Published online Sep 24, 2024. doi: 10.5306/wjco.v15.i9.1232
Revised: July 22, 2024
Accepted: August 15, 2024
Published online: September 24, 2024
Processing time: 177 Days and 21.7 Hours
Metastatic colorectal cancer (mCRC) treatment has been evolving and increasingly driven by tumor biology and gene expression analysis. Rechallenge with epider
Our patient was diagnosed with stage IV RAS-wt, microsatellite-stable rectosig
Our case provides real-world data to support cetuximab rechallenge in later lines of RAS-wt mCRC treatment.
Core Tip: In RAS-wild type patients treated with anti-epidermal growth factor receptor inhibitors, the emergence of RAS mutations leads to resistance to further anti-epidermal growth factor receptor treatment. However, resistant clones seem to decay over time upon treatment withdrawal. Liquid biopsy-driven cetuximab rechallenge can be effective in later lines of treatment of metastatic colorectal cancer. Longer anti-epidermal growth factor receptor-free intervals may act as a surrogate for improved responses and survival outcomes.
- Citation: Guedes A, Silva S, Custódio S, Capela A. Successful cetuximab rechallenge in metastatic colorectal cancer: A case report. World J Clin Oncol 2024; 15(9): 1232-1238
- URL: https://www.wjgnet.com/2218-4333/full/v15/i9/1232.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i9.1232
According to the World Health Organization, colorectal cancer (CRC) is the third most commonly diagnosed cancer[1,2]. Metastatic CRC (mCRC) represents 25% to 30% of all cases of CRC, with 1.2 million estimated new cases every year and a 5-year overall survival below 20%[3-5]. The treatment of mCRC is evolving and has been increasingly driven by tumor biology and gene expression analysis[5-8]. KRAS mutations are found in up to 50% of sporadic mCRC cases and have been independently associated with a worse prognosis[9,10].
The activation of the epidermal growth factor receptor (EGFR) signaling cascade is a well-described pathway leading to colon tumorigenesis[11]. Considering the relevant role of EGFR and its downstream pathways in tumorigenesis and disease progression, it became a promising target for mCRC treatment[12]. Several studies have shown that EGFR and downstream signaling blocking by drugs such as cetuximab or panitumumab, combined with a doublet chemotherapy backbone, leads to tumor cell growth inhibition with clinical improvement[13-16]. Accordingly, in patients with RAS wild-type (RAS-wt) mCRC, EGFR monoclonal antibody plus chemotherapy is the standard option for treatment in the first-line (1 L) setting[17-19]. However, mutations within the RAS and BRAF oncogenes located downstream of EGFR lead to its constitutive activation, even if the EGFR is blocked. As such, patients with RAS-mutated (RAS-mut) mCRC do not benefit from agents targeting the EGFR[17-20]. In RAS-wt patients treated with anti-EGFR inhibitors based therapy, the emergence of RAS mutations, due to either late acquisition of mutations by cellular subclones or to the progressive selection of initially undetectable mutated subclones, can translate into resistance to further anti-EGFR treatment, in
A 52-year-old man presented to the emergency department complaining of abdominal colic pain, rectal bleeding, asthenia and anorexia.
Symptoms started in June 2014, when the patient began having abdominal pain, asthenia and anorexia, with significant weight loss (15% of body weight, corresponding to 15 kg in 6 months), associated with rectal bleeding. Due to progressive symptoms, he was taken to the emergency department. During hospitalization, an abdominal ultrasound revealed two hepatic nodules and a probable sigmoid neoformation.
The patient was obese and had corrective surgery for an inguinal hernia in 2010.
The patient reported a family case of CRC (maternal uncle, age unknown).
On physical examination, vital parameters were all within the normal range and cardiopulmonary auscultation was normal. The abdomen was a bit distended but painless on palpation and showed no signs of peritoneal irritation. The Eastern Cooperative Oncology Group performance status (ECOG-PS) was 1.
The patient had mild microcytic anemia (hemoglobin 12.1 g/dL) and a carcinoembryonic antigen level of 60 ng/mL. No other alterations were reported.
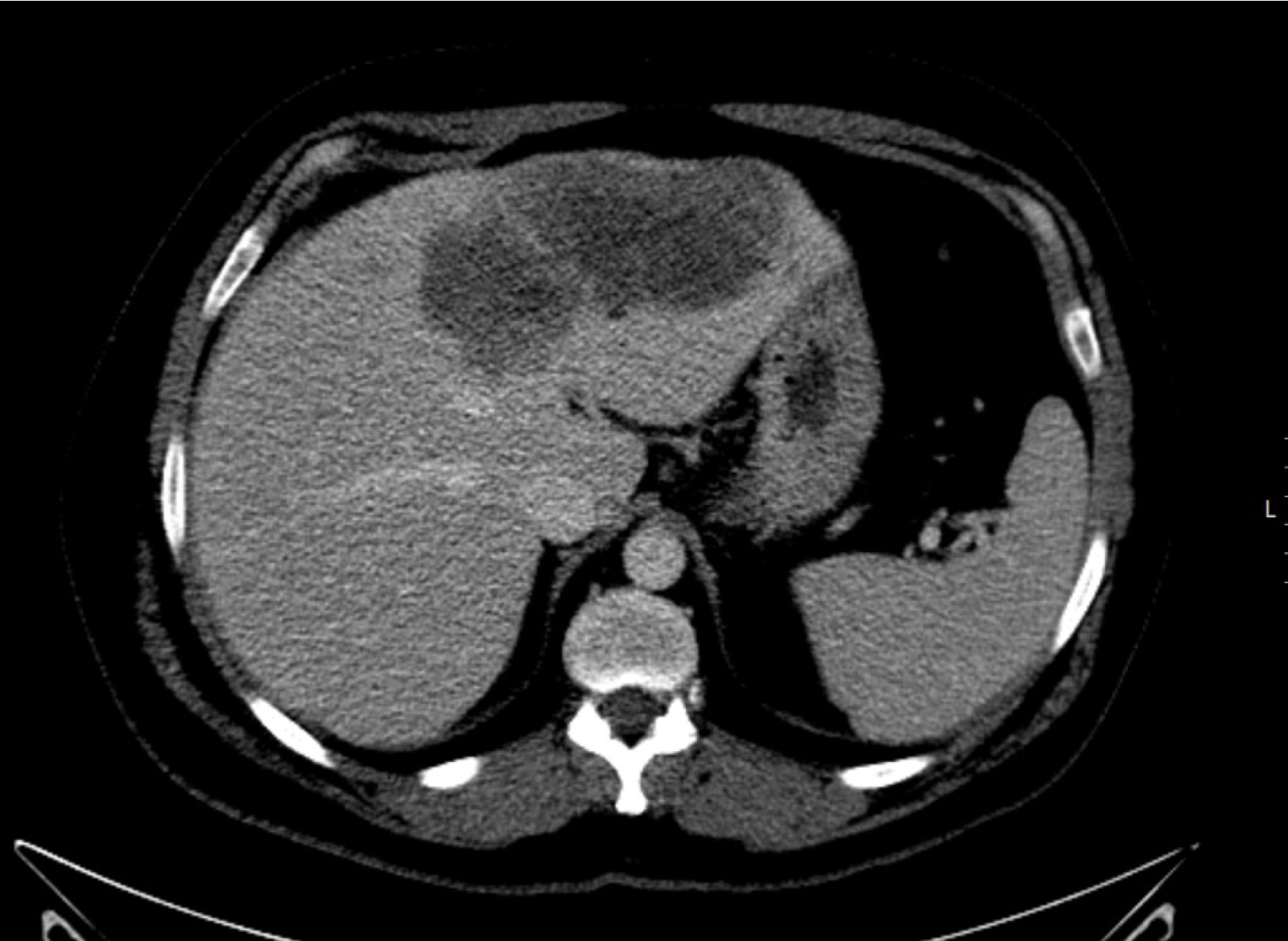
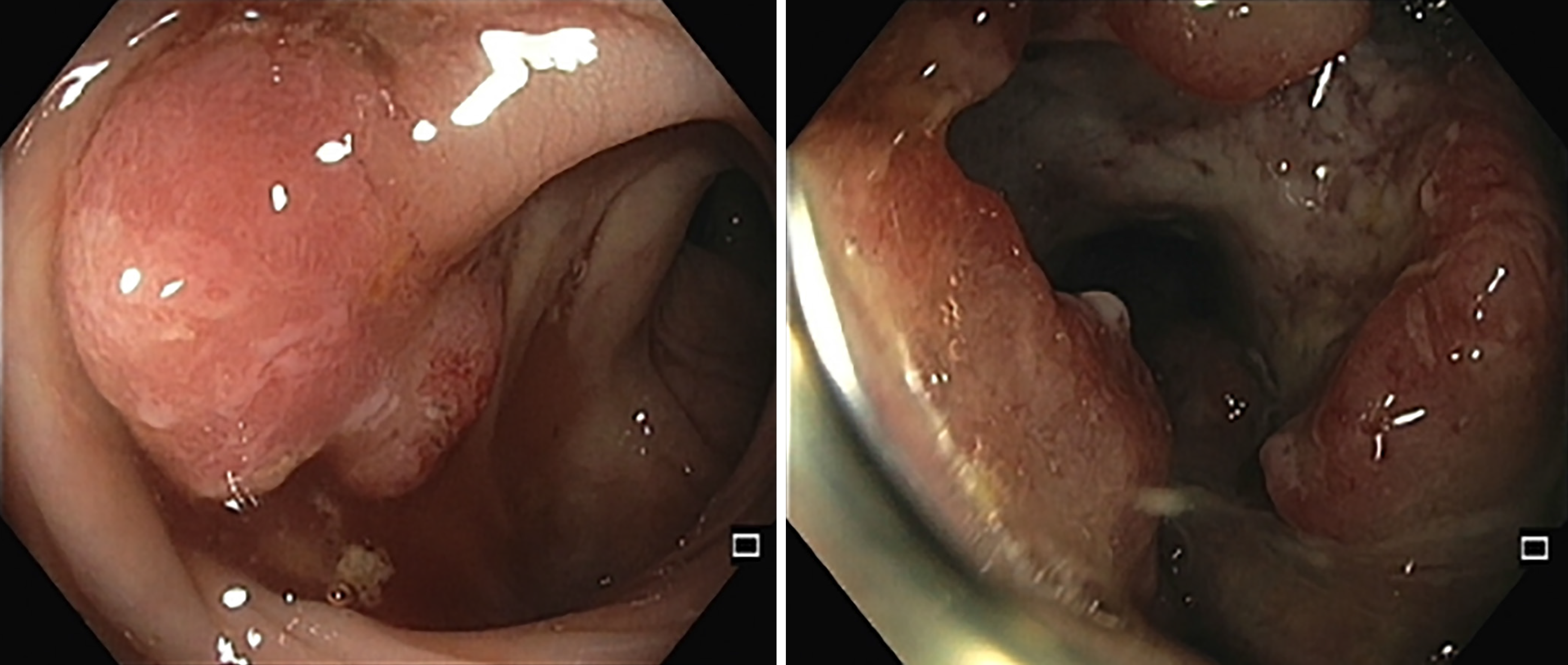
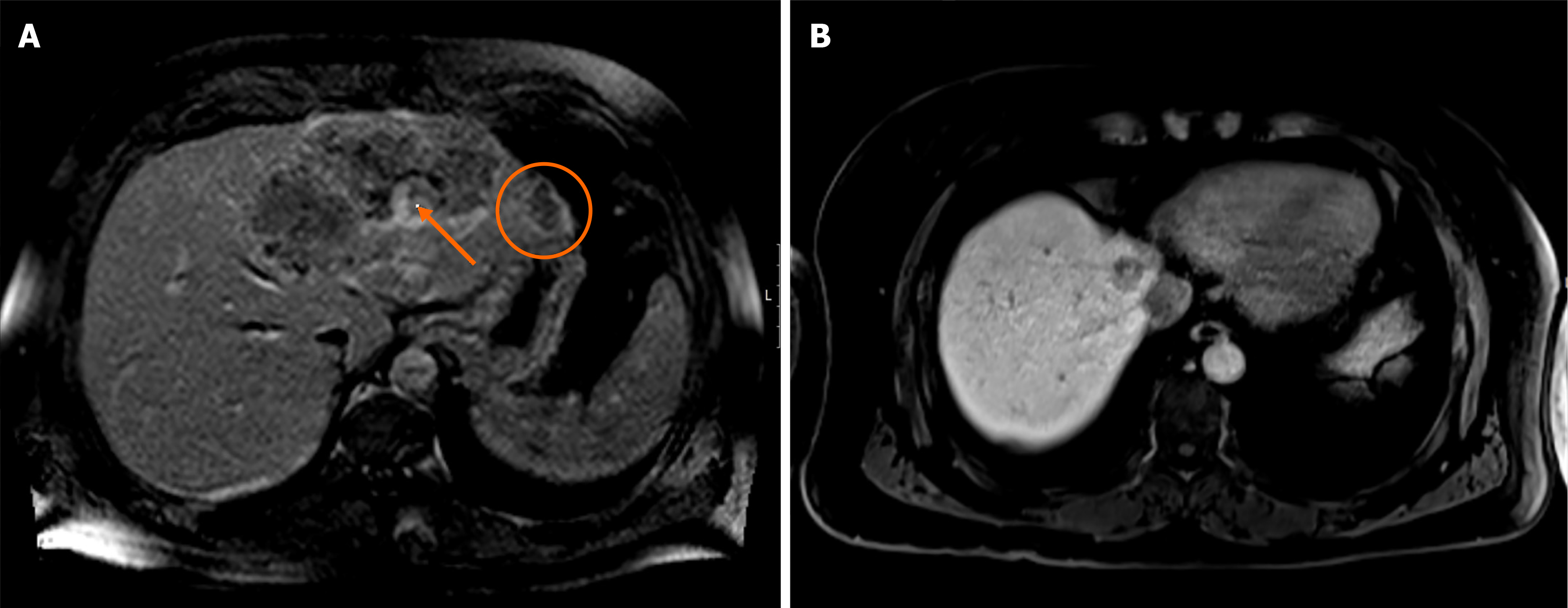
Imaging studies were obtained. Pelvic computed tomography (CT) revealed a 13 cm left hepatic nodule (Figure 1) and sigmoid mural thickening; thoracic CT did not show lung masses. Colonoscopy revealed a neoformative mass located 15 to 22 cm from the anal margin, with reduction of the lumen diameter (Figure 2). Hepatic magnetic resonance imaging revealed the presence of several confluent hepatic lesions in the left lobe, 16 cm wide (Figure 3A).
Endoscopic biopsy of the colorectal mass revealed a well-differentiated adenocarcinoma (grade 1); liver nodule biopsy confirmed metastasis of adenocarcinoma, intestinal type. RAS mutational status was wt and mismatch repair deficiency/microsatellite instability was excluded (mismatch repair-proficient/microsatellite-stable).
The final diagnosis was cT3N0M1a - stage IV rectosigmoid junction adenocarcinoma, RAS-wt with microsatellite stability.
The multidisciplinary team discussion decided on conversion treatment and the patient was started on FOLFIRI chemotherapy; 9 cycles were completed. After RAS mutation was excluded, cetuximab was added for the last 6 cycles (from September 2014 to January 2015). An acneiform rash grade 2-3 was reported and managed with doxycycline and topical steroids.
After conversion chemotherapy, the patient achieved partial response. In February 2015, the patient had a left hepa
In January 2016, the patient had hepatic and nodal relapse, with one de novo hepatic metastasis of segment VIII 23 mm wide and an adenopathy next to the caudate lobe (Figure 3B). ECOG-PS was still 1 and the patient was asymptomatic. Conversion chemotherapy (second-line) with FOLFOX and bevacizumab was started in February 2016, with partial re
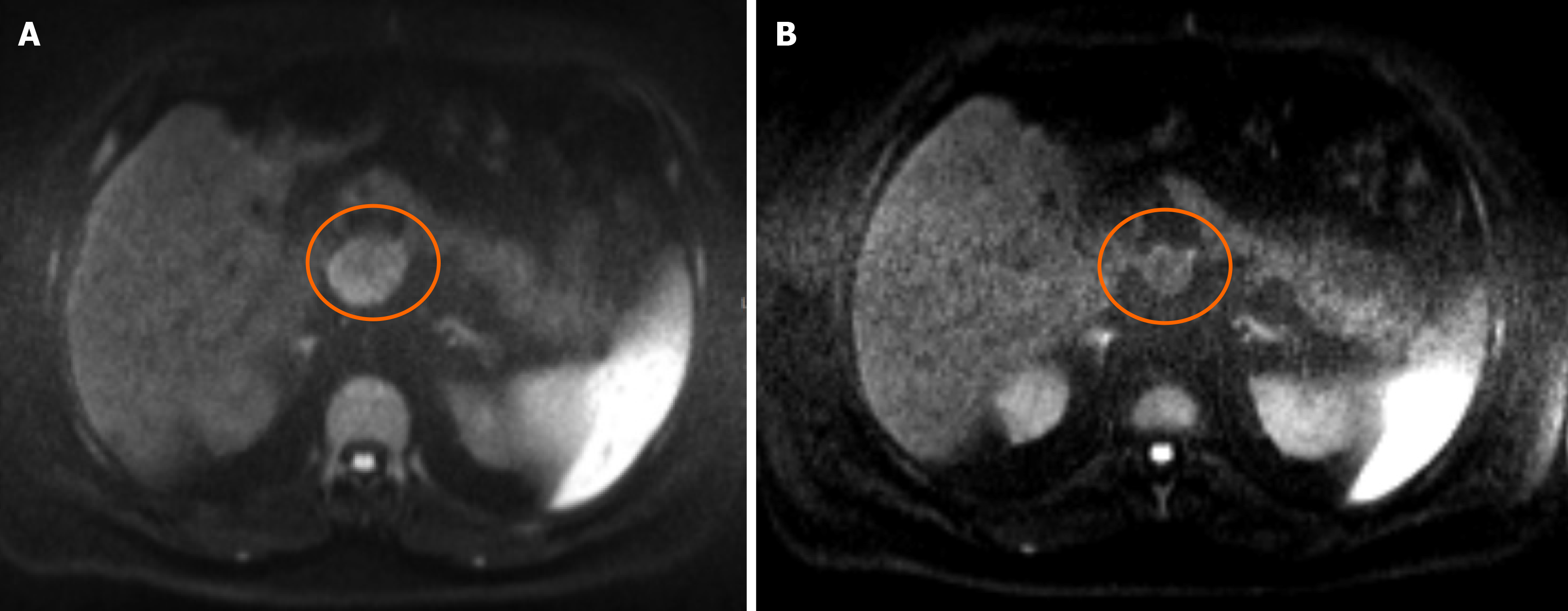
After 8 cycles (January 2017), the patient remained asymptomatic (ECOG-PS 1) but showed hepatic and nodal relapse with two de novo hepatic metastases in the right lobe and portal adenopathy (41 mm; Figure 4A). Palliative third-line (3 L) chemotherapy with FOLFIRI and cetuximab was started in February 2017, with partial response after 7 cycles (Figure 4B). In August 2017, the patient had a metastasectomy (segments I and VIII) and portal lymphadenectomy (R0). Complementary chemotherapy with 5-fluorouracil (DeGramont) was started in October 2017; 6 cycles were completed.
In June 2018, the patient presented with 1-week evolution of choluria and pruritus; hepatic dysfunction was evidenced by increased bilirubin, lactate dehydrogenase, aspartate aminotransferase, and alanine aminotransferase. Physical exa
Disease progression was detected in March 2019; the patient was asymptomatic with an ECOG-PS of 1. Liquid biopsy showed RAS-wt status. In this setting, fifth-line (5 L) treatment with FOLFOX plus cetuximab was started, in June 2019. Treatment was suspended due to disease progression with asthenia and anorexia; TAS-102 was started as sixth-line (6 L) treatment, in September 2019, but was soon interrupted due to clinical deterioration. The patient died in December 2019 with an overall survival of 5 years and 6 months.
In this paper, we describe the case of a mCRC RAS-wt patient who was treated with a combination of CT and cetuximab at 1 L, 3 L, 4 L and 5 L of treatment with clear clinical responses and prolonged survival. The patient showed partial response to 1 L treatment with FOLFIRI plus cetuximab with a progression-free survival (PFS) of 16 months after hepatectomy and primary resection. Upon disease progression, in the context of increasing resistance to anti-EGFR treatment, treatment with FOLFOX plus bevacizumab (2 L) followed by liver metastasectomy led to a PFS of 11 months. Overall, the patient had an anti-EGFR-free interval of 15 months between 1 L and 3 L. As described previously in the literature[26], we believe that the suspension of cetuximab enabled the falloff of resistant clones and resistance to anti-EGFR antibodies was decreased. This allowed for the reintroduction of cetuximab in 3 L, in combination with chemotherapy, obtaining a durable response with a PFS of 16 months. In fact, evidence suggests that a longer anti-EGFR-free interval correlates with improved PFS and OS[24].
Compared with the results of the CRICKET study, which showed a median PFS of 4 months after cetuximab recha
In this case, we also highlight the previously discussed importance of liquid biopsy to guide clinical decisions[8,24,27,29]. The evaluation of RAS mutational status of the patient at different disease stages allowed for a better understanding of tumoral behavior. In this case, the patient was initially RAS-wt and showed to be RAS-wt again after 4 L, in line with data that showed significantly better responses to anti-EGFR rechallenge and longer PFS in RAS-wt patients[27]. Had it been performed earlier (before the first rechallenge), it would also have probably shown RAS-wt status, given the great response to 3 L treatment. Regarding safety, the most relevant adverse events were gastrointestinal and dermatological. Grade 2-3 rash was initially managed with corticosteroid therapy but eventually led to treatment discontinuation after 4 L.
Our case confirmed that liquid biopsy-driven cetuximab rechallenge can be effective in later lines of treatment and that longer anti-EGFR free intervals may act as a surrogate for improved outcomes. Our patient achieved exceedingly good PFS and overall survival rates, highlighting the individual variability in mCRC treatment as well as the yet unexplored potential of the rechallenge strategy for these patients.
The authors thank Paula Pinto, PharmD, PhD (Pharmaceutical Medicine Academy) for providing medical writing and editorial assistance.
| 1. | World Health Organization. Colorectal cancer. [cited 10 January 2024]. Available from: https://www.iarc.who.int/cancer-type/colorectal-cancer/. |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64436] [Article Influence: 16109.0] [Reference Citation Analysis (176)] |
| 3. | Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14:89-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 1064] [Article Influence: 177.3] [Reference Citation Analysis (1)] |
| 4. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2268] [Cited by in RCA: 3263] [Article Influence: 652.6] [Reference Citation Analysis (2)] |
| 5. | Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, Seligmann J, De Baere T, Osterlund P, Yoshino T, Martinelli E; ESMO Guidelines Committee. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:10-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 810] [Article Influence: 405.0] [Reference Citation Analysis (34)] |
| 6. | Udagawa S, Ooki A, Shinozaki E, Fukuda K, Yamaguchi K, Osumi H. Circulating Tumor DNA: The Dawn of a New Era in the Optimization of Chemotherapeutic Strategies for Metastatic Colo-Rectal Cancer Focusing on RAS Mutation. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Modest DP, Pant S, Sartore-Bianchi A. Treatment sequencing in metastatic colorectal cancer. Eur J Cancer. 2019;109:70-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 229] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 8. | Gramaça J, Fernandes IG, Trabulo C, Gonçalves J, Dos Santos RG, Baptista A, Pina I. Emerging role of liquid biopsy in rat sarcoma virus mutated metastatic colorectal cancer: A case report. World J Gastrointest Oncol. 2024;16:234-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (3)] |
| 9. | Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 1425] [Article Influence: 356.3] [Reference Citation Analysis (0)] |
| 10. | Klein-Scory S, Wahner I, Maslova M, Al-Sewaidi Y, Pohl M, Mika T, Ladigan S, Schroers R, Baraniskin A. Evolution of RAS Mutational Status in Liquid Biopsies During First-Line Chemotherapy for Metastatic Colorectal Cancer. Front Oncol. 2020;10:1115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Krasinskas AM. EGFR Signaling in Colorectal Carcinoma. Patholog Res Int. 2011;2011:932932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Janani B, Vijayakumar M, Priya K, Kim JH, Prabakaran DS, Shahid M, Al-Ghamdi S, Alsaidan M, Othman Bahakim N, Hassan Abdelzaher M, Ramesh T. EGFR-Based Targeted Therapy for Colorectal Cancer-Promises and Challenges. Vaccines (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 13. | Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Williams R, Rong A, Wiezorek J, Sidhu R, Patterson SD. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1610] [Cited by in RCA: 1730] [Article Influence: 144.2] [Reference Citation Analysis (0)] |
| 14. | Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL, Kay E, Mitchell JK, Madi A, Jasani B, James MD, Bridgewater J, Kennedy MJ, Claes B, Lambrechts D, Kaplan R, Cheadle JP; MRC COIN Trial Investigators. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103-2114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 766] [Cited by in RCA: 763] [Article Influence: 54.5] [Reference Citation Analysis (2)] |
| 15. | Tejpar S, Stintzing S, Ciardiello F, Tabernero J, Van Cutsem E, Beier F, Esser R, Lenz HJ, Heinemann V. Prognostic and Predictive Relevance of Primary Tumor Location in Patients With RAS Wild-Type Metastatic Colorectal Cancer: Retrospective Analyses of the CRYSTAL and FIRE-3 Trials. JAMA Oncol. 2017;3:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 528] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 16. | Heinemann V, von Weikersthal LF, Decker T, Kiani A, Kaiser F, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl C, Seipelt G, Kullmann F, Moehler M, Scheithauer W, Held S, Miller-Phillips L, Modest DP, Jung A, Kirchner T, Stintzing S. FOLFIRI plus cetuximab or bevacizumab for advanced colorectal cancer: final survival and per-protocol analysis of FIRE-3, a randomised clinical trial. Br J Cancer. 2021;124:587-594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 17. | Gazzaniga P, Raimondi C, Urbano F, Cortesi E. Second line EGFR-inhibitors in RAS mutant metastatic colorectal cancer: The plasma RAS wild type “window of opportunity”. Ann Oncol. 2018;29:VIII183-VIII184. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2286] [Cited by in RCA: 2424] [Article Influence: 269.3] [Reference Citation Analysis (31)] |
| 19. | Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2901] [Cited by in RCA: 3122] [Article Influence: 195.1] [Reference Citation Analysis (1)] |
| 20. | Siravegna G, Mussolin B, Buscarino M, Corti G, Cassingena A, Crisafulli G, Ponzetti A, Cremolini C, Amatu A, Lauricella C, Lamba S, Hobor S, Avallone A, Valtorta E, Rospo G, Medico E, Motta V, Antoniotti C, Tatangelo F, Bellosillo B, Veronese S, Budillon A, Montagut C, Racca P, Marsoni S, Falcone A, Corcoran RB, Di Nicolantonio F, Loupakis F, Siena S, Sartore-Bianchi A, Bardelli A. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21:827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 21. | Bardelli A, Siena S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol. 2010;28:1254-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 533] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 22. | Santini D, Vincenzi B, Addeo R, Garufi C, Masi G, Scartozzi M, Mancuso A, Frezza AM, Venditti O, Imperatori M, Schiavon G, Bronte G, Cicero G, Recine F, Maiello E, Cascinu S, Russo A, Falcone A, Tonini G. Cetuximab rechallenge in metastatic colorectal cancer patients: how to come away from acquired resistance? Ann Oncol. 2012;23:2313-2318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 23. | Reddy TP, Khan U, Burns EA, Abdelrahim M. Chemotherapy rechallenge in metastatic colon cancer: A case report and literature review. World J Clin Oncol. 2020;11:959-967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Mariani S, Puzzoni M, Giampieri R, Ziranu P, Pusceddu V, Donisi C, Persano M, Pinna G, Cimbro E, Parrino A, Spanu D, Pretta A, Lai E, Liscia N, Lupi A, Giglio E, Palomba G, Casula M, Pisano M, Palmieri G, Scartozzi M. Liquid Biopsy-Driven Cetuximab Rechallenge Strategy in Molecularly Selected Metastatic Colorectal Cancer Patients. Front Oncol. 2022;12:852583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 25. | Ma J, Yang QL, Ling Y. Rechallenge and maintenance therapy using cetuximab and chemotherapy administered to a patient with metastatic colorectal cancer. BMC Cancer. 2017;17:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Cremolini C, Rossini D, Dell'Aquila E, Lonardi S, Conca E, Del Re M, Busico A, Pietrantonio F, Danesi R, Aprile G, Tamburini E, Barone C, Masi G, Pantano F, Pucci F, Corsi DC, Pella N, Bergamo F, Rofi E, Barbara C, Falcone A, Santini D. Rechallenge for Patients With RAS and BRAF Wild-Type Metastatic Colorectal Cancer With Acquired Resistance to First-line Cetuximab and Irinotecan: A Phase 2 Single-Arm Clinical Trial. JAMA Oncol. 2019;5:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 311] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 27. | Rossini D, Cremolini C, Conca E, Del Re M, Busico A, Pietrantonio F, Bergamo F, Danesi R, Cardellino G, Tamburini E, Dell'aquila E, Strippoli A, Masi G, Tonini G, Negri F, Corsi D, Pella N, Intini R, Falcone A, Santini D. Liquid biopsy allows predicting benefit from rechallenge with cetuximab(cet)+irinotecan(iri) in RAS/BRAF wild-type mCRC patients(pts) with resistance to 1st-line cet+iri: Final results and translational analyses of the CRICKET study by GONO. Ann Oncol. 2018;29:V102. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Cremolini C, Montagut C, Ronga P, Venturini F, Yamaguchi K, Stintzing S, Sobrero A. Rechallenge with anti-EGFR therapy to extend the continuum of care in patients with metastatic colorectal cancer. Front Oncol. 2022;12:946850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 29. | Valenzuela G, Burotto M, Marcelain K, González-Montero J. Liquid biopsy to detect resistance mutations against anti-epidermal growth factor receptor therapy in metastatic colorectal cancer. World J Gastrointest Oncol. 2022;14:1654-1664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Reference Citation Analysis (2)] |