Published online Sep 24, 2024. doi: 10.5306/wjco.v15.i9.1168
Revised: July 14, 2024
Accepted: August 2, 2024
Published online: September 24, 2024
Processing time: 105 Days and 5.6 Hours
Homeobox (HOX) C9, a member of the HOX family, is an important transcription factor, and it plays a significant role in various biological processes. This family of genes is highly valued for their essential roles in establishing and maintaining the body axis during embryonic development and adult tissues. Further, HOXC9 plays a central role in neuronal differentiation, angiogenesis, and adipose distribution, which are essential for the development of the nervous system, matu
Core Tip: This paper discusses the association between abnormal homeobox (HOX) C9 expression and the occurrence and progression of various tumors, the impact of HOXC9 on the clinical pathological characteristics and prognosis of cancer patients, the role of HOXC9 in central nervous system tumors, breast cancer, bladder cancer, gastric cancer, colon cancer, non-small cell lung cancer, and thyroid papillary carcinoma, and the molecular mechanisms underlying the biological functions of HOXC9, including its interaction with other proteins and its regulation through DNA methylation.
- Citation: Zhang Y, Li J. Recent advancements in understanding of biological role of homeobox C9 in human cancers. World J Clin Oncol 2024; 15(9): 1168-1176
- URL: https://www.wjgnet.com/2218-4333/full/v15/i9/1168.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i9.1168
The homeobox (HOX) genes were first discovered in Drosophila melanogaster, where they are referred to as the HOM-C genes and are part of the Hox gene family[1-6]. In humans, the Hox genes are categorized into two subfamilies. The first subfamily, referred to as the anterior-posterior (A-P) type, is clustered in chromosomes and expressed in an A-P manner. The second subfamily comprises the non-A-P-type HOX genes, which are dispersed across different chromosomes. Based on sequence similarity, these genes form distinct groups including the Emx, Pax, Msx, and Otx families of the HOX genes[1].
Structurally, the HOX genes form a substantial part of the HOX gene family. These genes have a common 180-bp sequence referred to as the HOX, which encodes a conserved 60 amino acid region known as the homeodomain[7,8]. Functionally, proteins derived from the HOX genes can be transcription factors that, together with upstream signaling molecules and downstream target genes, contribute to complex regulatory networks via positive and negative feedback loops. Moreover, these proteins can interact with other transcription factors, playing roles in morphogenesis, cell adhesion and migration, and cell cycle regulation[9].
Previous studies have revealed that HOXC9 is a pivotal transcription factor in embryonic development. That is, it plays roles in essential biological processes such as cell cycle, differentiation, and apoptosis[10-14]. Some studies have revealed dysregulation in HOXC9 expression in various malignant tumors. Thus, HOXC9 has both oncogenic and tumor-suppressive effects[14-19]. Further, its expression is closely associated with the clinicopathological characteristics and prognosis of patients with cancer.
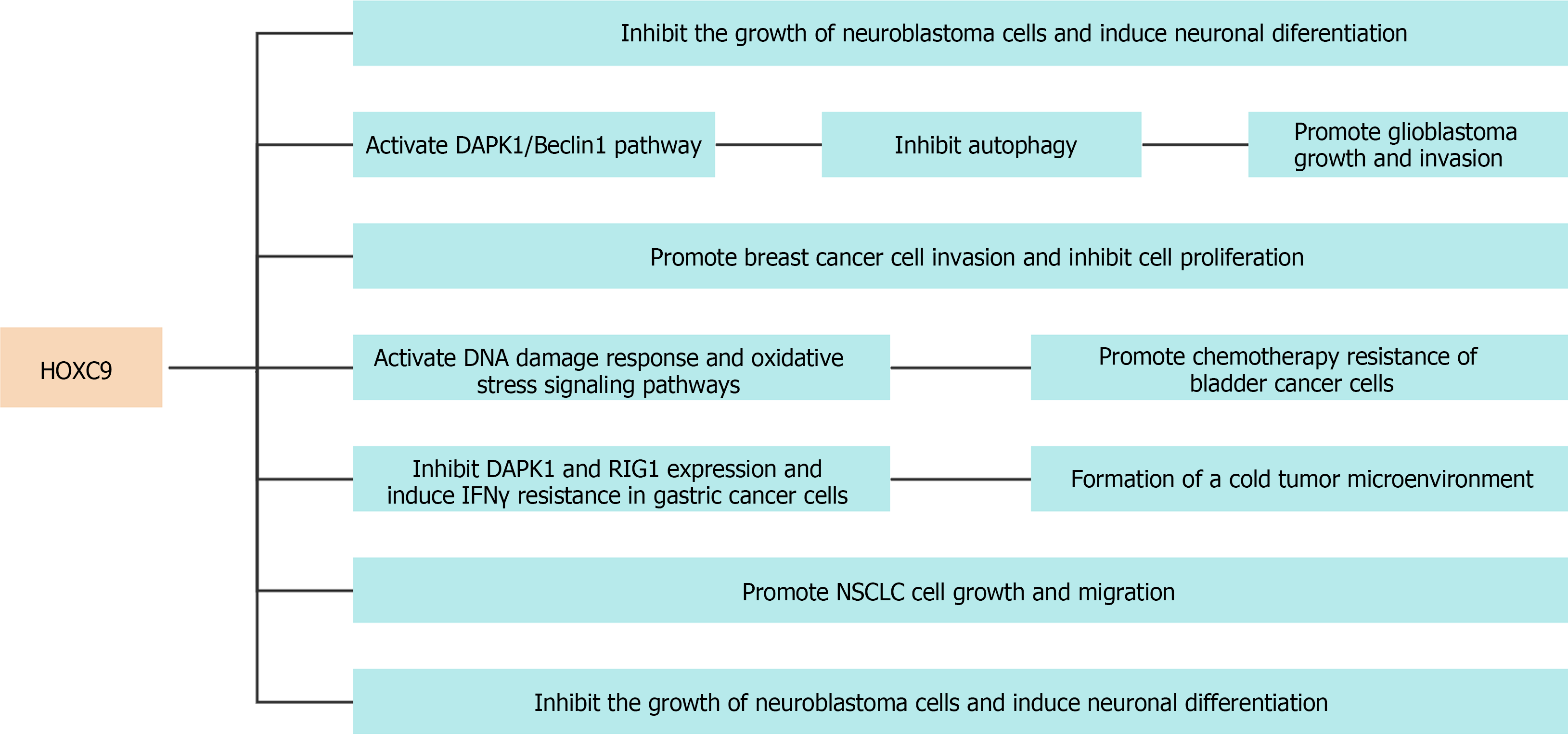
Neuroblastoma (NB) is an embryonic tumor of the sympathetic nervous system that originates from primitive neural crest cells and develops in infants and young children[20-24]. The inconspicuous onset and rapid progression of the tumor often lead to unsatisfactory clinical treatment outcomes, resulting in a poor prognosis in children with NB[25-28]. In addition, NB cells can differentiate to a sympathetic ganglion cell phenotype, indicating a disruption in the physiological molecular program governing neuroblast differentiation and growth control. In advanced-stage NBs, Mao et al[14] reported reduced HOXC9 expression, which is involved in cell cycle control and NB cell differentiation[14].
The differentiation state has a significant impact on the clinical outcomes of NB, with induced differentiation being utilized as a therapeutic method. An elevated HOXC9 expression level is associated with NB differentiation and thus a promising prognosis[19,29-33]. Growth arrest and neuronal differentiation are enhanced by HOXC9 by regulating genes associated with cell cycle progression and neuronal differentiation. Retinoic acid (RA) upregulates HOXC9 expression, and its downregulation results in resistance to RA-induced growth arrest and differentiation[34]. In addition, HOXC9 expression is epigenetically silenced in RA-resistant cells, and its overexpression inhibits cell proliferation and tumorigenesis[34]. HOXC9 expression was significantly decreased in the RA-resistant NB cell line SK-N-AS. Conversely, arsenic trioxide led to an increase in the quantity of neuronal synapses in SK-N-AS cells, thereby upregulating HOXC9 and HOXD8 levels while simultaneously downregulating PHOX2B and EZH2 levels[34].
Glioblastoma multiforme (GM), an exceedingly malignant primary neoplasm of the brain, is associated with a poor prognosis characterized by a 5-year survival rate of 5.8%[35-40]. High expression of HOXC9 in GM is a predictor of poor prognosis. Meanwhile, silencing HOXC9 inhibits the growth and invasion of GM cells. Mechanistically, HOXC9 directly inhibits DAPK1 gene transcription, activates the DAPK1/Beclin1 signaling pathway, and suppresses autophagy[18].
Breast cancer (BRCA) accounted for 24.2% of all female cancer cases in 2018. Therefore, it is an evident malignant neoplasm on a global scale[41-45]. Despite notable advancements in the diagnosis and management of patients aimed at improving both their quality of life and overall survival (OS), there are persistent obstacles in the form of tumor relapse and distant spread of cancer cells, which ultimately lead to an increased mortality rate[46-48]. Hur et al[15] investigated differences in HOXC9 expression between BRCA and normal tissues by utilizing publicly available databases. They evaluated the association between HOXC9 Levels and both disease- and distant metastasis-free survival in patients with BRCA[15]. The results indicated increased HOXC9 expression in BRCA tissues, which was associated with a negative prognosis in patients with lymph node metastasis. Subsequent in vitro studies revealed that the upregulation of HOXC9 in BT474 and MCF7 BRCA cell lines enhanced cell invasion while inhibiting cell proliferation. This suggests that HOXC9 has a potential role in driving the phenotypic transition of BRCA cells from a proliferative to an invasive state.
Bladder cancer (BLCA), a prevalent urinary system malignancy, is the fifth most common cancer among men in developed countries[49-51]. Surgical intervention can effectively treat BLCA in its initial stages. Nevertheless, individuals diagnosed with late-stage BLCA who are not suitable candidates for surgery have a worse prognosis[52-56]. Moreover, chemoresistance poses a substantial challenge for cancer researchers and clinicians, contributing to chemotherapy failure in patients with advanced-stage BLCA[57-60]. miR-193a-3p significantly contributes to promoting multiple chemoresistance in BLCA. Lv et al[61] showed that the HOXC9 gene is targeted directly by miR-193a-3p. The suppression of HOXC9 expression can possibly trigger the activation of DNA damage response and oxidative stress signaling pathways, thereby promoting resistance in cancer cells against chemotherapy[61].
Gastric cancer (GC), a common malignant neoplasm of the gastrointestinal system, is associated with factors including Helicobacter pylori infection, gastroesophageal reflux disease, and Barrett’s esophagus, which can increase susceptibility[41,62-65]. Diagnosing early-stage GC poses a significant challenge, frequently leading to delayed-stage diagnoses and an unfavorable prognosis. Previous studies have revealed that HOXC9 is overexpressed in GC tissues, thereby inhibiting immune response and the interferon gamma signaling pathway[66]. Mechanically, it elicits resistance to interferon gamma in GC cells by inhibiting DAPK1 and RIG1 expression, leading to the establishment of a tumor microenvironment characterized by immunological unresponsiveness. In addition, HOXC9 downregulation might be a potential indicator for the efficacy of programmed cell death protein 1 blockade in treating patients with GC.
In GC, HOXC9 expression is significantly correlated with the tumor metastasis ability and stem cell-like characteristics[17]. Previous studies have shown that HOXC9 can be a direct target of miR-26a[17]. The downregulation of miR-26a expression leads to the upregulation of HOXC9. Meanwhile, the restoration of miR-26a expression not only downregulates HOXC9 but also reverses the promoting effects of HOXC9 on metastasis and the stem cell-like phenotypes of GC cells.
Epidemiological investigations have revealed an increasing incidence of colorectal carcinoma in elderly individuals, particularly those aged ≥ 50 years[67-70]. The various risk factors associated with colon cancer include colon polyps, chronic colitis, and obesity[71]. The overexpression of HOXC9 in colon cancer tissues is strongly correlated with unfavorable clinical characteristics such as tumor-node-metastasis stage, distant metastasis, and venous invasion. Kaplan-Meier curve analysis showed that elevated HOXC9 levels are indicative of a worse OS and progression-free survival[72]. Further, gene set enrichment analysis revealed that HOXC9 overexpression in colon cancer significantly enriches pathways related to natural killer cell-mediated cytotoxicity, cell adhesion molecules, innate immune system, and interactions involving cytokine receptors.
Lung cancer is still a prominent contributor to cancer-related mortality worldwide, and non-small-cell lung cancer (NSCLC) accounts for approximately 85% of the cases[73-76]. Notably, 75% of diagnoses are made during advanced stages, consequently leading to a reduced 5-year survival rate[77,78]. In lung adenocarcinoma, HOXC9 overexpression is significantly correlated with OS and disease-free survival[79]. Bi et al[80] revealed that the circular RNA Hsa_circ_0020123 enhances the development of NSCLC via its function as a sponge for miR-495. In addition, upregulation of HOXC9 negates the suppressive effects on NSCLC cell proliferation and movement caused by miR-495[80]. Further, the methylation frequency of the HOXC9 gene is significantly higher in stage I NSCLC tissues than in adjacent normal tissues[81].
Thyroid papillary carcinoma (TPC) is a relatively prevalent type of thyroid malignancy, predominantly affecting women aged 30-45 years[82-85]. The clinical remission rate is significantly high, which is associated with a favorable prognosis[86]. Previous studies have revealed a decrease in HOXC9 expression levels within TPC, exhibiting considerable variations in expression compared with that in adjacent normal tissues. Moreover, HOXC9 downregulation is associated with Hashimoto’s thyroiditis and lymph node metastasis, thereby indicating its potential utility as a diagnostic and prognostic indicator of TPC[87].
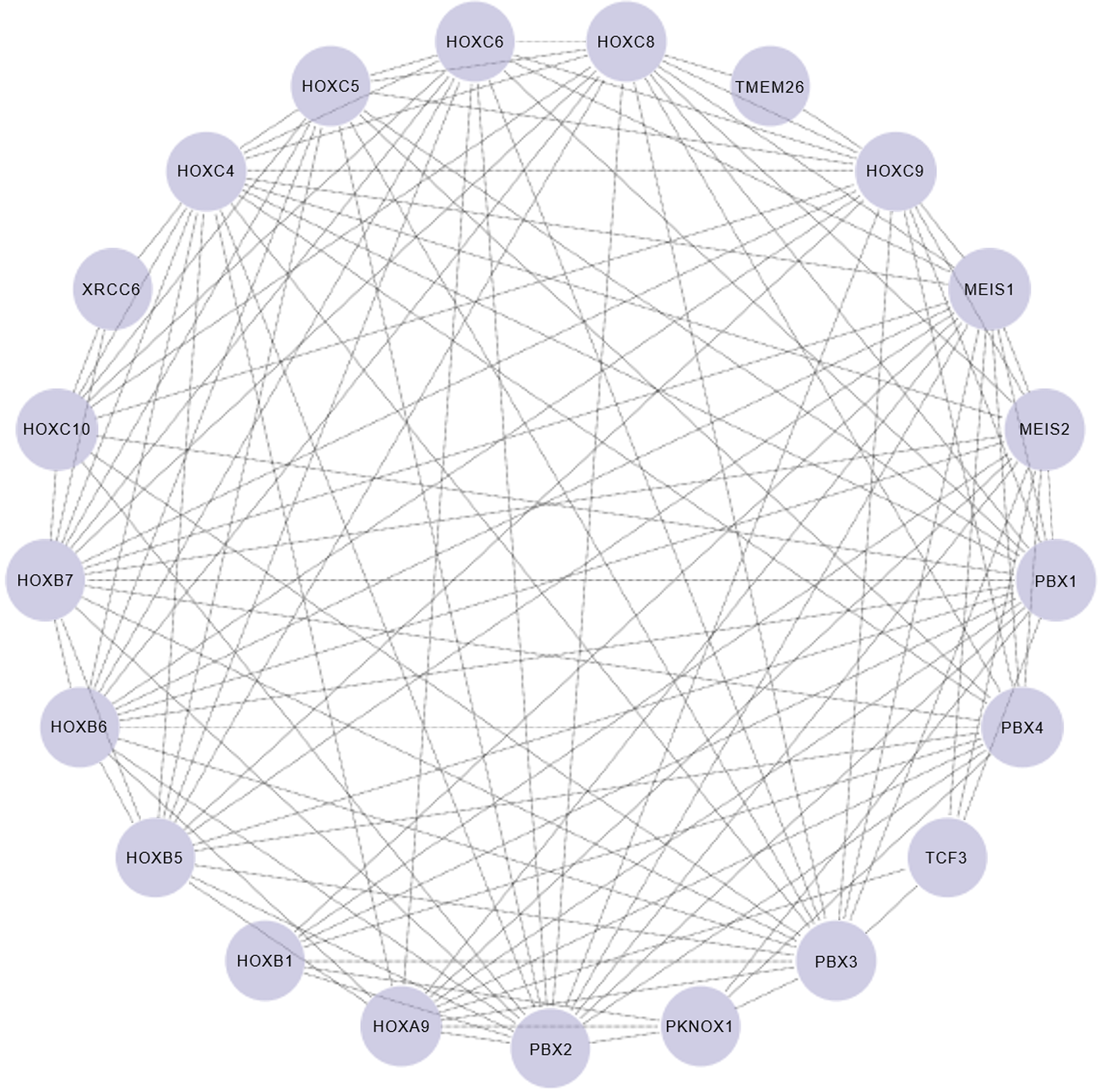
HOXC9, a pivotal embryonic development gene, effectively participates in different morphogenetic mechanisms including neuronal differentiation, vascular growth, and adipose tissue distribution[11-14] (Figure 1). Wang et al[10] performed a comprehensive analysis of the genome-wide impact of HOXC9 on neuronal differentiation[10], and identified an intricate network involving the transcriptional regulation of 2370 genes primarily associated with neuronal differentiation, cell cycle progression, and DNA damage response. Notably, HOXC9 was found to interact with the transcriptional repressor E2F6, thereby orchestrating its recruitment to cell cycle gene promoters to repress their expression. Figure 2 shows the protein-protein interaction network of HOXC9 based on data from the String database[88].
Previous studies have revealed the essential role of HOXC9 in regulating vascular morphology. HOXC9 overexpression or interleukin-8 knockout in zebrafish resulted in aberrant vascular development, marked by the loss or incompleteness of the dorsal aorta and intersegmental vessels[13]. This emphasizes the significance of HOXC9 as a transcriptional factor that promotes the quiescence of endothelial cells while suppressing angiogenesis, thereby influencing vascular morphogenesis via an interleukin-8-mediated process[13]. Moreover, HOXC9 plays an important role as a stimulator of Stab2, collectively contributing to the development of the thoracic duct. In vitro studies have validated the regulatory influence of Stab2 on endothelial cell movement and angiogenesis without affecting cell death.
Brune et al[89] performed an examination of HOXC9 mRNA expression in abdominal subcutaneous and omental fat tissues collected from 636 individuals. The results showed significantly elevated HOXC9 mRNA expression in the subcutaneous fat tissues compared with that in the omental fat tissues[89]. Moreover, in the subcutaneous adipose tissues, HOXC9 mRNA expression was significantly negatively correlated with adipocyte volume, body mass, and fasting plasma insulin levels. Further studies showed that the HOXC9 expression in the cells of the stromal vascular fraction was higher than that in adipocytes. Thus, HOXC9 is an important developmental gene that regulates fat distribution.
DNA methylation is a common epigenetic modification that is typically associated with the suppression of gene expression[90-92]. Lin et al[81] revealed that the HOXC9 gene is more frequently methylated in stage I NSCLC tissues than in adjacent normal tissues[81]. The methylation status of HOXC9 is correlated with specific types of cancer and clinical characteristics. Thus, it can be a biomarker for the diagnosis, prognosis, and prediction of response to immunotherapy. Therefore, the association between HOXC9 and methylation emphasizes the significant role of epigenetic regulation in gene expression, cell development, and disease development.
In addition, the biological effects of mutations in the HOXC9 gene are based on the type of mutation and its location within the gene. Suemori et al[93] presented mutations at the HOXC9 gene locus in mice using gene targeting techniques, with an aim to elucidate the function of the HOXC9 gene[93]. The results revealed that the HOXC9 gene determines the anterior-posterior axis of the body by regulating the formation of various body parts. Further, the HOXC9 gene can function by repressing HOXC8 gene expression. This cross-regulatory mechanism is conserved among multiple species and is significantly important in understanding the establishment of the body axis in vertebrates. From a disease perspective, mutations in the HOXC9 gene are associated with various developmental abnormalities and diseases, including congenital malformations and developmental delays. Therefore, understanding the normal function of the HOXC9 gene and the effects of its mutations is important for not only facilitating fundamental biological research but also identifying potential therapeutic methods and intervention strategies.
The HOXC9 gene plays an important role in embryonic development and cell differentiation, with recent research emphasizing its dual roles across various tumors. In NB, HOXC9 inhibits the cell cycle and promotes neuronal differentiation, and its elevated expression is significantly correlated with a better prognosis. Conversely, in BRCA, TPC, BLCA, GC, NSCLC, GM, and colon cancer, it functions as an oncogenic factor, and HOXC9 overexpression is significantly associated with a worse prognosis. In addition, DNA methylation is closely related to HOXC9 expression and can be a biomarker for the early diagnosis and prognosis of NSCLC. Elucidating the molecular mechanism of the HOXC9 gene in malignant tumors may provide a novel reference for targeted therapy in patients with malignant tumors.
| 1. | Duboule D. The vertebrate limb: a model system to study the Hox/HOM gene network during development and evolution. Bioessays. 1992;14:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 120] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Ponrathnam T, Saini R, Banu S, Mishra RK. Drosophila Hox genes induce melanized pseudo-tumors when misexpressed in hemocytes. Sci Rep. 2021;11:1838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Hubert KA, Wellik DM. Hox genes in development and beyond. Development. 2023;150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 46] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 4. | Joshi R, Sipani R, Bakshi A. Roles of Drosophila Hox Genes in the Assembly of Neuromuscular Networks and Behavior. Front Cell Dev Biol. 2021;9:786993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Buffry AD, McGregor AP. Micromanagement of Drosophila Post-Embryonic Development by Hox Genes. J Dev Biol. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Feng Y, Zhang T, Wang Y, Xie M, Ji X, Luo X, Huang W, Xia L. Homeobox Genes in Cancers: From Carcinogenesis to Recent Therapeutic Intervention. Front Oncol. 2021;11:770428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 7. | Levine M, Hoey T. Homeobox proteins as sequence-specific transcription factors. Cell. 1988;55:537-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 296] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1984] [Cited by in RCA: 1979] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 9. | Svingen T, Tonissen KF. Hox transcription factors and their elusive mammalian gene targets. Heredity (Edinb). 2006;97:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Wang X, Choi JH, Ding J, Yang L, Ngoka LC, Lee EJ, Zha Y, Mao L, Jin B, Ren M, Cowell J, Huang S, Shi H, Cui H, Ding HF. HOXC9 directly regulates distinct sets of genes to coordinate diverse cellular processes during neuronal differentiation. BMC Genomics. 2013;14:830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Stoll SJ, Bartsch S, Kroll J. HOXC9 regulates formation of parachordal lymphangioplasts and the thoracic duct in zebrafish via stabilin 2. PLoS One. 2013;8:e58311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Stoll SJ, Kroll J. HOXC9: a key regulator of endothelial cell quiescence and vascular morphogenesis. Trends Cardiovasc Med. 2012;22:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Stoll SJ, Bartsch S, Augustin HG, Kroll J. The transcription factor HOXC9 regulates endothelial cell quiescence and vascular morphogenesis in zebrafish via inhibition of interleukin 8. Circ Res. 2011;108:1367-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Mao L, Ding J, Zha Y, Yang L, McCarthy BA, King W, Cui H, Ding HF. HOXC9 links cell-cycle exit and neuronal differentiation and is a prognostic marker in neuroblastoma. Cancer Res. 2011;71:4314-4324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Hur H, Lee JY, Yang S, Kim JM, Park AE, Kim MH. HOXC9 Induces Phenotypic Switching between Proliferation and Invasion in Breast Cancer Cells. J Cancer. 2016;7:768-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Zhao XF, Yang YS, Park YK. HOXC9 overexpression is associated with gastric cancer progression and a prognostic marker for poor survival in gastric cancer patients. Int J Clin Oncol. 2020;25:2044-2054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Peng X, Kang Q, Wan R, Wang Z. miR-26a/HOXC9 Dysregulation Promotes Metastasis and Stem Cell-Like Phenotype of Gastric Cancer. Cell Physiol Biochem. 2018;49:1659-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Xuan F, Huang M, Liu W, Ding H, Yang L, Cui H. Homeobox C9 suppresses Beclin1-mediated autophagy in glioblastoma by directly inhibiting the transcription of death-associated protein kinase 1. Neuro Oncol. 2016;18:819-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Kocak H, Ackermann S, Hero B, Kahlert Y, Oberthuer A, Juraeva D, Roels F, Theissen J, Westermann F, Deubzer H, Ehemann V, Brors B, Odenthal M, Berthold F, Fischer M. Hox-C9 activates the intrinsic pathway of apoptosis and is associated with spontaneous regression in neuroblastoma. Cell Death Dis. 2013;4:e586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 20. | Morozova O, Vojvodic M, Grinshtein N, Hansford LM, Blakely KM, Maslova A, Hirst M, Cezard T, Morin RD, Moore R, Smith KM, Miller F, Taylor P, Thiessen N, Varhol R, Zhao Y, Jones S, Moffat J, Kislinger T, Moran MF, Kaplan DR, Marra MA. System-level analysis of neuroblastoma tumor-initiating cells implicates AURKB as a novel drug target for neuroblastoma. Clin Cancer Res. 2010;16:4572-4582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Ben Amar D, Thoinet K, Villalard B, Imbaud O, Costechareyre C, Jarrosson L, Reynaud F, Novion Ducassou J, Couté Y, Brunet JF, Combaret V, Corradini N, Delloye-Bourgeois C, Castellani V. Environmental cues from neural crest derivatives act as metastatic triggers in an embryonic neuroblastoma model. Nat Commun. 2022;13:2549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Ponzoni M, Bachetti T, Corrias MV, Brignole C, Pastorino F, Calarco E, Bensa V, Giusto E, Ceccherini I, Perri P. Recent advances in the developmental origin of neuroblastoma: an overview. J Exp Clin Cancer Res. 2022;41:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 103] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 23. | Jansky S, Sharma AK, Körber V, Quintero A, Toprak UH, Wecht EM, Gartlgruber M, Greco A, Chomsky E, Grünewald TGP, Henrich KO, Tanay A, Herrmann C, Höfer T, Westermann F. Single-cell transcriptomic analyses provide insights into the developmental origins of neuroblastoma. Nat Genet. 2021;53:683-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 173] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 24. | Mei S, Alchahin AM, Embaie BT, Gavriliuc IM, Verhoeven BM, Zhao T, Li X, Jeffries NE, Pepich A, Sarkar H, Olsen TK, Wickström M, Stenman J, Reina-Bedoya O, Kharchenko PV, Saylor PJ, Johnsen JI, Sykes DB, Kogner P, Baryawno N. Single-cell analyses of metastatic bone marrow in human neuroblastoma reveals microenvironmental remodeling and metastatic signature. JCI Insight. 2024;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 25. | Kamihara J, Bourdeaut F, Foulkes WD, Molenaar JJ, Mossé YP, Nakagawara A, Parareda A, Scollon SR, Schneider KW, Skalet AH, States LJ, Walsh MF, Diller LR, Brodeur GM. Retinoblastoma and Neuroblastoma Predisposition and Surveillance. Clin Cancer Res. 2017;23:e98-e106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 26. | Bhoopathi P, Mannangatti P, Emdad L, Das SK, Fisher PB. The quest to develop an effective therapy for neuroblastoma. J Cell Physiol. 2021;236:7775-7791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Mohlin SA, Wigerup C, Påhlman S. Neuroblastoma aggressiveness in relation to sympathetic neuronal differentiation stage. Semin Cancer Biol. 2011;21:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Lampis S, Raieli S, Montemurro L, Bartolucci D, Amadesi C, Bortolotti S, Angelucci S, Scardovi AL, Nieddu G, Cerisoli L, Paganelli F, Valente S, Fischer M, Martelli AM, Pasquinelli G, Pession A, Hrelia P, Tonelli R. The MYCN inhibitor BGA002 restores the retinoic acid response leading to differentiation or apoptosis by the mTOR block in MYCN-amplified neuroblastoma. J Exp Clin Cancer Res. 2022;41:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Zage PE, Huo Y, Subramonian D, Le Clorennec C, Ghosh P, Sahoo D. Identification of a novel gene signature for neuroblastoma differentiation using a Boolean implication network. Genes Chromosomes Cancer. 2023;62:313-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 30. | Chaudhry KA, Jacobi JJ, Gillard BM, Karasik E, Martin JC, da Silva Fernandes T, Hurley E, Feltri ML, Attwood KM, Twist CJ, Smiraglia DJ, Long MD, Bianchi-Smiraglia A. Aryl hydrocarbon receptor is a tumor promoter in MYCN-amplified neuroblastoma cells through suppression of differentiation. iScience. 2023;26:108303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 31. | Vernaza A, Cardus DF, Smith JL, Partridge V, Baker AL, Lewis EG, Zhang A, Zhao Z, Du L. Identification of CDKN3 as a Key Gene that Regulates Neuroblastoma Cell Differentiation. J Cancer. 2024;15:1153-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Epp S, Chuah SM, Halasz M. Epigenetic Dysregulation in MYCN-Amplified Neuroblastoma. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 33. | Fetahu IS, Taschner-Mandl S. Neuroblastoma and the epigenome. Cancer Metastasis Rev. 2021;40:173-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 34. | Li C, Feng C, Chen Y, Wu P, Li P, Xiong X, Peng X, Wang Z, Li Y. Arsenic trioxide induces the differentiation of retinoic acid-resistant neuroblastoma cells via upregulation of HoxC9. Adv Clin Exp Med. 2022;31:903-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 35. | Chehade G, Lawson TM, Lelotte J, Daoud L, Di Perri D, Whenham N, Duprez T, Tajeddine N, Tissir F, Raftopoulos C. Long-term survival in patients with IDH-wildtype glioblastoma: clinical and molecular characteristics. Acta Neurochir (Wien). 2023;165:1075-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 36. | Hertler C, Felsberg J, Gramatzki D, Le Rhun E, Clarke J, Soffietti R, Wick W, Chinot O, Ducray F, Roth P, McDonald K, Hau P, Hottinger AF, Reijneveld J, Schnell O, Marosi C, Glantz M, Darlix A, Lombardi G, Krex D, Glas M, Reardon DA, van den Bent M, Lefranc F, Herrlinger U, Razis E, Carpentier AF, Phillips S, Rudà R, Wick A, Tabouret E, Meyronet D, Maurage CA, Rushing E, Rapkins R, Bumes E, Hegi M, Weyerbrock A, Aregawi D, Gonzalez-Gomez C, Pellerino A, Klein M, Preusser M, Bendszus M, Golfinopoulos V, von Deimling A, Gorlia T, Wen PY, Reifenberger G, Weller M. Long-term survival with IDH wildtype glioblastoma: first results from the ETERNITY Brain Tumor Funders' Collaborative Consortium (EORTC 1419). Eur J Cancer. 2023;189:112913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 37. | Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger G, Weller M, Schackert G; German Glioma Network. Long-term survival with glioblastoma multiforme. Brain. 2007;130:2596-2606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 627] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 38. | Hartmann C, Hentschel B, Simon M, Westphal M, Schackert G, Tonn JC, Loeffler M, Reifenberger G, Pietsch T, von Deimling A, Weller M; German Glioma Network. Long-term survival in primary glioblastoma with versus without isocitrate dehydrogenase mutations. Clin Cancer Res. 2013;19:5146-5157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 39. | Șerban G, Tămaș F, Bălașa R, Manu D, Tămaș C, Bălașa A. Prognostic Factors of Survival in Glioblastoma Multiforme Patients-A Retrospective Study. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Kalita O, Sporikova Z, Hajduch M, Megova Houdova M, Slavkovsky R, Hrabalek L, Halaj M, Klementova Y, Dolezel M, Drabek J, Tuckova L, Ehrmann J Jr, Vrbkova J, Trojanec R, Vaverka M. The Influence of Gene Aberrations on Survival in Resected IDH Wildtype Glioblastoma Patients: A Single-Institution Study. Curr Oncol. 2021;28:1280-1293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55839] [Article Influence: 7977.0] [Reference Citation Analysis (132)] |
| 42. | Kirkham AA, Jerzak KJ. Prevalence of Breast Cancer Survivors Among Canadian Women. J Natl Compr Canc Netw. 2022;20:1005-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 43. | Álvarez-Bustos A, de Pedro CG, Romero-Elías M, Ramos J, Osorio P, Cantos B, Maximiano C, Méndez M, Fiuza-Luces C, Méndez-Otero M, Martín S, Cebolla H, Ruiz-Casado A. Prevalence and correlates of cancer-related fatigue in breast cancer survivors. Support Care Cancer. 2021;29:6523-6534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 44. | Breidenbach C, Heidkamp P, Hiltrop K, Pfaff H, Enders A, Ernstmann N, Kowalski C. Prevalence and determinants of anxiety and depression in long-term breast cancer survivors. BMC Psychiatry. 2022;22:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 45. | Möhl A, Orban E, Jung AY, Behrens S, Obi N, Chang-Claude J, Becher H. Comorbidity burden in long-term breast cancer survivors compared with a cohort of population-based controls from the MARIE study. Cancer. 2021;127:1154-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Heidary Z, Ghaemi M, Hossein Rashidi B, Kohandel Gargari O, Montazeri A. Quality of Life in Breast Cancer Patients: A Systematic Review of the Qualitative Studies. Cancer Control. 2023;30:10732748231168318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 47. | Inari H, Teruya N, Kishi M, Horii R, Akiyama F, Takahashi S, Ito Y, Ueno T, Iwase T, Ohno S. Clinicopathological features of breast cancer patients with internal mammary and/or supraclavicular lymph node recurrence without distant metastasis. BMC Cancer. 2020;20:932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Song YC, Kong J, Li N, Liu XL, Li XH, Zhu LY, Wang YW, Fang H, Jing H, Tang Y, Li YX, Wang XH, Zhang J, Wang SL. Comparison of supraclavicular surgery plus radiotherapy versus radiotherapy alone in breast cancer patients with synchronous ipsilateral supraclavicular lymph node metastasis: A multicenter retrospective study. Radiother Oncol. 2023;183:109639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 49. | Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8789] [Cited by in RCA: 9568] [Article Influence: 869.8] [Reference Citation Analysis (0)] |
| 50. | Pattenden T, Samaranayake D, Morton A, Thangasamy I. Bladder cancer in Aboriginal and Torres Strait Islander people living in Australia: a scoping review protocol. BMJ Open. 2022;12:e059144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Lobo N, Mount C, Omar K, Nair R, Thurairaja R, Khan MS. Landmarks in the treatment of muscle-invasive bladder cancer. Nat Rev Urol. 2017;14:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 52. | Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder Cancer: A Review. JAMA. 2020;324:1980-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 1064] [Article Influence: 212.8] [Reference Citation Analysis (0)] |
| 53. | Babjuk M, Böhle A, Burger M, Capoun O, Cohen D, Compérat EM, Hernández V, Kaasinen E, Palou J, Rouprêt M, van Rhijn BWG, Shariat SF, Soukup V, Sylvester RJ, Zigeuner R. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol. 2017;71:447-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1401] [Article Influence: 175.1] [Reference Citation Analysis (0)] |
| 54. | Chou R, Selph S, Buckley DI, Fu R, Griffin JC, Grusing S, Gore JL. Intravesical Therapy for the Treatment of Nonmuscle Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. J Urol. 2017;197:1189-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 55. | Dugbartey GJ, Relouw S, McFarlane L, Sener A. Redox System and Oxidative Stress-Targeted Therapeutic Approaches in Bladder Cancer. Antioxidants (Basel). 2024;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 56. | Stroggilos R, Frantzi M, Zoidakis J, Mokou M, Moulavasilis N, Mavrogeorgis E, Melidi A, Makridakis M, Stravodimos K, Roubelakis MG, Mischak H, Vlahou A. Gene Expression Monotonicity across Bladder Cancer Stages Informs on the Molecular Pathogenesis and Identifies a Prognostic Eight-Gene Signature. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 57. | Chen Y, Huang M, Lu J, Zhang Q, Wu J, Peng S, Chen S, Zhang Y, Cheng L, Lin T, Chen X, Huang J. Establishment of a prognostic model to predict chemotherapy response and identification of RAC3 as a chemotherapeutic target in bladder cancer. Environ Toxicol. 2024;39:509-528. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 58. | Woolbright BL, Choudhary D, Mikhalyuk A, Trammel C, Shanmugam S, Abbott E, Pilbeam CC, Taylor JA 3rd. The Role of Pyruvate Dehydrogenase Kinase-4 (PDK4) in Bladder Cancer and Chemoresistance. Mol Cancer Ther. 2018;17:2004-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 59. | Mun JY, Baek SW, Jeong MS, Jang IH, Lee SR, You JY, Kim JA, Yang GE, Choi YH, Kim TN, Chu IS, Leem SH. Stepwise molecular mechanisms responsible for chemoresistance in bladder cancer cells. Cell Death Discov. 2022;8:450. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 60. | Daga M, Pizzimenti S, Dianzani C, Cucci MA, Cavalli R, Grattarola M, Ferrara B, Scariot V, Trotta F, Barrera G. Ailanthone inhibits cell growth and migration of cisplatin resistant bladder cancer cells through down-regulation of Nrf2, YAP, and c-Myc expression. Phytomedicine. 2019;56:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 61. | Lv L, Li Y, Deng H, Zhang C, Pu Y, Qian L, Xiao J, Zhao W, Liu Q, Zhang D, Wang Y, Zhang H, He Y, Zhu J. MiR-193a-3p promotes the multi-chemoresistance of bladder cancer by targeting the HOXC9 gene. Cancer Lett. 2015;357:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 62. | Yang WJ, Zhao HP, Yu Y, Wang JH, Guo L, Liu JY, Pu J, Lv J. Updates on global epidemiology, risk and prognostic factors of gastric cancer. World J Gastroenterol. 2023;29:2452-2468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 193] [Article Influence: 96.5] [Reference Citation Analysis (13)] |
| 63. | Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014;20:4483-4490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 265] [Cited by in RCA: 315] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 64. | Lei ZN, Teng QX, Tian Q, Chen W, Xie Y, Wu K, Zeng Q, Zeng L, Pan Y, Chen ZS, He Y. Signaling pathways and therapeutic interventions in gastric cancer. Signal Transduct Target Ther. 2022;7:358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 164] [Article Influence: 54.7] [Reference Citation Analysis (1)] |
| 65. | Huang J, Lucero-Prisno DE 3rd, Zhang L, Xu W, Wong SH, Ng SC, Wong MCS. Updated epidemiology of gastrointestinal cancers in East Asia. Nat Rev Gastroenterol Hepatol. 2023;20:271-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 126] [Reference Citation Analysis (0)] |
| 66. | Tang Y, Wang T, Yu Y, Yan Y, Wu C. Upregulation of HOXC9 generates interferon-gamma resistance in gastric cancer by inhibiting the DAPK1/RIG1/STAT1 axis. Cancer Sci. 2021;112:3455-3468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 67. | Stoffel EM, Murphy CC. Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults. Gastroenterology. 2020;158:341-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 382] [Article Influence: 76.4] [Reference Citation Analysis (1)] |
| 68. | Burnett-Hartman AN, Lee JK, Demb J, Gupta S. An Update on the Epidemiology, Molecular Characterization, Diagnosis, and Screening Strategies for Early-Onset Colorectal Cancer. Gastroenterology. 2021;160:1041-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 174] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 69. | Healy MA, Thirumurthi S, You YN. Screening high-risk populations for colon and rectal cancers. J Surg Oncol. 2019;120:858-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 70. | Kim JH. Chemotherapy for colorectal cancer in the elderly. World J Gastroenterol. 2015;21:5158-5166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 117] [Cited by in RCA: 148] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 71. | Hou W, Yi C, Zhu H. Predictive biomarkers of colon cancer immunotherapy: Present and future. Front Immunol. 2022;13:1032314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 72. | Hu M, Ou-Yang W, Jing D, Chen R. Clinical Prognostic Significance of HOXC9 Expression in Patients with Colorectal Cancer. Clin Lab. 2019;65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 73. | Hu K, Ma C, Ma R, Zheng Q, Wang Y, Zhang N, Sun Z. Roles of Krüppel-like factor 6 splice variant 1 in the development, diagnosis, and possible treatment strategies for non-small cell lung cancer. Am J Cancer Res. 2022;12:4468-4482. [PubMed] |
| 74. | Padinharayil H, Varghese J, John MC, Rajanikant GK, Wilson CM, Al-Yozbaki M, Renu K, Dewanjee S, Sanyal R, Dey A, Mukherjee AG, Wanjari UR, Gopalakrishnan AV, George A. Non-small cell lung carcinoma (NSCLC): Implications on molecular pathology and advances in early diagnostics and therapeutics. Genes Dis. 2023;10:960-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 75. | Bracht JWP, Mayo-de-Las-Casas C, Berenguer J, Karachaliou N, Rosell R. The Present and Future of Liquid Biopsies in Non-Small Cell Lung Cancer: Combining Four Biosources for Diagnosis, Prognosis, Prediction, and Disease Monitoring. Curr Oncol Rep. 2018;20:70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 76. | Tartarone A, Rossi E, Lerose R, Mambella G, Calderone G, Zamarchi R, Aieta M. Possible applications of circulating tumor cells in patients with non small cell lung cancer. Lung Cancer. 2017;107:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Ma C, Ma RJ, Hu K, Zheng QM, Wang YP, Zhang N, Sun ZG. The molecular mechanism of METTL3 promoting the malignant progression of lung cancer. Cancer Cell Int. 2022;22:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 78. | Ma C, Hu K, Ullah I, Zheng QK, Zhang N, Sun ZG. Molecular Mechanisms Involving the Sonic Hedgehog Pathway in Lung Cancer Therapy: Recent Advances. Front Oncol. 2022;12:729088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 79. | Liu Y, Jing L, Zhang J. circRNA-mediated upregulation of HOXC9 is correlated with poor outcome and immune microenvironment infiltrates in LUAD. Biochem Biophys Res Commun. 2022;635:128-135. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 80. | Bi R, Wei W, Lu Y, Hu F, Yang X, Zhong Y, Meng L, Wang M, Jiang L, Xie X. High hsa_circ_0020123 expression indicates poor progression to non-small cell lung cancer by regulating the miR-495/HOXC9 axis. Aging (Albany NY). 2020;12:17343-17352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 81. | Lin Q, Geng J, Ma K, Yu J, Sun J, Shen Z, Bao G, Chen Y, Zhang H, He Y, Luo X, Feng X, Zhu J. RASSF1A, APC, ESR1, ABCB1 and HOXC9, but not p16INK4A, DAPK1, PTEN and MT1G genes were frequently methylated in the stage I non-small cell lung cancer in China. J Cancer Res Clin Oncol. 2009;135:1675-1684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 82. | Kim SY, Shin SJ, Lee DG, Yun HJ, Kim SM, Chang H, Chang HS, Shin H, Lee YS. Clinicopathological and Genetic Characteristics of Patients of Different Ages with Diffuse Sclerosing Variant Papillary Thyroid Carcinoma. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 83. | Pillai S, Gopalan V, Smith RA, Lam AK. Diffuse sclerosing variant of papillary thyroid carcinoma--an update of its clinicopathological features and molecular biology. Crit Rev Oncol Hematol. 2015;94:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 84. | Li W, Wang Y, Gao L, Feng R, Lv K, Wu X, Yang X, Cai S, Wang H, Li J. Sonographic characteristics of diffuse sclerosing variant of papillary thyroid carcinoma with histopathological correlation: a preliminary study. Orphanet J Rare Dis. 2024;19:136. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 85. | Vuong HG, Kondo T, Pham TQ, Oishi N, Mochizuki K, Nakazawa T, Hassell L, Katoh R. Prognostic significance of diffuse sclerosing variant papillary thyroid carcinoma: a systematic review and meta-analysis. Eur J Endocrinol. 2017;176:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 86. | Sugitani I. Active surveillance of low-risk papillary thyroid microcarcinoma. Best Pract Res Clin Endocrinol Metab. 2023;37:101630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 87. | Cao YM, Wen D, Qu N, Zhu YX. Prognostic and clinical significance of HOXC9 and HOXD10 in papillary thyroid cancer. Transl Cancer Res. 2021;10:3317-3325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 88. | Szklarczyk D, Kirsch R, Koutrouli M, Nastou K, Mehryary F, Hachilif R, Gable AL, Fang T, Doncheva NT, Pyysalo S, Bork P, Jensen LJ, von Mering C. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023;51:D638-D646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1815] [Cited by in RCA: 3487] [Article Influence: 1743.5] [Reference Citation Analysis (0)] |
| 89. | Brune JE, Kern M, Kunath A, Flehmig G, Schön MR, Lohmann T, Dressler M, Dietrich A, Fasshauer M, Kovacs P, Stumvoll M, Blüher M, Klöting N. Fat depot-specific expression of HOXC9 and HOXC10 may contribute to adverse fat distribution and related metabolic traits. Obesity (Silver Spring). 2016;24:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 90. | Kaplun DS, Kaluzhny DN, Prokhortchouk EB, Zhenilo SV. DNA Methylation: Genomewide Distribution, Regulatory Mechanism and Therapy Target. Acta Naturae. 2022;14:4-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 91. | Liang G, Weisenberger DJ. DNA methylation aberrancies as a guide for surveillance and treatment of human cancers. Epigenetics. 2017;12:416-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 92. | Luczak MW, Jagodziński PP. The role of DNA methylation in cancer development. Folia Histochem Cytobiol. 2006;44:143-154. [PubMed] |
| 93. | Suemori H, Takahashi N, Noguchi S. Hoxc-9 mutant mice show anterior transformation of the vertebrae and malformation of the sternum and ribs. Mech Dev. 1995;51:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 2.0] [Reference Citation Analysis (0)] |