Published online Aug 24, 2024. doi: 10.5306/wjco.v15.i8.1078
Revised: July 20, 2024
Accepted: July 29, 2024
Published online: August 24, 2024
Processing time: 147 Days and 18.6 Hours
Modern pharmacological studies have confirmed that plant-derived compounds from Puerariae flos (PF) has significant biological activities against liver damage, tumors and inflammation. Kakkatin is an isoflavone polyphenolic compound isolated from PF flower. However, the effect of kakkatin and its derivatives on anti-tumor has not been well explored.
To design and synthesize a kakkatin derivative [6-(hept-6-yn-1-yloxy)-3-(4-hydroxyphenyl)-7-methoxy-4H-chromen-4-one (HK)] to explore its anti-tumor biological activity.
Hept-6-yn-1-yl ethanesulfonate was introduced to replace hydrogen at the hy
Compared with kakkatin, the modified HK did not significantly increase the inhibitory activity of gastric cancer MGC803 cells, but the inhibitory activity of HCC SMMC-7721 cells was increased by about 30 times, with an IC50 value of 2.5 μM, and the tumor inhibition effect was better than cisplatin, which could significantly inhibit the cloning, invasion and metastasis of HCC SMMC-7721 cells, and induce apoptosis and G2/M cycle arrest. Its mechanism of action is mainly related to the upregulation of PDE3B and NFKB1 target proteins in the cAMP pathway.
HK have a significant inhibitory effect on HCC SMMC-7721 cells, and the targets of their action may be PDE3B and NFKB1 proteins in the cAMP pathway, making it a good lead drug for the treatment of HCC.
Core Tip: We synthesized a new kakkatin derivative [6-(hept-6-yn-1-yloxy)-3-(4-hydroxyphenyl)-7-methoxy-4H-chromen-4-one (HK)] and explored its anti-tumor activity against liver cancer, gastric cancer and its potential mechanism of action. It was found that the synthesized HK increased the anti-hepatoma activity by nearly 30 times compared with kakkatin, but the inhibitory effect on gastric cancer MGC803 cells was not much different from that of kakkatin, indicating that the anti-tumor activity of HK may be more specific. PDE3B and NFKB1 proteins in the cAMP pathway are the targets of the HK on HCC SMMC-7721 cells.
- Citation: Jiang YY, Dong HH, Zhou WT, Luo JZ, Wei X, Huang YQ. Preparation of kakkatin derivatives and their anti-tumor activity. World J Clin Oncol 2024; 15(8): 1078-1091
- URL: https://www.wjgnet.com/2218-4333/full/v15/i8/1078.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i8.1078
Hepatocellular carcinoma (HCC) is the most common primary liver cancer, and as the sixth leading cause of cancer worldwide, HCC has a high mortality rate and an increasing incidence year-on-year, with an estimated incidence of more than 1 million cases arising by 2025[1,2]. In the past decade, hepatic arterial perfusion chemoembolization has been the preferred first-line treatment for patients with advanced HCC, and the multi-kinase inhibitor sorafenib has become the mainstay of treatment[3]. However, studies have found that sorafenib only has a therapeutic effect on about 30% of HCC patients, and targeted drug resistance usually occurs within six months, and it can also cause toxic side effects in the body. If the disease is severe, sorafenib can also cause high blood pressure and abdominal pain, which can interrupt treatment, greatly reducing the effectiveness of sorafenib and limiting treatment options available to patients[4]. At present, the commonly used treatment methods for HCC give rise to problems such as toxic side effects, poor efficacy, ease of recurrence and metastasis after surgery, and a high drug resistance rate, which greatly reduce the quality of life of patients and adversely affects subsequent further treatment, making it necessary to develop new, effective anti-cancer treatment drugs.
In recent years, chemotherapy and prophylaxis using plant-derived compounds have achieved satisfactory results due to their high efficiency, effectiveness, and few side effects, and have become an attractive therapeutic strategy[5]. As a traditional Chinese medicine in China, Puerariae flos (PF) has been used for thousands of years to strengthen the spleen, clear the lungs, and relieve hangover; modern pharmacological studies have also confirmed that it has significant biological activities in ameliorating hangover, liver damage, tumors, inflammation, oxidation, etc., providing theoretical support for traditional medicine[6]. At the same time, Gehua Jiecheng decoction, which is based on PF flowers, has been found to inhibit the proliferation, invasion, migration, apoptosis, and epithelial-mesenchymal transformation of hepatocellular cancer cells, thereby exerting anti-tumor effects[7]. Kakkatin is an isoflavone isolated from PF flower and it is also a polyphenolic compound. Polyphenolic compounds exhibit different activities when they have different substituents on the same parent nucleus[8]. Therefore, we synthesized a new kakkatin derivative (6-(hept-6-yn-1-yloxy)-3-(4-hydro
In the present study, SMMC-7721, a human HCC cell line purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences, was used as the research object, and RPMI 1640 complete medium (KeyGEN BioTECH, Shanghai) containing 100 mL/L fetal bovine serum (KeyGEN BioTECH, Shanghai) and 10 mL/L penicillin-streptomycin bispecific antibody mixed solution (KeyGEN BioTECH, Shanghai) was placed in a humidified incubator at 37 °C and 50 mL/L CO2 at constant temperature. The cells in the logarithmic growth phase were used for subsequent experiments.
Firstly, kakkatin was dissolved in N, N-dimethylformamide, and K2CO3 was added with a molar ratio of potassium carbonate and kakkatin of 1.2:1, and stirred at room temperature for 15 minutes. Then, the substitution reaction was conducted, and the hept-6-yn-1-yl ethanesulphonate competing group was introduced into the phenolic hydroxyl group at the No. 6 position on the kakkatin structure [the molar ratio of the hept-6-yn-1-yl ethanesulphonate ester to kakkatin was 1:1, the components were stirred overnight at 50 °C, and the reaction was monitored by thin-layer chromatography and liquid chromatography-mass spectrometry (LCMS) until complete]. Finally, the specimen was purified, filtered, and passed through a reverse flash column to obtain a novel HK.
HCC SMMC-7721 cells and gastric cancer MGC803 cells (2 × 104 cells/well) were seeded in 96-well plates, and HK were added at working concentrations of 0.25 μM, 0.5 μM, 1 μM, 2 μM, 4 μM, 20 μM, 32 μM, and 64 μM, kakkatin, cisplatin (CDDP) and phosphate buffer saline (PBS) were set as control groups at the same concentration. After 24 hours of treatment, 10 μL of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (5 mg/mL, BioFroxx, Guangzhou) reagent was added to each well, mixed well and continued to incubate at 37 °C for 4 hours in the dark, the medium was removed, 100 μL of dimethyl sulfoxide solution (MACKLIN, Shanghai) was added to each well, and after shaking and mixing for 10 minutes, the absorbance at 490 nm was measured by microplate reader, and the cell viability and semi-inhibitory concentration of the drug (IC50) were calculated. The cell viability rate was given by [(As - Ab)]/[(Ac - Ab)] × 100 %, where As is a cell culture medium with drug wells, Ac is a cell culture medium without drug wells, and Ab is a medium with neither cells nor drugs. The experiment was repeated three times.
SMMC-7721 cells were seeded at a density of 700 cells per well in six-well cell plates, cultured for 24 hours, then treated with different concentrations of HK: The positive drug CDDP was used as a control and the specimens were incubated at 37 °C, at 50 mL/L CO2 and saturated humidity for 14 days. During this period, the corresponding drug-containing medium of each group was changed every two to three days, and the cell status observed; the specimens were finally fixed with 40 mL/L paraformaldehyde solution (Biosharp, Anhui), then subjected to 1 mL/L crystal violet staining (Macklin, Shanghai), PBS washing and drying, before the colony of the cells was cloned and counted the stained cells with ImageJ software.
After treating SMMC-7721 cells with different concentrations of HK for 24 hours and 48 hours, the cell culture medium was aspirated into a centrifuge tube for later use, the adherent cells were re-digested with trypsin, and then the collected cell culture medium was re-added, centrifuged at 1000 g for 5 minutes, the supernatant was discarded, PBS was washed three times and the cells were resuspended and counted, and the cell density was prepared to 1 × 105 cells/mL, centrifuged again at 1000 g for 5 minutes, and 195 μL of Annexin was added after discarding the supernatant. The cells were gently resuspended with Annexin V-fluorescein isothiocyanate (FITC) conjugate solution, mixed with 5 μL of Annexin V-FITC and 10 μL of propidium iodide (PI) staining solution, and incubated for 10-20 minutes at room temperature in the dark, followed by immediate detection of the rate of apoptosis of the cells using a flow cytometer.
SMMC-7721 cells were treated with different concentrations of HK for 24 hours, then trypsinized: The digested cells and culture medium were collected, centrifuged at 1000g for 3-5 minutes, the supernatant was discarded, the cells resu
SMMC-7721 cells in the logarithmic growth phase were seeded in a six-well plate (5 × 105 cells per well), incubated for 24 hours, and after the cells were full, they were evenly scratched in a vertical orientation in the wells with a sterile pipette from top to bottom, the cells were gently rinsed with PBS, and the serum-free medium containing different concentrations of HK was added, with CDDP used as the positive control group. The cells were incubated in a constant temperature incubator for 0 hours, 6 hours, 12 hours, 24 hours, 48 hours, and 72 hours, and the cell migration was recorded under an inverted microscope. The cell migration area at different times was calculated to assess the inhibitory effect of HK on the lateral migration of SMMC-7721 cells.
First, we removed the serum from the medium, starved SMMC-7721 cells for 12-24 hours, trypsinized, resuspended the cells with serum-free medium, and adjusted the cell density to 5 × 105 cells per well after counting. After sterilization, we placed the transwell insert in a 24-well plate with 600 μL of serum-containing medium per well in the lower insert and 200 μL of cell suspension per well in the upper insert. After 24 hours of treatment using different concentrations of HK, 40 mL/L paraformaldehyde and 1 mL/L crystal violet were used for fixed staining, and those purple transmembrane cells were observed and counted under an inverted microscope, and the specimens photographed.
First, the compound structure was mapped using the Swisstarget prediction platform (http://www.swisstargetpre
ADRB1, PDE3B, PRKACB, and NFKB1 were selected as the receptor proteins, and HK was selected as the ligand small molecule. The receptor proteins were imported into PyMOL 2.6.0 software for dehydration, deliganding, and then hydrogenated and non-polar hydrogen binding using AutoDockTools 1.5.7 software and converted to pdbqt format. The Grid function of the AutoDockTools 1.5.7 software was used to identify regions of the protein receptor that bind to the ligand, i.e., the active pocket. The protein data bank format ligand small molecule was imported into AutoDockTools 1.5.7, hydrogenated, calculated, and set the twist key. According to the docking pockets obtained in the previous step and the processed receptors and ligands, the molecular docking was performed using Autodock Vina1.1.2 software, and the affinity energy and hydrogen bonding of amino acid residues were analyzed by PyMOL.
The number of cells of HCC SMMC-7721 was adjusted to 1 × 106, and the cells were cultured for 12 hours. HK with a concentration of 10 times IC50 was added, and the cells were collected by centrifugation for 12 hours, the RNA was extracted with SevenFast Total RNA Extraction Kit for Cells reagent (SEVEN, China) after lysis, and the RNA was reverse transcribed into complementary DNA with a reverse transcription kit (MONPURE, China). Real-time PCR assays (RT-qPCR) was performed using LightCycler according to the kit (MONPURE, China) with pre-denaturation at 95 °C for 30 seconds, denaturation at 95 °C for 10 seconds, annealing and extension at 60 °C for 30 seconds (40 cycles). The 16S ribosomal RNA amplicon was used as an internal control for data normalization by applying a relative quantitative method to determine the corresponding level changes.
All obtained data were presented as mean ± SD, and statistical analyses were processed using GraphPad Prism 10.1.2 software, and statistical analyses were conducted using either a t test for two groups of comparisons or an analysis of variance for multiple group comparisons. P < 0.05 indicates a statistically significant difference. The smaller the P value, the greater the difference between the groups being compared.
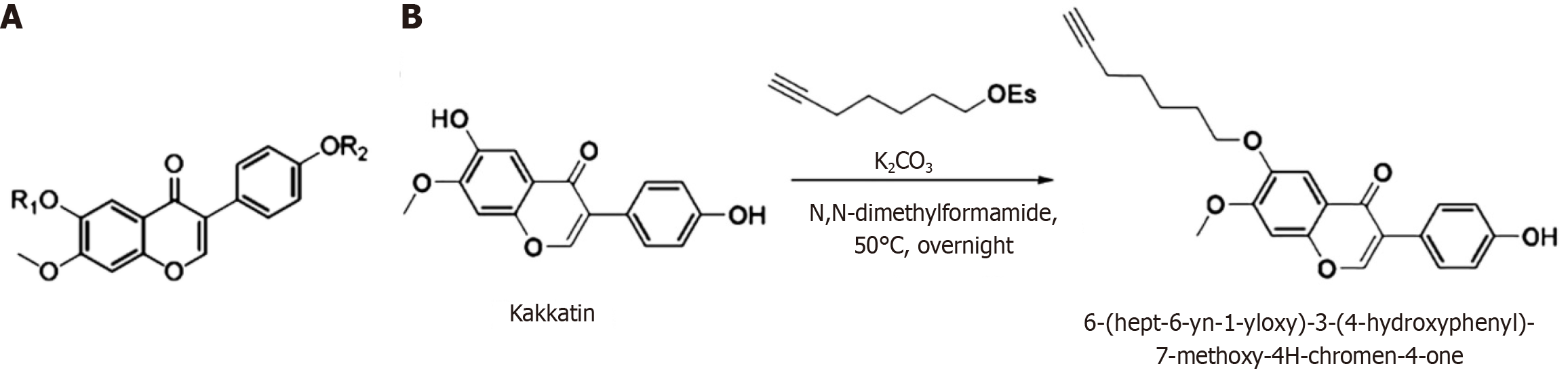
HK with a structural formula is shown in Figure 1A, where R1 and R2 are H, CH3, CH2F, CHF2, CF3, t-Bu, MOM, EE, THP, linear or branched alkanes with a carbon atomic number of 1-5, ester group [Ac, t-BuCO, PhCO, Ts, Ms, Tf, PO(OR') 2: R' = Alkyl or Ar, etc.], silicon ether (TMS, TES, TBS, TIPS, TBDPS, etc.), trifluoroalkanes or polyfluoroalkanes with carbon niches of 1-5, straight-chain terminal alkynes with carbon electron-counts of 1-6, n = 1, 2, 3, 4, or 5, m = 1, 2, 3, 4, 5, or 6, when one group is a terminal alkyne, the other two groups are alkanes or fluoroalkanes. Finally, we synthesized a white solid HK 3.22 mg in 12.09% yield, which was linked with hept-6-yn-1-yl ethanesulfonate at the phenolic hydroxyl position of kakkatin (Figure 1B).
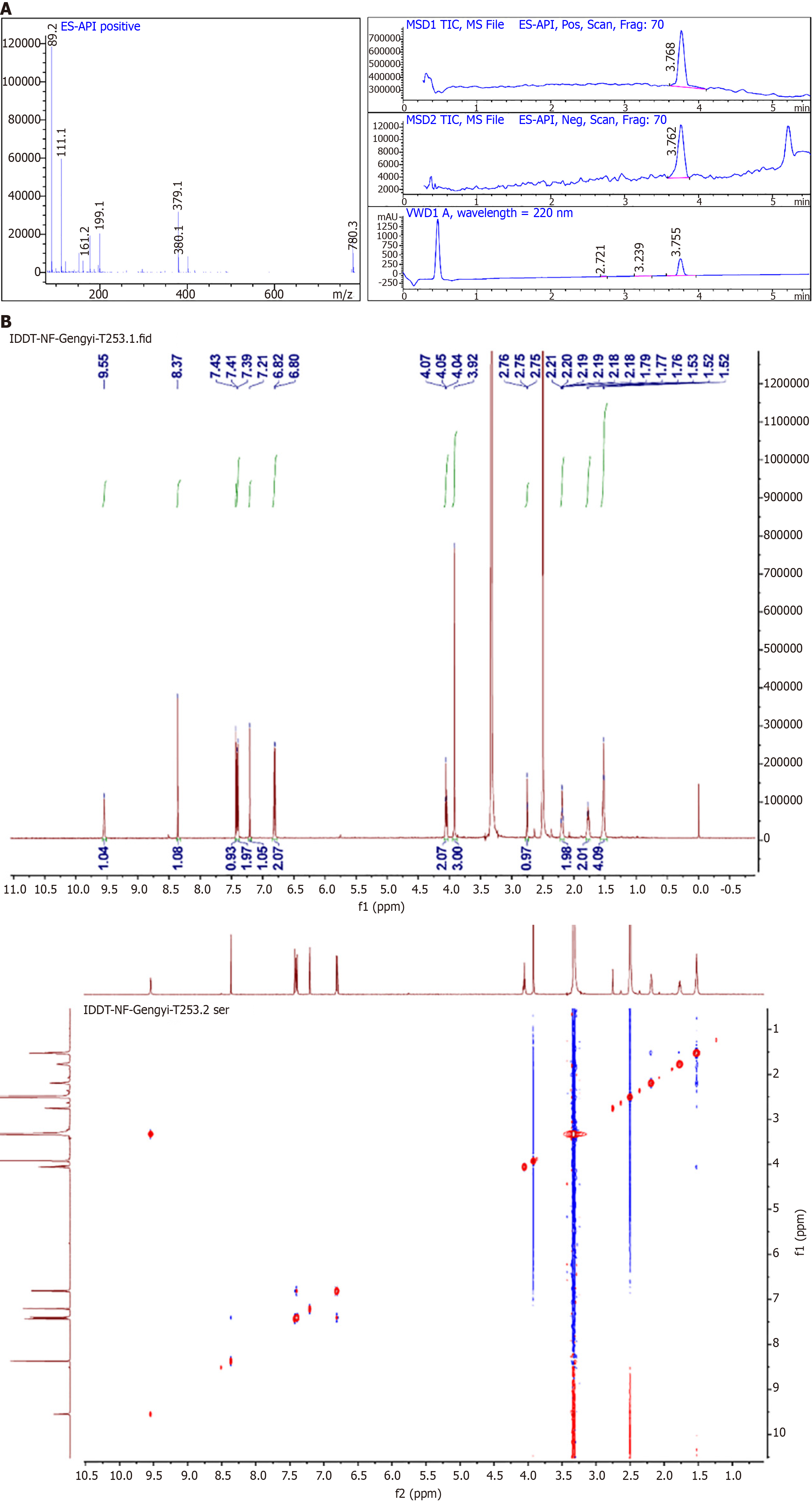
The structure of the HK was then characterized by liquid chromatography-mass spectrometry (LCMS, Figure 2A) and nuclear magnetic resonance (NMR) hydrogen spectroscopy (Figure 2B). 1H NMR (500 MHz, D app-region: None; font-family: 'Times New Roman'; line-height: 32px; text-align: Justify;" >) δ 9.55 (s, 1H), 8.37 (s, 1H), 7.37–7.45 (m, 3H), 7.21 (s, 1H), 6.77–6.83 (m, 2H), 4.06 (t, J = 6.5 Hz, 2H), 3.92 (s, 3H), 2.76 (t, J = 2.6 Hz, 1H), 2.16–2.23 (m, 2H), 1.74–1.83 (m, 2H), 1.49–1.57 (m, 4H). MS (ESI) C23H22O5, calculated thus: [M + H] + 379.1540; found: 378.9.
The anti-tumor activities of kakkatin and HK against HCC SMMC-7721 cells and gastric cancer MGC803 cells were evaluated (Table 1). As shown in Table 1, the IC50 value of the modified HK on SMMC-7721 cells decreased from 76.93 μM to 2.5 μM (c. 30-fold decrease), and the effect was superior to that of CDDP, with a decrease of about 7-fold compared with that of CDDP; the results indicated that the derivatives of kakkatin had a good inhibitory effect on SMMC-7721 cells, but the IC50 value of gastric cancer MGC803 cells was similar to that of kakkatin, suggesting that the anti-tumor activity of HK may be specific.
| Drug | SMMC-7721 cells | MGC803 cells |
| Cisplatin | 17.54 | 20.83 |
| Kakkatin | 76.93 | 38.24 |
| The 6-(hept-6-yn-1-yloxy)-3-(4-hydroxyphenyl)-7-methoxy-4H-chromen-4-one | 2.50 | 39.51 |
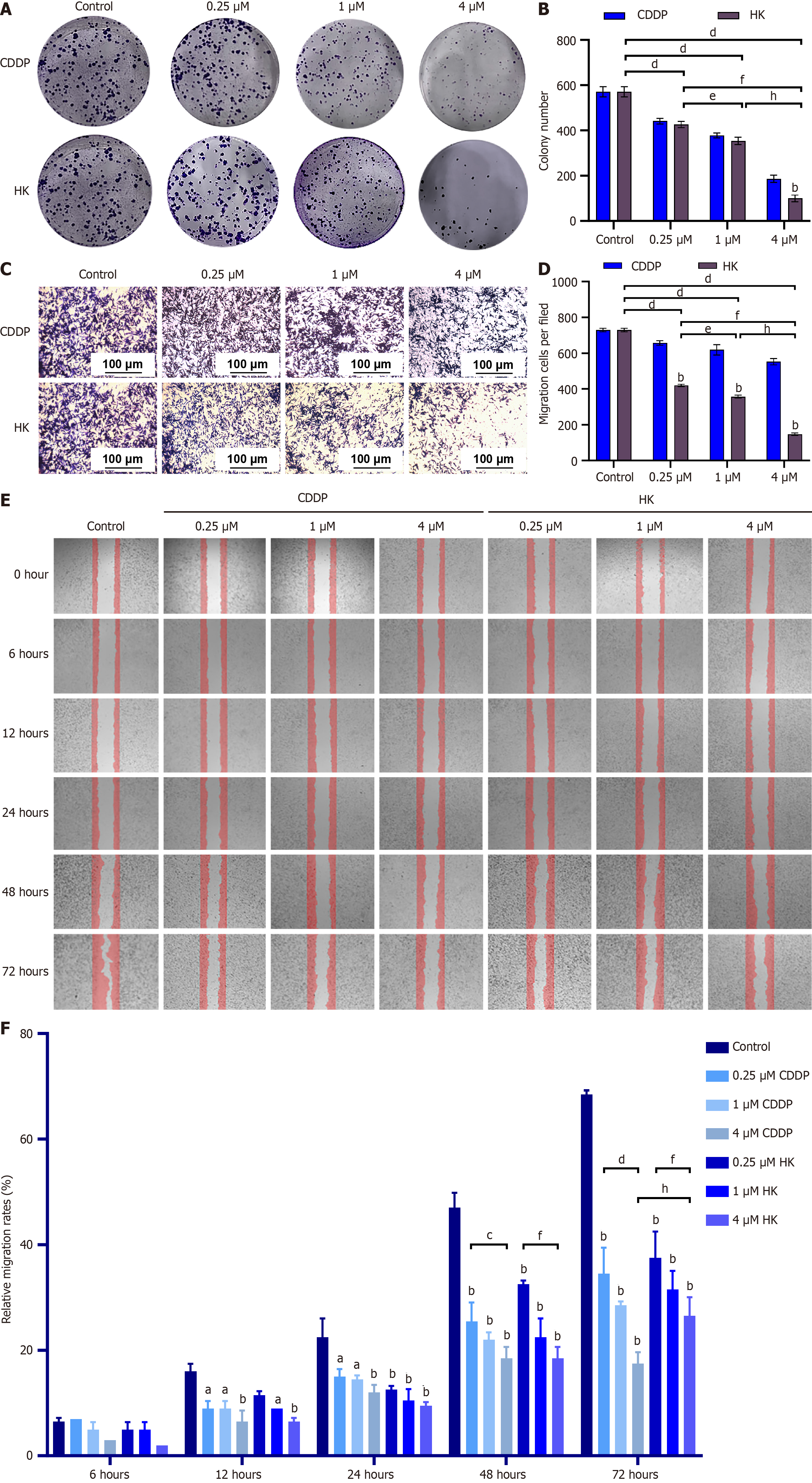
Plate cloning experiments showed that the colony formation of SMMC-7721 cells decreased significantly with the increase of the concentration of HK and the effect of HK in inhibiting cell proliferation was better than that of the positive control CDDP group (Figure 3A and B). The results of the transwell assay indicated that after 24 hurs of treatment of SMMC-7721 cells with different concentrations of HK, the number of cells in the lower compartment decreased with the increase of the concentration of HK and the cell migration and invasion ability were significantly inhibited (Figure 3C and D). The cell scratch assay showed that, compared with the CDDP group, the migration of SMMC-7721 cells was inhibited after 6 hours and the wound-healing rate was significantly reduced in a time and dose-dependent manner at 24 hours, 48 hours, and 72 hours. (Figure 3E and F).
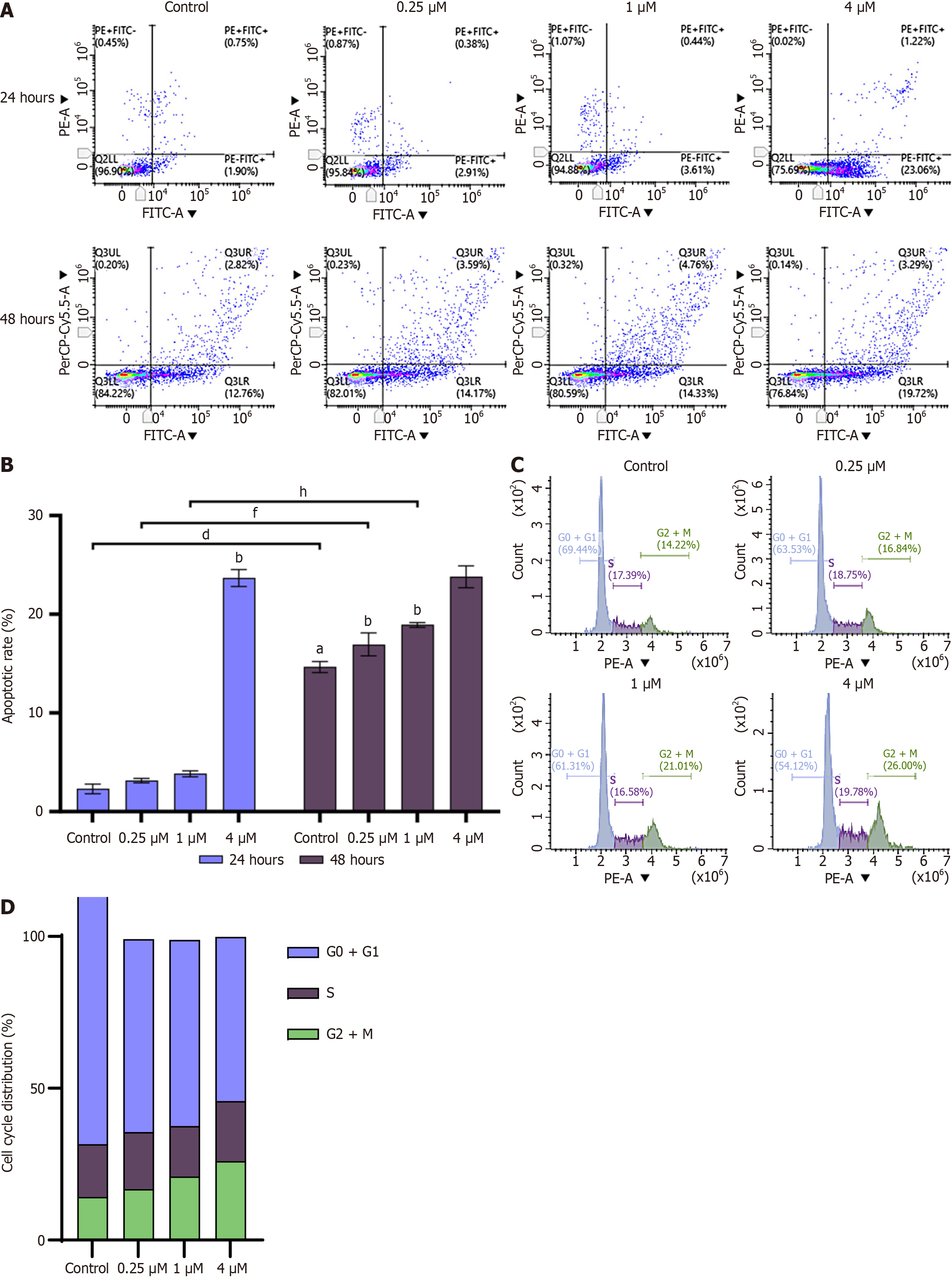
The further to investigate the inhibitory effect of HK on cell growth, apoptosis and changes in cell cycle stages were analyzed using flow cytometry. After treating SMMC-7721 cells with 0.25 μM, 1 μM, and 4 μM HK for 24 hours and 48 hours, respectively, early and late apoptotic and necrotic cells increased with increasing concentration compared to control and these concentrations of HK promoted apoptosis in a dose and time-dependent manner (Figure 4A and B). After 24 hours of treatment of HCC SMMC-7721 cells, the proportion of cells in the G2/M phase and the distribution of cells in the G0/G1 phase were significantly increased. It was concluded that the HK inhibited the growth of HCC SMMC-7721 cells by inducing partial G2/M cell cycle arrest in a dose-dependent manner to achieve an anti-tumor effect (Figures 4C and D).
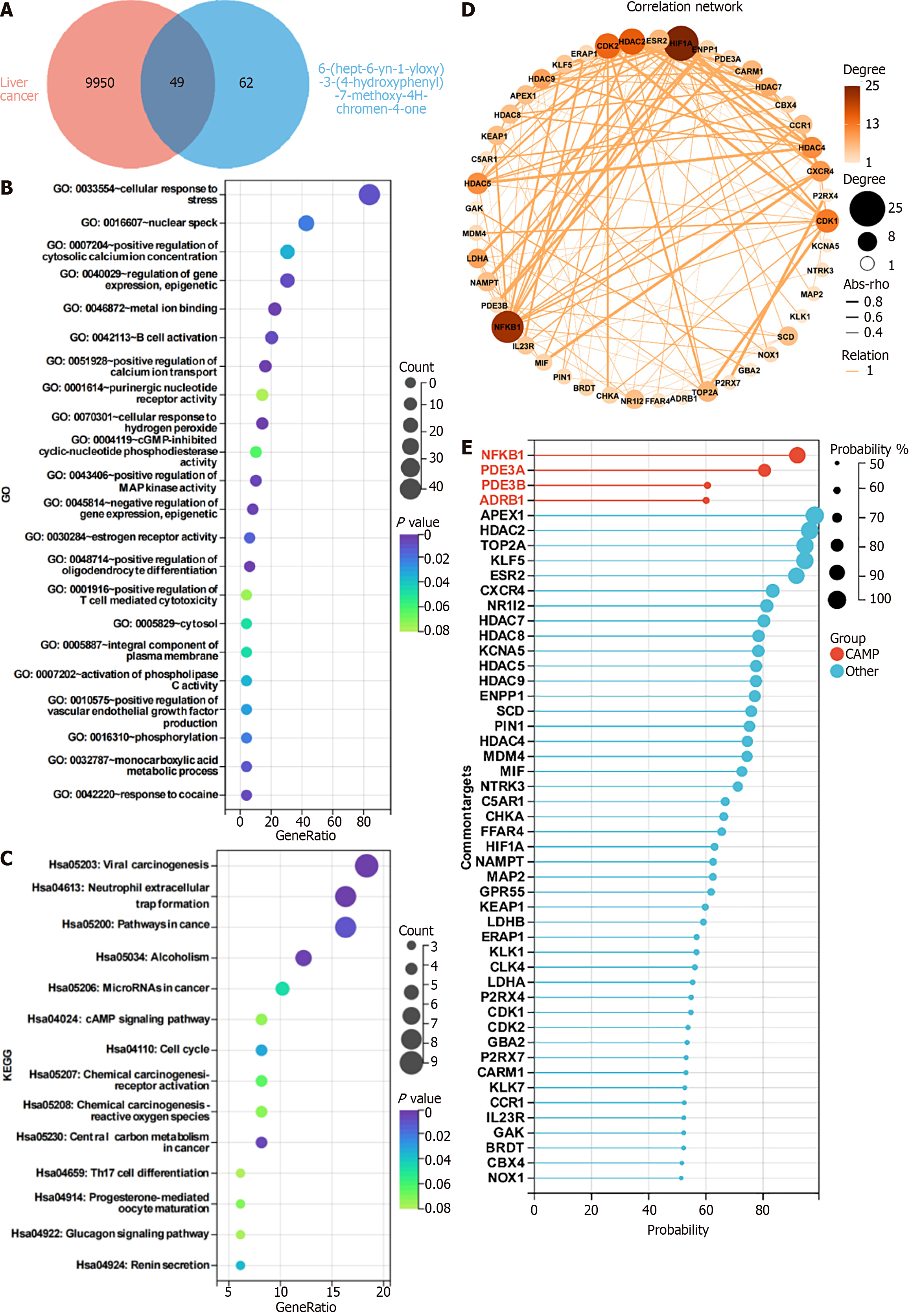
Network pharmacology analysis of the targets of HK: By searching the SwissTargetPrediction database, a total of 62 targets of HK were obtained. A total of 9950 relevant targets of known HCC SMMC-7721 cells were screened in the GeneCards database, of which 49 targets were shared, as shown in Figure 5A. Enrichment analysis was performed on these 49 targets and the results of GO analysis showed that HK may bind metal ions, while providing positive regulation of calcium ion transport and cellular response to hydrogen peroxide. It is related to the positive regulation of MAP kinase activity and other functions, as shown in Figure 5B. The results of KEGG analysis implied that HK may be related to the cAMP signaling pathway and neutrophil extracellular trap formation, as shown in Figure 5C. At the same time, the network diagram shows 49 potential targets for SMMC-7721, as illustrated in Figure 5D. NFKB1, as one of the strongly associated targets, has been studied to confirm the inhibitory effect on liver cancer and points to the cAMP pathway, as shown in Figure 5E, so we selected NFKB1, ADRB1, and PDE family PDE3B in the cAMP pathway, and the well-known PRKACB in the PKA family downstream of the cAMP pathway as affected thereby for subsequent validation.
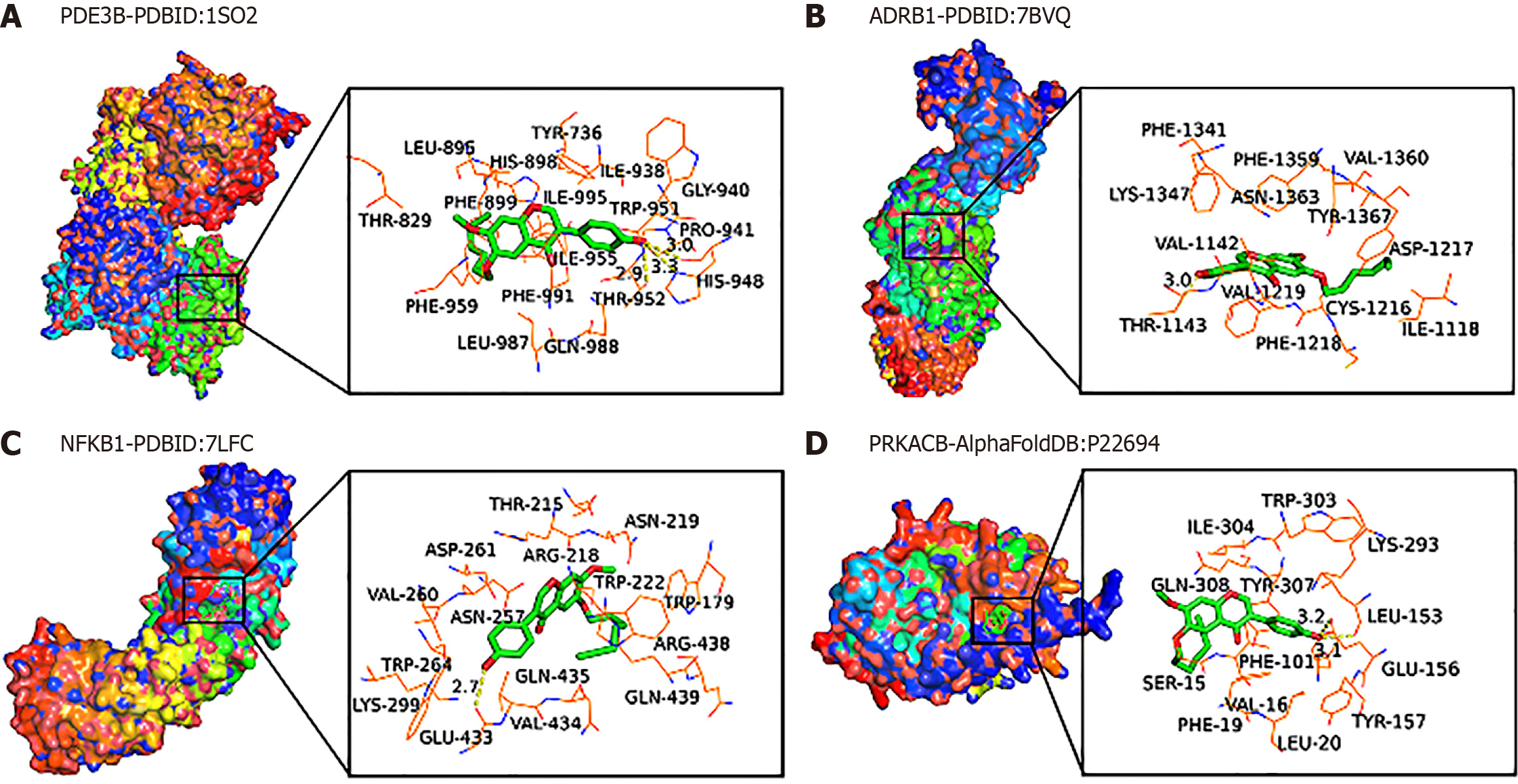
Molecular docking mimics the target protein of HK: To characterize the interaction between the protein of interest and HK, AutoDock Tools software was used to perform molecular docking of some core targets and target-related active components in the protein network. In practice, binding energies are often used to assess the degree of receptor-ligand affinity. It is generally believed that the binding energy is less than -4.25 kcal/mol, -5.0 kcal/mol, or -7.0 kcal/mol (1 kcal = 4.2 kJ), indicating that there is a certain, good, or strong binding activity between the receptor and the ligand, respectively. The lower the binding energy, the higher the affinity of the receptor to the ligand, and the more stable the conformation. The results of this study are displayed in Table 2, and the results of molecular docking of HK are less than -5.0 kcal/moL, indicating that these four target genes have good binding activity with HK, and the binding energies of ADRB1 and PDE3B are -9.6 kcal/mol and -9.5 kcal/mol, respectively, indicating that these two target genes can form a relatively stable conformation with HK (Figure 6) and thus play a role, therefore, RT-qPCR experiments were conducted to verify the presence and effect of these four genes.
| Complex | Gene | Affinity (kcal/mol) |
| 7bvq-molecule1-1 | ADRB1 | -9.6 |
| 1so2-molecule1-1 | PDE3B | -9.5 |
| P22694-molecule1-1 | PRKACB | -7.4 |
| 7 Lfc-molecule1-1 | NFKB1 | -5.9 |
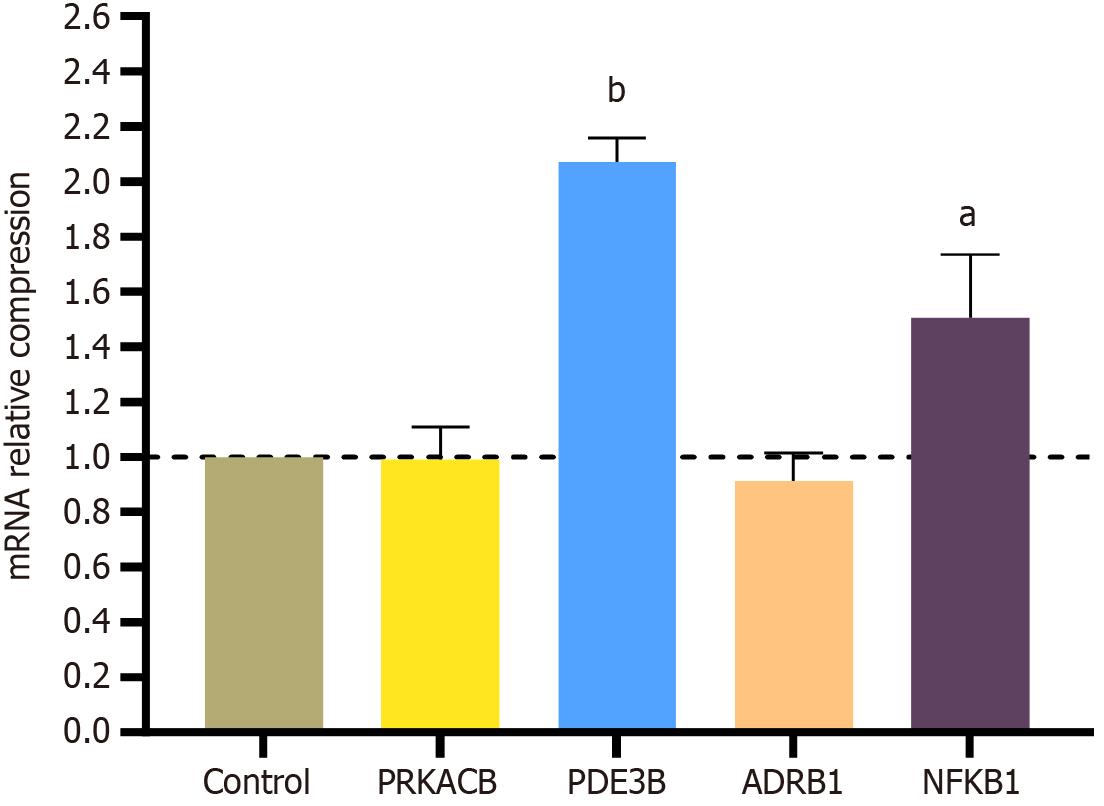
In the RT-qPCR validation results (Figure 7), PDE3B and NFKB1 were significantly upregulated in the drug group. Concetti et al[12] stated that NFKB1 is an inhibitor of HCC. The experimental results of Feng et al[13] found that PDE3B, as a cuproptosis-related gene, can reduce cancer invasion and migration.
Aiming at the various pharmacological activities of isoflavones in PF, HK was designed and synthesized on the basis of the structure of kakkatin. The molecular structure of HK was confirmed through the corresponding LCMS and 1H NMR characterization, which laid a foundation for further exploration of its biological activity. In the present study, the inhibitory activity of kakkatin and HK on gastric cancer MGC803 cells and HCC SMMC-7721 cells was screened by the MTT method. The results showed that the IC50 values of kakkatin and HK on gastric cancer MGC803 cells were similar, but the IC50 value of HK on HCC SMMC-7721 cells was reduced by nearly 30 times compared with kakkatin, and was less than the IC50 of the positive drug CDDP, which significantly improved the inhibitory activity of HCC SMMC-7721 cells. Therefore, in response to this result, the effects of HK on the proliferation, invasion and migration ability, apoptosis and cycle arrest of HCC SMMC-7721 cells were further analyzed.
The literature states that the spread and metastasis of cancer cells remains the leading cause of cancer-related deaths worldwide and our inability to identify tumor cells that have metastasized and colonized distant sites hinders the development of anti-metastatic therapies, so inhibition of cancer cell proliferation and metastasis is important for cancer treatment[14]. Cell cloning experiments indicated that the HK inhibited the colony formation of HCC SMMC-7721 cells in a dose-dependent manner at 0.25 μg/mL, 1 μg/mL, and 4 μg/mL, thereby suppressing the proliferation and growth of cancer cells. Cell scratch assay and transwell assay showed that different concentrations of HK could reduce the lateral and longitudinal migration ability of cells, and the inhibition effect was better than CDDP. Traditionally, apoptosis has been thought to be a spontaneous process, but recent studies at the animal level have found that apoptotic cells play an important role in the growth, phagocytosis, proliferation and mechanical stimulation of surrounding cells[15]. HCC SMMC-7721 cells were treated with different concentrations of HK for 24 hours and 48 hours, respectively, and the number of early and late apoptotic and necrotic cells gradually increased in a time and concentration-dependent manner. An abnormal cell cycle is prevalent in cancer and plays an important role in the development of cancer metabolism, immunity, and metastasis[16]. Flow cytometry results implied that the process of the cell cycle could be changed after 24 hours of treatment and the cell cycle was arrested at the G2/M phase.
The further to understand the functions and pathways of the core targets, GO function enrichment and pathway analysis were conducted on 49 core targets. The results of GO analysis suggested that HK mainly regulated metal ion binding, positive regulation of calcium ion transport, cellular response to hydrogen peroxide and positive regulation of MAP kinase activity. The results of KEGG pathway enrichment analysis showed that the effect of HK on HCC SMMC-7721 cells mainly involved the cAMP signaling pathway and the formation of neutrophil extracellular traps. These results indicated that HK inhibited the activity of HCC SMMC-7721 cells by regulating multiple channels.
Network pharmacology showed that NFKB1 was a strongly associated target and pointed to the cAMP pathway, indicating that HK mainly exerted tumor suppressive activity against HCC SMMC-7721 cells through targets related to the cAMP pathway, such as PDE3B, ARBD1, NFKB1 and PRKACB[17]. Therefore, the molecular docking of HK with the four targets showed that their binding energies were less than -5 kcal/mol, indicating that the binding energy of HK was stable with the core target proteins. Finally, RT-qPCR results showed that PDE3B and NFKB1 were significantly up-regulated after HK was applied to HCC SMMC-7721 cells. The role of NFKB1 in liver cancer has been extensively studied and it has been confirmed to be a suppressor of liver cancer and its upregulation plays an important role in the pre
The further to understand the functions and pathways of the core targets, GO function enrichment and pathway analysis were conducted on 49 core targets. The results of GO analysis suggested that HK mainly regulated metal ion binding, positive regulation of calcium ion transport, cellular response to hydrogen peroxide and positive regulation of MAP kinase activity. The results of KEGG pathway enrichment analysis showed that the effect of HK on HCC SMMC-7721 cells mainly involved the cAMP signaling pathway and the formation of neutrophil extracellular traps. These results indicated that HK inhibited the activity of HCC SMMC-7721 cells by regulating multiple channels.
Network pharmacology showed that NFKB1 was a strongly associated target and pointed to the cAMP pathway, indicating that HK mainly exerted tumor suppressive activity against HCC SMMC-7721 cells through targets related to the cAMP pathway, such as PDE3B, ARBD1, NFKB1 and PRKACB[17]. Therefore, the molecular docking of HK with the four targets showed that their binding energies were less than -5 kcal/mol, indicating that the binding energy of HK was stable with the core target proteins. Finally, RT-qPCR results showed that PDE3B and NFKB1 were significantly up-regulated after HK was applied to HCC SMMC-7721 cells. The role of NFKB1 in liver cancer has been extensively studied and it has been confirmed to be a suppressor of liver cancer and its upregulation plays an important role in the pre
In summary, a derivative of kakkatin was prepared and it was found that HK could promote apoptosis, induce cell cycle arrest, and enhance the inhibitory activity of HCC SMMC-7721 cells in a concentration-dependent manner. Network prediction, molecular docking, and RT-qPCR experiments showed that HK inhibited the proliferation and migration of HCC SMMC-7721 cells by upregulating PDE3B and NFKB1 targets in the cAMP pathway. In conclusion, the HK had better inhibitory effects on HCC SMMC-7721 cells and their target was PDE3B and NFKB1 proteins in the cAMP pathway, which provided a good lead drug for the treatment of liver cancer.
The authors are thankful to The Youjiang Medical University for Nationalities for their great help during the research.
| 1. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3887] [Article Influence: 971.8] [Reference Citation Analysis (3)] |
| 2. | Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 423] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 3. | Huang A, Yang XR, Chung WY, Dennison AR, Zhou J. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther. 2020;5:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 473] [Cited by in RCA: 495] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 4. | Tang W, Chen Z, Zhang W, Cheng Y, Zhang B, Wu F, Wang Q, Wang S, Rong D, Reiter FP, De Toni EN, Wang X. The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct Target Ther. 2020;5:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 701] [Article Influence: 140.2] [Reference Citation Analysis (0)] |
| 5. | Liu C, Yang S, Wang K, Bao X, Liu Y, Zhou S, Liu H, Qiu Y, Wang T, Yu H. Alkaloids from Traditional Chinese Medicine against hepatocellular carcinoma. Biomed Pharmacother. 2019;120:109543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 6. | Chen C, Li X, Kano Y, Yuan D, Qu J. Oriental traditional herbal Medicine--Puerariae Flos: A systematic review. J Ethnopharmacol. 2023;306:116089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Li J, An MY, He JX, Yang Z. Effects of Gehua Jiecheng on the proliferation, invasion, migration, apoptosis and epithelial-mesenchymal transformation of hepatocellular carcinoma cells[J]. Zhongguo Laonianxue Zazhi. 2023;43:2704-2708. [DOI] [Full Text] |
| 8. | Goyal J, Verma PK. An Overview of Biosynthetic Pathway and Therapeutic Potential of Rutin. Mini Rev Med Chem. 2023;23:1451-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 9. | Daina A, Michielin O, Zoete V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019;47:W357-W364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 760] [Cited by in RCA: 2251] [Article Influence: 450.2] [Reference Citation Analysis (0)] |
| 10. | Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y, Kaplan S, Dahary D, Warshawsky D, Guan-Golan Y, Kohn A, Rappaport N, Safran M, Lancet D. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr Protoc Bioinformatics. 2016;54:1.30.1-1.30.33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1289] [Cited by in RCA: 2902] [Article Influence: 322.4] [Reference Citation Analysis (0)] |
| 11. | Sherman BT, Hao M, Qiu J, Jiao X, Baseler MW, Lane HC, Imamichi T, Chang W. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022;50:W216-W221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2064] [Cited by in RCA: 3238] [Article Influence: 1079.3] [Reference Citation Analysis (0)] |
| 12. | Concetti J, Wilson CL. NFKB1 and Cancer: Friend or Foe? Cells. 2018;7:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 13. | Feng Y, Huang Z, Song L, Li N, Li X, Shi H, Liu R, Lu F, Han X, Ding Y, Ding Y, Wang J, Yang J, Jia Z. PDE3B regulates KRT6B and increases the sensitivity of bladder cancer cells to copper ionophores. Naunyn Schmiedebergs Arch Pharmacol. 2024;397:4911-4925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Pascual G, Domínguez D, Benitah SA. The contributions of cancer cell metabolism to metastasis. Dis Model Mech. 2018;11:dmm032920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Kawamoto Y, Nakajima YI, Kuranaga E. Apoptosis in Cellular Society: Communication between Apoptotic Cells and Their Neighbors. Int J Mol Sci. 2016;17:2144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Cheung AH, Hui CH, Wong KY, Liu X, Chen B, Kang W, To KF. Out of the cycle: Impact of cell cycle aberrations on cancer metabolism and metastasis. Int J Cancer. 2023;152:1510-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Palencia-Campos A, Aoto PC, Machal EMF, Rivera-Barahona A, Soto-Bielicka P, Bertinetti D, Baker B, Vu L, Piceci-Sparascio F, Torrente I, Boudin E, Peeters S, Van Hul W, Huber C, Bonneau D, Hildebrand MS, Coleman M, Bahlo M, Bennett MF, Schneider AL, Scheffer IE, Kibæk M, Kristiansen BS, Issa MY, Mehrez MI, Ismail S, Tenorio J, Li G, Skålhegg BS, Otaify GA, Temtamy S, Aglan M, Jønch AE, De Luca A, Mortier G, Cormier-Daire V, Ziegler A, Wallis M, Lapunzina P, Herberg FW, Taylor SS, Ruiz-Perez VL. Germline and Mosaic Variants in PRKACA and PRKACB Cause a Multiple Congenital Malformation Syndrome. Am J Hum Genet. 2020;107:977-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |