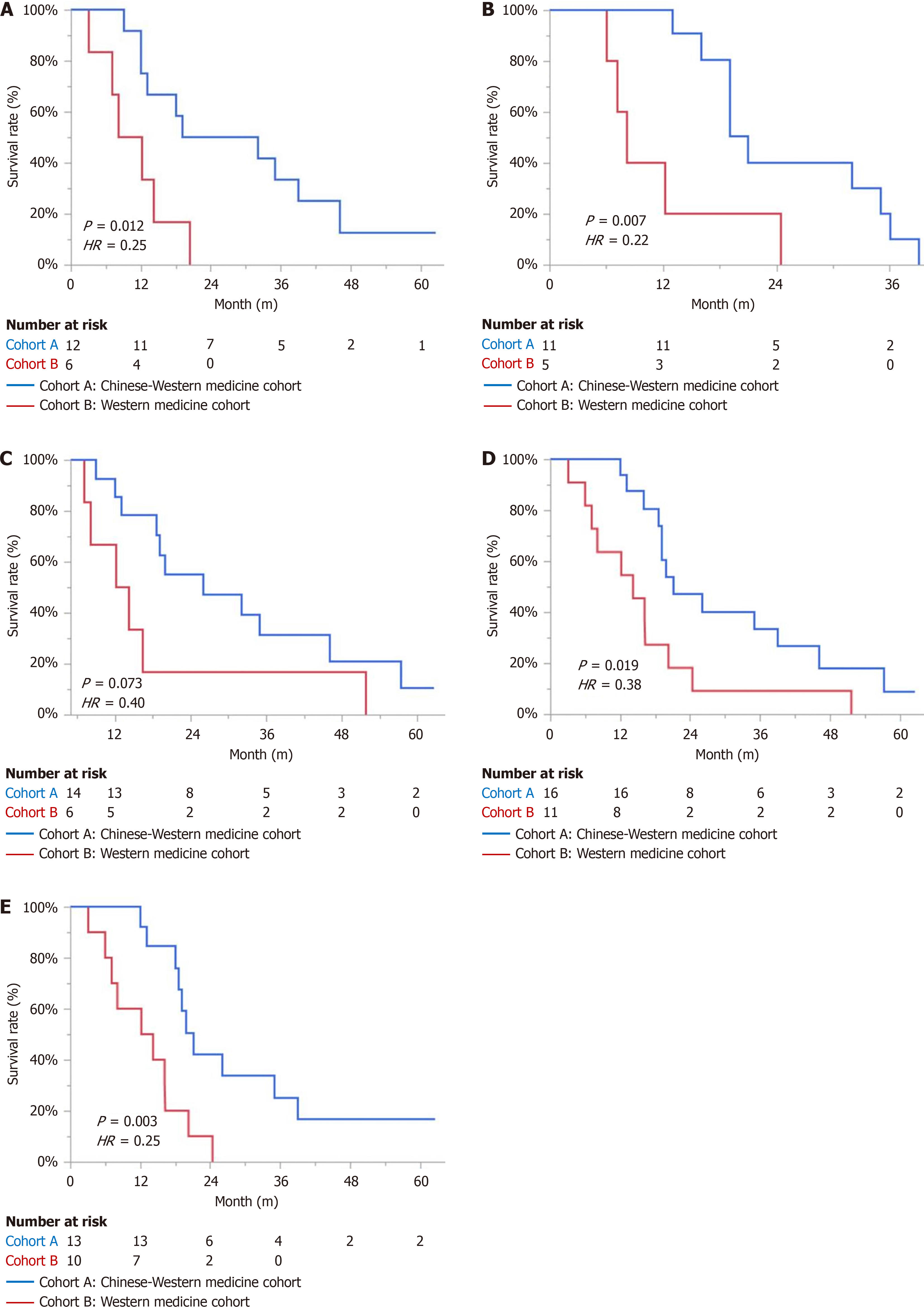
In the subgroup analysis of right-sided colon, liver metastasis, chemotherapy, and first-line treatment, there was a statistically significant difference in mOS in both cohorts. In the targeted therapy subgroup, the cohort A showed an extension of 12.8 months in mOS compared to the cohort B. However, due to the small sample size, the difference in mOS did not reach statistical significance (Table 2 and Figure 3).
| Subgroup | mOS (month) | χ2 | P value | HR (95%CI) | |
| Cohort A | Cohort B | ||||
| Right-side colon | 25.5 | 10.15 | 6.335 | 0.012 | 0.25 (0.07, 0.83) |
| Liver metastasis | 21 | 8.1 | 7.192 | 0.007 | 0.22 (0.06, 1.80) |
| Targeted therapy | 26 | 13.2 | 3.918 | 0.073 | 0.40 (0.14, 1.20) |
| Chemotherapy | 21 | 14.2 | 5.475 | 0.019 | 0.38 (0.17, 0.90) |
| First-line treatment | 21 | 13.2 | 8.597 | 0.003 | 0.25 (0.09, 0.67) |
No liver and kidney function damage, abnormal electrocardiogram, and allergic reaction related to Chinese medicine treatment were found. The incidence of adverse events related to Chinese medicine treatment: diarrhea and oral mu
The survival of mCRC patients with BRAF V600E mutant is significantly worse than that of patients without this mu
Why can the combination of Chinese medicine treatment achieve benefits across "all populations" without being limited by population characteristics? The essence of Chinese medicine treatment lies in its holistic approach and syndrome differentiation and treatment. It adjusts the relationship between the body's “pathogenic factors” and “vital energy” through the method of "reducing excess and supplementing deficiency", aiming to achieve or approach a balanced state of "Yin and Yang in harmony". However, in addition to the disease itself, treatment factors can also cause the body's functions to deviate from their normal state. Chinese medicine treatment can promptly and dynamically correct this "deviation".
The combination of Chinese medicine treatment with Western medicine treatment in this study follows the treatment concept of “staged treatment” in Chinese medicine. This approach involves adjusting the balance between “supporting the righteous” and “dispelling the evil” in Chinese medicine treatment based on the principles of traditional Chinese medicine syndrome differentiation, as well as considering the impact of Western medicine conventional treatment methods (such as surgery, chemotherapy, targeted therapy, and immunotherapy) and the disease itself on the body’s yin and yang balance. By incorporating the concept of “preventing and treating diseases” in Chinese medicine, staged treatment aims to correct the imbalance of Yin and Yang and help the body achieve or restore a state of “harmonious yin and yang”. This approach enhances the effectiveness of Chinese medicine treatment and contributes to achieving better therapeutic outcomes. During the induction chemotherapy phase, Professor Yang Yufei proposed the Jianpi Bushen Sequential Formula, which primarily focuses on supporting the “vital energy”, specific usage is as follows: “Staged treatment” in Chinese medicine consists of three stages. Stage 1: This stage begins one day before chemotherapy and lasts until day 6. The treatment principle is to invigorate the spleen and stomach and stop vomiting, with the aim of alleviating chemotherapy-related gastrointestinal reactions such as nausea and vomiting. A commonly used prescription during this stage is modified Liu Jun An Wei Fang [11,12]. Stage 2: This stage starts from day 7 and continues until day 20 of che
The selected evaluation indicators in this study are objective and quantifiable, which helps minimize the risk of bias. However, as a retrospective study, there are significant difficulties in collecting comprehensive information on outpatient patients. This could potentially lead to data omissions and other limitations. Additionally, the study only investigated patients from two hospitals and the sample size of the study is relatively limited, which may limit the generalizability of the findings. Conducting surveys in multiple medical institutions would provide a more diverse and representative sample.
Chinese medicine and Western medicine have two distinct theoretical systems and development approaches, both dedicated to improving the survival of cancer patients. They can complement each other and achieve win-win coo
This study employed a retrospective cohort design to examine the survival benefits of integrated Chinese and Western medicine compared to solely Western medicine treatment in patients with BRAF V600E mutant mCRC in the real world. The findings of the study confirmed that the integrated Chinese and Western medicine significantly extended the OS of BRAF V600E mutant mCRC patients, in comparison to chemotherapy and/or targeted therapy. At the same time, integrated Chinese and Western medicine reduced the risk of death in these patients, with more pronounced benefits observed in patients with right colon involvement, liver metastasis, combined chemotherapy, and first-line treatment. In conclusion, this study provides more clinical treatment options for the patients with BRAF V600E mutant mCRC and provides evidence-based medical evidence for the clinical application of integrated Chinese and Western medicine in the treatment of these patients.
Thanks to all authors for their efforts in this work.
| 1. | Yaeger R, Chatila WK, Lipsyc MD, Hechtman JF, Cercek A, Sanchez-Vega F, Jayakumaran G, Middha S, Zehir A, Donoghue MTA, You D, Viale A, Kemeny N, Segal NH, Stadler ZK, Varghese AM, Kundra R, Gao J, Syed A, Hyman DM, Vakiani E, Rosen N, Taylor BS, Ladanyi M, Berger MF, Solit DB, Shia J, Saltz L, Schultz N. Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell. 2018;33:125-136.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 613] [Article Influence: 87.6] [Reference Citation Analysis (35)] |
| 2. | Beypinar I, Demir H, Sakin A, Taskoylu BY, Sakalar T, Ergun Y, Korkmaz M, Ates O, Eren T, Turhal S, Artac M. The Real-Life Data of BRAF Mutation on the Treatment of Colorectal Cancer: a TOG Study. J Gastrointest Cancer. 2021;52:932-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (16)] |
| 3. | Tang W, Liu Y, Ji M, Liu T, Chen Y, Zhuang A, Mao Y, Chang W, Wei Y, Ren L, Xu J. Association of RAS/BRAF Status and Prognosis of Metastatic Colorectal Cancer: Analysis of 1002 Consecutive Cases. Ann Surg Oncol. 2022;29:3593-3603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (35)] |
| 4. | Kayhanian H, Goode E, Sclafani F, Ang JE, Gerlinger M, Gonzalez de Castro D, Shepherd S, Peckitt C, Rao S, Watkins D, Chau I, Cunningham D, Starling N. Treatment and Survival Outcome of BRAF-Mutated Metastatic Colorectal Cancer: A Retrospective Matched Case-Control Study. Clin Colorectal Cancer. 2018;17:e69-e76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (35)] |
| 5. | Zhang T, Yang YF, He B, Yi DH, Hao J, Zhang D. Efficacy and Safety of Quxie Capsule () in Metastatic Colorectal Cancer: A Double-Blind Randomized Placebo Controlled Trial. Chin J Integr Med. 2018;24:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (35)] |
| 6. | Zhang T, Xu Y, Sun LY, He B, Hao J, Zhang D, Yang YF. Efficacy of Quxie Capsule in Metastatic Colorectal Cancer: Long-Term Survival Update of A Double-Blind, Randomized, Placebo Controlled Trial. Chin J Integr Med. 2022;28:971-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (35)] |
| 7. | Zhang T, He WT, Zi MJ, Song G, Yi DH, Yang YF. Cohort Study on Prognosis of Patients with Metastatic Colorectal Cancer Treated with Integrated Chinese and Western Medicine. Chin J Integr Med. 2018;24:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (35)] |
| 8. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:329-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 956] [Article Influence: 239.0] [Reference Citation Analysis (16)] |
| 9. | 9 Ciombor KK, Strickler JH, Bekaii-Saab TS, Yaeger R. BRAF-Mutated Advanced Colorectal Cancer: A Rapidly Changing Therapeutic Landscape. J Clin Oncol. 2022;40:2706-2715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (35)] |
| 10. | Lakatos G, Köhne CH, Bodoky G. Current therapy of advanced colorectal cancer according to RAS/RAF mutational status. Cancer Metastasis Rev. 2020;39:1143-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (35)] |
| 11. | Zhang T, Fei YT, Xu Y, Sun LY, He B, Yan SH, Tang M, Yan YZ, Mao J, Yang YF. Effect of Jianpi Bushen Sequential Formula on Adjuvant Chemotherapy of Colon Cancer: Study Protocol for a Randomized Controlled Trial. Chin J Integr Med. 2021;27:891-895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Dai LL, Chen DM, Zhou SM, Zhao N, Si WT, Cao Y, Zeng BZ, Yang YF. ["Two-stage three-step method" for the prevention and treatment of adverse reactions to chemotherapy in colorectal cancer]. Zhongyi Zazhi. 2019;60:982-985. [DOI] [Full Text] |
| 13. | Zhang T, Liu JP, Xu Y, Fei YT, Wang XC, Wang JB, Yao JT, Wu J, Li Y, Cao Y, Liu SY, Yang YF. [Guidelines for Traditional Chinese Medicine Diagnosis and Treatment of Metastatic Colorectal Cancer]. Zhongguo Shiyan Fangjixue Zazhi. 2023;29:24-31. [DOI] [Full Text] |