Published online Sep 24, 2024. doi: 10.5306/wjco.v15.i9.1177
Revised: July 12, 2024
Accepted: July 31, 2024
Published online: September 24, 2024
Processing time: 160 Days and 5 Hours
Hemorrhage, which is not a rare complication in patients with gastric cancer (GC)/gastroesophageal junction cancer (GEJC), can lead to a poor prognosis. However, no study has examined the effectiveness and safety of chemotherapy as an initial therapy for GC/GEJC patients with overt bleeding (OB).
To investigate the impact of OB on the survival and treatment-related adverse events (TRAEs) of GC/GEJC patients.
Patients with advanced or metastatic GC/GEJC who received systematic treat
After 1:2 PSM analysis, 93 patients were assessed, including 32 patients with OB before treatment (OBBT) and 61 patients without OBBT. The disease control rate was 90.6% in the group with OBBT and 88.5% in the group without OBBT, and this difference was not statistically significant. There was no difference in the incidence of TRAEs between the group with OBBT and the group without OBBT. The median overall survival (mOS) was 15.2 months for patients with OBBT and 23.7 months for those without OBBT [hazard ratio (HR) = 1.101, 95% confidence interval (CI): 0.672-1.804, log rank P = 0.701]. The mOS was worse for patients with OB after treatment (OBAT) than for those without OBAT (11.4 months vs 23.7 months, HR = 1.787, 95%CI: 1.006-3.175, log rank P = 0.044).
The mOS for GC/GEJC patients with OBBT was similar to that for those without OBBT, but the mOS for patients with OBAT was worse than that for those without OBAT.
Core Tip: Overt bleeding (OB) is a dangerous condition in patients with advanced or metastatic gastric cancer (GC)/gastroe
- Citation: Yao YH, Zhang H, Xiao Y, Liu ZT, Shi YY, Yu JY, Li Q, Cao BS. Systematic treatment in gastric cancer patients with overt bleeding: A propensity score matching analysis. World J Clin Oncol 2024; 15(9): 1177-1187
- URL: https://www.wjgnet.com/2218-4333/full/v15/i9/1177.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i9.1177
Gastric cancer (GC) was the fifth most common cancer and ranked fourth in terms of cancer mortality worldwide in 2020[1]. Hemorrhage, which is not a rare complication of GC/gastroesophageal junction cancer (GEJC), affects approximately 15% of patients with GC/GEJC[2]. Bleeding not only reduces the quality of life of GC/GEJC patients but also leads to a poor prognosis, and such bleeding can affect anticancer treatment and may even be life-threatening if it fails to stop in a timely manner[3,4].
The risk factors for bleeding in patients with GC/GEJC vary and include age, smoking history, chronic disease and so on[2,5,6]. Radical gastrectomy is ideal for early-stage GC/GEJC with overt bleeding (OB)[7], but there is rarely a curable chance for patients with advanced or metastatic GC/GEJC[8]. Successful hemostasis via endoscopic therapy strategies is achieved in 67%-100% of GC patients with OB. However, the rebleeding rate is high, ranging from 16% to 80%, and the median overall survival (mOS) is poor, ranging from 1.0 to 6.5 months[3,9,10]. Endoscopic hemostasis is prone to failure for bleeding lesions larger than 2 cm[11]. The success rate of hemostasis by transcatheter embolotherapy in GC patients with bleeding is 40%-100%. The rebleeding rate is 16%-41%, and the mOS is no more than three months for unresectable disease[10,11]. Prior studies have reported that the rate of successful hemostasis by palliative radiotherapy ranges from 50% to 80%. The response duration and the mOS are 0.9-3.7 months and 2.1-5.3 months, respectively, for unresectable GC[10,12,13]. Above all, the rebleeding rate is high, and survival is poor after local hemostasis therapy for unresectable GC with bleeding. Systematic therapy is the preferred treatment strategy for advanced or metastatic GC/GEJC. However, there are no reports about the efficacy and safety of chemotherapy as an initial therapy for GC/GEJC patients with OB.
This retrospective study aimed to investigate the impact of OB on treatment-related adverse events (TRAEs) in GC/GEJC patients receiving systematic anticancer treatment, evaluate the risk factors for OB after treatment (OBAT), and assess the influence of OB before treatment (OBBT) and OBAT on OS.
We retrospectively enrolled patients who were diagnosed with advanced or metastatic GC/GEJC from January 1, 2013 to December 31, 2021, at the Department of Medical Oncology and Radiation Sickness of Peking University Third Hospital. The patient eligibility criteria were as follows: (1) Were older than 18 years; (2) Had a histologically confirmed diagnosis of advanced or metastatic GC/GEJC; (3) Received systematic therapy as initial anticancer treatment, including chemo
The patients’ information collected from the database was as follows: Age, sex, height, weight, Eastern Corporative Oncology Group Performance Status (ECOG-PS), chronic disease status, drinking history, smoking history, primary tumor location, clinical stage according to the 8th edition of the American Joint Committee on Cancer (AJCC) cancer staging manual, OB status, therapeutic strategy, best response to treatment, TRAEs and so on.
The diagnosis of OB was that the following conditions occurred within one month before the first dose of systematic treatment: (1) Melena, he
Age, sex, smoking history, comorbidities, nutritional status, and lesion status are reported to be risk factors for gastric bleeding in patients with gastrointestinal cancer. Therefore, we analyzed the relationships between rebleeding and seve
We used SPSS version 25.0 (International Business Machines, New York, NY, United States) to analyze the data and considered that differences to be statistically significant if the two-sided P values were less than 0.05. The classified va
Propensity score matching (PSM) analysis was performed between the group with OBBT and the group without OBBT to reduce the impact of bias. The propensity score model was estimated via a logistic regression model that adjusted for variables including sex, age, BMI, weight loss, smoking history, drinking history, chronic disease status, ECOG-PS score, primary tumor location, and cancer stage. PSM was performed via a 1:2 matching method and nearest-neighbour mat
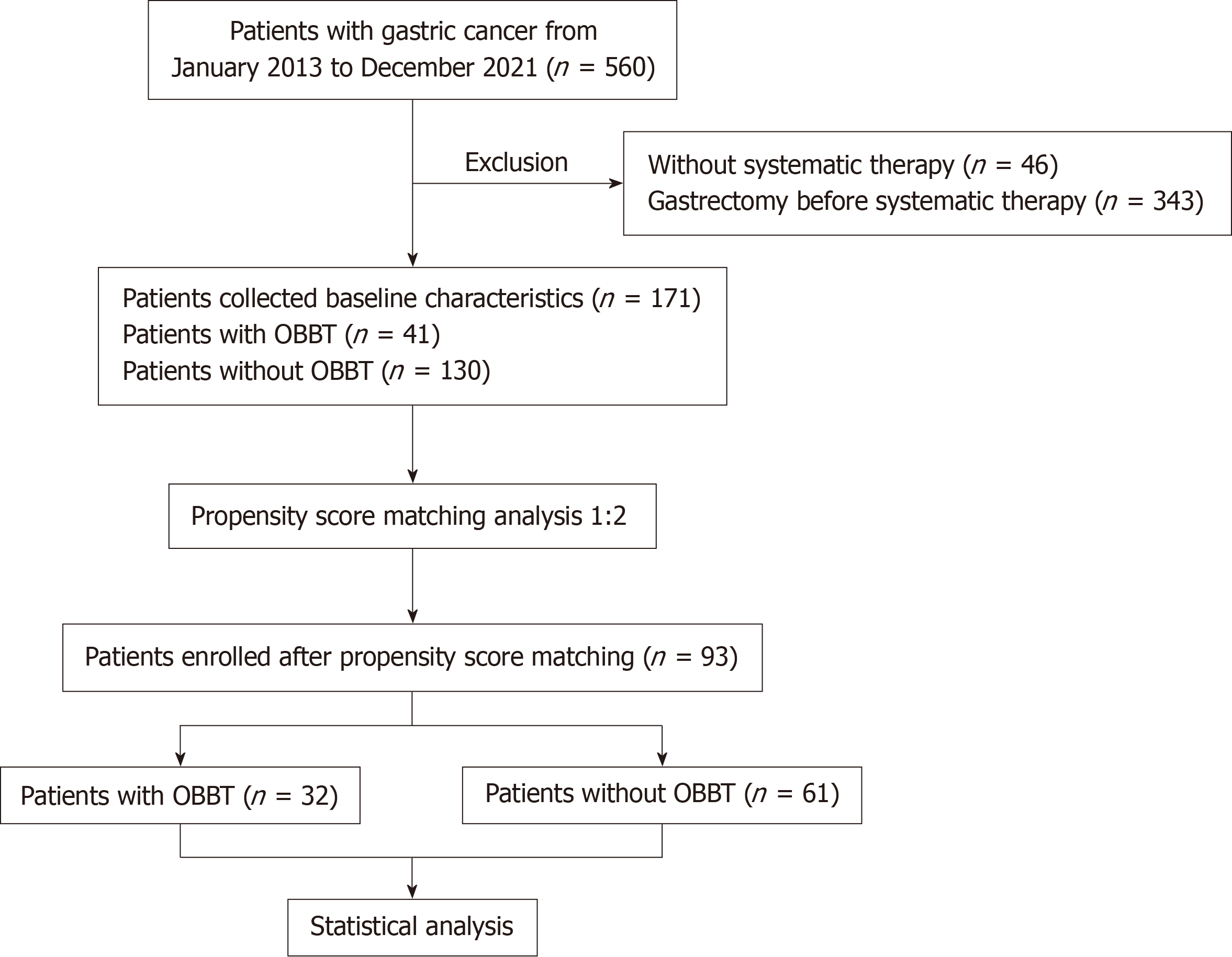
From 560 patients with GC/GEJC from January 1, 2013 to December 31, 2021, 171 patients were enrolled in the study (Figure 1). The most common reason for exclusion was radical gastrectomy at an early stage. The characteristics of the included patients are listed in Table 1. Approximately three-quarters (129/171) of the patients were at stage IV, and the others were at stage III. Forty-one (24.0%) of the 171 included patients had OBBT. The patient characteristics in the group with OBBT and the group without OBBT were not balanced (Table 1). After a 1:2 PSM analysis, there were 32 patients in the group with OBBT and 61 patients without OBBT, for which the patient characteristics were well balanced (Table 2). The median follow-up period was 17.1 months in the group with OBBT and 25.2 months in the group without OBBT.
| Variables | Patients with OBBT (n = 41) | Patients without OBBT (n = 130) | P value | SMD |
| Gender | ||||
| Male | 30 (73.2) | 94 (72.3) | 0.914 | 0.019 |
| Female | 11 (26.8) | 36 (27.7) | ||
| Age (range, years) | 65 (47-85) | 64 (29-83) | 0.003 | 0.486 |
| BMI (range, kg/m2) | 21.0 (15.2-27.2) | 22.0 (16.0-33.6) | 0.153 | 0.259 |
| Weight loss (range, %) | 8.3 (0-37.4) | 5.7 (0-26.0) | 0.044 | 0.399 |
| Smoking history | ||||
| Yes | 15 (36.6) | 60 (46.2) | 0.282 | 0.195 |
| No | 26 (63.4) | 70 (53.8) | ||
| Drinking history | ||||
| Yes | 7 (17.1) | 17 (13.1) | 0.521 | 0.112 |
| No | 34 (82.9) | 113 (86.9) | ||
| 1Chronic disease | ||||
| Yes | 16 (39.0) | 42 (32.3) | 0.428 | 0.141 |
| No | 25 (61.0) | 88 (67.7) | ||
| ECOG-PS | ||||
| 0 | 1 (2.5) | 5 (3.8) | 0.010 | 0.473 |
| 1 | 32 (78.0) | 119 (91.5) | ||
| 2 | 8 (19.5) | 6 (4.6) | ||
| Primary tumor location | ||||
| Body | 15 (36.6) | 74 (56.9) | 0.061 | 0.429 |
| Pylorus | 13 (31.7) | 24 (18.5) | ||
| Cardia | 13 (31.7) | 32 (24.6) | ||
| Cancer stage (AJCC 8th) | ||||
| Stage III | 9 (22.0) | 33 (25.4) | 0.656 | 0.081 |
| Stage IV | 32 (78.0) | 97 (74.6) |
| Variables | Patients with OBBT (n = 32) | Patients without OBBT (n = 61) | P value | SMD |
| Gender | ||||
| Male | 23 (71.9) | 39 (63.9) | 0.440 | 0.171 |
| Female | 9 (28.1) | 22 (36.1) | ||
| Age (range, years) | 64.5 (47-80) | 67 (29-81) | 0.629 | 0.100 |
| BMI (range, kg/m2) | 22.8 (15.9-27.2) | 21.8 (16.0-28.1) | 0.757 | 0.066 |
| Weight loss (range, %) | 7.5 (0-33.3) | 6.5 (0-23.80) | 0.671 | 0.092 |
| Smoking history | ||||
| Yes | 12 (37.5) | 22 (36.1) | 0.891 | 0.030 |
| No | 20 (62.5) | 39 (63.9) | ||
| Drinking history | ||||
| Yes | 5 (15.6) | 8 (13.1) | 0.986 | 0.072 |
| No | 27 (84.4) | 53 (86.9) | ||
| 1Chronic disease | ||||
| Yes | 13 (40.6) | 25 (41.0) | 0.973 | 0.007 |
| No | 19 (59.4) | 36 (59.0) | ||
| ECOG-PS | ||||
| 0 | 1 (3.1) | 2 (3.3) | 0.943 | 0.076 |
| 1 | 29 (90.6) | 54 (88.5) | ||
| 2 | 2 (6.3) | 5 (8.2) | ||
| Primary tumor location | ||||
| Body | 12 (37.5) | 26 (42.6) | 0.891 | 0.105 |
| Pylorus | 9 (28.1) | 16 (26.2) | ||
| Cardia | 11 (34.4) | 19 (31.1) | ||
| Cancer stage (AJCC 8th) | ||||
| Stage III | 6 (18.8) | 13 (21.3) | 0.771 | 0.064 |
| Stage IV | 26 (81.2) | 48 (78.7) |
All the patients received systematic anticancer treatment, including a chemotriplet regimen (fluorouracil, platinum and taxane), a chemodoublet-based regimen (chemodoublet regimen plus/minus targeted therapy or ICI), and fluorouracil or ICI monotherapy. Among patients with OBBT, 75.0% (24/32) received a chemodoublet-based regimen, 18.8% (6/32) received a triplet regimen, and 6.3% (2/32) received fluorouracil or ICI monotherapy. In the group without OBBT, a che
| Characteristics | Total (n = 93) | Patients with OBBT (n = 32) | Patients without OBBT (n = 61) | P value |
| Therapeutic regimen | ||||
| Chemotriplet regimen | 36 (38.7) | 6 (18.8) | 30 (49.2) | 0.006 |
| 1Chemodoublet-based regimen | 54 (58.1) | 24 (75.0) | 30 (49.2) | |
| 2Monotherapy | 3 (3.2) | 2 (6.3) | 1 (1.6) | |
| Radiographic best response | ||||
| CR or PR | 21 (22.6) | 11 (34.4) | 10 (16.4) | 0.157 |
| SD/no CR or no PR | 62 (66.7) | 18 (56.2) | 44 (72.1) | |
| PD | 7 (7.5) | 3 (9.4) | 4 (6.6) | |
| NA | 3 (3.2) | 0 | 3 (4.9) | |
| Radical surgery after systematic treatment | ||||
| Yes | 14 (15.1) | 5 (15.6) | 9 (14.8) | 0.911 |
| No | 79 (84.9) | 27 (84.4) | 52 (85.2) |
In this study, the ORR in the group with OBBT was greater than that in the group without OBBT [34.4% (11/32) vs 16.4% (10/61), P = 0.049]. The DCR was 90.6% in the group with OBBT and 88.5% in the group without OBBT, and this difference was not statistically significant. The rates of radical surgery after systematic therapy were 15.6% in the group with OBBT and 14.8% in the group without OBBT (Table 3).
The most common TRAEs were gastrointestinal disorders (nausea, vomiting, decreased appetite, constipation, and diarrhea) and hematological toxicity (leukopenia/neutropenia and thrombocytopenia). The incidence of all-grade and grade ≥ 3 TRAEs did not differ between the two groups (Table 4).
| Patients with OBBT (n = 32) | Patients without OBBT (n = 61) | |||
| Any grade | Grade ≥ 3 | Any grade | Grade ≥ 3 | |
| Any TRAEs | 32 (100) | 13 (40.6) | 55 (90.2) | 23 (37.7) |
| Leukopenia/Neutropenia | 23 (71.9) | 5 (15.6) | 39 (64.0) | 13 (21.3) |
| Constipation | 18 (56.3) | 0 | 33 (54.1) | 0 |
| Peripheral sensory neuropathy | 17 (53.1) | 1 (3.1) | 30 (49.2) | 3 (4.9) |
| Fatigue | 16 (50.0) | 0 | 30 (49.2) | 0 |
| Decreased appetite | 14 (43.8) | 1 (3.1) | 29 (47.5) | 0 |
| Thrombocytopenia | 13 (40.6) | 6 (18.8) | 16 (26.2) | 2 (3.3) |
| Nausea | 12 (37.5) | 1 (3.1) | 27 (44.3) | 4 (6.5) |
| Vomiting | 4 (12.5) | 2 (6.3) | 13 (21.3) | 4 (6.5) |
| Diarrhea | 4 (12.5) | 2 (6.3) | 14 (23.0) | 5 (8.2) |
| Infection | 3 (9.4) | 0 | 5 (8.2) | 0 |
| Oral mucosal ulcer | 2 (6.3) | 0 | 6 (9.8) | 2 (3.3) |
| Febrile neutropenia | 1 (3.1) | 1 (3.1) | 6 (9.8) | 6 (9.8) |
| Liver injury | 1 (3.1) | 0 | 4 (6.5) | 1 (1.6) |
In the matched population, 17.2% (16/93) of patients experienced OBAT, including ten patients with OBBT and six without OBBT. χ2 tests (Table 5) revealed that OBBT [62.5% (10/16) vs 28.6% (22/77), P = 0.009] was a risk factor for OBAT. After adjusting for factors whose P value was less than 0.25, including drinking history, chronic disease, primary tumor location, OBBT status, and radiographic best response to systematic therapy, logistic regression analysis revealed that drinking history (P = 0.027), tumors located in the body (P = 0.028), presence of OBBT (P = 0.006) and radiographic best response PD (P = 0.038) were independent, positive influencing factors for OBAT (Table 5).
| Characteristics | χ2 test | Binary logistic analysis | |||
| Patients with OBAT (n = 16) | Patients without OBAT (n = 77) | P value | OR (95%CI) | P value | |
| Gender | |||||
| Male | 12 (75.0) | 50 (64.9) | 0.565 | ||
| Female | 4 (25.0) | 27 (35.1) | |||
| Age (year) | |||||
| < 65 | 7 (43.8) | 35 (45.5) | 0.901 | ||
| ≥ 65 | 9 (56.3) | 42 (54.5) | |||
| Smoking history | |||||
| No | 12 (75.0) | 47 (61.0) | 0.396 | ||
| Yes | 4 (25.0) | 30 (39.0) | |||
| Drinking history | |||||
| No | 12 (75.0) | 68 (88.3) | 0.228 | Reference | |
| Yes | 4 (25.0) | 9 (11.7) | 8.512 (1.279-56.655) | 0.027 | |
| 1Chronic disease | |||||
| No | 7 (43.8) | 48 (62.3) | 0.169 | Reference | |
| Yes | 9 (56.3) | 29 (37.7) | 3.929 (0.974-15.855) | 0.055 | |
| ECOG-PS | |||||
| 0 or 1 | 14 (87.5) | 72 (93.5) | 0.346 | ||
| 2 | 2 (12.5) | 5 (6.5) | |||
| BMI (kg/m2) | |||||
| ≤ 18.4 | 2 (12.5) | 9 (11.7) | 0.668 | ||
| 18.5-23.9 | 8 (50.0) | 47 (61.0) | |||
| ≥ 24.0 | 6 (37.5) | 21 (27.3) | |||
| Weight loss | |||||
| < 5% | 8 (50.0) | 31 (40.3) | 0.472 | ||
| ≥ 5% | 8 (50.0) | 46 (59.7) | |||
| Primary tumor location | |||||
| Pylorus | 2 (12.5) | 23 (29.9) | 0.168 | Reference | |
| Body | 10 (62.5) | 28 (36.4) | 10.104 (1.287-79.302) | 0.028 | |
| Cardia | 4 (25.0) | 26 (33.8) | 1.773 (0.209-15.072) | 0.600 | |
| Cancer stage (AJCC 8th) | |||||
| Stage III | 4 (25.0) | 15 (19.5) | 0.734 | ||
| Stage IV | 12 (75.0) | 62 (80.5) | |||
| OBBT status | |||||
| No | 6 (37.5) | 55 (71.4) | 0.009 | Reference | |
| Yes | 10 (62.5) | 22 (28.6) | 7.015 (1.726-28.505) | 0.006 | |
| Therapeutic regimen | |||||
| Chemotriplet regimen | 5 (31.3) | 31 (40.3) | 0.761 | ||
| 2Chemodouble- based regimen | 11 (68.8) | 43 (55.8) | |||
| 3Monotherapy | 0 | 3 (3.9) | |||
| Grade 3/4 TRAEs | |||||
| No | 10 (62.5) | 47 (61.0) | 0.913 | ||
| Yes | 6 (37.5) | 30 (39.0) | |||
| Radiographic best response | |||||
| CR or PR | 2 (12.5) | 19 (24.7) | 0.149 | Reference | |
| SD/no CR or no PR | 10 (62.5) | 52 (67.5) | 2.763 (0.462-16.504) | 0.265 | |
| PD | 3 (18.8) | 4 (5.2) | 14.039 (1.158-170.260) | 0.038 | |
| NA | 1 (6.3) | 2 (2.6) | 32.043 (1.248-822.685) | 0.038 | |
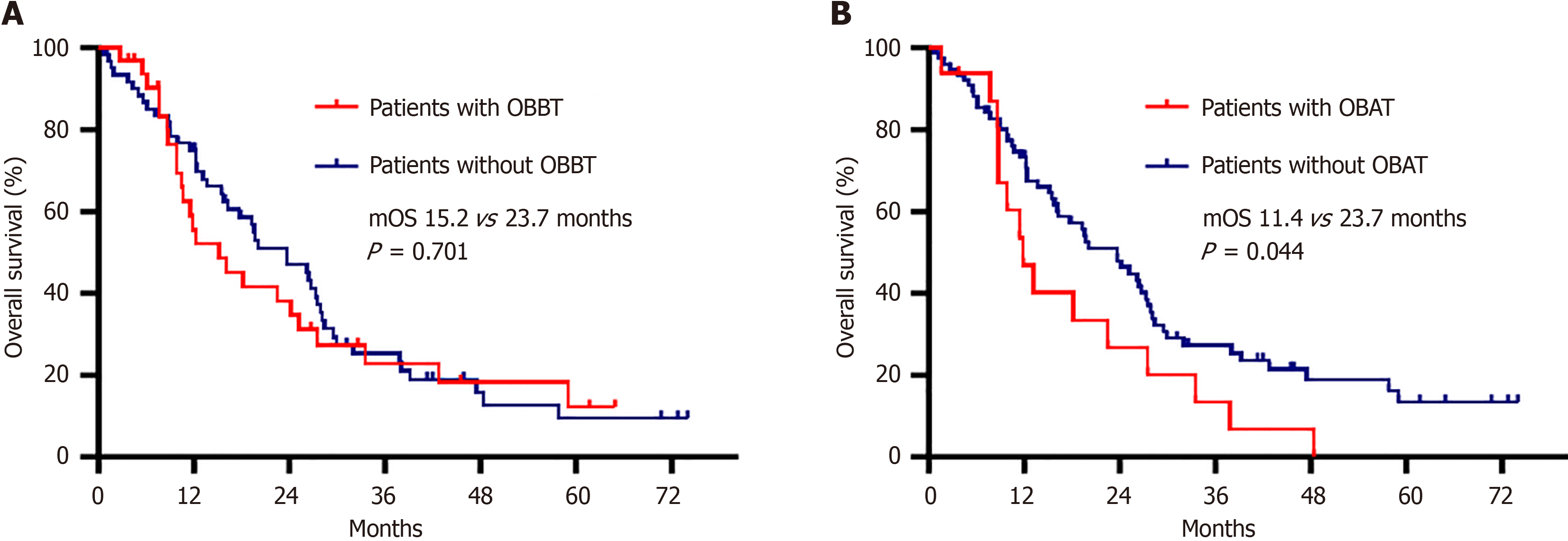
As of June 20, 2024, 71 (76.3%) deaths had occurred, and four patients were lost to follow-up. The mOS was 15.2 months for patients with OBBT and 23.7 months for those without OBBT (HR = 1.101, 95%CI: 0.672-1.804, P = 0.701) (Figure 2A). The mOS was worse for the GC/GEJC patients with OBAT than for those without OBAT (11.4 months vs 23.7 months, P = 0.044) (Figure 2B), and the HR for death in the group with OBAT, as compared with the group without OBAT, was 1.787 (95%CI: 1.006-3.175).
OB in patients with advanced or metastatic GC/GEJC patients is a severe and potentially dangerous event that may affect anticancer treatment. Although many studies have reported several treatment modalities for GC/GEJC with OB, the analysis of chemotherapy efficacy and long-term survival for the patients still needs to be met. Our study showed that the safety of chemotherapy for hemostatic GC/GEJC patients was similar to that of patients without OB. Controlled bleeding after chemotherapy did not affect survival in patients with OBBT, but bleeding or rebleeding after treatment decreased the survival.
Our research revealed that the occurrence of TRAEs was similar to that reported in previous studies[16,17], and the incidence of all grade and grade 3-4 TRAEs in the group with OBBT was not greater than that in the group without OBBT, which was better than expected. Most patients with OBBT received intravenous injection treatment with a chemodoublet regimen. For the chemodoublet regimen strategy, the incidence of Grade3-4 TRAEs was lower than that for the chemo
The mOS of patients with OB in the present study was longer than that of patients who received hemostatic strategies such as endoscopic hemostasis, transcatheter embolotherapy, and radiotherapy in several other studies[3,4,10,21]. On the one hand, systematic therapy may be more effective than local therapy for advanced or metastatic GC/GEJC. On the other hand, this study enrolled patients receiving systematic therapy who were usually hemostatic or hemodynamically stable and had a better performance status.
Neoplasms usually result in bleeding from diffuse mucosal erosion or ulceration or from cancer invasion into under
Prior studies has shown that an ECOG-PS ≤ 1 was associated with better chemotherapy tolerance than an ECOG-PS = 2[25]. The ECOG-PS of most patients was 0 or 1 in this study, and only a few patients had an ECOG-PS = 2. However, the rate of ECOG-PS = 2 was significantly greater in the group with OBBT than in the group without OBBT (19.5% vs 4.6%, P = 0.010) before PSM. To reduce the bias of the ECOG-PS on TRAEs in this study, we added the ECOG-PS score as a matc
The rebleeding rate was 31.3% (10/32) for patients with OBBT in the current study, similar to other studies[3,10]. The bleeding rate varied across different primary tumor locations in the study. A previous study reported similar phenomena[2]. The difference could be related to unequal erosion caused by the acidic medium of the stomach, which was different in each stomach part. Because all patients in this study were at AJCC stage III-IV (T3-T4 and N +), we did not analyze the relationships between the bleeding rate and the T, N or tumor stage categories.
The occurrence of OBAT in patients with OBBT was greater than that in patients without OBBT [31.3% (10/32) vs 9.8% (6/61), P = 0.009]. Although OBBT was a risk factor for OBAT, only 31.3% of patients with OBBT experienced OBAT, and most patients with OBBT achieved hemostasis after systematic therapy. This may explain why OBAT was associated with shorter survival, whereas OBBT did not affect the mOS.
There were several shortcomings in this study. First, it was retrospective, and there may have been omissions in data collection and selection bias. Second, the study was conducted at a single center and the sample size was small. Finally, the various regimens for the patients may have led to analytical bias with respect to TRAEs and survival. Ultimately, we look forward to conducting a prospective study in GC patients with OB to identify more effective treatments.
In conclusion, this study revealed that the mOS for GC/GEJC patients who received systematic treatment with OBBT was similar to that for those without OBBT, but the mOS for GC/GEJC patients with OBAT was worse than that for GC/GEJC patients without OBAT. The DCR and TRAEs were similar for patients with and without OBBT. Therefore, we should actively control bleeding and choose appropriate treatment plans for GC/GEJC patients with OBBT. It is also essential to prevent bleeding in GC/GEJC patients after systematic treatment in order to prolong their survival.
We thank the patients and their families, all the clinicians and nurses at the Department of Medical Oncology and Ra
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64681] [Article Influence: 16170.3] [Reference Citation Analysis (177)] |
| 2. | Minhem MA, Nakshabandi A, Mirza R, Alsamman MA, Mattar MC. Gastrointestinal hemorrhage in the setting of gastrointestinal cancer: Anatomical prevalence, predictors, and interventions. World J Gastrointest Endosc. 2021;13:391-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (7)] |
| 3. | Song IJ, Kim HJ, Lee JA, Park JC, Shin SK, Lee SK, Lee YC, Chung H. Clinical Outcomes of Endoscopic Hemostasis for Bleeding in Patients with Unresectable Advanced Gastric Cancer. J Gastric Cancer. 2017;17:374-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Takeda K, Sakayauchi T, Kubozono M, Katagiri Y, Umezawa R, Yamamoto T, Ishikawa Y, Takahashi N, Suzuki Y, Kishida K, Jingu K. Palliative radiotherapy for gastric cancer bleeding: a multi-institutional retrospective study. BMC Palliat Care. 2022;21:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 5. | Bosch FTM, Mulder FI, Huisman MV, Zwicker JI, Di Nisio M, Carrier M, Segers A, Verhamme P, Middeldorp S, Weitz JI, Grosso MA, Duggal A, Büller HR, Wang TF, Garcia D, Kamphuisen PW, Raskob GE, van Es N. Risk factors for gastrointestinal bleeding in patients with gastrointestinal cancer using edoxaban. J Thromb Haemost. 2021;19:3008-3017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Pucheanu X, Beuran M. Bleeding gastric cancer in young and elderly patients. J Med Life. 2015;8:356-360. [PubMed] |
| 7. | Wang L, Wang XA, Hao JQ, Zhang LN, Li ML, Wu XS, Weng H, Lv WJ, Zhang WJ, Chen L, Xiang HG, Lu JH, Liu YB, Dong P. Long-term outcomes after radical gastrectomy in gastric cancer patients with overt bleeding. World J Gastroenterol. 2015;21:13316-13324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 961] [Article Influence: 320.3] [Reference Citation Analysis (0)] |
| 9. | Kim YI, Choi IJ. Endoscopic management of tumor bleeding from inoperable gastric cancer. Clin Endosc. 2015;48:121-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Kawabata H, Hitomi M, Motoi S. Management of Bleeding from Unresectable Gastric Cancer. Biomedicines. 2019;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Meehan T, Stecker MS, Kalva SP, Oklu R, Walker TG, Ganguli S. Outcomes of transcatheter arterial embolization for acute hemorrhage originating from gastric adenocarcinoma. J Vasc Interv Radiol. 2014;25:847-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Lee J, Byun HK, Koom WS, Lee YC, Seong J. Efficacy of radiotherapy for gastric bleeding associated with advanced gastric cancer. Radiat Oncol. 2021;16:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Tanaka O, Sugiyama A, Omatsu T, Tawada M, Makita C, Matsuo M. Hemostatic radiotherapy for inoperable gastric cancer: a pilot study. Br J Radiol. 2020;93:20190958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9:373-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 893] [Article Influence: 223.3] [Reference Citation Analysis (0)] |
| 15. | Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2908] [Cited by in RCA: 3842] [Article Influence: 274.4] [Reference Citation Analysis (0)] |
| 16. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5328] [Article Influence: 355.2] [Reference Citation Analysis (3)] |
| 17. | Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 1902] [Article Influence: 475.5] [Reference Citation Analysis (1)] |
| 18. | Salehifar E, Avan R, Janbabaei G, Mousavi SK, Faramarzi F. Comparison the Incidence and Severity of Side Effects Profile Of FOLFOX and DCF Regimens in Gastric Cancer Patients. Iran J Pharm Res. 2019;18:1032-1039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Hacibekiroglu I, Kodaz H, Erdogan B, Turkmen E, Esenkaya A, Onal Y, Uzunoglu S, Cicin I. Comparative analysis of the efficacy and safety of modified FOLFOX-6 and DCF regimens as first-line treatment in advanced gastric cancer. Mol Clin Oncol. 2015;3:1160-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Rothenberg ML, Cox JV, Butts C, Navarro M, Bang YJ, Goel R, Gollins S, Siu LL, Laguerre S, Cunningham D. Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/folinic acid plus oxaliplatin (FOLFOX-4) as second-line therapy in metastatic colorectal cancer: a randomized phase III noninferiority study. Ann Oncol. 2008;19:1720-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Hashimoto K, Mayahara H, Takashima A, Nakajima TE, Kato K, Hamaguchi T, Ito Y, Yamada Y, Kagami Y, Itami J, Shimada Y. Palliative radiation therapy for hemorrhage of unresectable gastric cancer: a single institute experience. J Cancer Res Clin Oncol. 2009;135:1117-1123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Laszkowska M, Rodriguez S, Kim J, Hur C. Heavy Alcohol Use Is Associated With Gastric Cancer: Analysis of the National Health and Nutrition Examination Survey From 1999 to 2010. Am J Gastroenterol. 2021;116:1083-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | He Z, Zhao TT, Xu HM, Wang ZN, Xu YY, Song YX, Ni ZR, Xu H, Yin SC, Liu XY, Miao ZF. Association between alcohol consumption and the risk of gastric cancer: a meta-analysis of prospective cohort studies. Oncotarget. 2017;8:84459-84472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Han X, Xiao L, Yu Y, Chen Y, Shu HH. Alcohol consumption and gastric cancer risk: a meta-analysis of prospective cohort studies. Oncotarget. 2017;8:83237-83245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Xu Y, Ding L, Zhang YQ. Predictors of chemotherapy tolerance and survival benefit in a geriatric patient population with advanced solid tumors. Indian J Cancer. 2021;58:583-589. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |