Published online Jul 24, 2024. doi: 10.5306/wjco.v15.i7.945
Revised: May 21, 2024
Accepted: June 5, 2024
Published online: July 24, 2024
Processing time: 126 Days and 21.7 Hours
Epidermal growth factor receptor (EGFR) mutation and c-ros oncogene 1 (ROS1) rearrangement are key genetic alterations and predictive tumor markers for non-small cell lung cancer (NSCLC) and are typically considered to be mutually exc
Herein, we report the case of a 64-year-old woman diagnosed with lung adenocarcinoma, with concomitant EGFR L858R mutation and ROS1 rearrangement. The patient received two cycles of chemotherapy after surgery, but the disease prog
The efficacy of EGFR tyrosine kinase inhibitors and crizotinib was vastly different in this NSCLC patient with EGFR/ROS1 co-mutation. This report will aid future treatment of such patients.
Core Tip: Over the past two decades, molecular targeted therapies have improved clinical outcomes significantly for non-small cell lung cancer (NSCLC) patients with either epidermal growth factor receptor (EGFR) mutation or c-ros oncogene 1 (ROS1) fusion. Nevertheless, EGFR/ROS1 co-mutation is a rare event in NSCLC, and the standard treatment for such cases is still equivocal. We report an EGFR/ROS1 co-mutation in an NSCLC patient who remained clinically stable after 53 months of treatment with crizotinib. This is the longest progression-free survival reported in the literature. This case suggested that crizotinib may be a potential choice for NSCLC patients with such EGFR/ROS1 co-mutations.
- Citation: Peng GQ, Song HC, Chen WY. Concomitant epidermal growth factor receptor mutation/c-ros oncogene 1 rearrangement in non-small cell lung cancer: A case report. World J Clin Oncol 2024; 15(7): 945-952
- URL: https://www.wjgnet.com/2218-4333/full/v15/i7/945.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i7.945
Lung cancer has the second-highest incidence of all cancers and the highest cancer-related death rate in the world[1]. Non-small cell lung cancer (NSCLC) accounts for about 85% of all lung cancers. Lung adenocarcinoma is the most common histopathological subtype of NSCLC, accounting for about 55% of cases. The mutation rate of epidermal growth factor receptor (EGFR) in Asian patients with lung adenocarcinoma is approximately 51%, and the mutation rate of c-ros oncogene 1 (ROS1) in NSCLC is 2%-3%[2-4]. EGFR/ROS1 co-mutation is relatively rare, and the incidence of concomitant EGFR mutations in patients with ROS1 fusion genes is less than 24%[5-7].
Early studies have shown that tyrosine kinase inhibitors (TKIs) targeting EGFR and ROS1 are the first-line treatment for patients with EGFR mutations or ROS1 rearrangement[8-11]. However, there is currently no consensus on the optimal management of patients with these co-mutations. This article reports the treatment of an NSCLC patient with EGFR and ROS1 co-mutation to provide a reference for the treatment and management of other NSCLC patients with these co-mutations.
A 64-year-old Chinese woman presented on November 1, 2018 with a complaint of cough and sputum production for 1 wk.
Symptoms started 1 wk before presentation, with cough and sputum production.
An appendectomy due to appendicitis had been performed approximately 30 years prior to the presentation. Cho
The patient denied any family history of malignant tumors. The patient also had no history of smoking.
On physical examination, the vital signs were as follows: Body temperature, 36.6 °C; blood pressure, 136/75 mmHg; heart rate, 74 beats per min; and respiratory rate, 20 breaths per min.
Levels of serum tumor markers were normal. Carcinoembryonic antigen was < 0.5 ng/mL (normal range: 0-5 ng/mL), squamous cell carcinoma antigen was 0.6 ng/mL (normal range: 0-1.5 ng/mL), and cytokeratin 19 fragment was 1.57 ng/mL (normal range: 0-2.08 ng/mL). No abnormality was found in the routine blood and urine analyses.
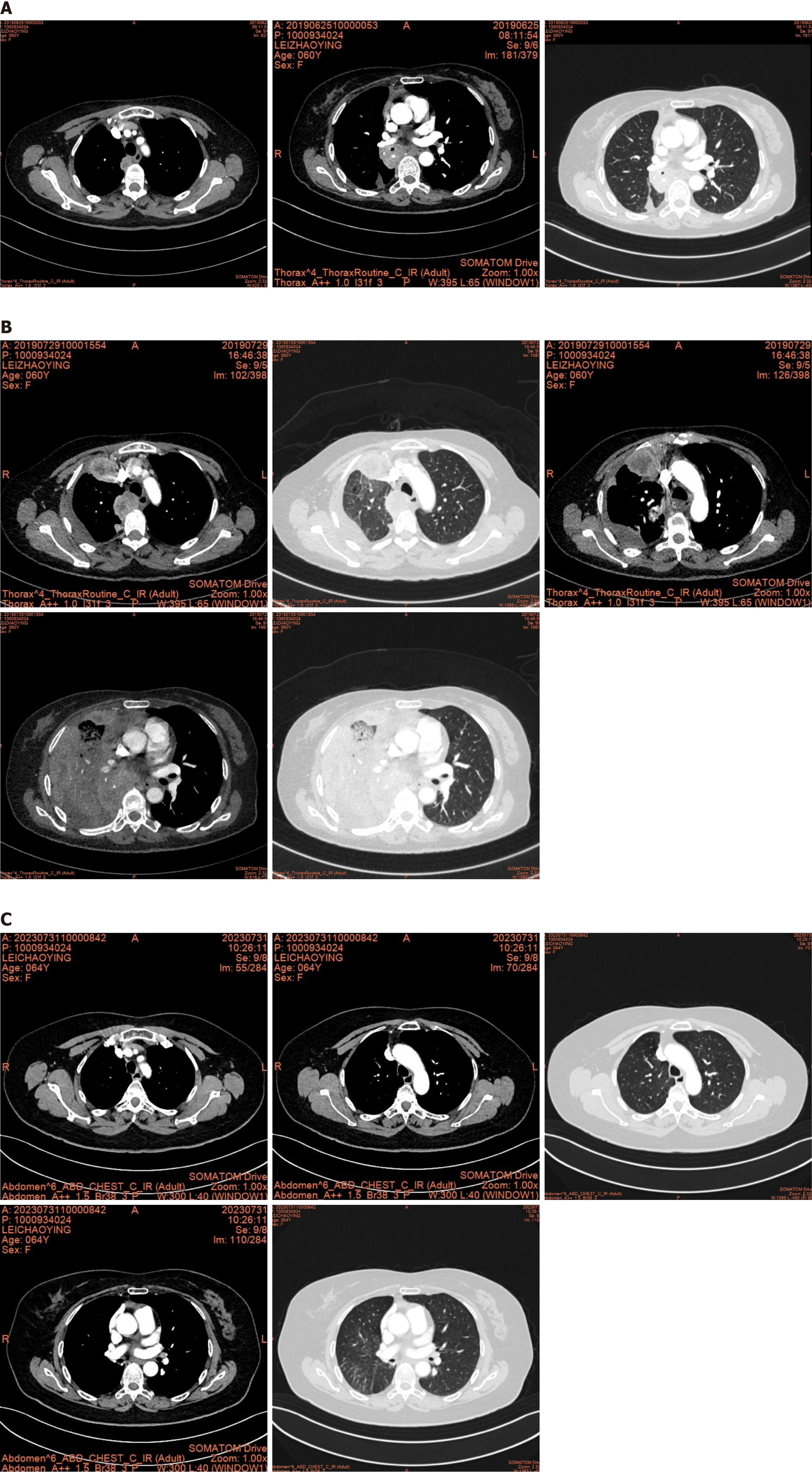
On November 2, 2018, chest computed tomography (CT) showed a 2.8 cm × 1.8 cm patchy shadow in the inferior lobe of the right lung. The scan also showed multiple ground-glass nodules in bilateral lungs. The patient was prescribed oral anti-tuberculosis drugs. She discontinued the medication after 1 month due to intolerance. On March 25, 2019, the patient visited our hospital again. Chest CT showed a 4.0 cm × 2.7 cm shadow in the lower lobe of the right lung. Due to the significant growth, the possibility of a neoplastic lesion was considered.
Combined with the patient’s medical history, the final diagnosis was determined to be NSCLC.
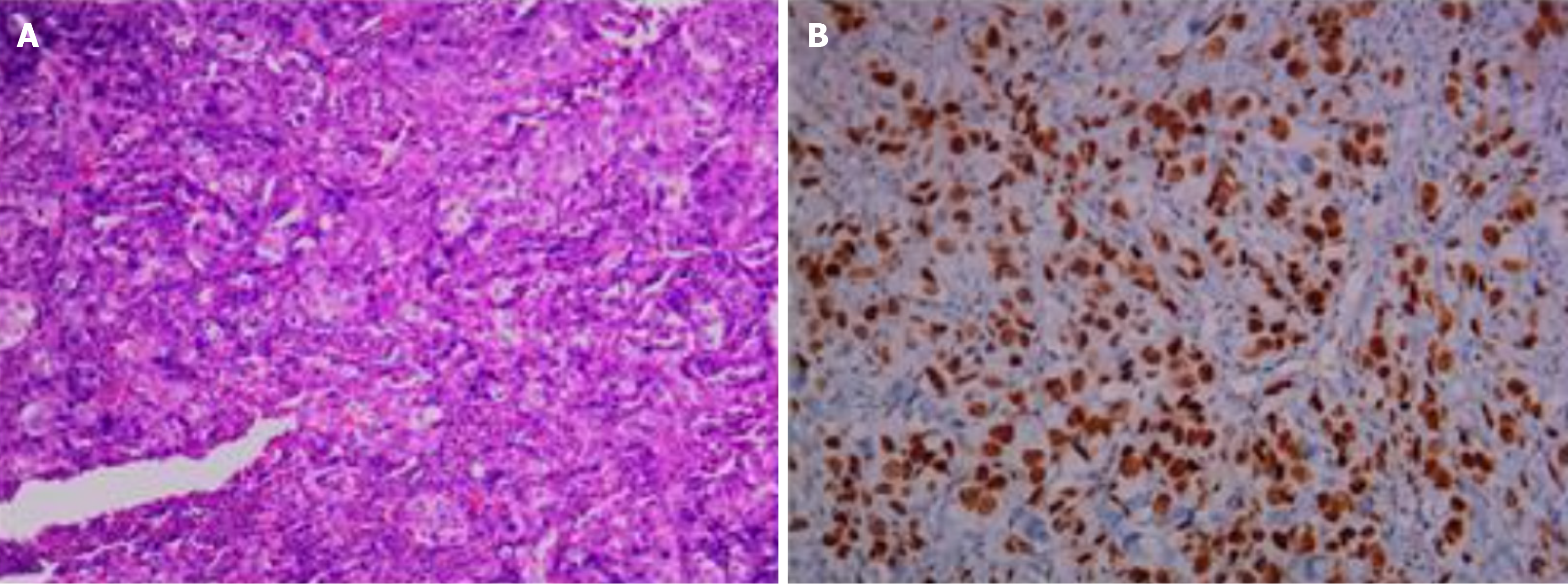
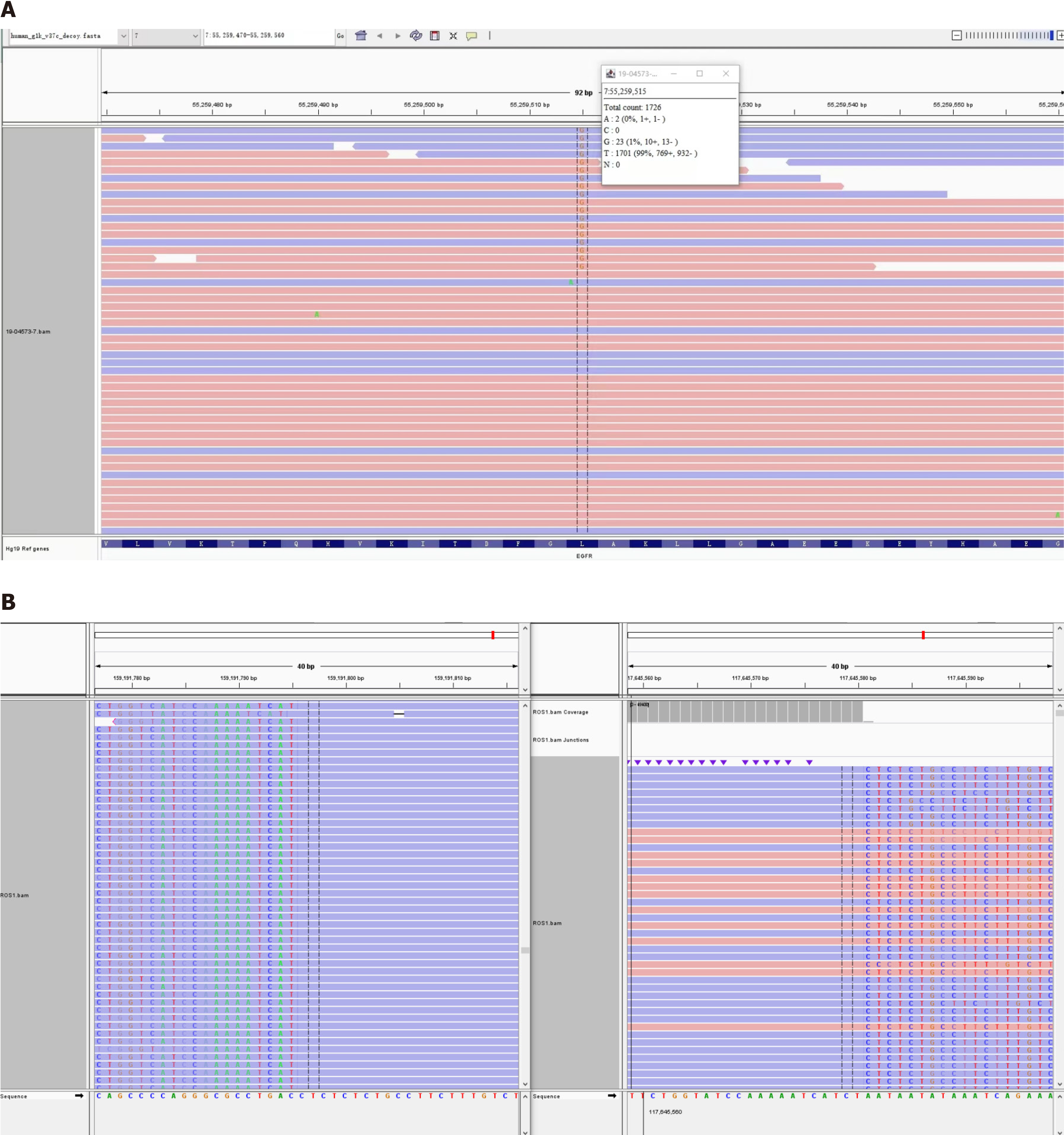
On April 3, 2019, thoracoscopic radical resection of the lower right lung cancer and pleural adhesion cauterization was performed under general anesthesia. Postoperative pathological diagnosis was acinar adenocarcinoma grade Ⅲ, micropapillary adenocarcinoma (accounting for about 5% of tumor tissue) in the local area, and tumor thrombus in the alveolar cavity (Figure 1). ROS1 rearrangement and EGFR L858R mutation were detected by next-generation sequencing of 13 lung cancer driver genes. The frequency of the EGFR mutation found in this patient was only 1.3% (Figure 2).
After thoracoscopic radical resection, the patient received two cycles of chemotherapy (pemetrexed 690 mg D1 + cisplatin 50 mg D1-2; on May 14, 2019 and June 5, 2019). Comparison of the CT film obtained on June 25, 2019 with the previous film (May 7, 2019) showed progression of the lesion. Multiple lymph nodes in the mediastinum and right hilum were also enlarged, suggesting metastasis (Figure 3).
Gefitinib was administered for 1 month starting on June 26, 2019. During this period, the patient developed drug-induced diarrhea, which improved after treatment with loperamide. Chest CT on July 30, 2019 revealed that multiple lymph nodes in the mediastinum, right cardiophrenic angle, and right hilum were enlarged, indicating progression of the disease (Figure 3).
On August 7, 2019, crizotinib was administered. The efficacy of crizotinib was evaluated after 1 month, which revealed stable disease. Since then, the patient has been treated with crizotinib for over 53 months and continues to have stable disease (Figure 3).
After 53 months of treatment with crizotinib, the patient is still alive.
Genetic mutations are typically considered to be mutually exclusive[12-15]. A recent study[16] demonstrated that patients with co-mutations have a poor progression-free survival. Patients with both ROS1 fusion and EGFR mutations are exceedingly rare in comparison to the co-occurrence of EGFR/PIK3CA, ALK/KRAS, and EGFR/MET mutations[16-18]. However, using highly sensitive methods such as the amplification-refractory mutation system analysis leads to an increase in the detection rate of co-mutations of KRAS or BRAF mutations and ALK or ROS1 rearrangements[13]. Studies[13-15] have shown that the incidence of concomitant gene mutations in lung adenocarcinoma was 3.8%, and EGFR/ROS1 and EML4-ALK were among the mutations detected. There is no consensus on the clinical characteristics, treatment options, and prognosis of NSCLC patients with co-mutations.
The mechanism of EGFR/ROS1 co-mutation remains unclear. Wu et al[19] hypothesized that two gene alterations can be detected within the same tumor cells in NSCLC, which might result in cancer development through co-action. Won et al[20] proposed that genetic instability in cancer cells leads to genetic and phenotypic heterogeneity in tumors, indicating that different genetic mutations might occur in tumor cells rather than in a single clone. Accumulating evidence shows co-existence of classical oncogenes, including EGFR, ALK, ROS1, and MET, in lung adenocarcinoma patients, especially in young females with no history of smoking[21]. In this case report, the patient was an elderly woman and the pathology suggested adenocarcinoma, which was consistent with the literature reports.
Cases of EGFR/ROS1 co-mutation have been infrequently reported in NSCLC, creating a lack of clear treatment standards. Zhang et al[22] reported a patient with an EGFR exon 21 L858R mutation. The disease progressed after gefitinib treatment for 11 months. The gene test showed EGFR exon 21 L858R mutation (abundance 36.62%), EGFR exon 20 T790M (7.95%), and ROS1 fusion (15.81%), as well as EGFR, HER2, and BRAF amplification. The second-line treatment included osimertinib, and the disease progressed after 7 months. The third-line chemotherapy included two cycles of pemetrexed and carboplatin, which did not prevent disease progression.
Another study reported the cases of three stage IA NSCLC patients with EGFR/ROS1 co-mutation who received ch
According to Zhuang et al[21], the progression-free survival of patients with an EGFR co-mutation treated with first-line TKIs was better than that of patients treated with first-line chemotherapy. A recent study described the case of a 48-year-old woman diagnosed with NSCLC with an EGFR 19 deletion/ROS1 rearrangement who achieved a favorable outcome[19]. Following 1 month of treatment with a combination of almonertinib and crizotinib, there was a significant reduction in the primary mass and all lymph nodes. Almonertinib was subsequently replaced by furmonertinib due to elevated levels of creatine kinase. The patient received furmonertinib and crizotinib treatment for 7 months, and stable disease was achieved throughout the follow-up period.
It appears that the clinical efficacy of EGFR TKIs and crizotinib treatment is quite different based on these clinical reports and our experience. The best choice for first-line treatment remains unclear. It has been reported, though, that the efficacy of TKIs can be predicted by the phosphorylation levels of EGFR and ALK in patients with EGFR/ALK co-mutation[25].
Nevertheless, there are still several challenges in predicting the efficacy of treatment in patients with EGFR/ROS1 co-mutations. First, current genetic testing is unable to determine the dominant oncogene aberration in a single tumor cell. Second, there is limited research on the relationship between the abundance of a ROS1 mutation and lung cancer prognosis. Finally, there have been no reports on whether EGFR phosphorylation levels and ROS1 mutation abundance can predict the effectiveness of TKIs. In our report, we found that the patient had a very low EGFR mutation frequency (1.3%), which may explain the poor response to the gefitinib treatment. Therefore, it is possible that low EGFR pho
Liu et al[26] observed that TKI combination therapy was more effective than single TKI treatment in patients with EGFR/ALK co-mutations. Wu et al[19] demonstrated similar outcome in one patient with an EFGR 19 deletion/ROS1 rearrangement upon treatment with a combination of third-generation EGFR TKIs and crizotinib. Combination therapy appears to exhibit a favorable efficacy. However, not all patients are able to tolerate the toxicity associated with TKI co
There is no overall survival difference between patients with single EGFR mutations and those with concomitant ALK/ROS1 mutations (21.0 months vs 23.0 months, respectively, P = 0.196)[27]. However, concomitant EGFR mutation and ALK/ROS1 mutation reduced the therapeutic effect of EGFR TKIs in patients. Patients with co-mutations had a significantly shorter progression-free survival than those with a single EGFR mutation (6.6 months vs 10.7 months, respectively, P = 0.004)[27]. In our report, the patient remained stable after 53 months of crizotinib treatment, which is the longest reported progression-free survival of a patient with an EGFR/ROS1 co-mutation.
EGFR/ROS1 co-mutation is rare in patients with NSCLC. Due to its rarity, the best treatment approach is unclear. TKI therapy can be used as the first-line option, but the clinical efficacy of EGFR-TKIs and crizotinib therapy appears to vary significantly between patients. The level of EGFR phosphorylation may play a crucial role in the selection of therapeutic drugs. Further investigation is required to examine the correlation between ROS1 mutation frequency and the prognosis of lung cancer.
We are grateful to Dr. Xiao-Xiao Wang for her help with the artwork.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64697] [Article Influence: 16174.3] [Reference Citation Analysis (177)] |
| 2. | Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G, Yang PC. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9:154-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 917] [Cited by in RCA: 1140] [Article Influence: 103.6] [Reference Citation Analysis (0)] |
| 3. | Han B, Tjulandin S, Hagiwara K, Normanno N, Wulandari L, Laktionov K, Hudoyo A, He Y, Zhang YP, Wang MZ, Liu CY, Ratcliffe M, McCormack R, Reck M. EGFR mutation prevalence in Asia-Pacific and Russian patients with advanced NSCLC of adenocarcinoma and non-adenocarcinoma histology: The IGNITE study. Lung Cancer. 2017;113:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 4. | Kohno T, Nakaoku T, Tsuta K, Tsuchihara K, Matsumoto S, Yoh K, Goto K. Beyond ALK-RET, ROS1 and other oncogene fusions in lung cancer. Transl Lung Cancer Res. 2015;4:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 133] [Reference Citation Analysis (0)] |
| 5. | Lin JJ, Ritterhouse LL, Ali SM, Bailey M, Schrock AB, Gainor JF, Ferris LA, Mino-Kenudson M, Miller VA, Iafrate AJ, Lennerz JK, Shaw AT. ROS1 Fusions Rarely Overlap with Other Oncogenic Drivers in Non-Small Cell Lung Cancer. J Thorac Oncol. 2017;12:872-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 6. | Scheffler M, Schultheis A, Teixido C, Michels S, Morales-Espinosa D, Viteri S, Hartmann W, Merkelbach-Bruse S, Fischer R, Schildhaus HU, Fassunke J, Sebastian M, Serke M, Kaminsky B, Randerath W, Gerigk U, Ko YD, Krüger S, Schnell R, Rothe A, Kropf-Sanchen C, Heukamp L, Rosell R, Büttner R, Wolf J. ROS1 rearrangements in lung adenocarcinoma: prognostic impact, therapeutic options and genetic variability. Oncotarget. 2015;6:10577-10585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Wiesweg M, Eberhardt WEE, Reis H, Ting S, Savvidou N, Skiba C, Herold T, Christoph DC, Meiler J, Worm K, Kasper S, Theegarten D, Hense J, Hager T, Darwiche K, Oezkan F, Aigner C, Welter S, Kühl H, Stuschke M, Schmid KW, Schuler M. High Prevalence of Concomitant Oncogene Mutations in Prospectively Identified Patients with ROS1-Positive Metastatic Lung Cancer. J Thorac Oncol. 2017;12:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5906] [Cited by in RCA: 6478] [Article Influence: 404.9] [Reference Citation Analysis (0)] |
| 9. | Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, Hu C, Hu C, Luo Y, Chen L, Ye M, Huang J, Zhi X, Zhang Y, Xiu Q, Ma J, Zhang L, You C. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2700] [Cited by in RCA: 3262] [Article Influence: 233.0] [Reference Citation Analysis (0)] |
| 10. | Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, Riely GJ, Varella-Garcia M, Shapiro GI, Costa DB, Doebele RC, Le LP, Zheng Z, Tan W, Stephenson P, Shreeve SM, Tye LM, Christensen JG, Wilner KD, Clark JW, Iafrate AJ. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1502] [Cited by in RCA: 1506] [Article Influence: 136.9] [Reference Citation Analysis (0)] |
| 11. | Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, Okamoto I, Zhou C, Cho BC, Cheng Y, Cho EK, Voon PJ, Planchard D, Su WC, Gray JE, Lee SM, Hodge R, Marotti M, Rukazenkov Y, Ramalingam SS; FLAURA Investigators. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:113-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2556] [Cited by in RCA: 3682] [Article Influence: 526.0] [Reference Citation Analysis (0)] |
| 12. | Cardarella S, Ortiz TM, Joshi VA, Butaney M, Jackman DM, Kwiatkowski DJ, Yeap BY, Jänne PA, Lindeman NI, Johnson BE. The introduction of systematic genomic testing for patients with non-small-cell lung cancer. J Thorac Oncol. 2012;7:1767-1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Takeuchi K, Soda M, Togashi Y, Suzuki R, Sakata S, Hatano S, Asaka R, Hamanaka W, Ninomiya H, Uehara H, Lim Choi Y, Satoh Y, Okumura S, Nakagawa K, Mano H, Ishikawa Y. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18:378-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 927] [Cited by in RCA: 1034] [Article Influence: 79.5] [Reference Citation Analysis (1)] |
| 14. | Sun Y, Ren Y, Fang Z, Li C, Fang R, Gao B, Han X, Tian W, Pao W, Chen H, Ji H. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol. 2010;28:4616-4620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 282] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 15. | Yoshida A, Kohno T, Tsuta K, Wakai S, Arai Y, Shimada Y, Asamura H, Furuta K, Shibata T, Tsuda H. ROS1-rearranged lung cancer: a clinicopathologic and molecular study of 15 surgical cases. Am J Surg Pathol. 2013;37:554-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 16. | Li W, Chang F, Zhang H, Meng F, Ke Z, Zhang Y. Clinical Pathological Characteristics and Prognosis of Multigene Co-Mutations in Elderly Patients With Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Med Insights Oncol. 2023;17:11795549231211505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | Sakamoto T, Matsubara T, Takahama T, Yokoyama T, Nakamura A, Tokito T, Okamoto T, Akamatsu H, Oki M, Sato Y, Tobino K, Ikeda S, Mori M, Mimura C, Maeno K, Miura S, Harada T, Nishimura K, Hiraoka M, Kenmotsu H, Fujimoto J, Shimokawa M, Yamamoto N, Nakagawa K. Biomarker Testing in Patients With Unresectable Advanced or Recurrent Non-Small Cell Lung Cancer. JAMA Netw Open. 2023;6:e2347700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Chen H, Liu M, Dai Z, Li S, Luo Y, Wang Y, Su W, Cai W, Yang D, Huang J, Yang Z. Concomitant genetic alterations are associated with response to EGFR targeted therapy in patients with lung adenocarcinoma. Transl Lung Cancer Res. 2020;9:1225-1234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Wu Z, Zhang Z, Zhang D, Li Z. Remarkable response to third-generation EGFR-TKI plus crizotinib in a patient with pulmonary adenocarcinoma harboring EGFR and ROS1 co-mutation: a case report. Front Oncol. 2024;14:1357230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Won JK, Keam B, Koh J, Cho HJ, Jeon YK, Kim TM, Lee SH, Lee DS, Kim DW, Chung DH. Concomitant ALK translocation and EGFR mutation in lung cancer: a comparison of direct sequencing and sensitive assays and the impact on responsiveness to tyrosine kinase inhibitor. Ann Oncol. 2015;26:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 21. | Zhuang X, Zhao C, Li J, Su C, Chen X, Ren S, Li X, Zhou C. Clinical features and therapeutic options in non-small cell lung cancer patients with concomitant mutations of EGFR, ALK, ROS1, KRAS or BRAF. Cancer Med. 2019;8:2858-2866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 22. | Zhang S, Xu Z, Zhang W. OPRM1-ROS1 Fusion Detected by Next-Generation Sequencing with Circulating DNA in a Patient with EGFR Mutated Advanced NSCLC: A Case Report. Case Rep Oncol. 2022;15:700-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 23. | Zhang X, Jiang Y, Yu H, Xia H, Wang X. A comprehensive study on the oncogenic mutation and molecular pathology in Chinese lung adenocarcinoma patients. World J Surg Oncol. 2020;18:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Ju L, Han M, Zhao C, Li X. EGFR, KRAS and ROS1 variants coexist in a lung adenocarcinoma patient. Lung Cancer. 2016;95:94-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Yang JJ, Zhang XC, Su J, Xu CR, Zhou Q, Tian HX, Xie Z, Chen HJ, Huang YS, Jiang BY, Wang Z, Wang BC, Yang XN, Zhong WZ, Nie Q, Liao RQ, Mok TS, Wu YL. Lung cancers with concomitant EGFR mutations and ALK rearrangements: diverse responses to EGFR-TKI and crizotinib in relation to diverse receptors phosphorylation. Clin Cancer Res. 2014;20:1383-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 26. | Liu J, Mu Z, Liu L, Li K, Jiang R, Chen P, Zhou Q, Jin M, Ma Y, Xie Y, Xiang J, Li B, Ma Y, Mao X, Zhang L, Zhang T, Wu D. Frequency, clinical features and differential response to therapy of concurrent ALK/EGFR alterations in Chinese lung cancer patients. Drug Des Devel Ther. 2019;13:1809-1817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Mao Y, Wu S. ALK and ROS1 concurrent with EGFR mutation in patients with lung adenocarcinoma. Onco Targets Ther. 2017;10:3399-3404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |