Published online Sep 24, 2024. doi: 10.5306/wjco.v15.i9.1157
Revised: June 27, 2024
Accepted: July 31, 2024
Published online: September 24, 2024
Processing time: 102 Days and 2.8 Hours
Over the last decade, our knowledge of colorectal serrated polyps and lesions has significantly improved due to numerous studies on this group of precursor lesions. Serrated lesions were misleading as benign before 2010, but they are currently reclassified as precancerous lesions that contribute to 30% of colorectal cancer through the serrated neoplasia pathway. The World Health Organization updated the classification for serrated lesions and polyps of the colon and rectum in 2019, which is more concise and applicable in daily practice. The responsible authors prescribe that “colorectal serrated lesions and polyps are characterized by a serrated (sawtooth or stellate) architecture of the epithelium.” From a clinical standpoint, sessile serrated lesion (SSL) and SSL with dysplasia (SSLD) are the two most significant entities. Despite these advancements, the precise diagnosis of SSL and SSLD based mainly on histopathology remains challenging due to va
Core Tip: The “serrated neoplastic pathway”, a crucial and significant aspect of our comprehension of cancer, has seen substantial advancement over the past decade. While the literature has made significant strides in molecular biology and diagnostic criteria, there is room for improvement in achieving a consensus on terminology. This review, which could potentially revolutionize your practice, aims to offer an in-depth understanding of essential criteria, the intricacies involved in diagnosing sessile serrated lesion (SSL) and SSL with dysplasia, the two entities of utmost clinical importance, and the ongoing discussions related to terminology.
- Citation: Tran TH, Nguyen VH, Vo DT. How to "pick up" colorectal serrated lesions and polyps in daily histopathology practice: From terminologies to diagnostic pitfalls. World J Clin Oncol 2024; 15(9): 1157-1167
- URL: https://www.wjgnet.com/2218-4333/full/v15/i9/1157.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i9.1157
Since 1996, Torlakovic and Snover[1] have postulated that “serrated adenoma polyposis” should have been distinguished from true hyperplastic polyposis, given a possible association with adenocarcinoma. This proposal put a milestone as a new entity, termed “sessile serrated adenoma (SSA)” with distinct morphological and genetic features, in a fundamental original article published in 2003[2].
Large lesions with abnormal proliferation, dilated distorted crypts, and dilation at the crypt base, with or without focal nuclear atypia with pseudostratification, were not diagnosed as simply “hyperplastic polyps (HP)” but should be given a different designation as “SSA” as neoplastic lesions for practical purposes. Since then, a series of studies on sessile serrated lesion (SSL) have emerged and increasingly shed more light on the nature of this lesion[3-5]. The diagnostic criteria and nomenclature for SSLs have undergone multiple changes, repeatedly causing confusion among pathologists and gastroenterologists[6]. The World Health Organization (WHO) classification of tumours of the digestive system, 4th edition, approved various terms for serrated polyp subgroups in the colon and rectum in 2010[7].
The “serrated pathway” has been recognized and accepted as the second pathway in colorectal cancer, summarized in a review article by Rex et al[8] in 2012. The hallmarks of the main “serrated pathway”[7] were pathological adenocarcinomas demonstrating frequent hypermethylation [CpG island methylator phenotype (CIMP)] and resulting in the loss of the MLH1 function and expression, BRAF V600E mutations, and microsatellite instability (MSI) with an often characteristic “MSI-like” tumor morphology.
SSL and traditional serrated adenoma (TSA) are known precursors to carcinoma, although HP, particularly proximal microvesicular HPs (MVHP), are probably a precursor to SSL. Goblet cell HPs and some MVHP may also give rise to TSA. The progression of SSL to carcinoma proceeds through the development of overt epithelial dysplasia (SSLD) due to progressive CpG island methylation. Overt dysplasia and low levels of CpG methylation also occur in TSA during its progression to carcinoma[6]. A large serrated polyp also increases the risk of colorectal carcinoma. Nevertheless, small distal HPs have no substantial malignant potential and do not affect colonoscopic surveillance intervals. Index SSL, TSA, and large (≥ 1 cm) serrated polyps, regardless of histology, increase the risk of large metachronous serrated polyps[9]. As a result, follow-up intervals are based on low levels of evidence and vary by country[8,10,11]. Irrespective of a better understanding of this genetically and pathologically, the accurate diagnosis of SSL and SSLD based mainly on his
There were controversies over the terminology of serrated lesions and polyps in the colon-rectum region between members of the WHO classification of gastrointestinal tumors board in 2010[7]. Considerable authors advocated using the term “SSA” to describe these lesions due to their potential for the development of malignancy, similar to conventional adenomas. Nonetheless, the structural changes in SSL primarily involve glandular architecture without cytological dysplasia, as seen in conventional adenomas. Thus, “adenoma” might mislead clinicians that these lesions have cytological dysplasia. The remaining authors suggested the term “adenoma with cytologic dysplasia,” but this again created a disarray because “adenoma” inherently implies the presence of dysplasia. As a result, the term “polyp” was proposed to obtain a more “neutral” biological perspective, and that was respected as less incoherent by fellow gastroenterologists[7,12]. Unfortunately, the term “polyp” was not entirely accurate either as most SSLs are not truly polypoid, but typically flat, making them hard to distinguish from normal mucosa with poorly defined boundaries. The committee members ultimately had to vote according to their perspectives, and as expected, the decision was asserted, and thus, as a compromise, the term “SSA/polyp (SSA/P)” was coined.
It was addressed that over the next decade, the term “SSA/P” was not widely accepted among pathologists and gastroenterologists[6,12]. This hardship was primarily due to the mentioned reasons and partially because of diagnoses translated into at least three other variants, namely, sessile serrated polyp (SSP)/A, SSA, and SSP. Consequently, the updated WHO classification presented a remarkable challenge to authors drafting the fifth edition. Their task was to correct past mistakes and accomplish two goals: (1) Unify the past terminologies into widely accepted ones for research and practice purposes; and (2) Design substitution terms that should be as biologically and pathologically precise as possible. Therefore, “injury” was a suggested term, which seems to meet both goals. The neutral term “lesion,” instead of adenoma or polyp, is defined as “an area of abnormal tissue change that may be benign or malignant.” This term is applicable to confound the dilemma of “adenoma” and “polyp,” as discussed earlier. When maintaining a neutral attitude in diagnostic pathology, “SSA/P” is commonly employed instead of “SLL” in classifying colorectal serrated lesions/(or) polyps.
SSL is the currently recommended terminology for a type of serrated colorectal polyp characterized by disordered maturation, abnormal basal crypt growth, and propensity for developing cytologic dysplasia and, ultimately, carcinoma by the WHO 2019[6,13]. The diagnostic criteria of SSLs include at least one “unequivocal” architecturally distorted crypt defined as “horizontal growth along the muscularis mucosae, dilation of the crypt base, serrations extending into the crypt base, and asymmetrical proliferation”[2,6,13,14]. The term “unequivocal” is noteworthy because crypts with only subtle architectural abnormalities should not be regarded as diagnostic of SSL[6,15]. These lesions are frequently found in the proximal colon, and size variations are possibly more significant than 5 mm. Nevertheless, location and size should be considered only when orientation or ambiguity complicates the diagnosis[6].
Although numerous organizations support the new term SSL, a survey conducted by Ono et al[16] in late 2019 revealed a significant disagreement among pathologists from North America. Only 29% (79 out of 274) of them intended to use the term SSL, compared to 78% (31 out of 40) and 70% (21 out of 30) of pathologists from Europe and Asia, respectively. In contrast, 85% (186 out of 219) of those who refused to use SSL cited that it might confuse or disappoint gastroenterologists. A pathologist from America, Nagtegaal and Snover[17] raised her view of using SSL over SSA and published a review in 2022, proposing not to use the term “adenoma” in this scenario since there is no cytological dysplasia in SSL, making it incorrect.
Furthermore, the definition of “adenoma”[17] does not necessarily require cytological dysplasia. For instance, adenomas from other organs, such as the liver, adrenal gland, bile duct, and thyroid, do not exhibit cytological dysplasia. Despite the absence of cytological dysplasia in TSA, the term “adenoma” is still considered relevant, and if not, the term “TSA” would also need to be reconsidered. Moreover, the term “lesion” is often vague and does not fully capture the precancerous nature of “adenoma.” These ongoing debates highlight the demand for a unified understanding of SSL’s histopathological characteristics and its role in colorectal carcinogenesis. While there is a broad consensus on its clinical implications, the applicable terminologies continue to vary across geographical regions, with Europe and Asia more accepting of the term “SSL.” In contrast, America tends to use the term “SSA.”
SSLD is the abrupt transition lesion from a typical SSL to a dysplastic one. Most SSLs harbor the BRAF gene mutation and exhibit a high level of CpG island methylation. The methylation process leads to a down-regulation of gene expression, including the MLH1 gene[18-20]. Consequently, SSLD is constantly associated with the loss of MLH1 expression. In SSLD, several studies have categorized dysplastic components into low and high grades[21-23], while other studies have classified varied dysplastic patterns[24,25], including three recent landmark studies (Table 1). In their study, Cenaj et al[23] used three categories: Serrated dysplasia, intestinal dysplasia, and a mixture of serrated and intestinal dysplasia. Liu et al[24] used four categories: Serrated dysplasia, adenomatous dysplasia, minimal deviation dysplasia, and dysplasia, not otherwise specified. Finally, an American Society of Clinical Pathology (ASCP) study[25] used five categories: Dysplasia as a superficial cap, dysplasia not as a superficial cap, serrated type, minimal deviation type, and not otherwise specified. Currently, the WHO 2019 classification[6] recommends using only the term SSLD, not reco
| Cenaj et al[23] | Liu et al[24] | ASCP (2021)[25] | WHO (2019)[6] | ||||
| Categories of dysplasia | MLH1 loss (%) | Categories of dysplasia | MLH1 loss (%) | Categories of dysplasia | MLH1 loss (%) | Categories of dysplasia | MLH1 loss (%) |
| Intestinal dysplasia: Low-grade; High-grade | NA | Adenomatous dysplasia | 5 | SSLDad1 | 6.1 | NA1 | NA2 |
| Serrated dysplasia: Low-grade; High-grade | NA | Serrated dysplasia | 13 | SSLDser | 59.1 | ||
| Mixture of a serrated and intestinal dysplasia: Low-grade; High-grade | NA | Dysplasia NOS | 83 | SSLDnos SSLDad2 | 35 24.3 | ||
| Minimal deviation dysplasia | 91 | SSLDmin | 50 | ||||
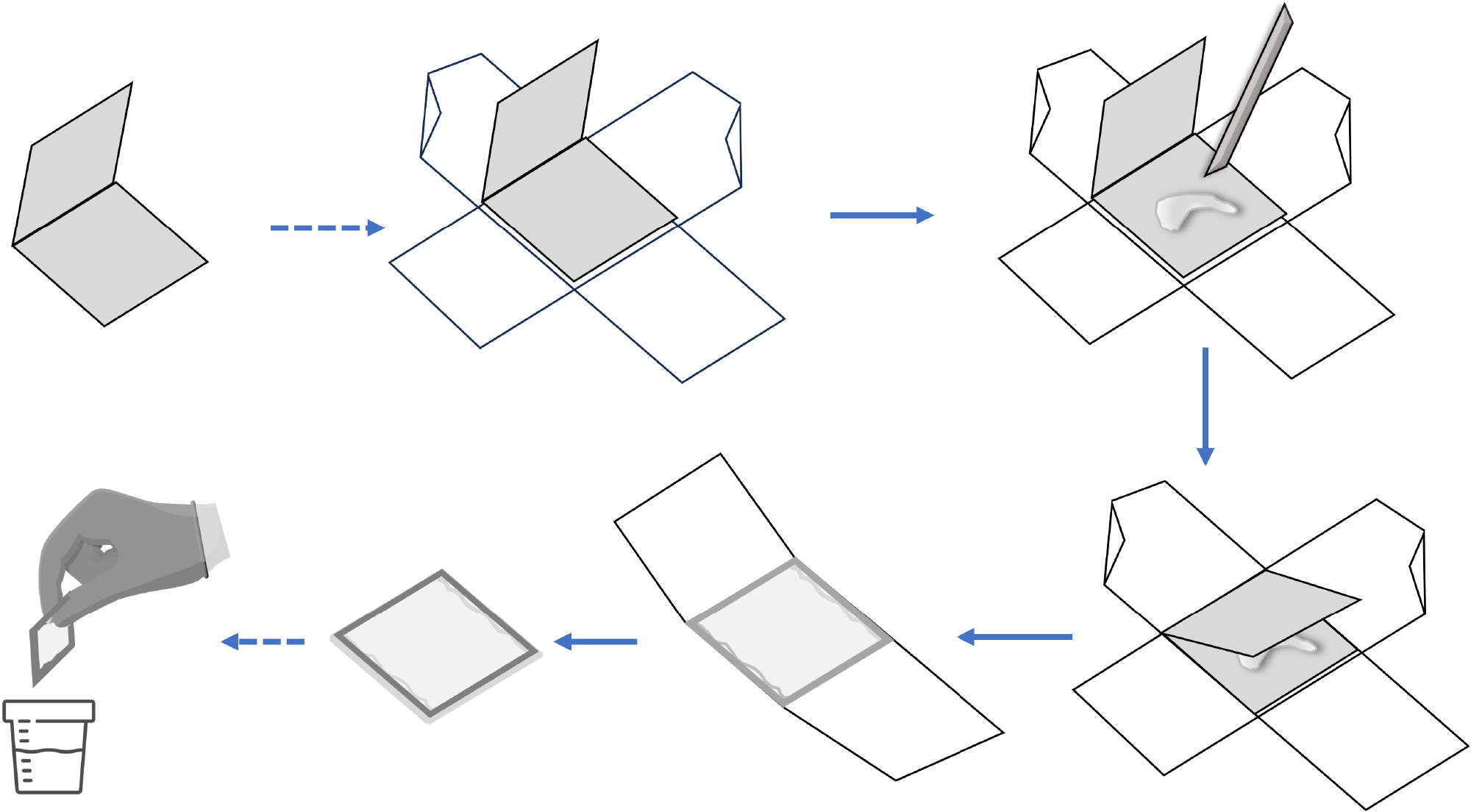
SSLs[26] are diagnosed primarily by architectural features, including deep crypt serration, crypt dilation, and abnormal shape at the crypt base. Nevertheless, the crypts’ bases are usually not visible in routine practice when the slides are not histologically well-oriented, such as tangential or horizontal cutting, leading to challenges in assessing architectural features. It had been observed that when flat right-colon polyps were placed directly into formalin, they sometimes “curled up,” which can later make it difficult for histologic technicians to orient polyp specimens properly in daily histopathological routine[26]. To address this problem, we can use an envelope consisting of a 2 cm × 4 cm piece of construction paper folded in half[26,27]. After performing a polypectomy, the assistant transfers the specimen onto the envelope’s bottom flap, with two pieces of standard adhesive tape attached perpendicularly for easy retrieval. Use a toothpick or a plastic utensil to gently flatten the specimens, of which the mucosal surface would face up or down. The top flap was then gently folded over the polyp and taped shut. Because tape affixed to construction paper may detach when immersed in formalin, the tape completely encircled the envelope such that tape was affixed to tape. The perpendicular application prevented the specimen from falling out through the sides of the envelope. The corners of the envelope were left untaped, which allowed formalin to enter both the envelope and the specimen. This complex was then placed in a jar of formalin. For piecemeal endoscopic resection, the fragments were kept in separate envelopes before being combined in a jar of formalin (Figure 1). The modified protocol with an improved orientation gave pathologists a better representation of mucosal architecture and crypt bases[26]. It increased inter-pathologist concordance from 62.8% to 77.0%, decreased pathologists’ requests for deeper tissue sections from 31.8% to 11.1%, and increased the SSL diagnosis rate from 40.3% to 75.6%.
In a local retrospective study by our group[27] virtually supported by Bateman’s review[28], we also examined the frequencies of cross-sectional, longitudinal, and tangential cuts found in 53 cases of MVHPs, 17 cases of SSLs, and 12 cases of SSLDs (using general biopsy-specimen processing techniques). As a result, the frequencies of the cross-sectional samples in the MVHP and SSL groups accounted for greater than one-third of the cases, which is a possible cause of the difficulty in distinguishing between MVHP and SSL among these cases. In the group of tangential cut samples, SSL misdiagnosis was possible if deeper sections were undone for re-evaluation. In the SSLD group, no cross-sectional samples were recorded partly due to either the larger lesion size or the sample eradicated by endoscopic tissue removal supporting a better orientation.
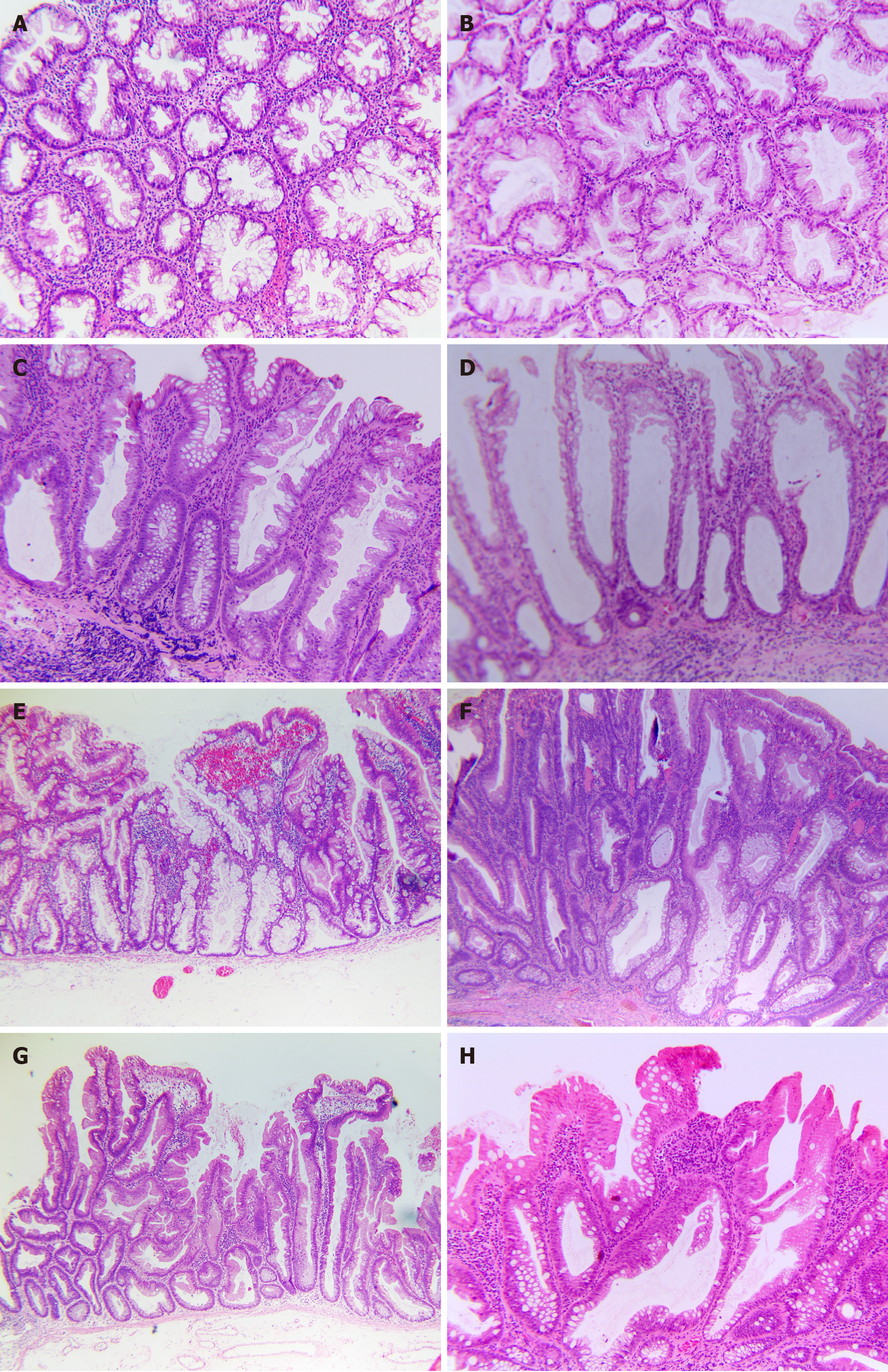
Vice versa, what should we do when a sample has poor orientation? If a sample has been tangentially sectioned, pathologists may request deeper sections to expose features at the crypt base. In a study by Jaravaza and Rijby[29], 103 cases initially diagnosed as HP were re-examined by having eight additional slices. The result yielded 11 cases of SSL, all less than 5 mm and representing 14.3% of proximal polyps and 10.4% of distal polyps. On average, it takes 3.6 serial sections to reverse a diagnosis. If the sample is cross-sectioned, it can be challenging for pathologists (Figure 2A). In such cases, rotating the block 90° to recut it longitudinally may not provide enough material for further evaluation, especially if the recent specimen is from a small biopsy. According to our report, pathologists must rely on other features rather than structural changes at the crypt base[27]. However, we can instead utilize asymmetrical crypt growth and clinical features, such as size and location, to support the diagnosis. For example, asymmetrical proliferation, location in the proximal colon, and size > 5 mm suggest SSL. Alternatively, if a minimal SSLD lesion (Figure 2B) is suspected, staining with MLH1 may support the diagnosis.
Over time, the definitions for SSLs and HPs have changed, leading to changes in the prevalence of diagnostic categories. The term “SSLs” was later replaced by “SSA/Ps” in the WHO classification 2010, defined as “serrated polyp with structural deformation from 2 to 3 continuous glands.” Several studies have shown that 8%-19% of HPs were reclassified as SSLs[30-32]. Applying the “1 crypt” rule in the WHO classification 2019 would likely increase the sensitivity of detecting SSLs. A study by Bettington et al[14] reported that applying the “at least one crypt” rule resulted in a 7% increase in the ratio of serrated polyps reclassified as SSLs.
Of the 1000 cases extracted from the archival data of diagnosed HP admitted to our hospital from 2019 to 2021[27], our survey reviewed 64 HPs with equivocal histological features, 13 over 64 were reclassified as 08 SSLs, 03 SSLDs, and 02 TSAs (a total of 21.5% actual serrated lesions and polyps) according to WHO 2019. Meanwhile, reviewing 118 cases diagnosed with serrated lesions/polyps, but not HP, 71 cases met the WHO 2019 criteria, including 25 HPs, 09 SSLs, 09 SSLDs, 27 TSAs, 01 unclassified serrated adenoma, and 02 cases of mixed polyps. The remaining 45 (38.1%) cases do not truly belong to a serrated group (i.e., inflammatory polyps, conventional tubular adenoma with mucosa prolapse with and without intramucosal carcinoma components). As a result, we suggest the following issues: (1) Dilation of the crypt base can be a subjective appraisal when it is the only one present without accompanying features (Figure 2C and D), leading to a significant variation in diagnosis among pathologists; (2) The presence of one or more crypts with horizontal growth along the muscularis mucosae forming an L or inverted T shape is considered the most objective and convincing feature (Figure 2E); (3) Serrations are often a conspicuous feature in SSL, but do not consistently extend to the crypt base; and (4) Asymmetrical proliferation constantly accompanies other features.
Therefore, a comprehensive assessment of these features is required in diagnostic practice, balancing subjective and objective features to reach the most appropriate and accurate diagnosis.
Difficulty distinguishing between HP and SSL can arise due to several factors. As mentioned earlier[28], when the singular feature present is dilation of the crypt base, which is subjective, it can lead to varying diagnoses between HP and SSL (Figure 2D). This subjectivity creates ambiguity, contributing to diagnostic inconsistency.
The poor orientation of samples is an essential and commonly encountered reason for difficulty in diagnostic his
Overall, comprehensive training, awareness of evolving diagnostic criteria, and a combination of objective and subjective feature assessments are crucial in distinguishing between these two types of polyps.
In a study by Cenaj et al[23], the authors divided SSLD into three dysplastic forms: (1) Serrated dysplasia (58%); (2) Intestinal dysplasia (26%); and (3) Mixture of a serrated and intestinal dysplasia (16%).
Intestinal dysplasia: Intestinal dysplasia is similar to conventional adenomas[23]. Low-grade intestinal dysplasia is characterized by an epithelium that lacks structural abnormalities (there are no back-to-back glands, cribriform, or gland-in-gland formations) and does not exhibit any high-grade cytologic features, as outlined below. By contrast, high-grade intestinal dysplasia presents high-grade cytologic features, which may or may not be accompanied by structural abnormalities.
Serrated dysplasia: Serrated dysplasia is composed of epithelial cells that have nuclei ranging from oval to slightly extended in shape, with a clear chromatin pattern and conspicuous nucleoli[23]. The nuclei are smaller in size than in intestinal dysplasia and typically show a less pronounced stratification with smooth nuclear membranes. The cytoplasm is eosinophilic. There may also be a varying number of goblet cells present. A defining architectural characteristic of serrated dysplasia is the existence of luminal serration or a saw-toothed growth pattern, which is typically more noticeable in the upper parts of the crypts and surface epithelium than at the bases of the crypts. There may also be an increase in mitotic figures, including atypical ones. High-grade serrated dysplasia is characterized by high-grade cytologic and/or architectural abnormalities. These nuclear alterations are often linked with architectural changes, such as the formation of back-to-back glands and gland-in-gland structures, with the possibility of a hyperserrated growth pattern.
A mixture of serrated and intestinal dysplasia: Dysplasia is characterized as lesions that encompass both types of morphologies within the same lesion.
Compared with conventional adenomas, SSLDs showed a significantly higher risk of malignant transformation within the polyp. SSLDs exhibiting intestinal dysplasia show a greater likelihood of cancer association compared to those with serrated dysplasia. This observation suggests that intestinal dysplasia is a notably high-risk feature in SSLD. Further investigations also pathogenetically support SSL’s low- to high-grade dysplasia sequencing to carcinoma.
In 2017, Liu et al[24] performed a morphological analysis of 266 SSLD with concurrent MLH1 immunohistochemistry to better determine the spectrum of dysplasia observed in these lesions. The patterns of dysplasia are correlated with the MLH1 expression. It was discovered that dysplasia can be morphologically categorized into four primary patterns, namely: (1) Minimal deviation (making up 19% of cases); (2) Serrated (accounting for 12%); (3) Adenomatous (comprising 8%); and (4) Not otherwise specified (making up the majority at 79%).
Minimal deviation dysplasia: This pattern was the single dysplasia pattern in the group[24]. It exhibits minimal architectural and cytological changes and is difficult to identify histologically. There is mild crypt disorganization, crypt crowding, and reduced luminal serration at low magnification compared with the background SSL. The cells frequently have a hypermucinous appearance with compressed, basally located nuclei showing mild hyperchromasia compared with the nuclei of the adjacent SSA component. This pattern was associated with loss of the MLH1 expression in 91%. In seven cases, the MLH1 expression was retained with intense MLH1 staining in lesions with other dysplasia patterns.
Serrated dysplasia: This less common dysplasia pattern was also a single pattern of the group[24]. An eosinophilic appearance at a low power with tightly packed crypts is characteristic. It shows closely packed small glands that occupy the entire thickness of the mucosa with an occasional cribriform growth. Architectural serration is subtle. The cells are cuboidal to low columnar with evident dysplasia, containing round vesicular nuclei, prominent nucleoli, and abundant eosinophilic cytoplasm. Mitoses are frequent, extend to the luminal surface, and can be atypical. The serrated dysplasia pattern had a solid predilection to retain staining for MLH1 (loss of the MLH1 expression in 13%).
Adenomatous dysplasia: This distinctive dysplasia pattern was identified as a single dysplasia pattern in all but one lesion[24]. The main characteristics of adenomatous dysplasia are the predominant location of the dysplastic component on the surface (“top-down” dysplasia) with preserved non-dysplastic SSA at the base of the lesion and complete similarity to the dysplasia of conventional adenomas without serration. The cells are columnar with at least focal goblet cell differentiation, elongated nuclei, and pseudostratification. This pattern also had a solid predilection to retain staining for MLH1 (loss of the MLH1 expression in 5%).
Dysplasia not otherwise specified: This pattern[24], which was the most prevalent, encompassed all instances that did not conform to the criteria for any of the distinct dysplasia patterns previously described. Therefore, the morphological manifestations within this pattern are diverse, but include the most readily identifiable and quintessential examples of SSLD. Architectural dysplasia includes crypt elongation, crypt crowding, excessive serration, and complex branching or cribriform growth. Cytological dysplasia is evident at low magnification and occupies the total thickness of the epithelium. The columnar dysplastic cells have basal hyperchromatic nuclei with pseudostratification, increased mitotic activity, and loss of polarity and cytoplasm, ranging from eosinophilic to amphophilic. Despite encompassing a “wastebasket” of patterns of dysplasia, this group was united by a high rate of MLH1 expression loss (83%).
The MLH1 immunohistochemistry is a useful supplementary test to aid in the diagnosis of dysplasia in SSAs under certain circumstances, but it should not be applied to every lesion. In the case of SSL with clear architectural and cytological dysplasia, the status of the MLH1 expression does not change the final diagnosis. It is advised in the following scenarios[24]: (1) Ambiguous cytological atypia that could be due to inflammation, prolapse, or cross-cut of the crypt bases; (2) Piecemeal resection with separate fragments of dysplasia and SSL, where a loss of the MLH1 expression in the dysplastic fragments leans toward SSLD rather than a conventional adenoma mixed with SSL; and (3) Lesions with mild morphological alterations of minimal deviation dysplasia to corroborate the diagnosis of dysplasia. The most noteworthy discovery of this study is the identification of minimal deviation dysplasia. The precise diagnosis of these subtle lesions poses a challenge for pathologists, and the MLH1 expression should be utilized when suspected on routine stains.
In 2021, an ASCP study evaluating 349 cases of colorectal polyps classified five SSLD subtypes[25].
SSLDad1, “adenomatous type 1” (dysplasia as a superficial cap): SSADad1 appears similar to a conventional adenoma that is positioned on top of an SSL, similar to Liu et al’s adenomatous dysplasia[24].
SSLDad2, “adenomatous type 2” (dysplasia not as a superficial cap): This group demonstrated an SSL with dysplasia that resembled a conventional tubular adenoma throughout the dysplastic component (similar to ad1). It did not exhibit features of serrated dysplasia, and, unlike SSLDad1, it did not exist as a superficial cap.
SSLDser, “serrated type”: This was also defined by Liu et al[24]. Specifically, TSA-like serrated changes were not included in this group. High-grade serrated dysplasia, as defined by Cenaj et al[23], is likely in this group as well, but not their low-grade serrated dysplasia.
SSLDmin, “minimal deviation” type: This was also defined as per the Liu et al[24] concept, including SSL with subtle histologic abnormalities, but clonal loss of MLH1 immunostaining.
SSLDnos, “not otherwise specified”: This refers to instances of definitive SSAD that do not conform to any of the previously mentioned categories (Figure 2F). This includes both new, unclassifiable cases (which make up the vast majority) and those where a consensus could not be achieved.
SSL admixed with TSA (SSL/TSA): This refers to polyps that contain both an SSL component and a distinct area that appears clonal, which is typical of a TSA, as previously defined by others. To summarize: (1) The nuclei are slim or pencillate; (2) The cytoplasm is eosinophilic or possibly mucinous; (3) The mitotic activity is minimal; and (4) The serrations are slit-like. Often, there is the presence of ectopic crypt base formation (Figure 2G).
SSL mixed with TSA and conventional dysplasia (SSL/TSA/CD): This category is characterized by three separate elements: SSL, TSA, and any type of conventional dysplasia, all as previously defined.
SSL with enteric metaplasia (SSLem): As mentioned above, this is a potential mimic of SSLD, which contains a focal area of the eosinophilic epithelium with absorptive features (i.e., brush border, no atypia, and no mitotic activity) that mimics dysplasia (Figure 2H).
Serrated tubulovillous adenoma: This refers to another recently identified potential counterpart of SSLD incorporated as a diagnostic category in anticipation of such cases appearing in the search population prior to the description of this lesion. As per the definition provided by Bettington et al[33], these are characterized as villiform polyps with more than 50% serrations associated with conventional-type dysplasia and lacking significant TSA-type features. These TSA-type features include slit-like serrations, TSA-like cytology, and minimal or absent mitotic activity.
The morphological classification of dysplasia in SSLD presented by the ASCP[25] has similarities to Liu et al’s classification, although the statistical figures show some differences[24]. SSLDad1 resembles the two studies, with the proportion in the ASCP study and Liu et al’s study being 11.6% and 8%, respectively, and the MLH1 expression loss rate being 6.1% and 5% for both. The SSLDser rates were 6.3% and 12% in the study of Liu et al[24] (and possibly 9% in Cenaj et al’s study[23]), but the rate of MLH1 expression loss was different, that is, from 59% to 13%. According to the ASCP, this distinction implies that SSADser with MLH1 loss is probably following the CIMP/MSI pathway of colon cancer. This observation certainly warrants further investigation.
SSLDmin by the ASCP only detected two cases (1%)[25]. The authors explained that this classification was only proposed by Liu et al[24]; hence, during the research sampling period (2007-2012), these lesions can be classified as mixed polyps. SSLDnos and SSLDad2 are considered included in Liu et al’s SSLDnos group (Figure 2F). The proportions of these two groups were 33.8% and 31.2%, respectively, almost similar to 79% in Liu et al’s study. Nevertheless, the rate of the MLH1 expression loss was 35% and 24.3% by the ASCP study[25] vs 83% by Liu et al[13]. SSL/TSA in the ASCP classification corresponds to the WHO[6] classification of TSA that originates from a precursor lesion, such as SSL. Many TSAs in adjacent or underlying regions contain lesions like HP (often MVHP) or SSL, which are considered precursor lesions. Several studies[33-36] have shown that approximately 30%-50% of TSAs arise from these precursor lesions. The recognition of such lesions as a transitioning TSA rather than an SSL is critical (Figure 2G).
SSL with enteric metaplasia, termed as “SSLem” in the ASCP classification 2022[25], refers to an SSL lesion with areas of eosinophilic cytoplasmic changes, which can be mistaken for SSLD, a controversial topic (Figure 2H). The visual characteristics of these cells are similar to the absorptive cells typically found in the small intestine, with narrow slits along the epithelium and a brush border on the apical surface[37]. Due to these features, some researchers refer to this phenotype as enteric metaplasia, which exhibits traits of aging cells rather than dysplastic cells[7]. However, there are others who believe that this cell type in a serrated polyp is a form of serrated dysplasia and should be classified as a SSP with TSA-like dysplasia or low-grade serrated dysplasia. Despite the eosinophilic appearance, these cytoplasmic changes in SSLem should not be mistaken for dysplasia with the view point of ASCP[25] and Pai et al[38]. Indeed, additional research is required to determine whether SSL with TSA-like cytology or flat TSA that originates from a SSP possesses the molecular characteristics of TSA. This would support the hypothesis that these lesions are a variant of TSA. Alternatively, they might represent a subtype of advanced SSL. This is an important area of study to enhance our understanding of these conditions.
The SSL/TSA/CD lesions showing mixed morphological features are not uncommonly encountered in diagnostic practice. Debate continues as to whether these represent “collision lesions,” that is, the coalescence of two closely situated, but independently arising lesions, or the result of distinct differentiation patterns occurring within a single lesion. Several studies recommend[39-41] that such cases should be termed as “mixed polyps,” and each component of lesion within the polyp should be reported individually.
The WHO classification 2019 for colorectal serrated lesions and polyps emphasizes that the dysplastic component in SSLD can be morphologically heterogeneous, with multiple dysplastic patterns coexisting in one lesion[6]. The MLH1 expression loss can be helpful in cases of minimal dysplasia[6]. So far, since it may be difficult and not reproducible due to the heterogeneity of morphological changes and the lack of correlation with the MLH1 expression loss, the current consensus does not either address a specific categorization of dysplasia patterns within SSLD, either recommend stratifying dysplasia into low and high grades as in conventional adenomas.
Pathologists use diagnostic terminology as a professional language to effectively communicate with clinicians. Therefore, it is necessary to reach a consensus on using terminology related to SSL such that data can be shared consistently and accurately. Diagnosing SSLs with endoscopic detection and eradication allows sample processing for a completely vertical orientation in parallel with updated terminology and diagnostic criteria. The lack of knowledge and ill experience with this diagnostic histopathology can cause difficulties at any step of the process. A well-processed sample combined with strict criteria will enhance the diagnostic skills of serrated lesions. Studies have shown that dysplasia morphology in SSLD is complex and heterogeneous, much more significant than conventional adenomas. Hence, there is no consensus on classifying these dysplastic lesions. Nevertheless, SSLD is associated with the MLH1 expression loss, which might be helpful in diagnosing SSLD in individual cases. Further studies are necessary to describe dysplastic patterns in SSLD. Hopefully, the next principle classification will reach a consensus on this issue.
The authors would like to thank Mr. Thinh Phuc NGO, a histotechnologist from the Department of Pathology, University Medical Center HCMC, for his diligent work of optimizing H&E slides from varying conditions of the formalin-fixed paraffin embedded blocks obtained from several local medical laboratories. We are grateful for the beautifully captured figures printed in this review.
| 1. | Torlakovic E, Snover DC. Serrated adenomatous polyposis in humans. Gastroenterology. 1996;110:748-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 239] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27:65-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 428] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 3. | Goldstein NS, Bhanot P, Odish E, Hunter S. Hyperplastic-like colon polyps that preceded microsatellite-unstable adenocarcinomas. Am J Clin Pathol. 2003;119:778-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Farris AB, Misdraji J, Srivastava A, Muzikansky A, Deshpande V, Lauwers GY, Mino-Kenudson M. Sessile serrated adenoma: challenging discrimination from other serrated colonic polyps. Am J Surg Pathol. 2008;32:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 5. | Pai RK, Hart J, Noffsinger AE. Sessile serrated adenomas strongly predispose to synchronous serrated polyps in non-syndromic patients. Histopathology. 2010;56:581-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Pai RK, Mäkinen MJ, Rosty C. Colorectal serrated lesions and polyps. In: Nagtegaal ID, Arends MJ, Odze RD, Lam AK. WHO Classification of Tumours of the Digestive System. Lyon: IARC Press, 2019: 163-169. |
| 7. | Snover DC, Ahnen DJ, Burt RW, Odze RD. Serrated polyps of the colon and rectum and serrated polyposis. In: Nagtegaal ID, Arends MJ, Odze RD, Lam AK. WHO Classification of Tumours of the Digestive System. Lyon: IARC Press, 2010: 160-165. |
| 8. | Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, Goldblum JR, Guillem JG, Kahi CJ, Kalady MF, O'Brien MJ, Odze RD, Ogino S, Parry S, Snover DC, Torlakovic EE, Wise PE, Young J, Church J. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315-29; quiz 1314, 1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 828] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 9. | Anderson JC, Butterly LF, Robinson CM, Weiss JE, Amos C, Srivastava A. Risk of Metachronous High-Risk Adenomas and Large Serrated Polyps in Individuals With Serrated Polyps on Index Colonoscopy: Data From the New Hampshire Colonoscopy Registry. Gastroenterology. 2018;154:117-127.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 10. | Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1439] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 11. | East JE, Atkin WS, Bateman AC, Clark SK, Dolwani S, Ket SN, Leedham SJ, Phull PS, Rutter MD, Shepherd NA, Tomlinson I, Rees CJ. British Society of Gastroenterology position statement on serrated polyps in the colon and rectum. Gut. 2017;66:1181-1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 201] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 12. | Odze RD. "Sessile Serrated Lesion": The Art and Science of Naming a Disorder. Arch Pathol Lab Med. 2021;145:1190-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Booth AL, Taggart MW, Ono Y, Gonzalez RS. From Mixed Hyperplastic/Adenomatous Polyp to Sessile Serrated Lesion: A Long and Winding Road for Long and Winding Crypts. Arch Pathol Lab Med. 2021;145:1289-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Bettington M, Walker N, Rosty C, Brown I, Clouston A, Wockner L, Whitehall V, Leggett B. Critical appraisal of the diagnosis of the sessile serrated adenoma. Am J Surg Pathol. 2014;38:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Chetty R, Bateman AC, Torlakovic E, Wang LM, Gill P, Al-Badri A, Arends M, Biddlestone L, Burroughs S, Carey F, Cowlishaw D, Crowther S, Da Costa P, Dada MA, d'Adhemar C, Dasgupta K, de Cates C, Deshpande V, Feakins RM, Foria B, Foria V, Fuller C, Green B, Greenson JK, Griffiths P, Hafezi-Bakhtiari S, Henry J, Jaynes E, Jeffers MD, Kaye P, Landers R, Lauwers GY, Loughrey M, Mapstone N, Novelli M, Odze R, Poller D, Rowsell C, Sanders S, Sarsfield P, Schofield JB, Sheahan K, Shepherd N, Sherif A, Sington J, Walsh S, Williams N, Wong N. A pathologist's survey on the reporting of sessile serrated adenomas/polyps. J Clin Pathol. 2014;67:426-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Ono Y, Chiu K, Yantiss RK, Gonzalez RS. Attitudes Regarding the World Health Organization-Recommended Term Sessile Serrated Lesion: Results From an International Survey. Arch Pathol Lab Med. 2021;145:1189-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Nagtegaal ID, Snover DC. Head to head: should we adopt the term 'sessile serrated lesion'? Histopathology. 2022;80:1019-1025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 18. | Bettington M, Walker N, Rosty C, Brown I, Clouston A, McKeone D, Pearson SA, Leggett B, Whitehall V. Clinicopathological and molecular features of sessile serrated adenomas with dysplasia or carcinoma. Gut. 2017;66:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 157] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 19. | Lee JA, Park HE, Yoo SY, Jeong S, Cho NY, Kang GH, Kim JH. CpG Island Methylation in Sessile Serrated Adenoma/Polyp of the Colorectum: Implications for Differential Diagnosis of Molecularly High-Risk Lesions among Non-dysplastic Sessile Serrated Adenomas/Polyps. J Pathol Transl Med. 2019;53:225-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Vu NTH, Le HM, Vo DT, Vu HA, Le NQ, Ho DDQ, Quach DT. Prevalence, risk factors, and BRAF mutation of colorectal sessile serrated lesions among Vietnamese patients. World J Clin Oncol. 2024;15:290-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 21. | Lash RH, Genta RM, Schuler CM. Sessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol. 2010;63:681-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 235] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 22. | Yang JF, Tang SJ, Lash RH, Wu R, Yang Q. Anatomic distribution of sessile serrated adenoma/polyp with and without cytologic dysplasia. Arch Pathol Lab Med. 2015;139:388-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Cenaj O, Gibson J, Odze RD. Clinicopathologic and outcome study of sessile serrated adenomas/polyps with serrated versus intestinal dysplasia. Mod Pathol. 2018;31:633-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Liu C, Walker NI, Leggett BA, Whitehall VL, Bettington ML, Rosty C. Sessile serrated adenomas with dysplasia: morphological patterns and correlations with MLH1 immunohistochemistry. Mod Pathol. 2017;30:1728-1738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Batts KP, Cinnor B, Kim A, Stickney E, Burgart LJ. Sessile Serrated Adenoma With Dysplasia of the Colon. Am J Clin Pathol. 2022;157:180-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Morales SJ, Bodian CA, Kornacki S, Rouse RV, Petras R, Rouse NA, Cohen LB, Bamji ND, Miller KM, Soetikno RM, Kaltenbach T, Aisenberg J. A simple tissue-handling technique performed in the endoscopy suite improves histologic section quality and diagnostic accuracy for serrated polyps. Endoscopy. 2013;45:897-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Diem VTN, Thai TH, Thinh NP, Phuong HT. Histological classification on serrated lesion/poly by WHO 2019. Vietnam Med J. 2023;529:322-326. |
| 28. | Bateman AC. The spectrum of serrated colorectal lesions-new entities and unanswered questions. Histopathology. 2021;78:780-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Jaravaza DR, Rigby JM. Hyperplastic polyp or sessile serrated lesion? The contribution of serial sections to reclassification. Diagn Pathol. 2020;15:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Schramm C, Kaiser M, Drebber U, Gruenewald I, Franklin J, Kuetting F, Bowe A, Hoffmann V, Gatzke S, Toex U, Steffen HM. Factors associated with reclassification of hyperplastic polyps after pathological reassessment from screening and surveillance colonoscopies. Int J Colorectal Dis. 2016;31:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Singh H, Bay D, Ip S, Bernstein CN, Nugent Z, Gheorghe R, Wightman R. Pathological reassessment of hyperplastic colon polyps in a city-wide pathology practice: implications for polyp surveillance recommendations. Gastrointest Endosc. 2012;76:1003-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Lin YC, Chiu HM, Lee YC, Shun CT, Wang HP, Wu MS. Hyperplastic polyps identified during screening endoscopy: reevaluated by histological examinations and genetic alterations. J Formos Med Assoc. 2014;113:417-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Bettington ML, Walker NI, Rosty C, Brown IS, Clouston AD, McKeone DM, Pearson SA, Klein K, Leggett BA, Whitehall VL. A clinicopathological and molecular analysis of 200 traditional serrated adenomas. Mod Pathol. 2015;28:414-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 34. | Chetty R, Hafezi-Bakhtiari S, Serra S, Colling R, Wang LM. Traditional serrated adenomas (TSAs) admixed with other serrated (so-called precursor) polyps and conventional adenomas: a frequent occurrence. J Clin Pathol. 2015;68:270-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Kim KM, Lee EJ, Kim YH, Chang DK, Odze RD. KRAS mutations in traditional serrated adenomas from Korea herald an aggressive phenotype. Am J Surg Pathol. 2010;34:667-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 36. | Kim MJ, Lee EJ, Suh JP, Chun SM, Jang SJ, Kim DS, Lee DH, Lee SH, Youk EG. Traditional serrated adenoma of the colorectum: clinicopathologic implications and endoscopic findings of the precursor lesions. Am J Clin Pathol. 2013;140:898-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Yokoo H, Usman I, Wheaton S, Kampmeier PA. Colorectal polyps with extensive absorptive enterocyte differentiation: histologically distinct variant of hyperplastic polyps. Arch Pathol Lab Med. 1999;123:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Pai RK, Bettington M, Srivastava A, Rosty C. An update on the morphology and molecular pathology of serrated colorectal polyps and associated carcinomas. Mod Pathol. 2019;32:1390-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 39. | Aust DE, Baretton GB; Members of the Working Group GI-Pathology of the German Society of Pathology. Serrated polyps of the colon and rectum (hyperplastic polyps, sessile serrated adenomas, traditional serrated adenomas, and mixed polyps)-proposal for diagnostic criteria. Virchows Arch. 2010;457:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | Quirke P, Risio M, Lambert R, von Karsa L, Vieth M. Quality assurance in pathology in colorectal cancer screening and diagnosis—European recommendations. Virchows Arch. 2011;458:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 41. | Hashimoto T, Tanaka Y, Ogawa R, Mori T, Yoshida H, Taniguchi H, Hiraoka N, Kojima M, Oono Y, Saito Y, Sekine S. Superficially serrated adenoma: a proposal for a novel subtype of colorectal serrated lesion. Mod Pathol. 2018;31:1588-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |