Published online Aug 24, 2024. doi: 10.5306/wjco.v15.i8.1048
Revised: June 10, 2024
Accepted: June 27, 2024
Published online: August 24, 2024
Processing time: 146 Days and 19.1 Hours
Celiac disease (CeD) is an autoimmune disorder triggered by the immune res
To investigate the prevalence of MN in hospitalized CeD patients in the United States.
Using data from the National Inpatient Sample spanning two decades, from January 2000 to December 2019, we identified 529842 CeD patients, of which 78128 (14.75%) had MN. Propensity score matching, based on age, sex, race, and calendar year, was employed to compare CeD patients with the general non-CeD population at a 1:1 ratio.
Positive associations were observed for several malignancies, including small intestine, lymphoma, nonmelanoma skin, liver, melanoma skin, pancreas myelodysplastic syndrome, biliary, stomach, and other neuroendocrine tumors (excluding small and large intestine malignant carcinoid), leukemia, uterus, and testis. Conversely, CeD patients exhibited a reduced risk of respiratory and secondary malignancies. Moreover, certain malignancies showed null associations with CeD, including head and neck, nervous system, esophagus, colorectal, anus, breast, malignant carcinoids, bone and connective tissues, myeloma, cervix, and ovary cancers.
Our study is unique in highlighting the detailed results of positive, negative, or null associations between different hematologic and solid malignancies and CeD. Furthermore, it offers insights into evolving trends in CeD hospital outcomes, shedding light on advancements in its management over the past two decades. These findings contribute valuable information to the understanding of CeD’s impact on health and healthcare utilization.
Core Tip: This study from National Inpatient Sample database presents one of the largest studied cohort of celiac disease (CeD) and present significant insights into the association between CeD and various malignancies, including gastrointestinal, genitourinary, hematologic, and gynecologic cancers. Interestingly, CeD patients exhibit a reduced risk of respiratory malignancies. Variations in hospital outcomes, such as length of stay, cost of care, and inpatient mortality, highlight the complex relationship between CeD and malignancies. The study underscores the importance of recognizing this relationship, emphasizing the need for vigilant screening in CeD patients, particularly for specific malignancies like small intestine, lymphoma, and skin cancers. These findings contribute to refining CeD management and understanding broader healthcare trends over two decades.
- Citation: Haider MB, Al Sbihi A, Reddy SN, Green P. Prevalence of malignant neoplasms in celiac disease patients - a nationwide United States population-based study. World J Clin Oncol 2024; 15(8): 1048-1060
- URL: https://www.wjgnet.com/2218-4333/full/v15/i8/1048.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i8.1048
Celiac disease (CeD) is an autoimmune, inflammatory condition developed in genetically predisposed individuals due to the immune response to the gluten component of wheat[1]. The most recent global prevalence of CeD is 1.4% based on serological markers and 0.7% confirmed through histological examination[2]. This disease is characterized by the presence of specific autoantibodies in the bloodstream and distinct pathological changes in the small intestine, including villous atrophy, crypt hypertrophy, and an increase in intraepithelial lymphocytes[3]. What sets CeD apart is that by avoiding gluten, its progression can be halted, and mucosal damage can even be reversed[4].
Individuals with CeD are at an elevated risk of developing other autoimmune conditions, such as autoimmune thyroiditis, type 1 diabetes mellitus, Addison’s disease, and various other disorders[5]. Furthermore, there is a well-established heightened risk of malignancies among CeD patients. Recent research has shown that CeD increases the likelihood of developing specific cancers, including gastrointestinal, lymphomas, skin cancers, and others[6,7]. Studies have indicated that the risk of cancer is most pronounced within the first year after diagnosis and subsequently decreases, likely due to better adherence to a gluten-free diet. Therefore, early diagnosis and gluten avoidance may reduce the risk of such complications[8,9].
Geographic differences in the occurrence of malignancies among individuals with CeD have been observed[10]. However, there is a lack of population-based studies on the connection between CeD and cancer in the United States. In this study, we investigate the prevalence of malignant neoplasms (MN) in CeD patients admitted to hospitals in the United States. We analyze the significance of the association between MN and CeD, categorized by the type of cancer, using a national inpatient database comprising 529842 CeD patients. The second part of our research explores hospital outcomes, including detailed mortality data, length of hospital stays, and the cost of care in CeD patients both with and without MN.
We employed data from the National (Nationwide) Inpatient Sample (NIS) database spanning a two-decade period from January 2000 to December 2019. The NIS is an integral component of the Healthcare Cost and Utilization Project (HCUP), a collaborative initiative established through a Federal-State partnership and financially supported by the Agency for Healthcare Research and Quality (AHRQ). This database stands as the most extensive publicly accessible repository of inpatient care information, encompassing over seven million hospital admissions and representing a 20% stratified sample of all hospital discharges across the United States[11].
Within this dataset, a comprehensive array of patient demographic details, clinical information (including diagnoses and procedure codes), and data pertaining to hospital utilization and outcomes are included. The diagnostic coding system employed in the dataset adhered to the International Classification of Diseases, 9th Edition (ICD-9) until the third quarter of 2015, after which it transitioned to the ICD-10 system in September 2015[12]. Importantly, it is noteworthy that HCUP databases align with the definition of limited datasets, and in accordance with the Health Insurance Portability and Accountability Act, no International Review Board review is necessitated for limited datasets[13].
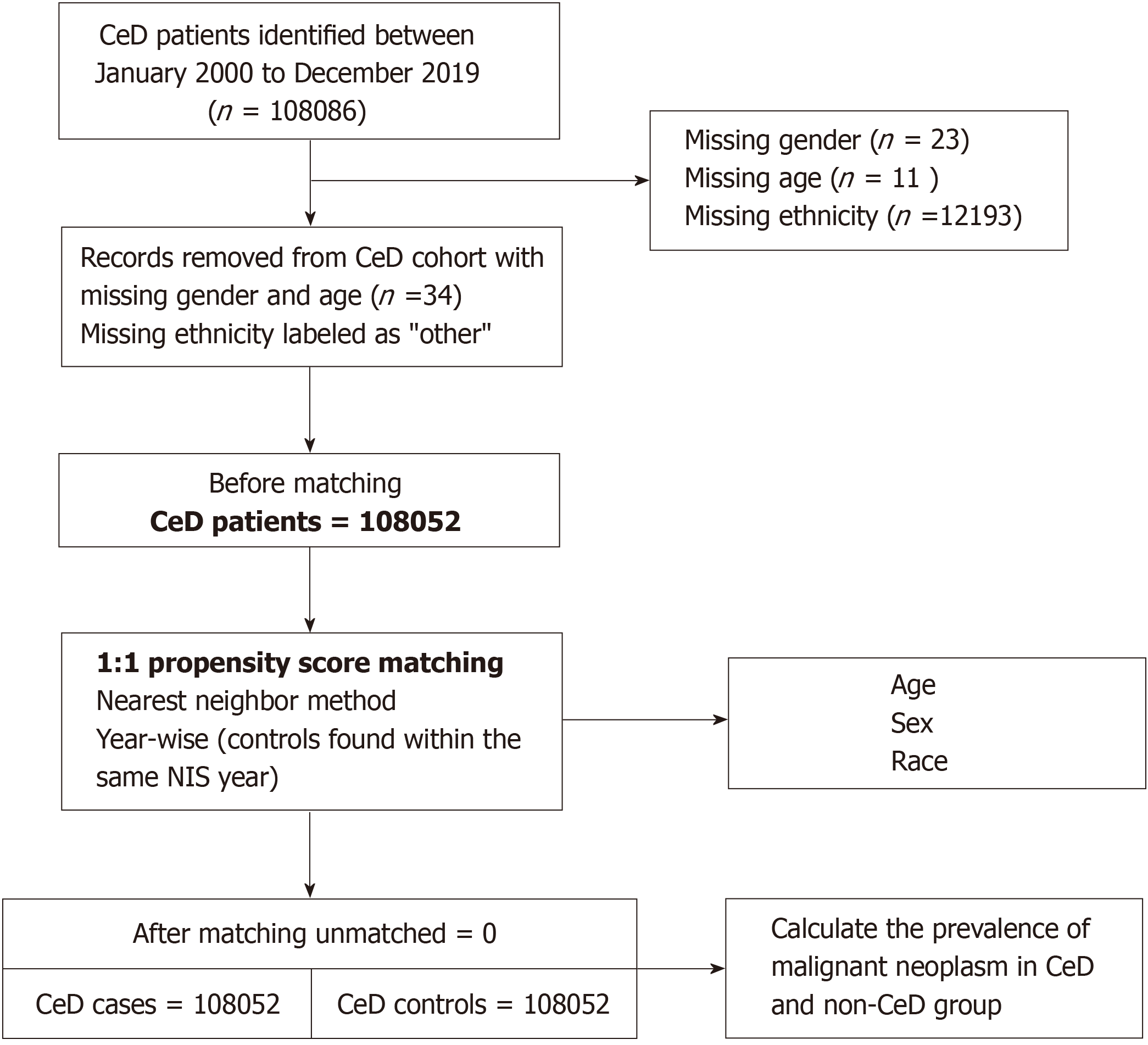
Patient and hospital characteristics, along with outcomes and resource utilization data, were retrieved from the NIS database using ICD codes (see Supplementary Table 1). Individuals who were admitted with either the primary or secondary diagnosis of CeD, as indicated by ICD-9 code 579.0 or ICD-10 code K90.0, were included in the case group. The case group underwent matching, aligning with individuals from the non-CeD general population in a 1:1 ratio based on age, sex, race, and calendar year, utilizing the nearest neighbor propensity score method. Detailed information about the progression of cases and controls to compare the prevalence of MN is shown in Figure 1.
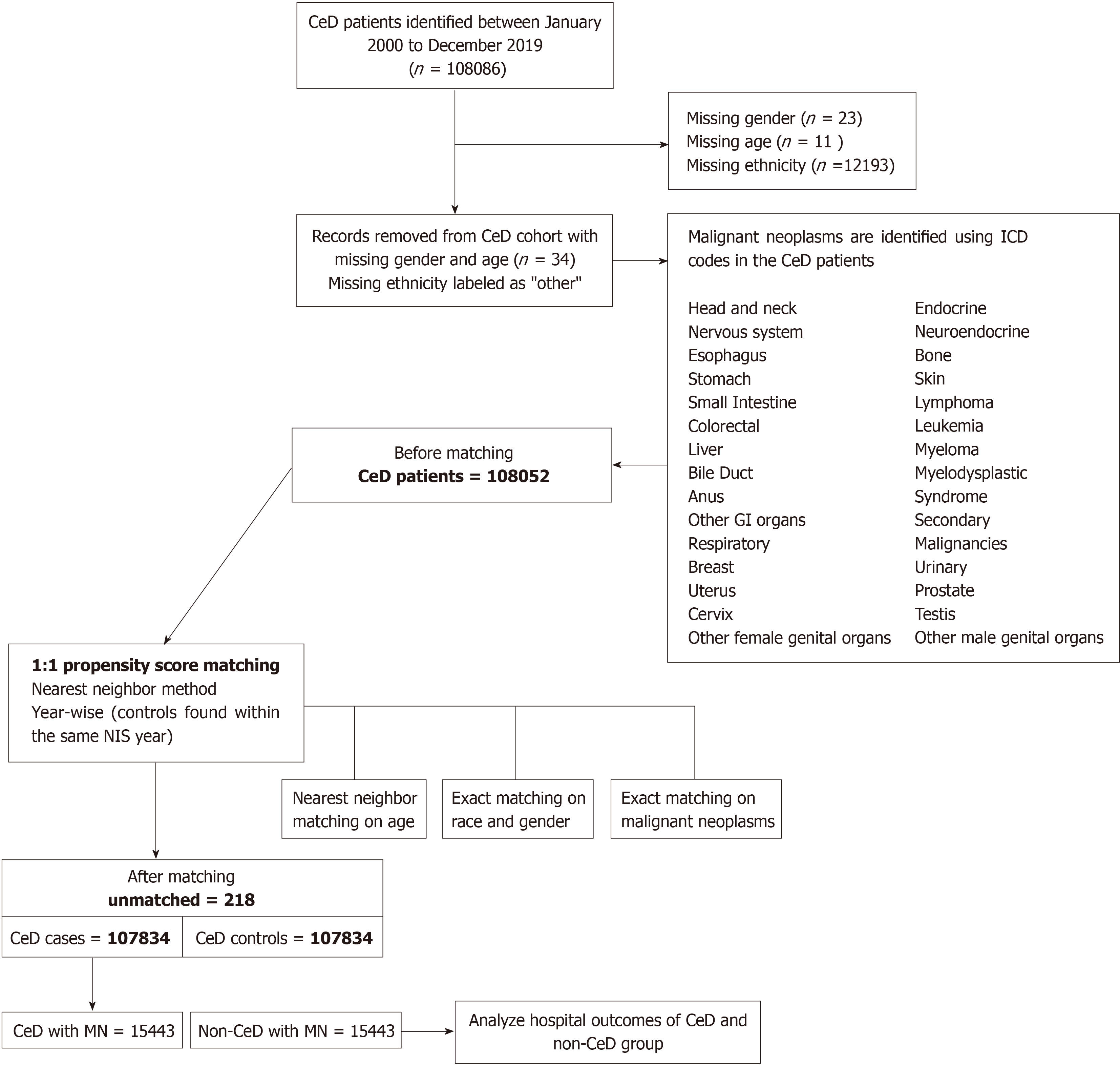
Additionally, we conducted a comparison of hospital outcomes between individuals with MN who had CeD and those without it. The group without CeD but with MN was carefully chosen from the general population, employing a 1:1 nearest neighbor propensity score matching technique based on age, sex, race, calendar year, and precise matching regarding the type of malignant neoplasm. Figure 2 provides a visual representation of the process outlining the development of cohorts with and without CeD in the context of MN.
We conducted a comparison of the occurrence of MN in individuals with CeD, referred to as cases, against a matched group without CeD, referred to as controls. We examined the demographic characteristics of CeD patients, including age, sex, race, and socioeconomic status, in the context of the presence or absence of MN. The NIS dataset included socioeconomic status information, which was categorized by dividing the median household income in the patient’s zip code into quartiles for each year.
Furthermore, we conducted a comparison of hospital-related outcomes among individuals with CeD who had MN and matched them against those without CeD. The matching process was based on age, sex, race, year, and the specific profile of MN. We assessed various aspects of hospital outcomes, which encompassed inpatient mortality, the length of hospital stays, and the overall charges incurred. To ensure accuracy, the total cost of care was adjusted using the Consumer Price Index from the United States Bureau of Labor Statistics[14].
Data processing was conducted using R (Studio 1.4), and statistical analyses were performed using SAS (SAS Institute, Cary, NC, United States). We executed one-to-one propensity score matching employing the “Matchit” package in R, employing nearest neighbor and exact matching techniques. Nominal variables were presented using frequency distributions, while continuous variables were summarized with means and standard deviations.
To compare the prevalence of MN in CeD patients with and without CeD, we utilized the χ2 test. Group comparisons of continuous variables were carried out using the Student t-test and the Rao-Scott χ2 test, accounting for the weighted sample in the analysis. We adhered to the year-specific AHRQ recommendations and adjusted the weights for years up to 2012[15]. Age was categorized into five groups for group-level comparisons: < 18; 18-49; 49-59; 59-69; and ≥ 70 years. In instances where race and socioeconomic status data were missing, they were categorized as “other”.
For assessing temporal trends, we employed the Cochran-Armitage trend test for nominal variables and Poisson regression with a log link for continuous variables. Outliers and missing values in the length of stay (LOS) and total charges were excluded from the hospital outcomes analysis. Hypothesis testing was conducted with a two-tailed approach, and statistical significance was established at a P value < 0.05.
The baseline characteristics of the CeD patients with and without MN are shown in Table 1. We found 529842 CeD patients from January 2000 to December 2019 in weighted NIS, of which 78128 (14.75%) had MN. Among the CeD patients, those who had MN were older, with a mean age of 68 (± 16) years as compared to a mean age of 53 (± 22) years in CeD without MN with P value < 0.0001, more males (36% among CeD with MN vs 28% among CeD without MN) than females (64% among CeD with MN vs 72% among CeD without MN) with P value < 0.0001, frequent in the Caucasian race (84% among CeD with MN vs 79% among CeD without MN) than African American race (1.90% among CeD with MN vs 2.96% among CeD without MN) with P value < 0.0001.
| Overall CeD patients | CeD without MN, weighted, n (%) | CeD with MN, weighted, n (%) | P value | |
| Weighted total | 529842 | 451714 (85.25) | 78128 (14.75) | |
| Sex | < 0.00012 | |||
| Female | 374102 (70.61) | 323904 (71.70) | 50199 (64.25) | |
| Male | 155739 (29.39) | 127810 (28.30) | 27929 (35.75) | |
| Age (year), mean ± SD | 55.34 ± 22.17 | 53.08 ± 22.40 | 67.66 ± 15.94 | < 0.00011 |
| Age groups (year) | < 0.00013 | |||
| < 18 | 30807 (5.82) | 29910 (6.62) | 898 (1.15) | |
| 18-49 | 168411 (31.79) | 159531 (35.31) | 8880 (11.35) | |
| 49-59 | 75661 (14.28) | 65080 (14.42) | 10581 (13.54) | |
| 59-69 | 85753 (16.18) | 68589 (15.18) | 17164 (21.96) | |
| ≥ 70 | 169209 (31.93) | 128604 (28.47) | 40605 (51.97) | |
| Race/ethnicity | < 0.00013 | |||
| White | 421882 (79.64) | 356333 (78.88) | 65549 (83.90) | |
| Black | 14876 (2.81) | 13386 (2.96) | 1490 (1.90) | |
| Hispanic | 20834 (3.93) | 18657 (4.13) | 2177 (2.78) | |
| Asian or Pacific Islander | 2746 (0.51) | 2462 (0.55) | 284 (0.36) | |
| Native American | 1698 (0.32) | 1501 (0.33) | 197 (0.25) | |
| Other | 67806 (12.79) | 59375 (13.14) | 8431 (10.79) | |
| Median household income for patient’s zip code | < 0.00013 | |||
| 0-25th percentile | 91079 (17.19) | 79152 (17.52) | 11927 (15.26) | |
| 26th to 50th percentile (median) | 125043 (23.60) | 107046 (23.70) | 17997 (23.05) | |
| 51st to 75th percentile | 143974 (27.17) | 122501 (27.12) | 21472 (27.48) | |
| 76th to 100th percentile | 160727 (30.34) | 135206 (29.93) | 25521 (32.66) | |
| Other | 9019 (1.70) | 7807 (1.73) | 1211 (1.55) |
Table 2 compared the prevalence of MN in CeD (cases) and matched (age, sex, and race) non-CeD patients in NIS from 2000 to 2019. As compared to non-CeD patients, CeD patients are at heightened risk of small intestine [odds ratio (OR) = 7.71; 95% confidence interval (CI): 5.0-11.9; P < 0.0001], lymphoma (OR = 2.06; 95%CI: 1.90-2.23; P < 0.0001), other gastrointestinal (GI) organs (OR = 2.02; 95%CI: 1.41-2.92; P < 0.0001), nonmelanoma skin (OR = 1.97; 95%CI: 1.81-2.16; P < 0.0001), thyroid (OR = 1.84; 95%CI: 1.58-2.13; P < 0.0001), liver (OR = 1.83; 95%CI: 1.49-2.24; P < 0.0001), melanoma skin (OR = 1.54; 95%CI: 1.35-1.76; P < 0.0001), pancreas (OR = 1.53; 95%CI: 1.29-1.80; P < 0.0001), myelodysplastic syndrome (OR = 1.84; 95%CI: 1.55-2.17; P < 0.0001), biliary (OR = 1.39; 95%CI: 1.01-1.91; P = 0.045), stomach (OR = 1.35; 95%CI: 1.08-1.69; P < 0.0.005), other neuroendocrine tumors (excluding small and large intestine malignant carcinoid) (OR = 1.48; 95%CI: 1.03-2.12; P = 0.031), leukemia (OR = 1.13; 95%CI: 1.02-1.25; P = 0.02), uterus (OR = 1.17; 95%CI: 1.03-1.32; P = 0.013), prostate (OR = 1.14; 95%CI: 1.06-1.23; P < 0.001), and testis (OR = 1.50; 95%CI: 1.07-2.10; P = 0.018). The bar chart in Supplementary Figure 1 also showed the prevalence comparison of MN positively associated with CeD.
| Neoplasm type | CeD cases | CeD controls | OR (95%CI) | P value |
| Unweighted (n = 108052), 50%, n (%) | Unweighted (n = 108052), 50%, n (%) | |||
| All malignant neoplasm | 15884 (14.70) | 14125 (13.07) | 1.15 (1.12-1.18) | < 0.0001 |
| Positive association | ||||
| Stomach | 179 (0.17) | 133 (0.12) | 1.35 (1.08-1.69) | 0.0092 |
| Small intestine | 177 (0.16) | 23 (0.02) | 7.71 (5.0-11.9) | < 0.0001 |
| Liver | 261 (0.24) | 143 (0.13) | 1.83 (1.49-2.24) | < 0.0001 |
| Biliary | 90 (0.08) | 65 (0.06) | 1.39 (1.01-1.91) | 0.0446 |
| Other GI organs | 87 (0.08) | 43 (0.04) | 2.02 (1.41-2.92) | < 0.0001 |
| Thyroid | 474 (0.44) | 259 (0.24) | 1.84 (1.58-2.13) | < 0.0001 |
| Pancreas | 358 (0.33) | 235 (0.22) | 1.53 (1.29-1.80) | < 0.0001 |
| Melanoma skin | 552 (0.51) | 359 (0.33) | 1.54 (1.35-1.76) | < 0.0001 |
| Nonmelanoma skin (all other skin excluding melanoma) | 1441 (1.33) | 735 (0.68) | 1.97 (1.81-2.16) | < 0.0001 |
| Other neuroendocrine tumors | 74 (0.07) | 50 (0.05) | 1.48 (1.03-2.12) | 0.0311 |
| Lymphoma | 1861 (1.72) | 912 (0.84) | 2.06 (1.90-2.23) | < 0.0001 |
| Leukemia | 835 (0.77) | 742 (0.69) | 1.13 (1.02-1.25) | 0.0187 |
| Myelodysplastic syndrome | 397 (0.37) | 216 (0.20) | 1.84 (1.55-2.17) | < 0.0001 |
| Uterus | 562 (0.52) | 482 (0.45) | 1.17 (1.03-1.32) | 0.0131 |
| Prostate | 1421 (1.32) | 1247 (1.15) | 1.14 (1.06-1.23) | 0.0007 |
| Testis | 84 (0.08) | 56 (0.05) | 1.50 (1.07-2.10) | 0.0179 |
| Negative association | ||||
| Respiratory | 1244 (1.15) | 1813 (1.68) | 0.68 (0.63-0.73) | < 0.0001 |
| Secondary malignancies | 2199 (2.04) | 2846 (2.63) | 0.76 (0.73-0.81) | < 0.0001 |
| Urinary | 952 (0.88) | 1053 (0.97) | 0.90 (0.83-0.99) | 0.0234 |
| Null association | ||||
| Head and neck | 272 (0.25) | 314 (0.29) | 0.87 (0.74-1.02) | 0.0823 |
| Nervous system | 227 (0.21) | 257 (0.24) | 0.88 (0.74-1.06) | 0.1722 |
| Esophagus | 152 (0.14) | 125 (0.12) | 1.22 (0.96-1.54) | 0.1045 |
| Colorectal | 1752 (1.62) | 1774 (1.64) | 0.99 (0.93-1.06) | 0.7087 |
| Anus | 21 (0.02) | 26 (0.02) | 0.81 (0.45-1.44) | 0.4658 |
| Breast | 2844 (2.63) | 2919 (2.70) | 0.98 (0.92-1.03) | 0.3166 |
| Other endocrine (excluding thyroid and pancreas) | 25 (0.02) | 30 (0.03) | 0.83 (0.49-1.41) | 0.5001 |
| Malignant carcinoid tumor - small intestine | 14 (0.01) | 13 (0.01) | 1.08 (0.51-2.29) | 0.8474 |
| Malignant carcinoid tumor - large intestine | 5 (0.005) | 6 (0.005) | 0.83 (0.25-2.73) | 0.7630 |
| Bone and connective tissues | 134 (0.12) | 161 (0.15) | 0.83 (0.66-1.04) | 0.1157 |
| Myeloma | 313 (0.29) | 274 (0.25) | 1.14 (0.97-1.34) | 0.1070 |
| Cervix | 448 (0.41) | 418 (0.39) | 1.07 (0.94-1.22) | 0.3070 |
| Ovary | 472 (0.44) | 430 (0.40) | 1.10 (0.96-1.25) | 0.1611 |
| Other female genital organs | 126 (0.12) | 139 (0.13) | 0.91 (0.71-1.15) | 0.4242 |
| Other male genital organs | 11 (0.01) | 4 (0.004) | 2.75 (0.88-8.63) | 0.0707 |
Conversely, individuals with CeD exhibit a notably reduced risk of developing respiratory malignancies (OR = 0.68; 95%CI: 0.63-0.73; P < 0.0001) and secondary malignancies (OR = 0.76; 95%CI: 0.73-0.81; P < 0.0001). Furthermore, our analysis did not reveal any significant association between CeD and the occurrence of malignancies affecting the head and neck, nervous system, esophagus, colorectal, anus, breast, malignant carcinoids, bone and connective tissues, myeloma, cervix, or ovary.
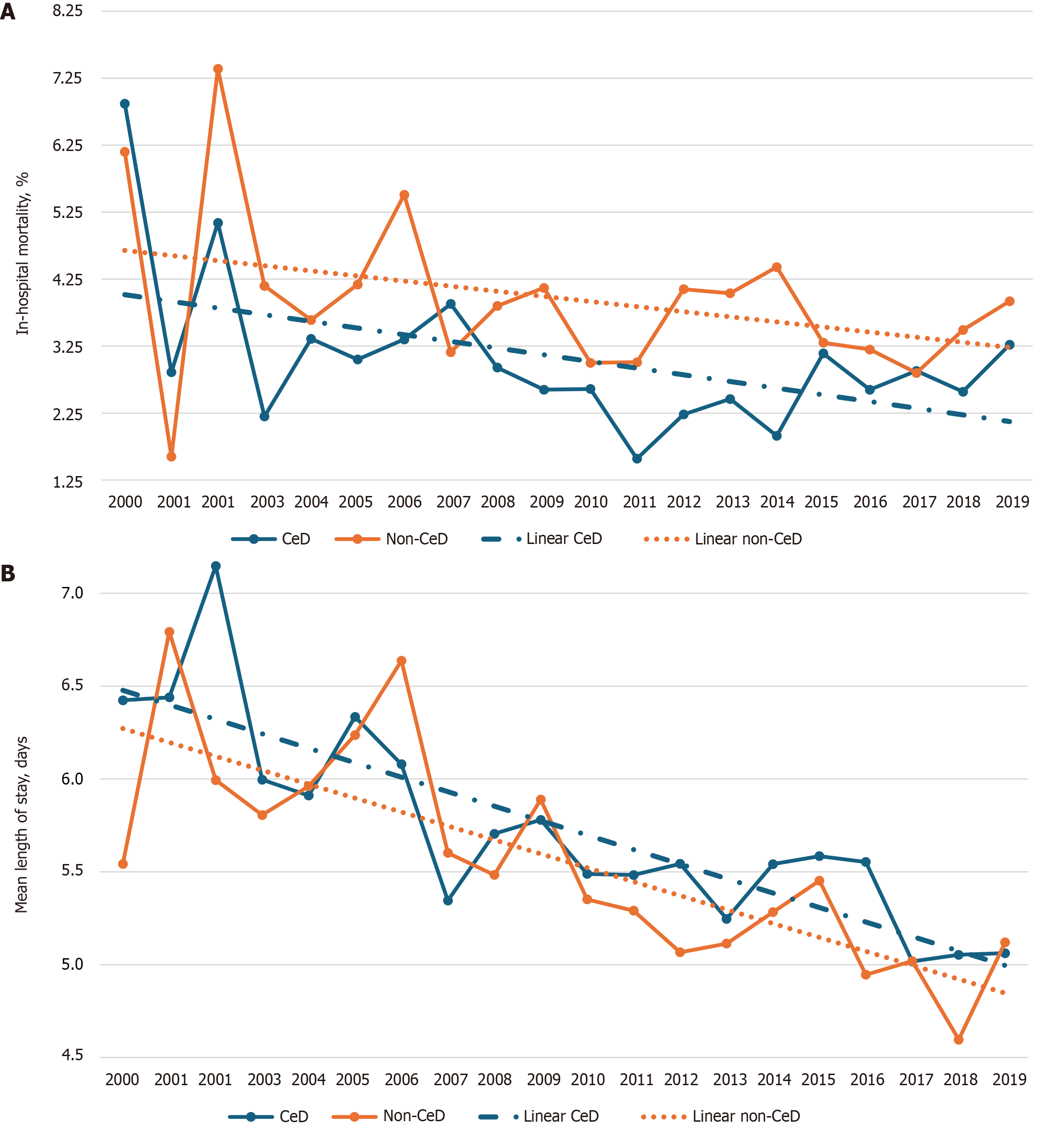
Figure 3 and Table 3, provide a comparative analysis of hospital outcomes and resource utilization. This assessment includes mortality rates, length of hospitalization, and the overall cost of care, and it contrasts patients with CeD who have MN with a matched group of patients without CeD but with MN. The matching process is based on age, sex, race, and the specific type of malignant neoplasm. The mean differences in length of the stay and total cost of care are found to be higher in CeD cohort (0.21 days; 99%CI: 0.05-0.38; P < 0.001) and ($3172; 99%CI: $1467-$4878; P < 0.001), respectively. However, the inpatient mortality is lower in CeD with MN than non-CeD with MN (0.72; 99%CI: 0.61-0.86; P < 0.001). Supplementary Figure 2 illustrated the trend in the total cost of care for hospitalized CeD patients with MN, compared to non-CeD patients with MN.
Utilizing the NIS dataset, which offers a representative sample of the United States population, our investigation revealed that individuals with CeD exhibited a notably heightened incidence of at least sixteen distinct MN. The most significant increase was observed in cases of small intestinal adenocarcinomas, with an OR of 7.7, followed by lymphomas (OR = 2.06) and other malignancies affecting the GI organs (OR = 2.01). Conversely, a reduced risk was identified for respiratory MNs. This study aligns with some findings from previous research while also highlighting notable disparities.
The initial documentation of malignancy in individuals with CeD dates back to 1965 when a case of small intestinal adenocarcinoma was reported in France[16]. Subsequently, during the late 1960s, research investigating the connection between CeD and the development of malignancies began to emerge[17,18]. Over the ensuing decades, a series of research studies conducted in Europe provided substantial evidence supporting the association between CeD and MN[19-22]. Likewise, a study conducted in the United States observed a heightened risk of MNs in a limited cohort of CeD patients compared to the general American population[23]. Various investigations have highlighted that the risk of MN development is notably higher in the early stages following the diagnosis of CeD. However, as time progresses, the Standardized Incidence Ratio (SIR) for MNs tends to decrease and may even become statistically nonsignificant after the initial year of diagnosis or in subsequent years[24-26]. Additionally, some studies have demonstrated that individuals with CeD experience elevated mortality rates in the initial years following diagnosis, potentially linked to malignancies[27-30]. Notably, adherence to a gluten-free diet emerges as a robust protective factor against the development of malignancies[8,31]. A study underscores that mortality rates are significantly higher among patients with delayed diagnoses or severe symptoms at the time of diagnosis[29].
The precise pathogenic mechanism underlying malignancy development in CeD remains an enigma[32]. Several factors, including but not limited to persistent inflammation, the release of proinflammatory cytokines, continual antigen stimulation, cytokine surges, heightened susceptibility to carcinogens, and nutritional deficiencies induced by the disease or the adoption of a gluten-free diet, have all been proposed as potential contributors to the onset of malignancies[23,33].
Among the malignancies exhibiting a positive association with CeD, our findings indicate that lymphomas have the highest prevalence and the second-highest OR within the CeD sample. The connection between CeD and the deve
Two Swedish studies, one involving roughly 11000 CeD patients and the other with approximately 11650 CeD patients, reported SIR of 6.3 (95%CI: 4.2-125)[31] and 6.6 (95%CI: 5.0-8.6) for NHL[38]. Furthermore, Elfström et al[39] research suggested that the risk of lymphoproliferative malignancies was similar between CeD patients with only positive serology and those without documented inflammation, compared to the general population. Additionally, earlier investigations have identified relative risks (RR) ranging from 3 to 100 for various lymphoma subtypes in the context of CeD[8,31,38,40,41]. The prevalence of enteropathy-associated T-cell lymphoma (EATL), a rare tumor, has attracted research attention alongside the increasing incidence of CeD in recent decades[42-44]. Typically, EATL has been linked to refractory CeD, which is characterized by the persistent or recurrent pathological manifestations of CeD despite stringent adherence to a gluten-free diet[45,46]. Notably, in regions of Northern Europe where CeD is more widespread, EATL type 1 (pleomorphic and anaplastic, typically CD56 negative, with gains in chromosomes 1q and 5q) prevails over EATL type 2 (medium-sized cancer cells, typically CD56 positive, with oncogene MYC gain), which is more commonly observed in Asian countries[47-50]. It is notable that there is no clear causality on the exact mechanism of how CeD patients develop lymphoma, the main hypothesis is that as an autoimmune disease with immunity overactivation, persistent inflammation, villous atrophy, and intestinal mucosal healing resulting in aberrant lymphocytes’ hyperproliferation can result in a possibility of malignant transformation of intraepithelial lymphocytes causing lymphomas[51-53]. Numerous studies have reported the occurrence of both T and B cell lymphomas in individuals with CeD[39,54-56]. In terms of prognosis, research has indicated that CeD patients face a roughly 0.15% elevated risk of NHL-related mortality in the decade following diagnosis[57]. It is also notable that T-cell lymphomas tend to exhibit a poorer prognosis in comparison to B-cell lymphomas[58,59].
Our findings indicate that small bowel carcinomas (SBC) exhibit the highest OR of 7.71 (95%CI: 5.0-11.9) among MN, demonstrating a positive correlation with CeD. SBC is a rare malignancy in the general population, and its association with CeD is firmly established. This connection was initially documented by Swinson et al[22], revealing a RR of 82.6 for SBC development in individuals with CeD. Over subsequent decades, several significant studies have reaffirmed this association. Elfström et al[60] in a prospective analysis encompassing more than 45000 CeD patients, reported an average hazard ratio (HR) for SBC development ranging from 2.22 to 4.67, stratified based on CeD marsh classification or positive serology. In 2014, Ilus et al[61] conducted a retrospective investigation involving 32439 CeD patients in Finland, unveiling a positive link between CeD and SBC development. The study reported an SIR of 5 in females, 3.47 in males, and 4.29 in all combined cases. Another substantial retrospective Swedish study led by Emilsson et al[62], encompassing more than 48000 CeD patients, reported an HR of 3.05, underscoring the affirmative association between CeD and SBC. Additionally, a sequential progression from adenoma to carcinoma has been suggested as a potential pathway for SBC development in CeD[63]. Notably, the survival rates for SBC in CeD patients are comparatively higher than those in individuals without CeD[64]. As prostate cancer is the most common cancer in males in general[65], our study shows a positive association between prostate cancer and CeD with an HR of 1.14 (95%CI: 1.06-1.23). Surprisingly, other studies that addressed this association did not show any significantly heightened risk of prostate cancer in CeD[25,31,61,66].
To our knowledge, this study stands as one of the most extensive investigations underscoring the positive link between nonmelanoma skin cancers and CeD. In contrast, the association between melanoma and CeD has been examined in three studies, with one study, also conducted in the United States, reporting a notably elevated SIR[23]. Conversely, two studies from Sweden failed to establish any significant connection between these two conditions[31,67]. Notably, the latter study, which involved 29028 patients, did not identify a significant association.
Our study reveals a positive correlation between pancreatic malignancies and CeD. However, it’s essential to note the divergence in findings across various studies concerning the association between CeD and pancreatic malignancies. For instance, Elfström et al[60] reported a substantially higher HR of 10.7 within the first year of follow-up, which subsequently decreased to 1.4. Lebwohl et al[26] conducted another large Swedish study, documented analogous outcomes with distinct HR. In contrast, a study utilizing a United States Veterans Affairs database reported an elevated RR for pancreatic cancer in individuals with CeD[68], while two separate European studies failed to identify any significant risk association[31,69].
Our results indicate an elevated incidence and a positive correlation between thyroid malignancies and CeD. It is worth mentioning that the existing literature has yielded conflicting outcomes in this regard. Specifically, two studies conducted in the United States and Italy have reported a positive association[70,71], whereas two other population-based studies in Sweden have reported a lack of significant association[31,72].
It is noteworthy that our study’s findings align with those of other research regarding the risk of lung cancer in CeD patients. Our study reveals a statistically significant negative correlation between respiratory malignancies and CeD. Similarly, the two largest studies conducted in Finland[61] and Sweden[26] also report a negative association. Conversely, several other studies have failed to identify a heightened risk of lung malignancies in individuals with CeD[23-25,31,69,73,74]. This lack of association could potentially be attributed to a lower prevalence of smoking among individuals with CeD[75,76]. Regarding colorectal cancer, our study demonstrates no significant association with CeD. This finding is consistent with a study by Lebwohl et al[77], which also reported no elevated risk of colorectal cancer. However, results from the study by Ilus et al[61], indicated an increased risk of colon cancer but not rectal cancer.
In the context of breast cancer risk in CeD, most available studies have reported a significantly decreased risk, including large-scale investigations by Lebwohl et al[26], Ilus et al[61], and Ludvigsson et al[78], as well as other relatively smaller studies with similar findings[31,25,69,79]. Our study supports this trend, reporting no increased risk of breast cancer in individuals with CeD, which aligns with several previous studies on this association[23,24,73,74,80].
Our study exhibits several notable strengths. First and foremost, it leverages an extensive database, including more than 108000 individuals diagnosed with CeD, which, when weighted, expands to encompass over 500000 individuals. This dataset stands as the most substantial cohort among comparable studies within the existing body of research, as far as our knowledge extends. Secondly, we investigated hospital outcomes, encompassing a variety of factors associated with mortality, LOS, and the burden on the healthcare system, spanning a substantial two-decade period. However, it is important to acknowledge several limitations in our study. Firstly, it is confined to inpatient populations, potentially limiting the generalizability of our findings to outpatient settings. Secondly, the NIS dataset lacks essential clinical details such as laboratory values, treatment modalities, and diagnostic procedures like histology and endoscopy findings that definitively confirm CeD. Thirdly, the NIS, as an administrative database, may be susceptible to selection bias and coding errors, which can occur without external validation. Lastly, it’s worth noting that the NIS does not track individual patients, meaning that if a patient is admitted multiple times, they may contribute to multiple entries in the database. Furthermore, we lack information about the level of adherence to a gluten-free diet among the patients and the degree to which their CeD is controlled. These limitations should be considered when interpreting our results.
Our study is unique in highlighting the detailed results of positive, negative, or null associations between different hematologic and solid malignancies and CeD. It also sheds light on data on hospitalized CeD patients with and without MN in terms of mortality, LOS, and related costs with trends shown over the last two decades, which have been understudied in this disease.
| 1. | Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet. 2018;391:70-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 666] [Article Influence: 95.1] [Reference Citation Analysis (0)] |
| 2. | Singh P, Arora A, Strand TA, Leffler DA, Catassi C, Green PH, Kelly CP, Ahuja V, Makharia GK. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:823-836.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 944] [Article Influence: 134.9] [Reference Citation Analysis (1)] |
| 3. | Kagnoff MF. Overview and pathogenesis of celiac disease. Gastroenterology. 2005;128:S10-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Parzanese I, Qehajaj D, Patrinicola F, Aralica M, Chiriva-Internati M, Stifter S, Elli L, Grizzi F. Celiac disease: From pathophysiology to treatment. World J Gastrointest Pathophysiol. 2017;8:27-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 191] [Cited by in RCA: 157] [Article Influence: 19.6] [Reference Citation Analysis (4)] |
| 5. | Denham JM, Hill ID. Celiac disease and autoimmunity: review and controversies. Curr Allergy Asthma Rep. 2013;13:347-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Pelizzaro F, Marsilio I, Fassan M, Piazza F, Barberio B, D'Odorico A, Savarino EV, Farinati F, Zingone F. The Risk of Malignancies in Celiac Disease-A Literature Review. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Al-Toma A, Volta U, Auricchio R, Castillejo G, Sanders DS, Cellier C, Mulder CJ, Lundin KEA. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J. 2019;7:583-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 598] [Article Influence: 99.7] [Reference Citation Analysis (1)] |
| 8. | Holmes GK, Prior P, Lane MR, Pope D, Allan RN. Malignancy in coeliac disease--effect of a gluten free diet. Gut. 1989;30:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 600] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 9. | Collin P, Reunala T, Pukkala E, Laippala P, Keyriläinen O, Pasternack A. Coeliac disease--associated disorders and survival. Gut. 1994;35:1215-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 318] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Rostami Nejad M, Aldulaimi D, Ishaq S, Ehsani-Ardakani MJ, Zali MR, Malekzadeh R, Rostami K. Geographic trends and risk of gastrointestinal cancer among patients with celiac disease in Europe and Asian-Pacific region. Gastroenterol Hepatol Bed Bench. 2013;6:170-177. [PubMed] |
| 11. | Agency for Healthcare Research and Quality. NIS overview. [cited 12 December 2023]. Available from: https://www.hcup-us.ahrq.gov/nisoverview.jsp. |
| 12. | Agency for Healthcare Research and Quality. Introduction to the HCUP National Inpatient Sample (NIS), 2015. [cited 12 October 2023]. Available from: www.hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2015.jsp. |
| 13. | Agency for Healthcare Research and Quality. DUA Training - Accessible Version. [cited 12 October 2023]. Available from: https://www.hcup-us.ahrq.gov/DUA/dua_508/DUA508version.jsp. |
| 14. | Agency for Healthcare Research and Quality. US Bureau of Labor Statistics. [cited 10 March 2023]. Available from: https://data.bls.gov/timeseries/CUUR0000SA0. |
| 15. | Agency for Healthcare Research and Quality. Trend weights for HCUP NIS data. [cited 15 December 2023]. Available from: https://www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp. |
| 16. | Kissel P, Rauber G, Barrucand D, Marchal C, Leclère J. [Adenocarcinoma of the small intestine in the evolution of a celiac disease in an adult]. Arch Mal Appar Dig Mal Nutr. 1965;54:945-951. [PubMed] |
| 17. | Austad WI, Cornes JS, Gough KR, McCarthy CF, Read AE. Steatorrhea and malignant lymphoma. The relationship of malignant tumors of lymphoid tissue and celiac disease. Am J Dig Dis. 1967;12:475-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 68] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Harris OD, Cooke WT, Thompson H, Waterhouse JA. Malignancy in adult coeliac disease and idiopathic steatorrhoea. Am J Med. 1967;42:899-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 284] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Evans IM. Coeliac disease and malignancy. Br Med J. 1977;2:1026-1027. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Cooper BT, Holmes GK, Ferguson R, Cooke WT. Celiac disease and malignancy. Medicine (Baltimore). 1980;59:249-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Swinson CM, Slavin G, Coles EC, Booth CC. Coeliac disease and malignancy. Lancet. 1983;1:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 264] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Green PH, Fleischauer AT, Bhagat G, Goyal R, Jabri B, Neugut AI. Risk of malignancy in patients with celiac disease. Am J Med. 2003;115:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 257] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 24. | Card TR, West J, Holmes GK. Risk of malignancy in diagnosed coeliac disease: a 24-year prospective, population-based, cohort study. Aliment Pharmacol Ther. 2004;20:769-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | West J, Logan RF, Smith CJ, Hubbard RB, Card TR. Malignancy and mortality in people with coeliac disease: population based cohort study. BMJ. 2004;329:716-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 243] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 26. | Lebwohl B, Green PHR, Emilsson L, Mårild K, Söderling J, Roelstraete B, Ludvigsson JF. Cancer Risk in 47,241 Individuals With Celiac Disease: A Nationwide Cohort Study. Clin Gastroenterol Hepatol. 2022;20:e111-e131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 27. | Grainge MJ, West J, Card TR, Holmes GK. Causes of death in people with celiac disease spanning the pre- and post-serology era: a population-based cohort study from Derby, UK. Am J Gastroenterol. 2011;106:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Cottone M, Termini A, Oliva L, Magliocco A, Marrone C, Orlando A, Pinzone F, Di Mitri R, Rosselli M, Rizzo A, Pagliaro L. Mortality and causes of death in celiac disease in a Mediterranean area. Dig Dis Sci. 1999;44:2538-2541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Corrao G, Corazza GR, Bagnardi V, Brusco G, Ciacci C, Cottone M, Sategna Guidetti C, Usai P, Cesari P, Pelli MA, Loperfido S, Volta U, Calabró A, Certo M; Club del Tenue Study Group. Mortality in patients with coeliac disease and their relatives: a cohort study. Lancet. 2001;358:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 385] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 30. | Peters U, Askling J, Gridley G, Ekbom A, Linet M. Causes of death in patients with celiac disease in a population-based Swedish cohort. Arch Intern Med. 2003;163:1566-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 200] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 31. | Askling J, Linet M, Gridley G, Halstensen TS, Ekström K, Ekbom A. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology. 2002;123:1428-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 307] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 32. | Dunne MR, Byrne G, Chirdo FG, Feighery C. Coeliac Disease Pathogenesis: The Uncertainties of a Well-Known Immune Mediated Disorder. Front Immunol. 2020;11:1374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 33. | Green PH, Jabri B. Celiac disease and other precursors to small-bowel malignancy. Gastroenterol Clin North Am. 2002;31:625-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Gale J, Simmonds PD, Mead GM, Sweetenham JW, Wright DH. Enteropathy-type intestinal T-cell lymphoma: clinical features and treatment of 31 patients in a single center. J Clin Oncol. 2000;18:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 216] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 35. | Egan LJ, Walsh SV, Stevens FM, Connolly CE, Egan EL, McCarthy CF. Celiac-associated lymphoma. A single institution experience of 30 cases in the combination chemotherapy era. J Clin Gastroenterol. 1995;21:123-129. [PubMed] |
| 36. | Nielsen OH, Jacobsen O, Pedersen ER, Rasmussen SN, Petri M, Laulund S, Jarnum S. Non-tropical sprue. Malignant diseases and mortality rate. Scand J Gastroenterol. 1985;20:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Catassi C, Fabiani E, Corrao G, Barbato M, De Renzo A, Carella AM, Gabrielli A, Leoni P, Carroccio A, Baldassarre M, Bertolani P, Caramaschi P, Sozzi M, Guariso G, Volta U, Corazza GR; Italian Working Group on Coeliac Disease and Non-Hodgkin's-Lymphoma. Risk of non-Hodgkin lymphoma in celiac disease. JAMA. 2002;287:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 201] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 38. | Smedby KE, Akerman M, Hildebrand H, Glimelius B, Ekbom A, Askling J. Malignant lymphomas in coeliac disease: evidence of increased risks for lymphoma types other than enteropathy-type T cell lymphoma. Gut. 2005;54:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 185] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 39. | Elfström P, Granath F, Ekström Smedby K, Montgomery SM, Askling J, Ekbom A, Ludvigsson JF. Risk of lymphoproliferative malignancy in relation to small intestinal histopathology among patients with celiac disease. J Natl Cancer Inst. 2011;103:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 40. | Leonard JN, Tucker WF, Fry JS, Coulter CA, Boylston AW, McMinn RM, Haffenden GP, Swain AF, Fry L. Increased incidence of malignancy in dermatitis herpetiformis. Br Med J (Clin Res Ed). 1983;286:16-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 91] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Sigurgeirsson B, Agnarsson BA, Lindelöf B. Risk of lymphoma in patients with dermatitis herpetiformis. BMJ. 1994;308:13-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Sharaiha RZ, Lebwohl B, Reimers L, Bhagat G, Green PH, Neugut AI. Increasing incidence of enteropathy-associated T-cell lymphoma in the United States, 1973-2008. Cancer. 2012;118:3786-3792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Catassi C, Kryszak D, Bhatti B, Sturgeon C, Helzlsouer K, Clipp SL, Gelfond D, Puppa E, Sferruzza A, Fasano A. Natural history of celiac disease autoimmunity in a USA cohort followed since 1974. Ann Med. 2010;42:530-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 251] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 44. | Rubio-Tapia A, Kyle RA, Kaplan EL, Johnson DR, Page W, Erdtmann F, Brantner TL, Kim WR, Phelps TK, Lahr BD, Zinsmeister AR, Melton LJ 3rd, Murray JA. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137:88-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 583] [Cited by in RCA: 519] [Article Influence: 32.4] [Reference Citation Analysis (1)] |
| 45. | Cellier C, Delabesse E, Helmer C, Patey N, Matuchansky C, Jabri B, Macintyre E, Cerf-Bensussan N, Brousse N. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet. 2000;356:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 460] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 46. | Rubio-Tapia A, Murray JA. Classification and management of refractory coeliac disease. Gut. 2010;59:547-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 217] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 47. | Delabie J, Holte H, Vose JM, Ullrich F, Jaffe ES, Savage KJ, Connors JM, Rimsza L, Harris NL, Müller-Hermelink K, Rüdiger T, Coiffier B, Gascoyne RD, Berger F, Tobinai K, Au WY, Liang R, Montserrat E, Hochberg EP, Pileri S, Federico M, Nathwani B, Armitage JO, Weisenburger DD. Enteropathy-associated T-cell lymphoma: clinical and histological findings from the international peripheral T-cell lymphoma project. Blood. 2011;118:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 233] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 48. | Chott A, Haedicke W, Mosberger I, Födinger M, Winkler K, Mannhalter C, Müller-Hermelink HK. Most CD56+ intestinal lymphomas are CD8+CD5-T-cell lymphomas of monomorphic small to medium size histology. Am J Pathol. 1998;153:1483-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 143] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 49. | Tse E, Gill H, Loong F, Kim SJ, Ng SB, Tang T, Ko YH, Chng WJ, Lim ST, Kim WS, Kwong YL. Type II enteropathy-associated T-cell lymphoma: a multicenter analysis from the Asia Lymphoma Study Group. Am J Hematol. 2012;87:663-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 50. | Deleeuw RJ, Zettl A, Klinker E, Haralambieva E, Trottier M, Chari R, Ge Y, Gascoyne RD, Chott A, Müller-Hermelink HK, Lam WL. Whole-genome analysis and HLA genotyping of enteropathy-type T-cell lymphoma reveals 2 distinct lymphoma subtypes. Gastroenterology. 2007;132:1902-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 51. | Nelson R. Celiac Disease and Lymphoma: What’s the Connection? [cited 10 June 2024]. Available from: https://www.patientpower.info/lymphoma/celiac-disease-and-lymphoma. |
| 52. | Lebwohl B. Celiac Disease and Lymphoma: Researcher Explains the Risk. [cited 10 June 2024]. Available from: https://www.beyondceliac.org/research-news/celiac-disease-and-lymphoma-researcher-explains-the-risk/. |
| 53. | Di Sabatino A, Biagi F, Gobbi PG, Corazza GR. How I treat enteropathy-associated T-cell lymphoma. Blood. 2012;119:2458-2468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 54. | Smedby KE, Hjalgrim H, Askling J, Chang ET, Gregersen H, Porwit-MacDonald A, Sundström C, Akerman M, Melbye M, Glimelius B, Adami HO. Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J Natl Cancer Inst. 2006;98:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 293] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 55. | Ansell P, Simpson J, Lightfoot T, Smith A, Kane E, Howell D, Newton R, McGonagle D, Jack A, Roman E. Non-Hodgkin lymphoma and autoimmunity: does gender matter? Int J Cancer. 2011;129:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Leslie LA, Lebwohl B, Neugut AI, Gregory Mears J, Bhagat G, Green PH. Incidence of lymphoproliferative disorders in patients with celiac disease. Am J Hematol. 2012;87:754-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Abdul Sultan A, Crooks CJ, Card T, Tata LJ, Fleming KM, West J. Causes of death in people with coeliac disease in England compared with the general population: a competing risk analysis. Gut. 2015;64:1220-1226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 58. | Halfdanarson TR, Rubio-Tapia A, Ristow KM, Habermann TM, Murray JA, Inwards DJ. Patients with celiac disease and B-cell lymphoma have a better prognosis than those with T-cell lymphoma. Clin Gastroenterol Hepatol. 2010;8:1042-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 59. | Daum S, Ullrich R, Heise W, Dederke B, Foss HD, Stein H, Thiel E, Zeitz M, Riecken EO. Intestinal non-Hodgkin's lymphoma: a multicenter prospective clinical study from the German Study Group on Intestinal non-Hodgkin's Lymphoma. J Clin Oncol. 2003;21:2740-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 162] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 60. | Elfström P, Granath F, Ye W, Ludvigsson JF. Low risk of gastrointestinal cancer among patients with celiac disease, inflammation, or latent celiac disease. Clin Gastroenterol Hepatol. 2012;10:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 61. | Ilus T, Kaukinen K, Virta LJ, Pukkala E, Collin P. Incidence of malignancies in diagnosed celiac patients: a population-based estimate. Am J Gastroenterol. 2014;109:1471-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 62. | Emilsson L, Semrad C, Lebwohl B, Green PHR, Ludvigsson JF. Risk of Small Bowel Adenocarcinoma, Adenomas, and Carcinoids in a Nationwide Cohort of Individuals With Celiac Disease. Gastroenterology. 2020;159:1686-1694.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 63. | Rampertab SD, Forde KA, Green PH. Small bowel neoplasia in coeliac disease. Gut. 2003;52:1211-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | Potter DD, Murray JA, Donohue JH, Burgart LJ, Nagorney DM, van Heerden JA, Plevak MF, Zinsmeister AR, Thibodeau SN. The role of defective mismatch repair in small bowel adenocarcinoma in celiac disease. Cancer Res. 2004;64:7073-7077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | Leslie SW, Soon-Sutton TL, R I A, Sajjad H, Skelton WP. Prostate Cancer. 2023 Nov 13. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 66. | Ludvigsson JF, Fall K, Montgomery S. Risk of prostate cancer in a population-based cohort of men with coeliac disease. Br J Cancer. 2012;106:217-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 67. | Lebwohl B, Eriksson H, Hansson J, Green PH, Ludvigsson JF. Risk of cutaneous malignant melanoma in patients with celiac disease: a population-based study. J Am Acad Dermatol. 2014;71:245-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Landgren AM, Landgren O, Gridley G, Dores GM, Linet MS, Morton LM. Autoimmune disease and subsequent risk of developing alimentary tract cancers among 4.5 million US male veterans. Cancer. 2011;117:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 69. | Goldacre MJ, Wotton CJ, Yeates D, Seagroatt V, Jewell D. Cancer in patients with ulcerative colitis, Crohn's disease and coeliac disease: record linkage study. Eur J Gastroenterol Hepatol. 2008;20:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 70. | Kent L, McBride R, McConnell R, Neugut AI, Bhagat G, Green PH. Increased risk of papillary thyroid cancer in celiac disease. Dig Dis Sci. 2006;51:1875-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 71. | Volta U, Vincentini O, Silano M; Collaborating Centers of the Italian Registry of Celiac Disease. Papillary cancer of thyroid in celiac disease. J Clin Gastroenterol. 2011;45:e44-e46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 72. | Ludvigsson JF, Lebwohl B, Kämpe O, Murray JA, Green PH, Ekbom A. Risk of thyroid cancer in a nationwide cohort of patients with biopsy-verified celiac disease. Thyroid. 2013;23:971-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 73. | Grainge MJ, West J, Solaymani-Dodaran M, Card TR, Logan RF. The long-term risk of malignancy following a diagnosis of coeliac disease or dermatitis herpetiformis: a cohort study. Aliment Pharmacol Ther. 2012;35:730-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 74. | Viljamaa M, Kaukinen K, Pukkala E, Hervonen K, Reunala T, Collin P. Malignancies and mortality in patients with coeliac disease and dermatitis herpetiformis: 30-year population-based study. Dig Liver Dis. 2006;38:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 75. | Vazquez H, Smecuol E, Flores D, Mazure R, Pedreira S, Niveloni S, Mauriño E, Bai JC. Relation between cigarette smoking and celiac disease: evidence from a case-control study. Am J Gastroenterol. 2001;96:798-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 76. | Suman S, Williams EJ, Thomas PW, Surgenor SL, Snook JA. Is the risk of adult coeliac disease causally related to cigarette exposure? Eur J Gastroenterol Hepatol. 2003;15:995-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 77. | Lebwohl B, Stavsky E, Neugut AI, Green PH. Risk of colorectal adenomas in patients with coeliac disease. Aliment Pharmacol Ther. 2010;32:1037-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 78. | Ludvigsson JF, West J, Ekbom A, Stephansson O. Reduced risk of breast, endometrial and ovarian cancer in women with celiac disease. Int J Cancer. 2012;131:E244-E250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 79. | Silano M, Volta U, Mecchia AM, Dessì M, Di Benedetto R, De Vincenzi M; Collaborating centers of the Italian registry of the complications of coeliac disease. Delayed diagnosis of coeliac disease increases cancer risk. BMC Gastroenterol. 2007;7:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 80. | Lohi S, Mäki M, Montonen J, Knekt P, Pukkala E, Reunanen A, Kaukinen K. Malignancies in cases with screening-identified evidence of coeliac disease: a long-term population-based cohort study. Gut. 2009;58:643-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |