Published online Sep 24, 2024. doi: 10.5306/wjco.v15.i9.1222
Revised: July 25, 2024
Accepted: July 29, 2024
Published online: September 24, 2024
Processing time: 183 Days and 19.4 Hours
Patients with neurofibromatosis type 1 (NF1) are exposed to a higher risk of developing neuroendocrine tumors (NETs). Periampullary neuroendocrine neoplasms (NENs) in NF1 patients primarily affect the duodenum and periampullary region.
A 50-year-old male patient was admitted to our hospital due to progressive skin and scleral yellowing for over 6 months. An abdominal contrast-enhanced computed tomography scan revealed a tumor in the periampullary region, which measured 1.2 cm × 1.4 cm in size and showed a progressive enhancement. Magnetic resonance cholangiopancreatography indicated the dilation of intrahepatic and extrahepatic bile ducts. The patient was diagnosed with an ampullary tumor with the possibility of malignancy. A Whipple procedure was performed. Microscopically, the duodenum tumor was found to invade the mucosa, sphincter, and muscular layer of the duodenal papilla. Histologic hematoxylin and eosin staining confirmed the presence of duodenal G1 NET. Subsequently, a bibliometric analysis was performed to evaluate the state of NEN research. Publications about periampullary NENs showed an annual increase, with most of them focusing on the treatment and diagnosis of NENs.
This article reported a case of periampullary duodenal NET in a patient with NF1, and a bibliometric analysis was conducted.
Core Tip: Patients with neurofibromatosis type 1 (NF1) are exposed to a higher risk of developing neuroendocrine tumors. Periampullary neuroendocrine neoplasms in NF1 patients primarily affect the duodenum and periampullary region. This article reported a case of periampullary duodenal neuroendocrine tumor in a patient with NF1. A bibliometric analysis was conducted on periampullary neuroendocrine neoplasms to determine the state of the research.
- Citation: Zhang XY, Yu JF, Li Y, Li P. Periampullary duodenal neuroendocrine tumor in a patient with neurofibromatosis-1: A case report. World J Clin Oncol 2024; 15(9): 1222-1231
- URL: https://www.wjgnet.com/2218-4333/full/v15/i9/1222.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i9.1222
Neurofibromatosis type 1 (NF1) is an autosomal dominant complex disease resulting from a germline mutation in one of the two alleles of the cancer suppressor gene NF1 on chromosome 17q11.2[1]. Clinically, NF1 affects 1 in 3000 newborns regardless of sex or race. NF1 primarily presents with nervous system and skin manifestations due to lesions in neural crest-derived tissues, involving Schwann cells and melanocytes[2]. Almost all patients with NF1 develop pigmentary lesions (café-au-lait macules, skinfold freckling, and Lisch nodules) and cutaneous neurofibromas. Others develop skeletal abnormalities (scoliosis, tibial pseudarthrosis, and orbital dysplasia), brain tumors (optic pathway gliomas and glioblastoma), peripheral nerve tumors (spinal neurofibromas, plexiform neurofibromas, and malignant peripheral nerve sheath tumors), learning disabilities, attention deficits, and social and behavioral problems, which negatively affect the quality of life[3].
NF1 is associated with an elevated risk of developing malignancies, with an estimated lifetime cancer risk of 59.6%[4]. Patients with NF1 have are exposed to a higher risk of developing gastrointestinal stromal tumors (GISTs), early-onset breast cancer, leukemia, and neuroendocrine tumors (NETs) (e.g., pheochromocytomas)[5]. Moreover, the NF1 gene encodes the protein neurofibromin, which is a tumor suppressor expressed in many cells. Mutations in the NF1 gene lead to uncontrolled cell proliferation and the development of benign and malignant tumors, including neuroendocrine neoplasms (NENs). NENs in NF1 patients primarily affect the duodenum and periampullary region[6]. NENs represent a diverse group of malignant diseases, ranging from benign to very malignant variants. NENs are typically divided into indolent NETs and aggressive neuroendocrine carcinomas[7]. This article reported a case of periampullary duodenal NET in a patient with NF1.
A 50-year-old male patient was admitted to our hospital for investigation of progressive skin and scleral yellowing for over 6 months.
Six months ago, the patient experienced skin and scleral yellowing, and the symptoms increased day by day. No other special discomforts were found.
The patient was in good health with no special medical history.
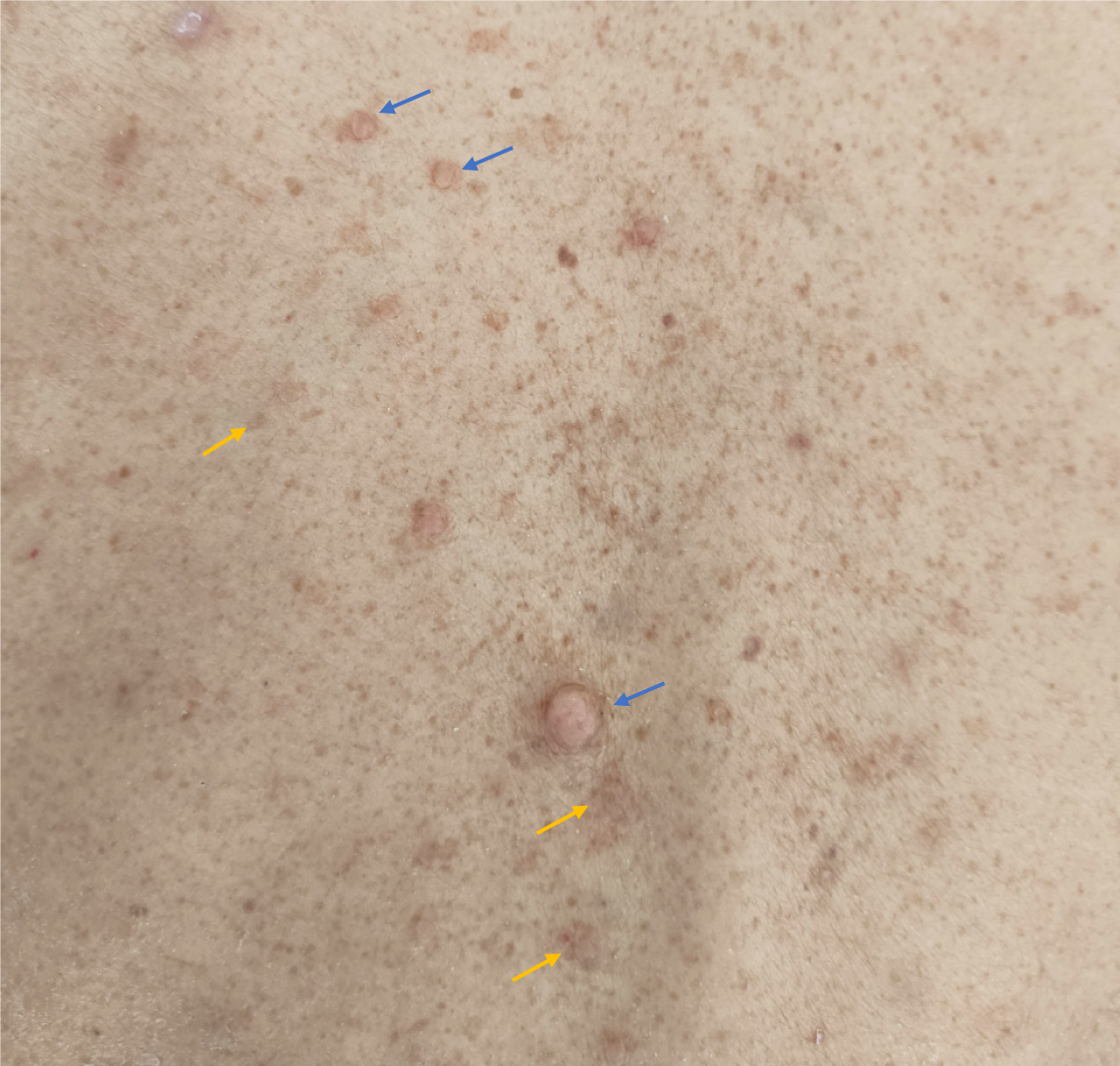
Upon admission, numerous café-au-lait macules and cutaneous neurofibromas were observed on his entire body (Figure 1). The patient reported having these cutaneous macules and tumors since the age of 7 and that his grandfather, father, and aunt all had the same condition. No other personal or family history was reported.
The patient’s blood pressure and heart rate were normal, and the electrocardiogram examination showed no abnormality.
Examination revealed impaired liver function with increased levels of aspartate aminotransferase (52.3 U/L; normal range: 8-40 U/L), alkaline phosphatase (477 U/L; normal range: 45-125 U/L), and gamma-glutamyl transferase (258 U/L; normal range: 10-60 U/L). High levels of bilirubin reflected the jaundice, with findings of total bilirubin 61.48 μmol/L (normal range: 3.4-17.1 μmol/L), indirect bilirubin 24.89 μmol/L (normal range: 0-12 μmol/L), and direct bilirubin 36.59 μmol/L (normal range: 0.85-6.8 μmol/L). Nonetheless, tumor marker levels, including carbohydrate antigen 19-9 (12.4 U/mL), carcinoembryonic antigen (1.86 ng/mL), and alpha-fetoprotein (1.27 ng/mL), were within normal limits.
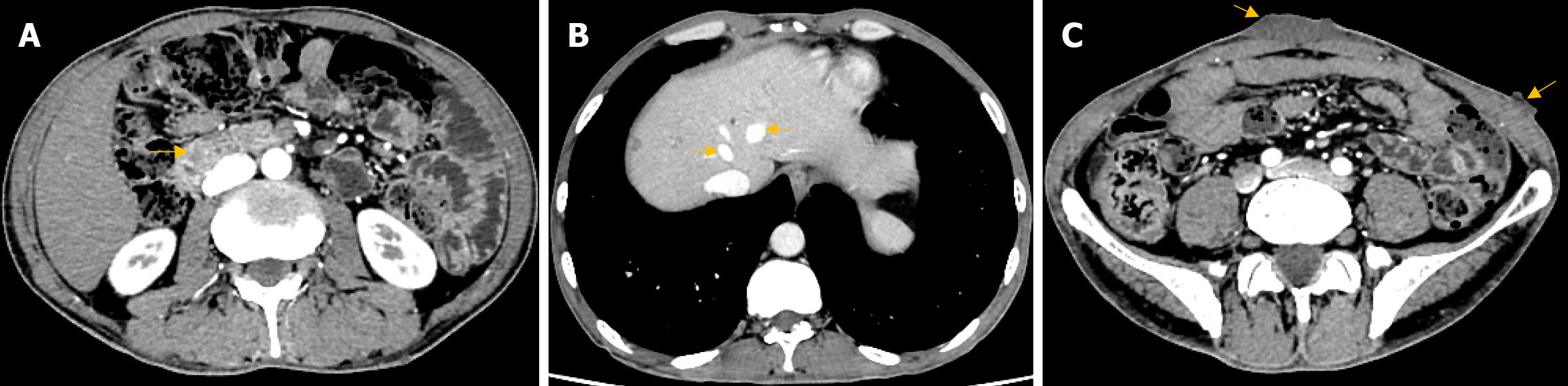
Upper gastrointestinal (GI) endoscopy showed a 5 cm × 5 cm spherical protruding lesion with solid consistency with normal mucosal coverage in the descending part of the duodenum. No peptic ulcer was detected in the stomach and duodenum. A colonoscopy revealed multiple colonic polyps, and the biopsy specimen of the lesion showed mixed hyperplastic adenomatous polyps. In addition, the abdominal contrast-enhanced computed tomography (CT) scan indicated a tumor in the periampullary region, which measured 1.2 cm × 1.4 cm in size and showed a progressive enhancement in the contrast-enhanced phase with CT values of 67 hounsfield unit (HU), 108 HU, 95 HU, and 99 HU (Figure 2A). The intrahepatic and extrahepatic bile ducts and pancreatic ducts above the level of the tumor were dilated. Multiple hemangiomas were found in the S8 segment of the liver, with the larger one measuring approximately 0.5 cm × 0.6 cm (Figure 2B). Multiple sections of the inferior abdominal surface showed localized thickening of the skin and subcutaneous tissue, with the larger one measuring approximately 1.1 cm × 5.1 cm with a CT value of 27 HU (Figure 2C). Thyroid ultrasound showed a cystic nodule in the midportion of the left lobe of the thyroid gland, measuring 4.6 mm × 3 mm × 4.1 mm and showing clear borders, punctate echogenic foci, and posterior acoustic enhancement without evident blood flow signals. No parathyroid nodule was found. Moreover, magnetic resonance cholangiopancreatography showed the dilation of intrahepatic and extrahepatic bile ducts and no filling defect.
Based on the patient’s symptoms and examinations, the patient was diagnosed with an ampullary tumor with the possibility of malignancy.
Therefore, a Whipple procedure was performed. The pancreatic head, part of the stomach, and duodenum were excised. A tissue of the cutaneous tumor was also excised for biopsy. No other abnormality was found during the laparotomy.
Macroscopically, a grayish-white and elastic hard tumor of 2 cm in maximum diameter was identified in the mucosal surface of the duodenum papilla. Another nodular lesion with a diameter of 1.3 cm was seen 2 cm away from the tumor, which was also grayish-white and elastic hard. In addition, a grayish-white and elastic hard tumor of 0.6 cm in maximum diameter was identified on the serous surface of the duodenum.
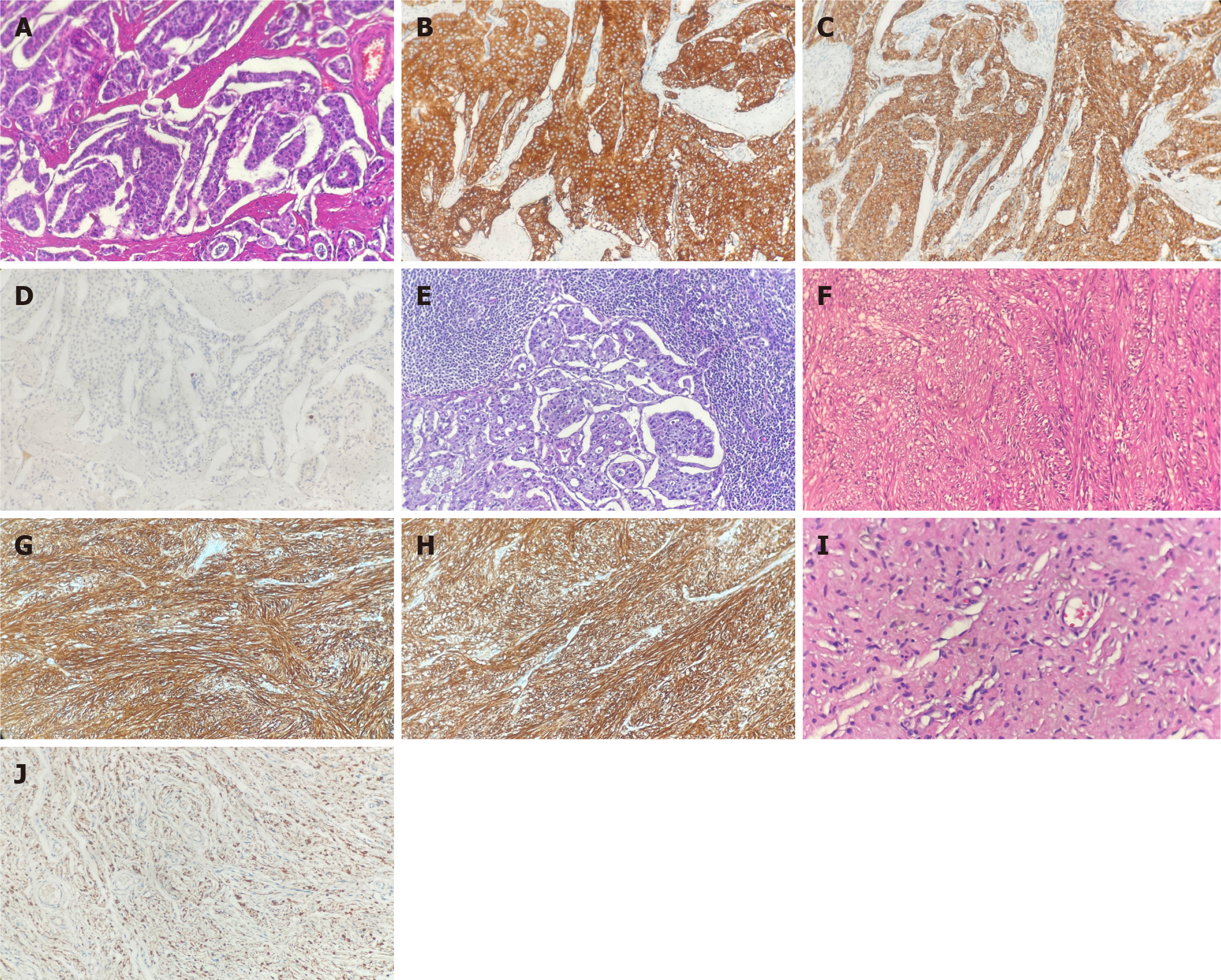
Microscopically, the duodenum tumor was found to invade the mucosa, sphincter, and muscular layer of the duodenal papilla. Histologic hematoxylin and eosin (HE) staining confirmed the presence of duodenal G1 NET (Figure 3A). Furthermore, the NET markers synaptophysin (Figure 3B) and chromogranin A (Figure 3C) were positive on immunohistochemical (IHC) staining. The Ki67 labeling index was 1%, with one mitosis per 10 high-power fields (Figure 3D). Additionally, metastatic tumors were observed in two of the two peripancreatic lymph nodes (Figure 3E), whereas no metastatic tumor was found in the pericholedochal lymph nodes (0/2) and lymph nodes near the lesser curvature of the stomach (0/2). The tumor in the serous surface of the duodenum was confirmed as a stromal tumor by HE staining (Figure 3F). Moreover, IHC staining revealed positive stromal tumor markers CD 117 (Figure 3G) and DOG-1 (Figure 3H). As for the cutaneous tissue, a grayish-yellow and soft tumor of 1.5 cm in maximum diameter was identified. The lesion was confirmed as neurofibroma by HE staining (Figure 3I), with IHC staining revealing positive neurofibroma marker S100 protein (Figure 3J). According to the American Joint Committee on Cancer 8th edition, the tumor was classified as pT2N1M0, G1, R0, stage III B. The patient had an uneventful postoperative recovery and was discharged from the hospital 20 days later.
Periampullary NENs arise from the duodenum, ampulla, and periampullary pancreas[8]. A bibliometric analysis was performed to understand the state of research on periampullary NENs.
For the bibliometric analysis, the Web of Science Core Collection database was systematically searched using the following strategy: TI = (“duodenal neuroendocrine tumors” OR “duodenal neuroendocrine tumor” OR “duodenal neuroendocrine carcinomas” OR “duodenal neuroendocrine carcinoma” OR “duodenal neuroendocrine neoplasms” OR “duodenal neuroendocrine neoplasm” OR “pancreatic neuroendocrine tumors” OR “pancreatic neuroendocrine tumor” OR “pancreatic neuroendocrine carcinomas” OR “pancreatic neuroendocrine carcinoma” OR “pancreatic neuroendocrine neoplasms” OR “pancreatic neuroendocrine neoplasm” OR “ampullary neuroendocrine tumors” OR “ampullary neuroendocrine tumor” OR “ampullary neuroendocrine carcinomas” OR “ampullary neuroendocrine carcinoma” OR “ampullary neuroendocrine neoplasms” OR “ampullary neuroendocrine neoplasm”) OR AB = (“duodenal neuroendocrine tumors” OR “duodenal neuroendocrine tumor” OR “duodenal neuroendocrine carcinomas” OR “duodenal neuroendocrine carcinoma” OR “duodenal neuroendocrine neoplasms” OR “duodenal neuroendocrine neoplasm” OR “pancreatic neuroendocrine tumors” OR “pancreatic neuroendocrine tumor” OR “pancreatic neuroendocrine carcinomas” OR “pancreatic neuroendocrine carcinoma” OR “pancreatic neuroendocrine neoplasms” OR “pancreatic neuroendocrine neoplasm” OR “ampullary neuroendocrine tumors” OR “ampullary neuroendocrine tumor” OR “ampullary neuroendocrine carcinomas” OR “ampullary neuroendocrine carcinoma” OR “ampullary neuroendocrine neoplasms” OR “ampullary neuroendocrine neoplasm”). The language of the publications was restricted to English to ensure proper interpretation of the results. Thereafter, fundamental analysis was performed on the website https://bibliometric.com. The bibliometric package (Version 4.1.2) in R software (Version 4.2.2) was used to analyze and visualize the bibliographic data from the selected publications as reported previously[9]. The search was performed on April 4, 2023.
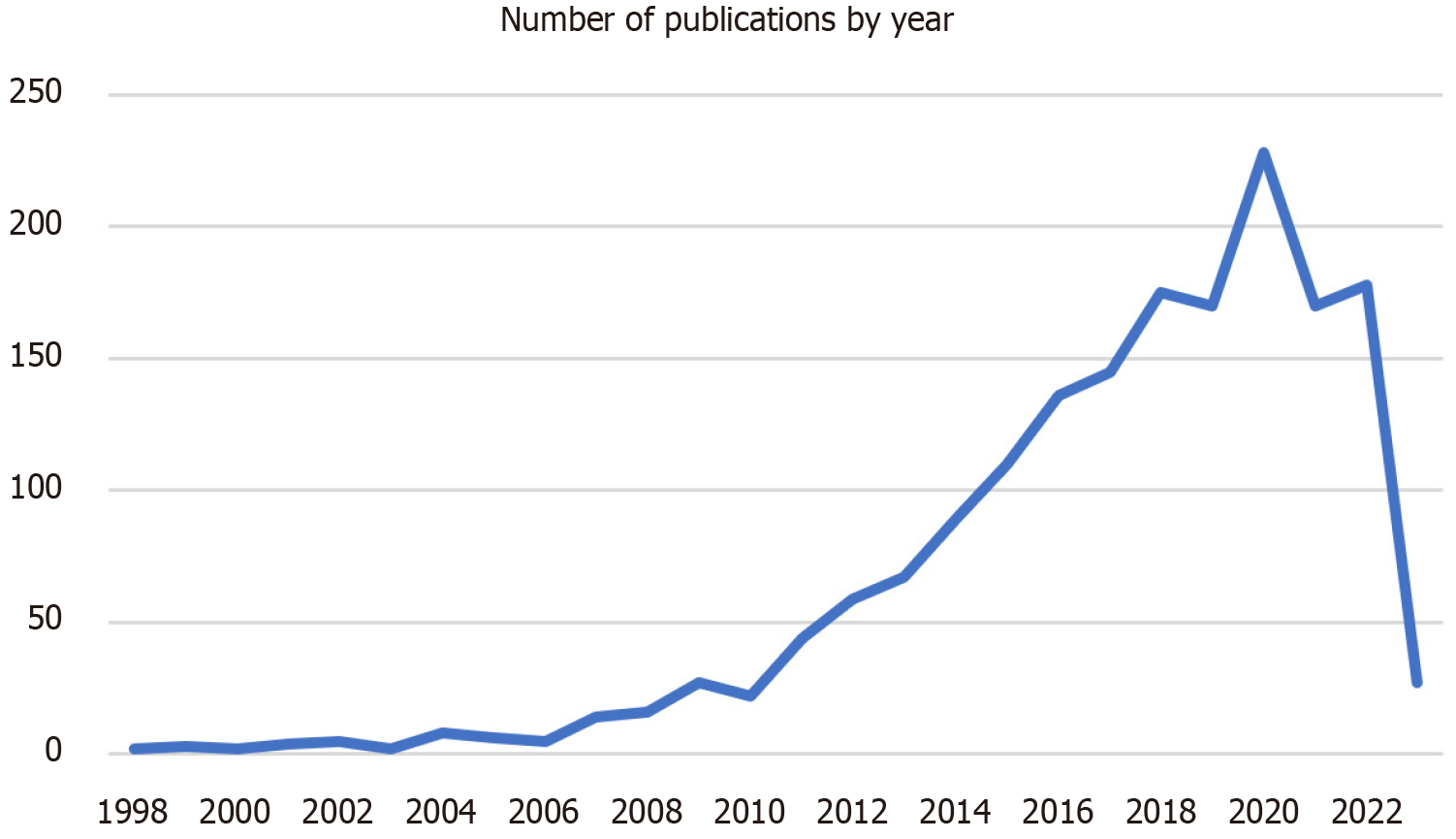
A total of 4494 publications were retrieved from Web of Science from 1998 to 2023, which were published in 619 journals and written by 16223 authors from 3769 affiliations in 57 countries/regions. The publications included 2153 (47.91%) research articles and 369 (8.21%) review articles, with an average of 14.52 citations per document. Overall, the number of publications showed an upward trend from 1998 to 2023, with an annual growth rate of 9.73% (Figure 4). The growth rate showed an acceleration after 2010, and the maximum productivity per year was observed in 2020 (n = 482, 10.73%).
The number of citations on duodenal and pancreatic NENs was counted based on the data on the Web of Science. However, the citation numbers showed significant variation, ranging from 2051 to 0. The top 10 most cited publications are listed in Supplementary Table 1. Most of the highly cited publications focused on clinical management, such as treating refractory patients, including with antiangiogenic and anti-PD-L1 therapy. Among the top 10 articles, only one article published in Science in 2011 focused on the pathogenesis of NETs, revealing the gene mutation profiles.
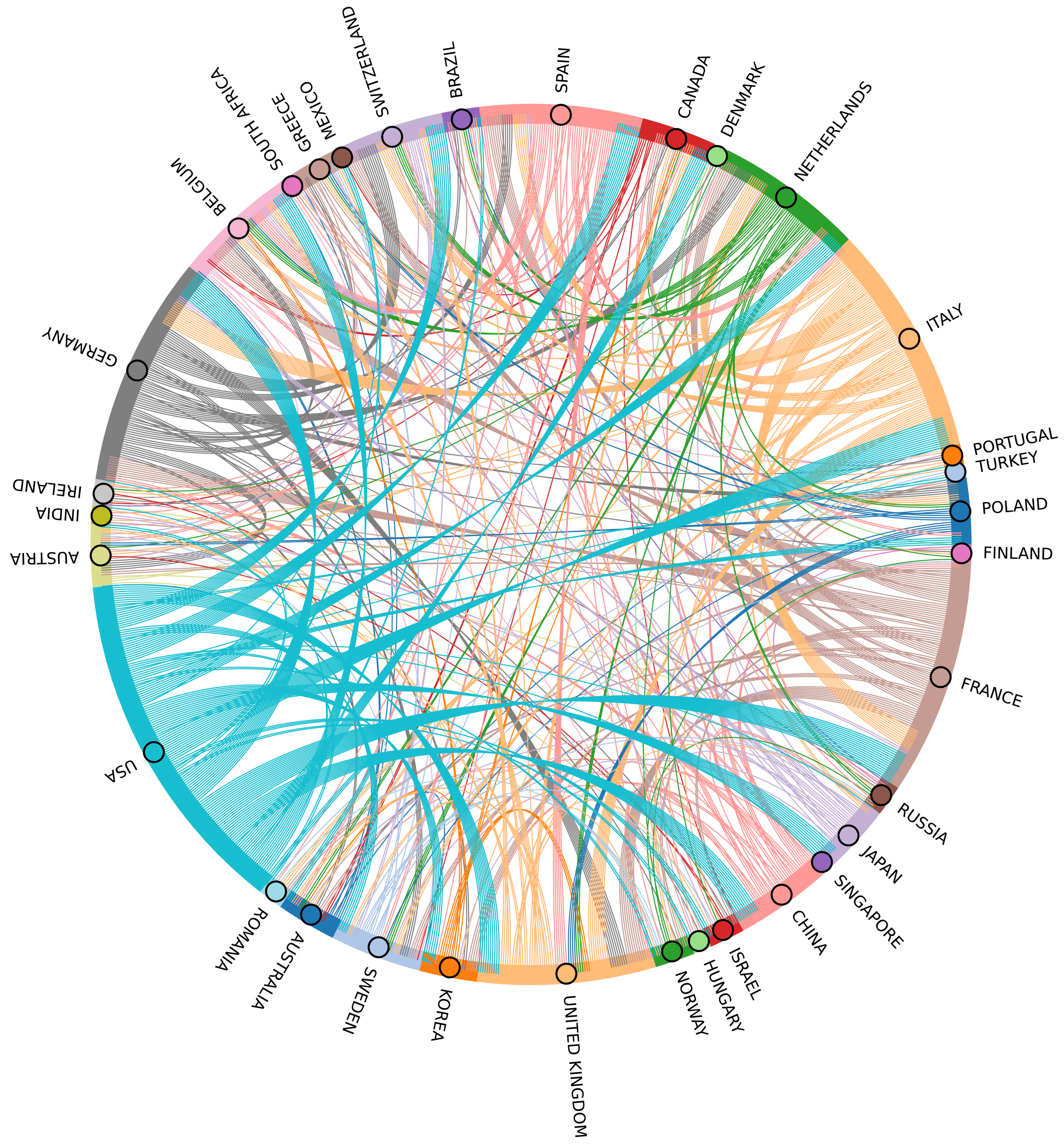
Furthermore, a total of 57 countries and regions contributed to the articles in this study. The top 10 countries with the most publications are shown in Table 1. The United States (n = 1182, 26.30%) accounted for the most publications, followed by China (n = 523, 11.64%) and Italy (n = 317, 7.05%). The Netherlands showed the highest proportion of publications involving collaboration with multiple countries (40.91%). In contrast, China and Japan showed the lowest ratio (6.88% and 6.54%, respectively), indicating less interaction with other countries. In this field, the most frequent collaboration involved the United States and France, with a total of 98 publications (Figure 5). The United States emerged as the most influential country, with the highest total citations (30336) and average article citations (25.7).
| Country | Articles | SCP | MCP | Frequency | MCP ratio, % | TC | Average article citations |
| United States | 1182 | 987 | 195 | 0.26302 | 16.50 | 30336 | 25.7 |
| China | 523 | 487 | 36 | 0.11638 | 6.88 | 4210 | 8.0 |
| Italy | 317 | 239 | 78 | 0.07054 | 24.61 | 6180 | 19.5 |
| Japan | 306 | 286 | 20 | 0.06809 | 6.54 | 3791 | 12.4 |
| Germany | 204 | 132 | 72 | 0.04539 | 35.29 | 3204 | 15.7 |
| France | 124 | 96 | 28 | 0.02759 | 22.58 | 3951 | 31.9 |
| Korea | 94 | 82 | 12 | 0.02092 | 12.77 | 1368 | 14.6 |
| Spain | 85 | 64 | 21 | 0.01891 | 24.71 | 869 | 10.2 |
| Netherlands | 66 | 39 | 27 | 0.01469 | 40.91 | 1367 | 20.7 |
| Canada | 49 | 36 | 13 | 0.01090 | 26.53 | 993 | 20.3 |
Moreover, three of the top ten most productive affiliations included the Fudan University, Memorial Sloan Kettering Cancer Center, and The University of Texas MD Anderson Cancer Center with 468, 246, and 231 publications, respectively (Table 2). These publications indicate the high level of research on NENs at these centers.
| Affiliation | Articles |
| Fudan University | 468 |
| Memorial Sloan Kettering Cancer Center | 246 |
| The University of Texas MD Anderson Cancer Center | 231 |
| The Ohio State University | 189 |
| Stanford University | 170 |
| The University of Iowa | 167 |
| Zhejiang University | 162 |
| Sichuan University | 152 |
| Johns Hopkins University | 148 |
| Sun Yat Sen University | 137 |
The 4494 retrieved articles were published in 619 journals, most of which were specialized in the surgery or clinical field. Table 3 shows the top 10 journals in terms of number of publications. Neuroendocrinology published the highest number of relevant studies, with 424 articles.
| Sources | Articles |
| Neuroendocrinology | 424 |
| Pancreas | 332 |
| Journal of Clinical Oncology | 149 |
| Annals of Surgical Oncology | 134 |
| Modern Pathology | 119 |
| Laboratory Investigation | 105 |
| Gastroenterology | 82 |
| Gastrointestinal Endoscopy | 81 |
| Surgery | 80 |
| American Journal of Gastroenterology | 78 |
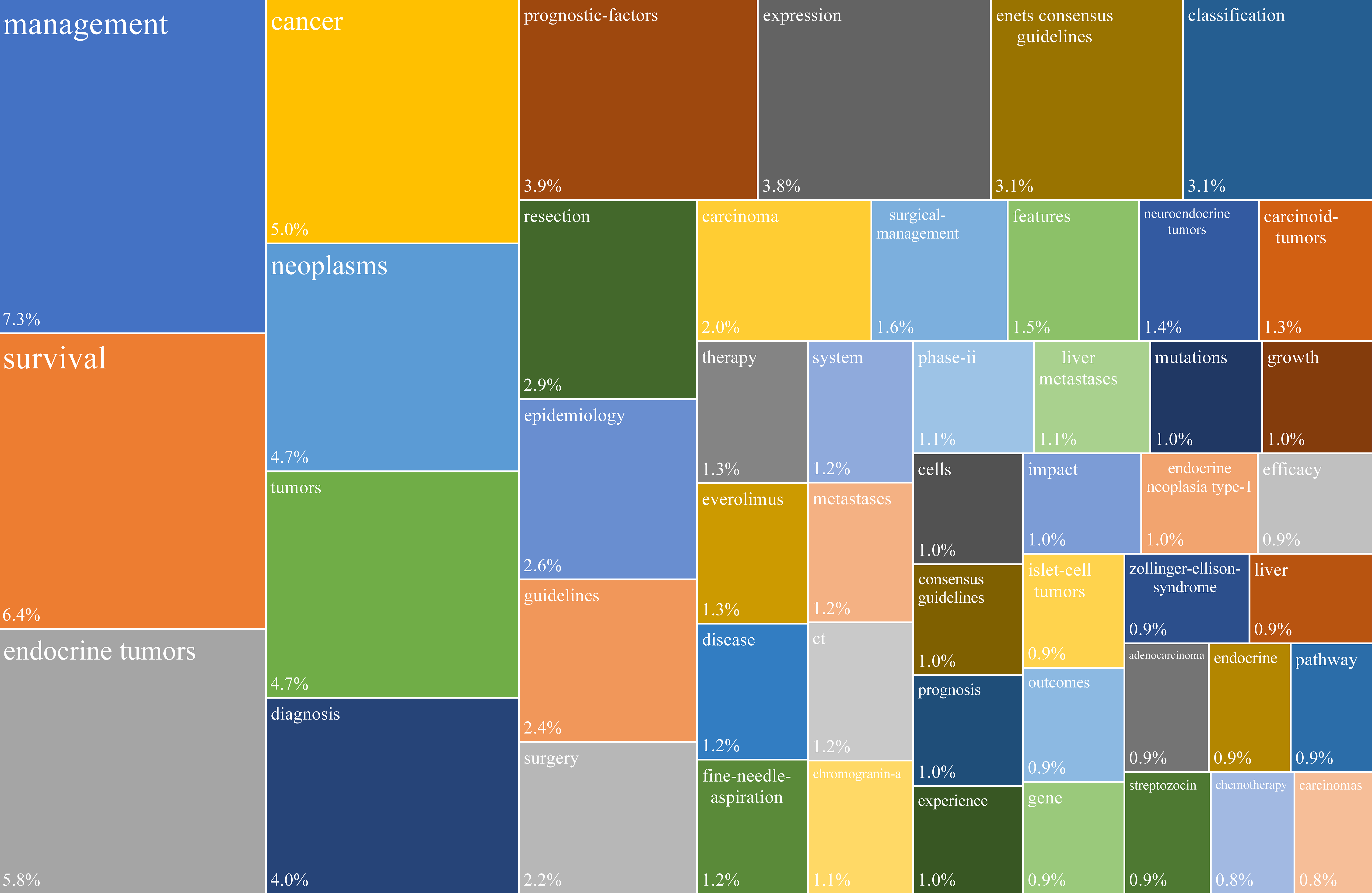
A total of 3805 keywords were captured in the “Keywords Plus” list, and the 50 most frequent terms were visualized with a tree map (Figure 6). The five most common keywords were “management,” “survival,” “endocrine tumors,” “cancer,” and “neoplasms.”
As shown by the bibliometric analysis, NEN research has grown over time, with most articles focusing on treatment and diagnosis. Over the past 40 years, advances in technologies such as endoscopy and blood biomarkers resulted in improved diagnostic levels of NENs. A retrospective, population-based study analyzing nationally representative data from the Surveillance, Epidemiology, and End Results program revealed a significant increase in the incidence and prevalence of NENs, with a six-fold increase in the incidence rate over the past 40 years[10]. As of 2012, the incidence rate of NENs was 6.98/100000, with an incidence rate of pancreatic NENs of 0.48/100000, an incidence rate of GI-NENs of 3.08/100000, and an incidence rate for gastroenteropancreatic neuroendocrine carcinoma of 0.4/100000. Gastroenteropancreatic NENs accounted for approximately 55%-70% of all NENs[11].
Furthermore, the advances in diagnosis promote improvements in treatment. Knowledge about the molecular drivers of tumorigenesis and advances in science and technology have increased the accuracy of molecular classification and led to the approval of more drugs for the treatment of advanced NENs. Once considered a rare group of cancers with no treatment options and managed with a nihilistic approach, NENs are now managed in centers with dedicated clinical programs and intensive research agendas[7]. Most NEN cases are sporadic, but several genetic syndromes should be considered, such as multiple endocrine neoplasia type 1 and type 2, von Hippel-Lindau syndrome, and NF1[12].
In 1964, Coskey and Tranquanda[13] first reported a patient with pancreatic NETs and NF1. In 1982, Cantor et al[14] first demonstrated the link between duodenal somatostatinoma and NF1. The link was later described as a frequent GI manifestation in NF1 patients[15,16]. A case report and literature review published in 2010 described 76 cases of periampullary and duodenal neoplasms associated with NF1[17]. In addition, the occurrence of NENs in NF1 patients infers the possibility of NF1 gene mutation being involved in the pathogenesis. Whole-exome sequencing of six NF1-associated duodenal NETs revealed the inactivation of somatic mutations of the NF1 gene in three of six tumors and deletion/loss of heterozygosity of chromosome 22 in 3 of 6 patients. IFNB1 was the only other altered gene in more than one tumor. These results confirmed the significance of somatic inactivation of the wild-type NF1 allele in developing NF1-associated duodenal NETs and suggested that loss of chromosome 22 plays an essential role in at least a subset of cases[18].
Considering the higher number of cases of NF1 patients with NENs being diagnosed, more attention should be paid to these patients. Sequencing data is required to analyze the gene mutation profile of NF1-associated NENs. Clarifying the pathogenesis and the role of inheritance factors may benefit the development of molecular therapy. However, a limited number of studies have investigated the differences between NENs in patients with or without NF1. Identifying differences in the presentation and behavior of NENs in patients with and without NF1 can provide insights into the underlying biology of these tumors and inform the development of new treatments.
GISTs in the duodenum were incidentally found during surgery in our patient. A high incidence of GISTs has been previously reported in patients with NF1[19]. In addition, the development of GISTs is associated with NET, especially in patients with NF1[20]. KIT and platelet-derived growth factor receptor-alpha mutations have also been reported to be sporadic events in NF1 GISTs. Furthermore, platelet-derived growth factor receptor-alpha can activate the RAS-MAPK pathway, which is associated with the inactivation of the NF1 gene and may play an essential role in developing GISTs in NF1 patients[21].
In summary, this article reported a case of periampullary duodenal NET and GIST in a patient with NF1. The bibliometric analysis showed a growing number of publications on periampullary NENs every year, with most studies focusing on the treatment and diagnosis of NENs. Further investigation is required to clarify the gene mutation profiles of NF1-associated periampullary NENs and the associations between periampullary NENs, GISTs, and NF1.
The authors thank the patient and his family for their interest and cooperation.
| 1. | Skuse GR, Kosciolek BA, Rowley PT. Molecular genetic analysis of tumors in von Recklinghausen neurofibromatosis: loss of heterozygosity for chromosome 17. Genes Chromosomes Cancer. 1989;1:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 81] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Klar N, Cohen B, Lin DDM. Neurocutaneous syndromes. Handb Clin Neurol. 2016;135:565-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Gutmann DH, Ferner RE, Listernick RH, Korf BR, Wolters PL, Johnson KJ. Neurofibromatosis type 1. Nat Rev Dis Primers. 2017;3:17004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 497] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 4. | Uusitalo E, Rantanen M, Kallionpää RA, Pöyhönen M, Leppävirta J, Ylä-Outinen H, Riccardi VM, Pukkala E, Pitkäniemi J, Peltonen S, Peltonen J. Distinctive Cancer Associations in Patients With Neurofibromatosis Type 1. J Clin Oncol. 2016;34:1978-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 262] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 5. | Walker L, Thompson D, Easton D, Ponder B, Ponder M, Frayling I, Baralle D. A prospective study of neurofibromatosis type 1 cancer incidence in the UK. Br J Cancer. 2006;95:233-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 146] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Nishi T, Kawabata Y, Hari Y, Imaoka H, Ishikawa N, Yano S, Maruyama R, Tajima Y. A case of pancreatic neuroendocrine tumor in a patient with neurofibromatosis-1. World J Surg Oncol. 2012;10:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Rizen EN, Phan AT. Neuroendocrine Tumors: a Relevant Clinical Update. Curr Oncol Rep. 2022;24:703-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 8. | Schmocker RK, Wright MJ, Ding D, Javed AA, Cameron JL, Lafaro K, Burns WR, He J, Wolfgang CL, Burkhart RA. Duodenal, ampullary, and pancreatic neuroendocrine tumors: Oncologic outcomes are driven by tumor biology and tissue of origin. J Surg Oncol. 2021;123:416-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Aria M, Cuccurullo C. bibliometrix: An R-tool for comprehensive science mapping analysis. JOI. 2017;11:959-975. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1736] [Cited by in RCA: 2249] [Article Influence: 281.1] [Reference Citation Analysis (0)] |
| 10. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3241] [Article Influence: 190.6] [Reference Citation Analysis (0)] |
| 11. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2472] [Article Influence: 309.0] [Reference Citation Analysis (4)] |
| 12. | Öberg K. The genetics of neuroendocrine tumors. Semin Oncol. 2013;40:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Coskey RL, Tranquada RE. Insulinoma and multiple neurofibromatosis: Report of a case. Metabolism. 1964;13:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Cantor AM, Rigby CC, Beck PR, Mangion D. Neurofibromatosis, phaeochromocytoma, and somatostatinoma. Br Med J (Clin Res Ed). 1982;285:1618-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Tanaka S, Yamasaki S, Matsushita H, Ozawa Y, Kurosaki A, Takeuchi K, Hoshihara Y, Doi T, Watanabe G, Kawaminami K. Duodenal somatostatinoma: a case report and review of 31 cases with special reference to the relationship between tumor size and metastasis. Pathol Int. 2000;50:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Cappelli C, Agosti B, Braga M, Cumetti D, Gandossi E, Rizzoni D, Agabiti Rosei E. Von Recklinghausen's neurofibromatosis associated with duodenal somatostatinoma. A case report and review of the literature. Minerva Endocrinol. 2004;29:19-24. [PubMed] |
| 17. | Relles D, Baek J, Witkiewicz A, Yeo CJ. Periampullary and duodenal neoplasms in neurofibromatosis type 1: two cases and an updated 20-year review of the literature yielding 76 cases. J Gastrointest Surg. 2010;14:1052-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Noë M, Pea A, Luchini C, Felsenstein M, Barbi S, Bhaijee F, Yonescu R, Ning Y, Adsay NV, Zamboni G, Lawlor RT, Scarpa A, Offerhaus GJA, Brosens LAA, Hruban RH, Roberts NJ, Wood LD. Whole-exome sequencing of duodenal neuroendocrine tumors in patients with neurofibromatosis type 1. Mod Pathol. 2018;31:1532-1538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Miettinen M, Fetsch JF, Sobin LH, Lasota J. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol. 2006;30:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 321] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 20. | Alabraba E, Bramhall S, O'Sullivan B, Mahon B, Taniere P. Pancreatic insulinoma co-existing with gastric GIST in the absence of neurofibromatosis-1. World J Surg Oncol. 2009;7:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Yamamoto H, Tobo T, Nakamori M, Imamura M, Kojima A, Oda Y, Nakamura N, Takahira T, Yao T, Tsuneyoshi M. Neurofibromatosis type 1-related gastrointestinal stromal tumors: a special reference to loss of heterozygosity at 14q and 22q. J Cancer Res Clin Oncol. 2009;135:791-798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |