Published online Aug 24, 2024. doi: 10.5306/wjco.v15.i8.1021
Revised: May 14, 2024
Accepted: July 9, 2024
Published online: August 24, 2024
Processing time: 145 Days and 18.6 Hours
Systemic inflammation and nutrition play pivotal roles in cancer progression and can increase the risk of delayed recovery after surgical procedures.
To assess the significance of inflammatory and nutritional indicators for the prognosis and postoperative recovery of patients with pancreatic cancer (PC).
Patients who were diagnosed with PC and underwent surgical resection at our hospital between January 1, 2019, and July 31, 2023, were enrolled in this retro
A total of 446 patients with PC met the inclusion criteria and were subsequently enrolled. Patients with early postoperative discharge tended to have higher PNI values and lower SII, NLR, and PLR values (all P < 0.05). Through multivariable logistic regression analysis, the SII value emerged as an independent risk factor influencing early recovery after surgery. Additionally, both univariable and mul
Preoperative systemic inflammatory-nutritional biomarkers may be capable of predicting short-term recovery after surgery as well as long-term patient outcomes.
Core Tip: This recent study investigated the predictive capacity of preoperative inflammatory-nutritional biomarkers for pos
- Citation: Lu JN, Zhou LS, Zhang S, Li JX, Xu CJ. Performance of nutritional and inflammatory markers in patients with pancreatic cancer. World J Clin Oncol 2024; 15(8): 1021-1032
- URL: https://www.wjgnet.com/2218-4333/full/v15/i8/1021.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i8.1021
Pancreatic cancer (PC) is recognized as an extremely aggressive malignancy, with a 5-year survival rate of approximately 13%[1]. The incidence of PC is increasing by 0.5% to 1.0% annually, and it is projected that PC will become the second most common cause of cancer-related death by 2030[2]. Surgery is the sole treatment with potential curative effects for PC and can increase the 5-year survival rate to more than 40%[1,3]. Despite this increase, the long-term survival rate is still considered suboptimal. Moreover, due to the intricate nature and physical trauma associated with surgery, patients who undergo surgery for PC typically have a prolonged recovery period; the postoperative hospitalization period usually exceeds 15 days, regardless of whether the surgery is performed through traditional open methods or minimally invasive laparoscopic techniques[4]. Therefore, a comprehensive understanding of PC biology, along with the discovery of readily applicable markers for early recovery and prognosis, would have great clinical benefit.
Noninvasive biomarkers have attracted considerable attention due to their ease of assessment without the need for invasive procedures such as tissue biopsy. Recently, nutritional and inflammatory markers have emerged as significant indicators for assessing the health status and prognosis of patients with various cancers, including colorectal cancer[5] and liver cancer[6]. These markers contribute critically to the understanding of inflammatory processes and nutritional balance. Although interest in these factors has been increasing, even in the context of PC[7-9], their roles in PC, especially in postoperative recovery, are incompletely understood. Further research is needed to fully elucidate the impacts of nutritional and inflammatory markers in the context of PC and to determine their potential as indicators for assessing postoperative recovery and long-term prognosis.
Therefore, the present study aimed to comprehensively evaluate the ability of seven markers, namely, the albumin-to-globulin ratio (AGR), prognostic nutritional index (PNI), systemic immune-inflammation index (SII), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), nutritional risk index (NRI), and geriatric nutritional risk index (GNRI), to predict postoperative recovery and prognosis in PC patients. Through this comprehensive evaluation, we aimed to provide valuable insights into the prognostic value of these markers and their utility in guiding patient management strategies.
This was a retrospective cohort study. A total of 446 PC patients who underwent PC-related surgery between January 1, 2019, and July 31, 2023, at the Second Affiliated Hospital, Zhejiang University School of Medicine were enrolled in our study. All patients were histopathologically diagnosed with PC, and patients were excluded if they met any of the following criteria: (1) The presence of other malignancies; (2) The presence of infectious diseases, such as lung infections, prior to surgery; or (3) The presence of autoimmune disorders.
All clinical data were collected retrospectively from the electronic medical record system and included the following main items: demographic characteristics, preoperative hematological parameters, tumor features, surgical information, etc. The demographic characteristics included age, sex, height, weight, smoking status, hypertension status and diabetes status. Body mass index (BMI) was calculated as weight (kg)/height (m)2. The main preoperative hematological parameters were lymphocyte, neutrophil, and platelet counts; albumin and globulin levels; and serum carbohydrate antigen 19-9 (sCA19-9) and carbohydrate antigen 125 (sCA125) levels. The tumor features included the tumor location, degree of differentiation, and stage according to the 8th edition of the American Joint Committee on Cancer TNM staging system. All patients were further classified into the early, middle or advanced subgroup according to their TNM stage. Moreover, the surgical procedure, method of surgery, time of surgery, and amount of bleeding were recorded as primary surgical information. Data collection and statistical analysis were conducted in accordance with the tenets of the Declaration of Helsinki and its subsequent amendments.
This study focused on seven nutritional and inflammatory markers: the AGR, PNI, SII, NLR, PLR, NRI, and GNRI. The formulas for calculating the AGR, PNI, SII, NLR, PLR, NRI, and GNRI are given in Supplementary Table 1.
Follow-up was carried out either in the outpatient clinic or by telephone. All patients were followed up on more than one occasion, and the most recent follow-up was conducted on January 1, 2024. Overall survival (OS) was defined as the period from pathological diagnosis to death or the last followup. Recurrence-free survival (RFS) was defined as the time from surgery to relapse or the last follow-up visit. In addition, the length of hospital stay was defined as the time from surgery to discharge. Early or delayed recovery was defined as a time to postsurgery discharge of less than or equal to 15 days. Rapid recovery, on the other hand, was defined as a time to postsurgery discharge of less than 10 days.
All data collection and assessment were performed by two or more clinicians and radiologists.
Continuous variables are expressed as mean ± SD or medians [interquartile ranges (IQRs)], while categorical variables are expressed as numbers (frequencies). Differences in continuous variables were analyzed using the independent samples t test, and differences in categorical variables were analyzed using the chi-squared test or Fisher's exact test. Nutritional and inflammatory markers were subjected to natural logarithmic transformation in the subsequent statistical analysis. Multivariable logistic regression models were used to calculate odds ratios (ORs) and to adjust for potentially confounding variables. The cutoff values for the associations between inflammatory–nutritional markers and early discharge were computed using receiver operating characteristic (ROC) curves and the Youden index. Differences in OS and RFS according to patient characteristics were assessed via the Kaplan-Meier method and the log-rank test. Univariable and multivariable Cox proportional hazards models were used to identify the prognostic factors associated with OS and RFS, adjusting for potentially confounding variables. The hazard ratio (HR) and the 95%CI were used to characterize the relative risk factors.
Data analyses were performed using IBM SPSS Statistics for Windows, version 27.0 (IBM Corp., Armonk, NY, United States), while violin plots and survival curves were generated with GraphPad Prism version 9.0 (GraphPad Software, San Diego, CA, United States). Cutoff values were chosen via X-tile software (version 3.6.1, Yale University, New Haven, CT, United States). Missing data were handled as missing values in the statistical analyses. A P value < 0.05 was considered to indicate statistical significance.
In the present study, we enrolled a cohort of 446 patients whose general characteristics are shown in Table 1. The cohort comprised 246 males (55.2%) and 200 females (44.8%), with an age of (65.09 ± 8.93) years and a BMI of (22.67 ± 3.00) kg/m2. Less than half of the patients had a history of smoking (37.2%), hypertension (46.9%), or diabetes (29.1%). The majority of the patients tested positive for sCA19-9 (≥ 37 U/mL) and negative for sCA125 (< 35 U/mL). Among the patients, 217 (48.7%) were diagnosed with carcinoma of the pancreatic head, and the tumors of 239 (53.6%) patients exhibited a moderate degree of differentiation upon pathological diagnosis. Among the patients, 198 patients (44.4%) had stage I disease, 139 (31.2%) had stage II disease, 58 (13.0%) had stage III disease, and 51 (11.4%) had stage IV disease. Due to the tumor location distribution, pancreaticoduodenectomy was the most common surgical procedure, conducted in 51.6% of the patients, with pancreatic caudectomy conducted in 42.8%. Of the enrolled patients, 284 (63.7%) underwent open surgery, whereas 162 (36.3%) underwent laparoscopic surgery. In this cohort, we further evaluated seven nutritional and inflammatory markers, which are displayed as the median (IQR) values in Table 1. Furthermore, at our hospital, the average hospital stay length was (17.68 ± 12.72) days.
| Characteristics | Total cohort (n = 446) |
| Demographics | |
| Age (years) | 65.09 ± 8.93 |
| Male | 246 (55.2%) |
| Female | 200 (44.8%) |
| BMI (kg/m2) | 22.67 ± 3.00 |
| Smoke | 166 (37.2%) |
| Hypertension | 209 (46.9%) |
| Diabetes | 130 (29.1%) |
| Tumor biomarkers | |
| sCA19-9 (U/mL) | |
| ≥ 37 | 336 (75.3%) |
| < 37 | 102 (22.9%) |
| Unknow | 8 (1.8%) |
| sCA125 (U/mL) | |
| ≥ 35 | 83 (18.6%) |
| < 35 | 341 (76.5%) |
| Unknow | 22 (4.9%) |
| Tumor features | |
| Location | |
| Head | 217 (48.7%) |
| Neck | 29 (6.5%) |
| Body | 69 (15.5%) |
| Tail | 58 (13.0%) |
| Both body and tail | 50 (11.2%) |
| Others | 23 (5.2%) |
| Degree of differentiation | |
| Poor | 142 (31.8%) |
| Moderate | 239 (53.6%) |
| Well | 43 (9.6%) |
| Unknow | 22 (4.9%) |
| TNM stage | |
| Ⅰ | 198 (44.4%) |
| Ⅱ | 139 (31.2%) |
| Ⅲ | 58 (13.0%) |
| Ⅳ | 51 (11.4%) |
| Surgical information | |
| Main surgical procedure | |
| Pancreaticoduodenectomy | 230 (51.6%) |
| Pancreaticocaudectomy | 191 (42.8%) |
| Others | 25 (5.6%) |
| Method of surgery | |
| Laparoscope | 162 (36.3%) |
| Open | 284 (63.7%) |
| Time of surgery (min) | 295 (225-395) |
| Bleeding (mL) | 200 (100-300) |
| Inflammatory-nutritional markers | |
| AGR | 1.42 (1.27-1.59) |
| PNI | 47.75 (43.89-51.11) |
| SII | 563.12 (367.88-894.94) |
| NLR | 2.74 (2.13-3.97) |
| PLR | 146.88 (107.55-195.98) |
| NRI | 104.02 (97.85-109.90) |
| GNRI | 102.86 (96.74-108.59) |
| Hospital stays (days) | 17.68 ± 12.72 |
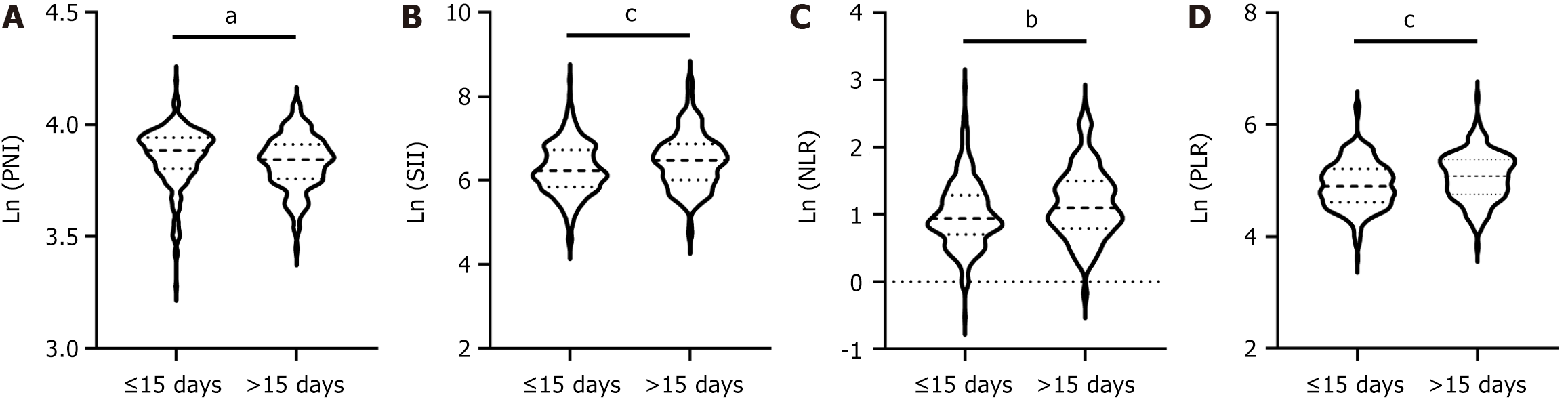
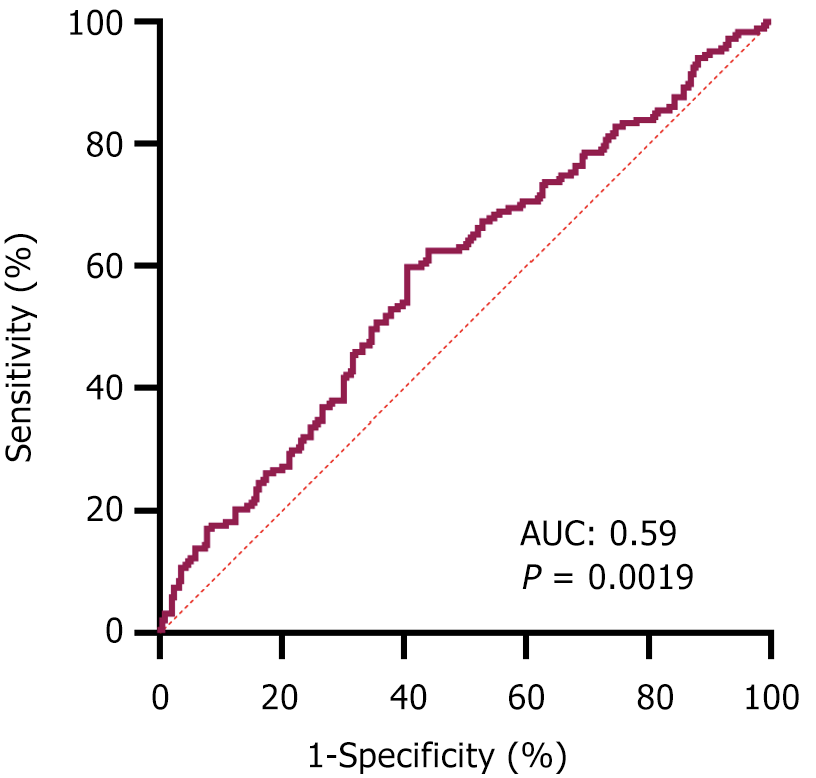
Identifying the preoperative factors that influence postoperative recovery is of particular importance. Thus, we further explored the relationship between preoperative nutritional and inflammatory markers and the length of postoperative hospital stay. Initially, we assessed the differences in preoperative nutritional and inflammatory scores between the early discharge group and the delayed discharge group using a cutoff postsurgery discharge time of 15 days. Several markers exhibited significant differences, including the PNI, SII, NLR, and PLR, all of which had P values of less than 0.05 (Table 2). Patients with early recovery were likely to have higher PNI values and lower SII, NLR, and PLR values (Figure 1). However, there were no significant differences in the AGR, NRI, or GNRI values between these groups (Table 2 and Supplementary Figure 1). Moreover, our findings indicated that age, hypertension status, tumor location, main surgical procedure, method of surgery, and time of surgery differed between the early discharge group and the delayed discharge group (all P values < 0.05, Table 2). Then, we employed multivariable logistic regression models to calculate the ORs and to adjust for potentially confounding variables. As shown in Table 3, age (OR = 1.037, P = 0003), surgical procedure (OR = 0.366, P < 0.001), time of surgery (OR = 1.172, P < 0.001), and SII (OR = 1.407, P = 0.046) were identified as the primary risk factors influencing early recovery. Moreover, based on the ROC curve (Figure 2) and Youden index, the optimal preoperative SII cutoff value was determined to be 574.69, and the area under the ROC curve for the SII was 0.59 (95%CI: 0.53-0.64, P = 0.0019).
| Characteristics | Early discharge cohort (n = 259) | Delayed discharge cohort (n = 187) | P value |
| Demographics | |||
| Age (years) | 64.01 ± 8.57 | 66.58 ± 9.23 | 0.003b |
| Gender (male/female) | 147/112 | 99/88 | 0.424 |
| BMI (kg/m2) | 22.76 ± 3.12 | 22.54 ± 2.81 | 0.453 |
| Smoke (yes/no) | 103/156 | 63/124 | 0.190 |
| Hypertension (yes/no) | 108/151 | 101/86 | 0.010a |
| Diabetes (yes/no) | 67/192 | 63/124 | 0.073 |
| Tumor features | |||
| Location | < 0.001c | ||
| Head | 90 | 127 | |
| Neck | 23 | 6 | |
| Body | 53 | 16 | |
| Tail | 45 | 13 | |
| Both body and tail | 35 | 15 | |
| Others | 13 | 10 | |
| Degree of differentiation | 0.320 | ||
| Poor | 85 | 57 | |
| Moderate | 132 | 107 | |
| Well | 30 | 13 | |
| Unknow | 12 | 10 | |
| TNM stage | 0.942 | ||
| Ⅰ | 116 | 82 | |
| Ⅱ | 80 | 59 | |
| Ⅲ | 32 | 26 | |
| Ⅳ | 31 | 20 | |
| Surgical information | |||
| Main surgical procedure | < 0.001c | ||
| Pancreaticoduodenectomy | 98 | 132 | |
| Pancreaticocaudectomy | 143 | 48 | |
| Others | 18 | 7 | |
| Method of surgery | < 0.001c | ||
| Laparoscope | 113 | 49 | |
| Open | 146 | 138 | |
| Time of surgery (min) | 282.61 ± 116.72 | 354.89 ± 124.67 | < 0.001c |
| Bleeding (mL) | 198.38 ± 168.88 | 218.29 ± 150.36 | 0.199 |
| Inflammatory-nutritional markers | |||
| AGR | 1.42 ± 0.24 | 1.44 ± 0.25 | 0.337 |
| PNI | 47.84 ± 5.72 | 46.43 ± 5.41 | 0.008b |
| SII | 643.27 ± 476.16 | 815.72 ± 660.21 | 0.003b |
| NLR | 3.13 ± 1.96 | 3.67 ± 2.20 | 0.007b |
| PLR | 149.93 ± 70.93 | 174.77 ± 79.25 | < 0.001c |
| NRI | 103.90 ± 9.50 | 102.44 ± 8.93 | 0.102 |
| GNRI | 102.69 ± 9.40 | 101.26 ± 8.82 | 0.103 |
In addition, we further adjusted the cutoff postsurgery discharge time to 10 days to explore the relationship between rapid recovery and the inflammatory-nutritional markers. We then conducted univariable logistic regression analyses. As indicated in Supplementary Table 2, six of the markers (PNI, SII, NLR, PLR, NRI, and GNRI), but not AGR, were significantly associated with rapid recovery. Due to the presence of shared components in the calculation of certain variables, we did not perform multivariable logistic regression analyses.
In summary, these results indicate potentially significant associations between preoperative inflammatory-nutritional biomarkers and postoperative recovery in patients with PC.
To identify the independent prognostic biomarkers, all the abovementioned variables, including demographic characteristics, tumor biomarkers, tumor characteristics, and inflammatory-nutritional markers, were incorporated into a Cox regression model. Univariable Cox regression analysis revealed that the degree of differentiation (P = 0.014), tumor stage (P < 0.001), and four markers - namely, PNI (P = 0.008), SII (P = 0.034), NLR (P = 0.008), and PLR (P = 0.031) were significant prognostic indicators for RFS, as detailed in Table 4. Furthermore, characteristics such as age (P = 0.004), BMI (P = 0.006), tumor location (P = 0.011), degree of differentiation (P = 0.003), tumor stage (P < 0.001) and main surgical procedure (P=0.007) were identified as significant prognostic indicators for OS, as shown in Table 5. Additionally, all seven biomarkers were determined to be associated with the prognosis of PC patients following surgery (all P values <0.05; Table 5).
| Characteristics | Univariable analysis | Multivariable analysis | ||
| HR | P value | HR | P value | |
| Demographics | ||||
| Age (years) | 1.001 (0.985-1.018) | 0.862 | ||
| Gender (male/female) | 1.071 (0.804-1.425) | 0.641 | ||
| BMI (kg/m2) | 0.973 (0.929-1.018) | 0.232 | ||
| Smoke (yes/no) | 1.007 (0.748-1.356) | 0.963 | ||
| Hypertension (yes/no) | 0.829 (0.623-1.104) | 0.199 | ||
| Type 2 diabetes (yes/no) | 0.923 (0.678-1.257) | 0.610 | ||
| Tumor biomarkers | ||||
| sCA19-9 (U/mL) ≥ 37 vs < 37 | 1.164 (0.821-1.652) | 0.394 | ||
| sCA125 (U/mL) ≥ 35 vs < 35 | 1.277 (0.892-1.828) | 0.181 | ||
| Tumor features | ||||
| Location (body/tail vs head/neck) | 0.854 (0.636-1.146) | 0.293 | ||
| Degree of differentiation | 0.014a | |||
| Poor | 2.455 (1.321-4.565) | 0.005e | ||
| Moderate | 1.908 (1.048-3.474) | 0.035d | ||
| Well | Reference | |||
| Tumor stage (TNM) | < 0.001c | < 0.001c | ||
| Early stage (Ⅰ) | 0.397 (0.253-0.621) | < 0.001f | 0.384 (0.241-0.612) | < 0.001f |
| Middle stage (Ⅱ/Ⅲ) | 0.640 (0.413-0.993) | 0.046d | 0.613 (0.388-0.967) | 0.035d |
| Advanced stage (Ⅳ) | Reference | |||
| Inflammatory-nutritional markers | ||||
| AGR | 0.887 (0.488-1.611) | 0.694 | ||
| Ln (PNI) | 0.203 (0.062-0.665) | 0.008b | 0.256 (0.076-0.863) | 0.028a |
| Ln (SII) | 1.276 (1.019-1.597) | 0.034a | ||
| Ln (NLR) | 1.439 (1.102-1.880) | 0.008b | ||
| Ln (PLR) | 1.443 (1.033-2.016) | 0.031a | ||
| Ln (NRI) | 0.299 (0.066-1.352) | 0.117 | ||
| Ln (GNRI) | 0.299 (0.066-1.353) | 0.117 | ||
| Characteristics | Univariable analysis | Multivariable analysis | ||||
| HR | P value | HR | P value | |||
| Demographics | ||||||
| Age (years) | 1.027 (1.009-1.046) | 0.004b | 1.021 (1.000-1.041) | 0.046a | ||
| Gender (male/female) | 1.148 (0.845-1.561) | 0.377 | ||||
| BMI (kg/m2) | 0.932 (0.886-0.980) | 0.006b | ||||
| Smoke (yes/no) | 1.280 (0.941-1.740) | 0.115 | ||||
| Hypertension (yes/no) | 0.843 (0.621-1.145) | 0.275 | ||||
| Type 2 diabetes (yes/no) | 0.996 (0.718-1.382) | 0.982 | ||||
| Tumor biomarkers | ||||||
| sCA19-9 (U/mL) ≥ 37 vs < 37 | 1.283 (0.880-1.871) | 0.195 | ||||
| sCA125 (U/mL) ≥ 35 vs < 35 | 1.413 (0.974-2.049) | 0.068 | ||||
| Tumor features | ||||||
| Location (body/tail vs head/neck) | 0.663 (0.482-0.911) | 0.011a | ||||
| Degree of differentiation | 0.003b | 0.012a | ||||
| Poor | 2.804 (1.433-5.487) | 0.003e | 2.369 (1.200-4.675) | 0.013d | ||
| Moderate | 1.899 (0.985-3.661) | 0.056 | 1.592 (0.822-3.084) | 0.168 | ||
| Well | Reference | |||||
| Tumor stage (TNM) | < 0.001c | 0.001c | ||||
| Early stage (Ⅰ) | 0.405 (0.260-0.632) | < 0.001f | 0.448 (0.281-0.715) | < 0.001f | ||
| Middle stage (Ⅱ/Ⅲ) | 0.725 (0.474-1.109) | 0.138 | 0.737 (0.473-1.149) | 0.178 | ||
| Advanced stage (Ⅳ) | Reference | |||||
| Inflammatory-nutritional markers | ||||||
| AGR | 0.415 (0.212-0.813) | 0.010a | ||||
| Ln (PNI) | 0.025 (0.008-0.079) | < 0.001c | 0.048 (0.015-0.155) | < 0.001c | ||
| Ln (SII) | 1.432 (1.122-1.829) | 0.004b | ||||
| Ln (NLR) | 1.788 (1.344-2.378) | < 0.001c | ||||
| Ln (PLR) | 1.839 (1.277-2.647) | 0.001b | ||||
| Ln (NRI) | 0.018 (0.004-0.078) | < 0.001c | ||||
| Ln (GNRI) | 0.018 (0.004-0.079) | < 0.001c | ||||
Multivariable analyses revealed that both tumor stage, specifically, early stage (P < 0.001, HR = 0.384) and middle stage (P = 0.035, HR = 0.613) compared to advanced stage and PNI (P = 0.028, HR = 0.256) were independent prognostic indicators for RFS, as shown in Table 4. Additionally, as shown in Table 5, age (P = 0.046, HR = 1.021), degree of differentiation (low vs high: P = 0.013, HR = 2.369), tumor stage (early vs advanced: P < 0.001, HR = 0.448), and ln(PNI) (P < 0.001, HR = 0.048) were identified as independent prognostic markers for OS.
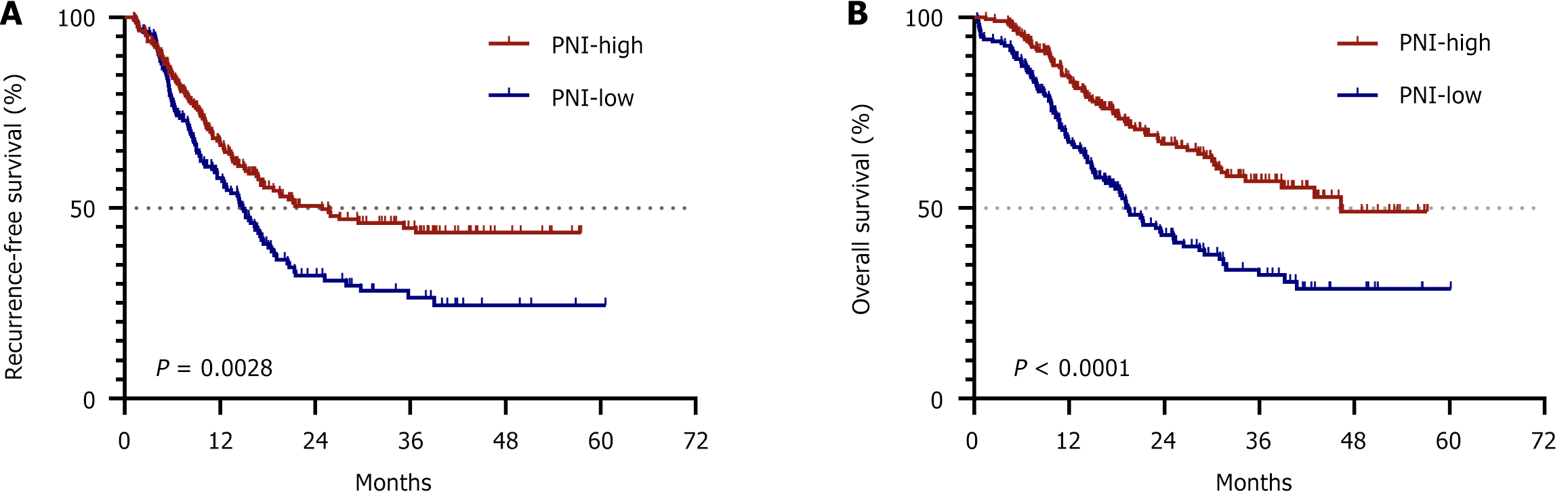
Ultimately, we assessed the prognostic significance of the PNI in PC patients who underwent surgical resection. Using X-tile software for analysis, we established a cutoff value for the PNI of 47.30. Accordingly, patients were categorized into the "PNI-low" group if their PNI value was below 47.30 and into the "PNI-high" group if their PNI value was equal to or greater than this threshold. The median RFS times of patients in the PNI-low and PNI-high groups were 14.80 months and 24.87 months, respectively, with a significant difference (P = 0.0028; Figure 3A). Furthermore, the OS times for patients in the PNI-low and PNI-high groups were 19.47 months and 46.30 months, respectively, and this difference was also statistically significant (P < 0.0001; Figure 3B).
Despite the availability of current treatments, PC remains a highly aggressive malignancy. We enrolled a cohort of 446 patients with PC, all of whom underwent surgical treatment at our center. We aimed to determine whether the preoperative nutritional and inflammatory statuses of patients could influence both their postoperative recovery and survival outcomes. To quantify this personal status, we utilized known inflammatory–nutritional indicators (AGR, PNI, SII, NLR, PLR, NRI, and GNRI). To our knowledge, our study is the first to reveal a correlation between preoperative inflammatory–nutritional markers and postoperative recovery. Additionally, we discovered that the preoperative PNI was the most reliable prognostic marker in PC patients undergoing surgical resection.
Alterations in the systemic inflammatory response and nutritional deterioration commonly occur in cancer patients, accompanying disease development and progression[10]. Thus, numerous prognostic markers based on the inflammatory and/or nutritional status have been developed to evaluate patients with cancer. Okadome et al[11] collected data from 337 patients with esophageal cancer who underwent curative resection and assessed the relationships among the PNI, the status of tumor-infiltrating lymphocytes (TILs), and clinical outcomes. Their findings revealed that both the PNI value and TIL score were associated with clinical outcomes in patients with esophageal cancer, reinforcing the potential roles of these indicators as prognostic biomarkers. In a study of patients who underwent pancreatectomy for PC with initial liver metastasis, the PNI and NLR were found to be associated with OS, while the SII and PNI were correlated with RFS[12]. Furthermore, recent studies have identified other inflammatory–nutritional markers, including the AGR[13], PLR[14], NRI[15], and GNRI[16], as having significant prognostic value in cancer patients. In the present study, we discovered significant associations between the PNI, SII, NLR, and PLR and RFS; additionally, the AGR, PNI, SII, NLR, PLR, NRI, and GNRI were significantly associated with OS. Because of the shared components in the calculation of these nutritional and inflammatory scores, our multivariable Cox regression analyses ultimately revealed that the preoperative PNI was the most reliable prognostic marker for both OS and RFS in patients with PC who underwent surgical resection.
The reasons that the PNI can predict the prognosis of cancer patients more accurately than other markers may be as follows: Lymphocytes play a pivotal role in the immune response, primarily by inhibiting tumor cell proliferation and metastasis. A decreased lymphocyte count can compromise the systemic immune system, allowing cancer cells to more readily evade immune surveillance[17]. Serum albumin remains the simplest and most effective parameter for assessing nutritional status in vivo and is a critical determinant of the immune response to cancer cells[18]. Therefore, the PNI, which incorporates the lymphocyte count and serum albumin level, may provide a comprehensive overview of both the inflammatory status and the nutritional status and thus could more accurately predict the prognosis of cancer patients.
Systemic inflammation and malnutrition significantly increase the risk of morbidity and mortality following gastrointestinal surgery[19]. Indeed, the preoperative inflammatory-nutritional status of patients has long been recognized as a critical determinant of the development of various complications after surgery. Thus, a body of research has focused on the potential of using markers such as the PNI, NLR, and NRI to predict postoperative complications[20-22]. Kryvoruchko et al[22] demonstrated that a high NLR and a low NRI were associated with an increased risk of postoperative complications. Moreover, they found that the NRI was also a predictor of 90-day mortality in patients following pancreatic surgery. Although it is acknowledged that postoperative complications can impact recovery to some extent, there is a notable paucity of published studies examining the relationship between preoperative inflammatory-nutritional markers and postoperative recovery as determined by the length of the postoperative hospital stay - a metric that may more accurately reflect the recovery trajectory of cancer patients than other commonly used metrics. In this study, we used a cutoff postsurgery discharge time of 15 days to divide the overall cohort into two groups: the early discharge group and the delayed discharge group. Patients who experienced faster recovery had higher PNI values and lower SII, NLR, and PLR values. Additionally, multivariable logistic regression analysis revealed that the SII was an independent risk factor affecting postoperative recovery outcomes in patients. Moreover, when the cutoff postsurgery discharge time was adjusted to 10 days, six of the preoperative nutritional and inflammatory markers were found to be discernible risk factors influencing postoperative recovery. Therefore, these results suggest a potential association between preoperative inflammatory-nutritional biomarkers and postoperative recovery outcomes in patients with PC.
The strength of this study is underscored by the substantial patient sample size and the simplicity, feasibility, and efficiency of these biomarkers as indicators for the regular assessment of patients with cancers. They hold promise for predicting not only the outcomes of patients with PC but also the likelihood of early postoperative recovery. However, the current study still has limitations. One of the most obvious limitations is the retrospective design. Additionally, our study did not address all possible inflammatory-nutritional biomarkers. Furthermore, the data for some patients were incomplete, and the missing values were thus categorized as such in the statistical analyses, potentially introducing selection bias.
The current study established that preoperative systemic inflammatory-nutritional biomarkers are linked to the prognosis of patients with PC, with the PNI exhibiting the highest prognostic value. Furthermore, these markers may be able to predict postoperative recovery outcomes.
| 1. | Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2279] [Cited by in RCA: 4680] [Article Influence: 4680.0] [Reference Citation Analysis (3)] |
| 2. | Park W, Chawla A, O'Reilly EM. Pancreatic Cancer: A Review. JAMA. 2021;326:851-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 798] [Cited by in RCA: 1079] [Article Influence: 269.8] [Reference Citation Analysis (0)] |
| 3. | Moore A, Donahue T. Pancreatic Cancer. JAMA. 2019;322:1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 4. | Wang M, Li D, Chen R, Huang X, Li J, Liu Y, Liu J, Cheng W, Chen X, Zhao W, Li J, Tan Z, Huang H, Li D, Zhu F, Qin T, Ma J, Yu G, Zhou B, Zheng S, Tang Y, Han W, Meng L, Ke J, Feng F, Chen B, Yin X, Chen W, Ma H, Xu J, Liu Y, Lin R, Dong Y, Yu Y, Liu J, Zhang H, Qin R; Minimally Invasive Treatment Group in the Pancreatic Disease Branch of China's International Exchange and Promotion Association for Medicine and Healthcare (MITG-P-CPAM). Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours: a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2021;6:438-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 166] [Article Influence: 41.5] [Reference Citation Analysis (1)] |
| 5. | Park JW, Chang HJ, Yeo HY, Han N, Kim BC, Kong SY, Kim J, Oh JH. The relationships between systemic cytokine profiles and inflammatory markers in colorectal cancer and the prognostic significance of these parameters. Br J Cancer. 2020;123:610-618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Wang D, Hu X, Xiao L, Long G, Yao L, Wang Z, Zhou L. Prognostic Nutritional Index and Systemic Immune-Inflammation Index Predict the Prognosis of Patients with HCC. J Gastrointest Surg. 2021;25:421-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 132] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 7. | Kawai M, Hirono S, Okada KI, Miyazawa M, Shimizu A, Kitahata Y, Kobayashi R, Ueno M, Hayami S, Tanioka K, Yamaue H. Low lymphocyte monocyte ratio after neoadjuvant therapy predicts poor survival after pancreatectomy in patients with borderline resectable pancreatic cancer. Surgery. 2019;165:1151-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Schlanger D, Popa C, Pașca S, Seicean A, Al Hajjar N. The role of systemic immuno-inflammatory factors in resectable pancreatic adenocarcinoma: a cohort retrospective study. World J Surg Oncol. 2022;20:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Hoshimoto S, Hishinuma S, Shirakawa H, Tomikawa M, Ozawa I, Ogata Y. Validation and clinical usefulness of pre- and postoperative systemic inflammatory parameters as prognostic markers in patients with potentially resectable pancreatic cancer. Pancreatology. 2020;20:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 754] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 11. | Okadome K, Baba Y, Yagi T, Kiyozumi Y, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Watanabe M, Baba H. Prognostic Nutritional Index, Tumor-infiltrating Lymphocytes, and Prognosis in Patients with Esophageal Cancer. Ann Surg. 2020;271:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 263] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 12. | Frigerio I, Malleo G, de Pastena M, Deiro G, Surci N, Scopelliti F, Esposito A, Regi P, Giardino A, Allegrini V, Bassi C, Girelli R, Salvia R, Butturini G. Prognostic Factors After Pancreatectomy for Pancreatic Cancer Initially Metastatic to the Liver. Ann Surg Oncol. 2022;29:8503-8510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 13. | Roberts WS, Delladio W, Price S, Murawski A, Nguyen H. The efficacy of albumin-globulin ratio to predict prognosis in cancer patients. Int J Clin Oncol. 2023;28:1101-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Zhong X, Su T, Yang Y, Ye L, Jiang L, Qi Y, Xie J, Jiang Y, Zhou W, Zhang C, Wu L, Zhu H, Ning G, Wang W. Platelet-Lymphocyte and Neutrophil-Lymphocyte Ratios Are Prognostic Markers for Pheochromocytomas and Paragangliomas. J Clin Endocrinol Metab. 2023;108:2230-2239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Lin F, Xia W, Chen M, Jiang T, Guo J, Ouyang Y, Sun H, Chen X, Deng W, Guo L, Lin H. A Prognostic Model Based on Nutritional Risk Index in Operative Breast Cancer. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 16. | Haas M, Lein A, Fuereder T, Brkic FF, Schnoell J, Liu DT, Kadletz-Wanke L, Heiduschka G, Jank BJ. The Geriatric Nutritional Risk Index (GNRI) as a Prognostic Biomarker for Immune Checkpoint Inhibitor Response in Recurrent and/or Metastatic Head and Neck Cancer. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 17. | Ruffell B, DeNardo DG, Affara NI, Coussens LM. Lymphocytes in cancer development: polarization towards pro-tumor immunity. Cytokine Growth Factor Rev. 2010;21:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 182] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 18. | Guo Y, Wei L, Patel SH, Lopez G, Grogan M, Li M, Haddad T, Johns A, Ganesan LP, Yang Y, Spakowicz DJ, Shields PG, He K, Bertino EM, Otterson GA, Carbone DP, Presley C, Kulp SK, Mace TA, Coss CC, Phelps MA, Owen DH. Serum Albumin: Early Prognostic Marker of Benefit for Immune Checkpoint Inhibitor Monotherapy But Not Chemoimmunotherapy. Clin Lung Cancer. 2022;23:345-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Lee B, Han HS. Tackling Surgical Morbidity and Mortality through Modifiable Risk Factors in Cancer Patients. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Paku M, Uemura M, Kitakaze M, Fujino S, Ogino T, Miyoshi N, Takahashi H, Yamamoto H, Mizushima T, Doki Y, Eguchi H. Impact of the preoperative prognostic nutritional index as a predictor for postoperative complications after resection of locally recurrent rectal cancer. BMC Cancer. 2021;21:435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Qi Q, Song Q, Cheng Y, Wang N. Prognostic Significance of Preoperative Prognostic Nutritional Index for Overall Survival and Postoperative Complications in Esophageal Cancer Patients. Cancer Manag Res. 2021;13:8585-8597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Kryvoruchko IA, Staikov P, Boyko VV, Sartelli M, Ivanova YV, Honcharov A, Gramatiuk S, Sargsyan K. Physiological stress level and screening for malnutrition as preoperative predictors of postoperative complications in pancreatic surgery: a retrospective study. BMC Surg. 2023;23:156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |