Published online Sep 24, 2024. doi: 10.5306/wjco.v15.i9.1215
Revised: July 28, 2024
Accepted: August 5, 2024
Published online: September 24, 2024
Processing time: 206 Days and 23.6 Hours
The development mechanisms of Lynch syndrome (LS)-related breast cancer (BC) and rectal cancer are complex and variable, leading to personalized variations in diagnosis and treatment plans.
This paper presents a comprehensive review of clinical diagnosis and treatment data from a patient with LS-associated BC and rectal cancer. Moreover, screening data and management guidelines, as well as relevant literature on LS, are inclu
Implementing early screening, prevention, and timely diagnosis and treatment measures is expected to reduce mitigate the incidence and mortality of LS-related BC and rectal cancer.
Core Tip: Lynch syndrome (LS) is the most common hereditary cancer syndrome and is characterized by a predisposition to a range of cancers, mainly colorectal cancer and endometrial cancer. In this case, a patient suffered from breast cancer 10 years ago and was subsequently diagnosed with rectal cancer, which is different from traditional LS development. Developing methods to diagnosis LS patients and giving their relatives corresponding genetic counselling advice can significantly reduce the incidence of LS-related tumours.
- Citation: Qin PF, Yang L, Hu JP, Zhang JY. Breast cancer and rectal cancer associated with Lynch syndrome: A case report. World J Clin Oncol 2024; 15(9): 1215-1221
- URL: https://www.wjgnet.com/2218-4333/full/v15/i9/1215.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i9.1215
Lynch syndrome (LS), also known as hereditary nonpolyposis colorectal cancer syndrome, is a rare hereditary cancer susceptibility syndrome. It is characterized by autosomal dominant genetic disorders resulting from germline mutations in DNA mismatch repair genes (MMR), with a penetrance rate of 80% to 85%. Roudko et al[1] proposed that LS predisposes individuals to a spectrum of cancers, primarily colorectal cancer and endometrial cancer, displaying familial aggregation. Patients with LS carry an allele mutation in the MMR gene, rendering all somatic cells potential precancerous cells. Sinicrope[2] proposed the subsequent somatic cell mutations pose a significant clinical risk across various cancer types, including endometrial cancer, colorectal cancer, ovarian cancer, breast cancer (BC), and gastric cancer. Accurate diagnosis of LS in patients and the provision of appropriate genetic counseling to their relatives can significantly reduce the incidence of LS-related tumors. In our retrospective study, we presented a rare case of LS observed at our hospital and discussed its pathogenesis in conjunction with the existing literature.
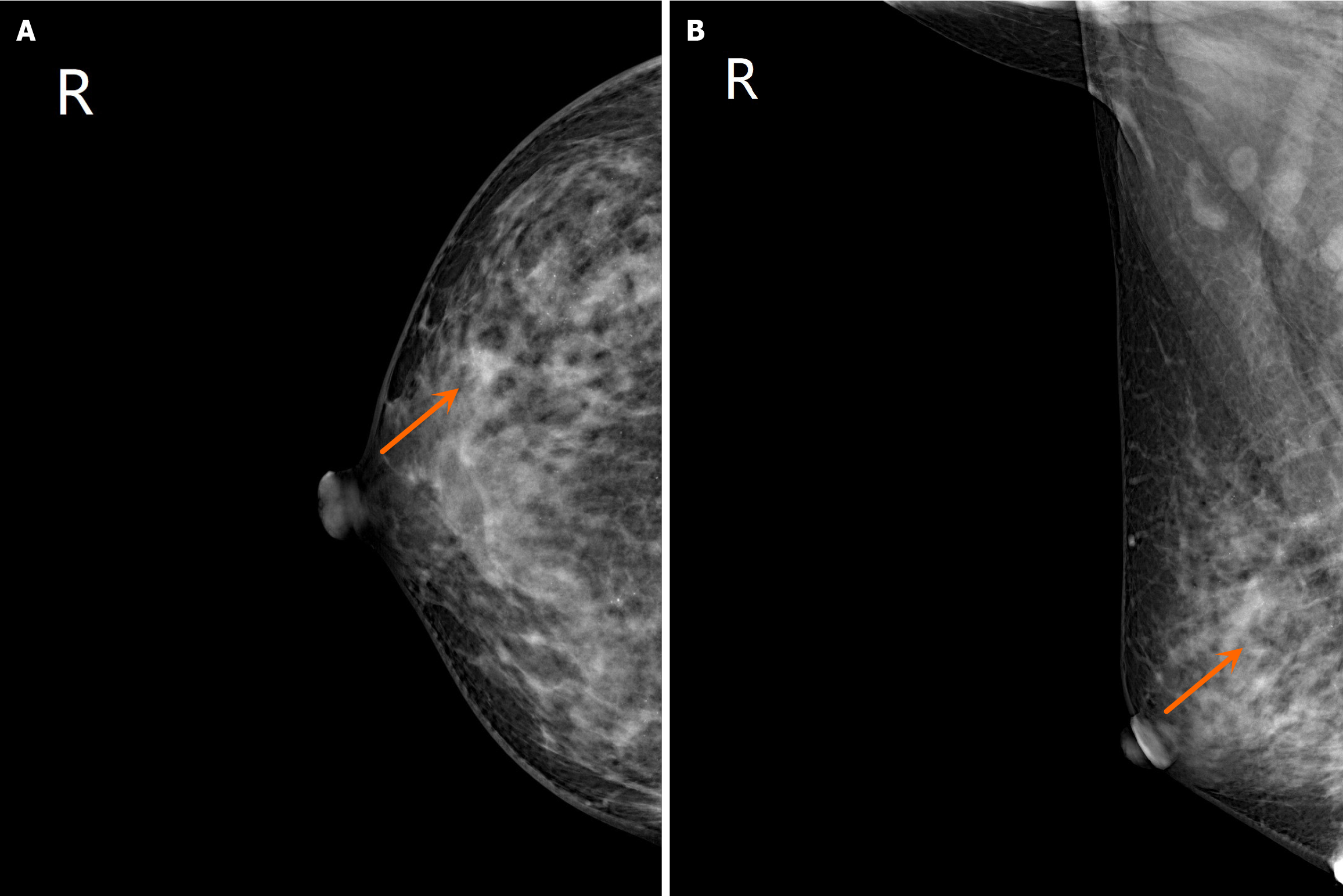
Case data of BC: The patient was a 39-year-old woman. In April 2013, a hard finger-shaped mass was discovered in the upper part of the right breast. There were no accompanying local swelling, pain, or nipple discharge at the time of admission. The patient reported no chest pain, cough, low-grade fever, or night sweats. B-mode ultrasound indicated multiple solid masses in the right breast (the largest one measures 2.1 cm × 1.2 cm) , leading to a provisional diagnosis of "right breast tumor". Following examinations and biopsy, the pathology results confirmed right breast invasive ductal carcinoma and partial ductal carcinoma in situ (stage IIa infiltrating ductal carcinoma of the right breast). In July 2013, the patient underwent a modified radical mastectomy under general anesthesia, which postoperative pathology indicating grade 2 invasive ductal carcinoma in the right breast, with localized fibrocystic changes observed in the surrounding breast tissue (Figure 1). No carcinoma infiltration was observed in the nipple and breast basal membrane. No evidence of metastatic tumor was found in seventeen lymph nodes in the rubber fossa lymph nodes. The patient's postoperative condition is good. They received 4 cycles of chemotherapy, along with antiemetic, acid suppression, and supportive therapy. In addition, immunohistochemical results showed that endoplasmic reticulum (ER) (++), progesterone receptor (PR) (+++), C-erbB-2 (+++), P53 (++), the DNA topoisomerase II (TOPO II) (++), Ki-67 (approximately 50%). Gene detection revealed medium-level expression of the oncogene (P53) and drug resistance gene (TOPO II), with proliferative cells displaying high activity.
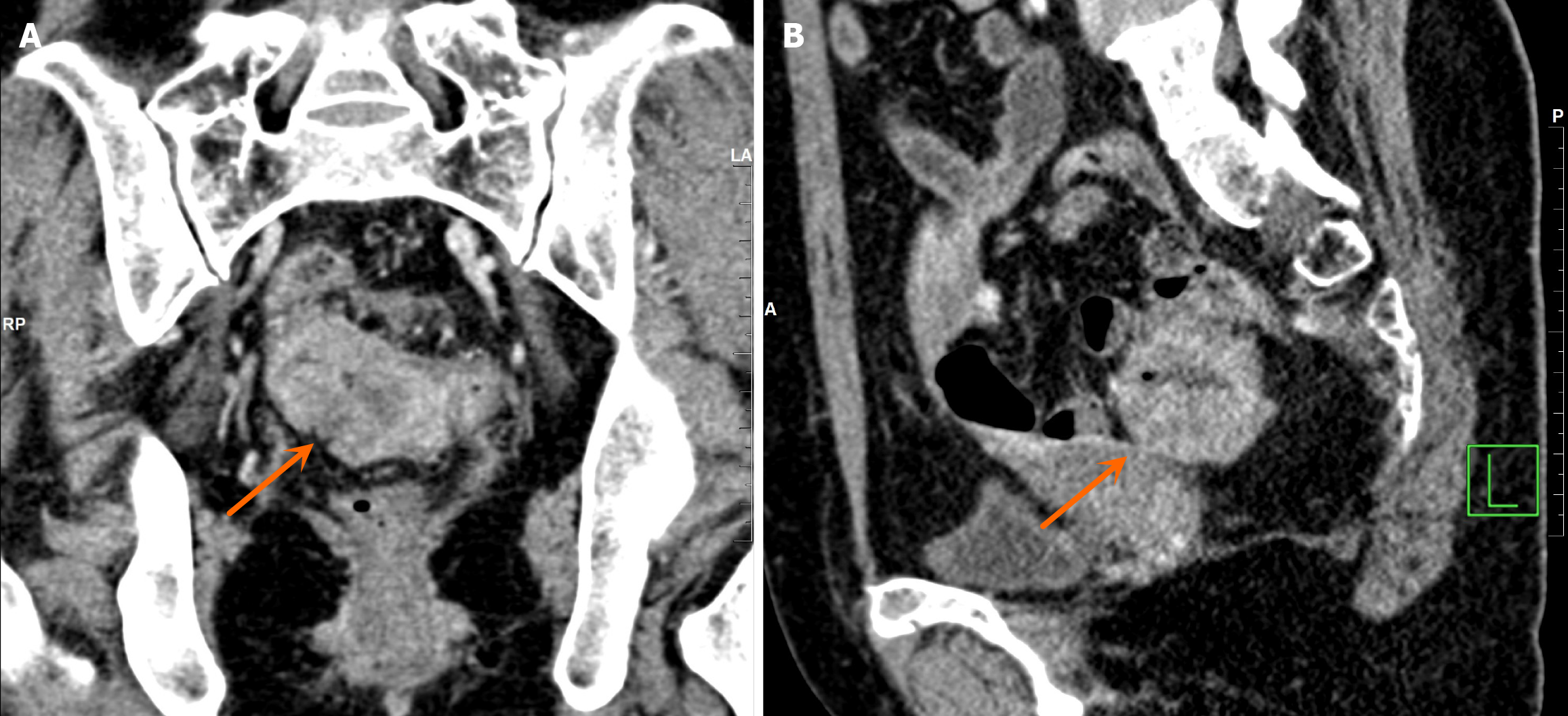
Case data of rectal cancer: The patient is a 49-year-old female who, since September 2023, has experienced unformed, loose stools without an apparent cause. These stools are often accompanied by small amounts of blood, mostly adherent to the surface, and occasionally mixed with mucus. She does not report tenesmus or diarrhea, and has a bowel movement frequency of 12-13 times every day. Notably, there were no other discernible abnormalities. Initially evaluated at an external hospital, the patient received a diagnosis of a sigmoidal mass with pelvic lymph nodes enlargement. Sub
The patient was diagnosed with rectal cancer.
The patient was diagnosed with BC.
The patient's family history included siblings affected by hyperthyroidism, with two sisters also diagnosed with BC. Remarkably, a decade later, the emergence of secondary rectal cancer with lymph node metastasis aligned with the prototypical features of LS.
B-mode ultrasound revealed multiple solid masses in the right breast (the largest mass measured 2.1 cm × 1.2 cm), leading to a provisional diagnosis of "right breast tumour". When initially evaluated at an external hospital, the patient received a diagnosis of a sigmoidal mass with pelvic lymph node enlargement. The patient was subsequently referred to our hospital for further investigations (Figure 2), which confirmed the presence of a rectal tumour.
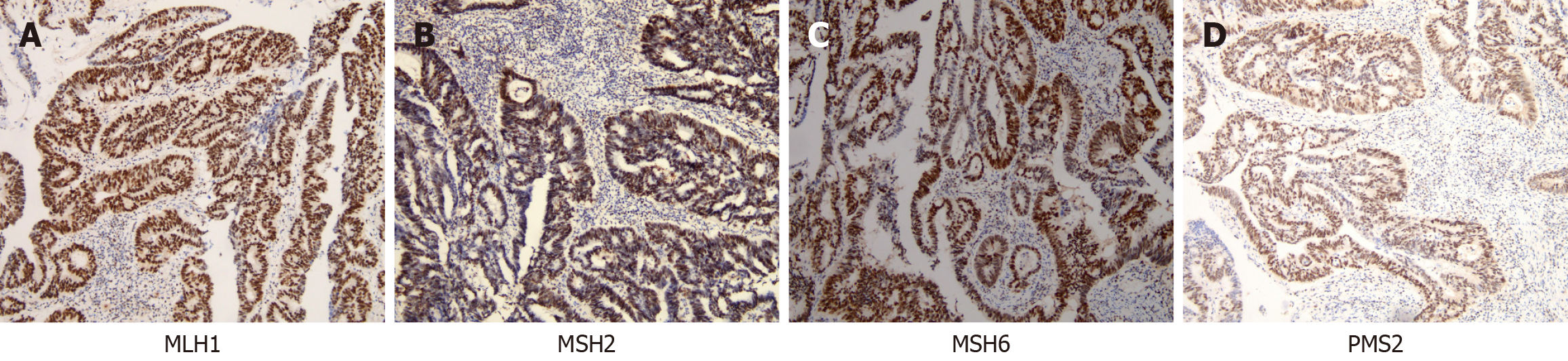
In addition, the immunohistochemical results revealed the following: ER (++), PR (+++), C-erbB-2 (+++), P53 (++), the TOPO II (++), and Ki-67 (approximately 50%). Immunohistochemical analysis provided further insights, revealing high expression levels of Ki-67 (approximately 80% of cells were positively stained), positive results for PMS2, MSH2, MSH6, and MLH1, and weakly positive results for Her-2 (Figure 3).
The patient received a diagnosis of a sigmoidal mass with pelvic lymph node enlargement.
Following examinations and biopsy, the pathology results confirmed right breast invasive ductal carcinoma and partial ductal carcinoma in situ (stage IIa infiltrating ductal carcinoma of the right breast). When initially evaluated at an external hospital, the patient received a diagnosis of a sigmoidal mass with pelvic lymph node enlargement. The patient was subsequently referred to our hospital for further investigations (Figure 2), which confirmed the presence of a rectal tumour.
The patient received 4 cycles of chemotherapy, along with antiemetics, acid suppressants, and supportive care.
The patient remained stable postoperatively, adhered to a structured rehabilitation regimen, and exhibited no signs of recurrence. Regular follow-up evaluations demonstrated that all clinical parameters remained within normal limits, with no evidence of tumour recurrence or metastasis, indicating a favourable prognosis.
LS has emerged as the most prevalent hereditary colorectal cancer syndrome and is associated primarily with mutations in key genes—MLH1, MSH2, MSH6, and PMS2-which are responsible for approximately 37%, 41%, 13%, and 9% of all LS cases, respectively. Additionally, Cerretelli et al[3] suggested that EPCAM, the upstream gene of MSH2, plays a crucial role, and its deletion will leads to hypermethylation of the MSH2 promoter, resulting in its expression being deleted. In the present case, the patient exhibited typical manifestations of LS, characterized by a history of high-risk factors for BC and the presence of abnormal breast nodules. During the operation, numerous extensive breast lesions were discovered, and postoperative pathological examination confirmed BC. The patient's family history included siblings affected by hyperthyroidism, with two sisters also diagnosed with BC. Remarkably, a decade later, the emergence of secondary rectal cancer with lymph node metastasis aligned with the prototypical features of LS. This case underscores the importance of vigilance in identifying similar cases in the future.
According to Abildgaard et al[4], LS has demonstrated a prevalence ranging from 1/100 to 1/180 in the population, constituting approximately 3% of patients with colorectal cancer. Individuals with LS typically present with colorectal cancer at a younger age than those with sporadic colorectal cancer, with a median age of 43 years vs 69 years. Adenomas are more prevalent in LS patients than in the general population but are less common than polyposis. The number of adenomatous polyps in LS-related colorectal cancer patients is usually fewer than 10, and these adenomas exhibit high-risk characteristics such as villous and poorly differentiated features[5]. The patient in this case, who was diagnosed with rectal cancer at the age of 49 years, aligned with the typical age presentation reported in the literature. Furthermore, the pathological diagnosis of adenocarcinoma with significant lymphocyte infiltration corresponds to documented characteristics of LS-related colorectal cancer. In the reports of Møller et al[6] and Davidson[7], there was a notable tendency predilection for right-sided colon cancer in LS patients, accounting for 60%-80%, whereas in individuals without LS, the incidence of right-sided colon cancer was only approximately 30%.
In female LS patients, an increased the elevated risk of colorectal cancer is accompanied by an increased susceptibility to gynaecological cancers, particularly endometrial and ovarian cancers. The risk of endometrial cancer in female LS patients ranges from 16% to 71%, whereas the risk of ovarian cancer is between 6% and 12%. In comparison, Liu et al[8], Chao et al[9], and Lim et al[10] suggested that the risk of endometrial cancer in the general population is 2.6%, and the risk of ovarian cancer is 1.4%. Notably, women with LS-associated endometrial cancer tend to be younger, with a median age of 49 years. According to Llach et al[11] and Gentry-Maharaj et al[12] the patient in this case was diagnosed with BC and then was diagnosed with rectal cancer a decade later; thus, it is imperative to closely monitor and screen for the potential occurrence of other diseases in the future. Special attention should be given to the screening and prevention of gynaecological diseases to achieve early detection, diagnosis, and treatment and combat the increased risk of cancer associated with LS.
According to Roberts et al[13], BC serves as the initial symptom in approximately 40%-60% of female patients with LS and is the most prevalent extraintestinal manifestation of LS, often regarded as the sentinel cancer of LS. In this case, the patient was initially diagnosed with BC, and subsequent discovery of rectal lesions, coupled with genetic testing results, led to the consideration of LS. Lotsari et al[14] focused on exploring LS-associated BC (LS-BC). LS-BC typically presents between the ages of 46 years and 54 years, featuring diverse pathological types with a tendency towards low differentiation. The patient, who was diagnosed with BC at the age of 39 years, exhibited adenocarcinoma, aligning with the characteristic features observed in LS-BC. Prior studies have extensively reported mutations in the MLH1 and MSH2 genes, with this patient exhibiting a pathogenic mutation in the MSH2 gene. According to Schwartz et al[15], MSH6 mutations are occasionally detected in LS, whereas PMS2 mutations are relatively rare. Diagnosing LS-BC can be challenging, leading experts to recommend comprehensive screening measures for all newly diagnosed BC patients. According to Adam et al[16], these measures include MMR gene detection, MSI analysis, immunohistochemistry, BRAF detection, and MLH1 promoter methylation analysis to screen for LS-BC. Given the elevated risk of BC in women with LS, it is crucial to establish the association between BC and LS. In this case, with the patient diagnosed with BC a decade prior and subsequently developing rectal cancer, suspicions of LS were raised on the basis of immunohistochemical results and family history. Consequently, the patient's family was recommended for related genetic counselling. Moving forward, enhancing awareness of LS, improving gastrointestinal endoscopy methods, and incorporating genetic testing into the diagnostic process are essential for achieving early diagnosis and treatment. Identifying LS-associated BC as not only aids in the early detection and prevention of other tumours, such as colorectal cancer but also, underscores the importance of proactive screening strategies in the broader context of LS-associated malignancies.
Despite differing pathogenesis and clinical manifestations from sporadic BC, LS-BC is managed with a treatment approach akin to sporadic BC. Notably, oestrogen therapy is not applicable. Staged surgery is recommended, and for patients with stage III disease or above, a combination of radiotherapy and chemotherapy is considered. Lymph node dissection is a crucial aspect of the treatment protocol, and radiotherapy may be considered in cases where surgical interventions are contraindicated. Importantly, for tumours exhibiting microsatellite instability-high (MSI-H) or MMR deficiency (MMR-D), immunotherapy, particularly programmed cell death 1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors, has demonstrated lasting efficacy. In Matsubayashi et al[17] and Suh et al[18], the United States Food and Drug Administration approved the use of these inhibitors in 2017 for the treatment of MSI-H or MMR-D solid tumors. The research has indicated that MSI-high BC is particularly responsive to anti-PD-1/PD-L1 immunotherapy. In this case, after the patient underwent surgery, chemotherapy, and targeted combined immunotherapy, a significant therapeutic effect was observed, with a substantial reduction in lesion size compared with the initial presentation. Moreover, prophylactic measures, including regular breast and ultrasound examinations, biopsies, and colonoscopies, can contribute to early detection and prevention. Prophylactic surgical resection of the breast has been shown to reduce tumour incidence. By implementing these comprehensive measures, early screening, diagnosis, and treatment of LS can be achieved, ultimately reducing the incidence and mortality associated with LS and its related tumours. This holistic approach highlights the importance of proactive management strategies in addressing the complexities of LS-BC.
The development of BC and rectal cancer related to LS is complex and changeable, and the diagnosis and treatment plan varies from person to person. Through the study of this case, we can learn from experience and lessons. Early screening, prevention and diagnosis and treatment are expected to reduce the incidence and mortality of LS-BC and rectal cancer.
| 1. | Roudko V, Cimen Bozkus C, Greenbaum B, Lucas A, Samstein R, Bhardwaj N. Lynch Syndrome and MSI-H Cancers: From Mechanisms to "Off-The-Shelf" Cancer Vaccines. Front Immunol. 2021;12:757804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Sinicrope FA. Lynch Syndrome-Associated Colorectal Cancer. N Engl J Med. 2018;379:764-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 175] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 3. | Cerretelli G, Ager A, Arends MJ, Frayling IM. Molecular pathology of Lynch syndrome. J Pathol. 2020;250:518-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 4. | Abildgaard AB, Nielsen SV, Bernstein I, Stein A, Lindorff-Larsen K, Hartmann-Petersen R. Lynch syndrome, molecular mechanisms and variant classification. Br J Cancer. 2023;128:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | International Mismatch Repair Consortium. Variation in the risk of colorectal cancer in families with Lynch syndrome: a retrospective cohort study. Lancet Oncol. 2021;22:1014-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 6. | Møller P, Seppälä TT, Bernstein I, Holinski-Feder E, Sala P, Gareth Evans D, Lindblom A, Macrae F, Blanco I, Sijmons RH, Jeffries J, Vasen HFA, Burn J, Nakken S, Hovig E, Rødland EA, Tharmaratnam K, de Vos Tot Nederveen Cappel WH, Hill J, Wijnen JT, Jenkins MA, Green K, Lalloo F, Sunde L, Mints M, Bertario L, Pineda M, Navarro M, Morak M, Renkonen-Sinisalo L, Valentin MD, Frayling IM, Plazzer JP, Pylvanainen K, Genuardi M, Mecklin JP, Moeslein G, Sampson JR, Capella G; Mallorca Group. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut. 2018;67:1306-1316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 401] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 7. | US Preventive Services Task Force, Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Krist AH, Kubik M, Li L, Ogedegbe G, Owens DK, Pbert L, Silverstein M, Stevermer J, Tseng CW, Wong JB. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:1965-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 1137] [Article Influence: 284.3] [Reference Citation Analysis (0)] |
| 8. | Liu YL, Cadoo KA, Maio A, Patel Z, Kemel Y, Salo-Mullen E, Catchings A, Ranganathan M, Kane S, Soslow R, Ceyhan-Birsoy O, Mandelker D, Carlo MI, Walsh MF, Shia J, Markowitz AJ, Offit K, Stadler ZK, Latham A. Early age of onset and broad cancer spectrum persist in MSH6- and PMS2-associated Lynch syndrome. Genet Med. 2022;24:1187-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Chao X, Li L, Wu M, Ma S, Tan X, Zhong S, Bi Y, Lang J. Comparison of screening strategies for Lynch syndrome in patients with newly diagnosed endometrial cancer: a prospective cohort study in China. Cancer Commun (Lond). 2019;39:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Lim N, Hickey M, Young GP, Macrae FA, Kelly C. Screening and risk reducing surgery for endometrial or ovarian cancers in Lynch syndrome: a systematic review. Int J Gynecol Cancer. 2022;32:646-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Llach J, Pellisé M, Monahan K. Lynch syndrome; towards more personalized management? Best Pract Res Clin Gastroenterol. 2022;58-59:101790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Gentry-Maharaj A, Karpinskyj C. Current and future approaches to screening for endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2020;65:79-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Roberts ME, Jackson SA, Susswein LR, Zeinomar N, Ma X, Marshall ML, Stettner AR, Milewski B, Xu Z, Solomon BD, Terry MB, Hruska KS, Klein RT, Chung WK. MSH6 and PMS2 germ-line pathogenic variants implicated in Lynch syndrome are associated with breast cancer. Genet Med. 2018;20:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 14. | Lotsari JE, Gylling A, Abdel-Rahman WM, Nieminen TT, Aittomäki K, Friman M, Pitkänen R, Aarnio M, Järvinen HJ, Mecklin JP, Kuopio T, Peltomäki P. Breast carcinoma and Lynch syndrome: molecular analysis of tumors arising in mutation carriers, non-carriers, and sporadic cases. Breast Cancer Res. 2012;14:R90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Schwartz CJ, da Silva EM, Marra A, Gazzo AM, Selenica P, Rai VK, Mandelker D, Pareja F, Misyura M, D'Alfonso TM, Brogi E, Drullinsky P, Razavi P, Robson ME, Drago JZ, Wen HY, Zhang L, Weigelt B, Shia J, Reis-Filho JS, Zhang H. Morphologic and Genomic Characteristics of Breast Cancers Occurring in Individuals with Lynch Syndrome. Clin Cancer Res. 2022;28:404-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Adam F, Fluri M, Scherz A, Rabaglio M. Occurrence of variants of unknown clinical significance in genetic testing for hereditary breast and ovarian cancer syndrome and Lynch syndrome: a literature review and analytical observational retrospective cohort study. BMC Med Genomics. 2023;16:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Matsubayashi H, Oishi T, Sasaki K, Abe M, Kiyozumi Y, Higashigawa S, Niiya F, Sato J, Ishiwatari H, Imai K, Hotta K, Kishida Y, Takada K, Ono H, Yamazaki K, Yasui H, Kenmotsu H, Kado N, Kagawa H, Shiomi A, Sugiura T, Bando E, Nishimura S, Hatakeyama K, Serizawa M, Harada R, Sugino T. Discordance of microsatellite instability and mismatch repair immunochemistry occurs depending on the cancer type. Hum Pathol. 2023;135:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 18. | Suh DH, Kim M, Lee KH, Eom KY, Kjeldsen MK, Mirza MR, Kim JW. Major clinical research advances in gynecologic cancer in 2017. J Gynecol Oncol. 2018;29:e31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |