Published online Jul 24, 2024. doi: 10.5306/wjco.v15.i7.920
Revised: May 21, 2024
Accepted: June 6, 2024
Published online: July 24, 2024
Processing time: 128 Days and 15 Hours
The association between tumor-infiltrating lymphocyte (TIL) levels and the res
To investigate the predictive potential of TIL levels for the response to NAT in TNBC patients.
A systematic search of the National Center for Biotechnology Information PubMed database was performed to collect relevant published literature prior to August 31, 2023. The correlation between TIL levels and the NAT pathologic com
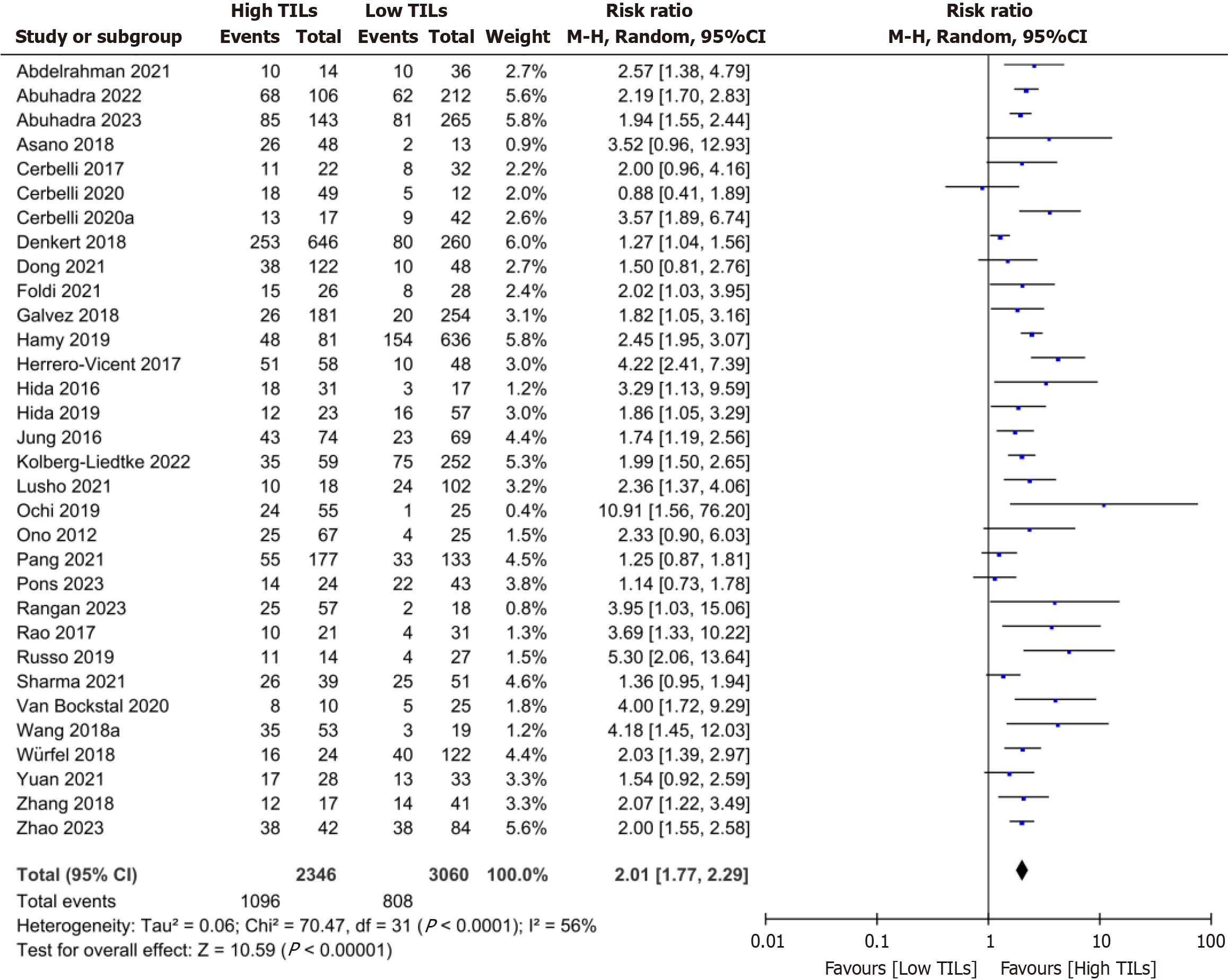
A total of 32 studies were included in this meta-analysis. The overall meta-ana
TILs can serve as a predictor of the response to NAT treatment in TNBC patients. TNBC patients with high levels of TILs exhibit a greater NAT pCR rate than those with low levels of TILs, and this predictive capability is con
Core Tip: The immune response status may have a significant impact on the effectiveness of chemotherapy. Tumor-infiltrating lymphocytes (TILs) can directly or indirectly participate in specific immune responses against tumor cells. However, the association between TIL levels and the response to neoadjuvant therapy (NAT) in patients with triple-negative breast cancer (TNBC) remains unclear. This systematic review and meta-analysis first investigated the relationship between TIL status and the response to NAT in TNBC patients. This systematic review and meta-analysis will provide clinical physicians with systematic evidence on the role of TILs to predict the response of TNBC patients to NAT.
- Citation: Sun HK, Jiang WL, Zhang SL, Xu PC, Wei LM, Liu JB. Predictive value of tumor-infiltrating lymphocytes for neoadjuvant therapy response in triple-negative breast cancer: A systematic review and meta-analysis. World J Clin Oncol 2024; 15(7): 920-935
- URL: https://www.wjgnet.com/2218-4333/full/v15/i7/920.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i7.920
Global Cancer Statistics 2020 reported that in 2020, breast cancer (BC) was becoming the most common malignant tumor globally[1]. Triple-negative BC (TNBC) is characterized by extremely aggressive biological behavior and has a high recurrence rate and poor survival[2,3]. Extensive investigations on early diagnosis, precision treatment, and prognostic prediction have been conducted to improve TNBC patient survival[4-6]. Neoadjuvant therapy (NAT) can effectively decrease the clinical stage of TNBC, and patients who attain pathologic complete response (pCR) following NAT have significantly prolonged event-free survival (EFS) and overall survival compared with those having residual infiltrative carcinoma. Consequently, NAT has been widely recommended as the preferred preoperative standard treatment moda
The immune response status may have a significant impact on the effectiveness of chemotherapy[9,10]. Research fin
Previously, a systematic review and meta-analysis on the correlation between TIL levels in different molecular sub
The present meta-analysis adhered to the reporting suggestions provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses[22].
The literature search was conducted on the National Center for Biotechnology Information PubMed (MEDLINE) database to identify pertinent articles published prior to August 31, 2023. The search strategy involved utilizing a combination of the following MeSH terms, title/abstract keywords, or full-text search terms: “breast cancer, or breast carcinoma” , “triple-negative, or TNBC” , “neoadjuvant therapy, or neoadjuvant” , and “tumor-infiltrating lymphocytes, T lympho
The studies eligible for this meta-analysis met the following criteria: (1) Pathological and immunohistochemical-based molecular subtyping confirming the diagnosis of TNBC; (2) Reported TIL levels by hematoxylin and eosin staining evaluation according to the standardized method presented by the International TILs Working Group in 2014 or other explicit assays; (3) Reported the number or rate of pCR events in TNBC patients based on different TIL levels; and (4) Were published in either English or Chinese.
Three researchers (Sun HK, Jiang WL, and Zhang SL) independently evaluated the titles and abstracts of the candidate studies, excluding those not pertinent to the topic. Subsequently, both researchers thoroughly examined the full texts to determine their eligibility for inclusion. In cases where uncertainty arose or disagreements occurred regarding inclusion, the researchers resorted to the study designer (Liu JB) for a review and discussion to achieve consensus. Furthermore, if multiple publications involved the same study population, priority was given to the publication with a larger sample size or the most recent study for eligibility in the meta-analysis.
Three researchers (Sun HK, Jiang WL, and Zhang SL) independently collected the relevant information and data for each study that met the inclusion criteria using a predesigned table. These included details such as the first author, geo
The meta-analysis was conducted in RevMan 5.4 software. The total cases of patients and the cases of patients who achieved pCR were recorded separately for the high TIL level group and low TIL level group in each study and input into RevMan software. The relative risk ratio (RR) and the associated 95% confidence interval (CI) were calculated per the following formula: The pCR rate in the high TIL level group divided by the pCR rate in the low TIL level group. RR > 1 and P < 0.05 indicated a greater pCR rate in the high TIL level subgroup than in the low TIL level subgroup.
In the meta-analysis, between-study heterogeneity was assessed using the I2 statistic (ranging from 0% to 100%). If an I2 value less than 50% or a P value greater than 0.05 indicated the absence or low between-study heterogeneity, a fixed-effects model was used for meta-analysis; otherwise, a random-effects model (REM) was used. Additionally, subgroup analysis was conducted to explore the source of between-study heterogeneity when significant heterogeneity was ob
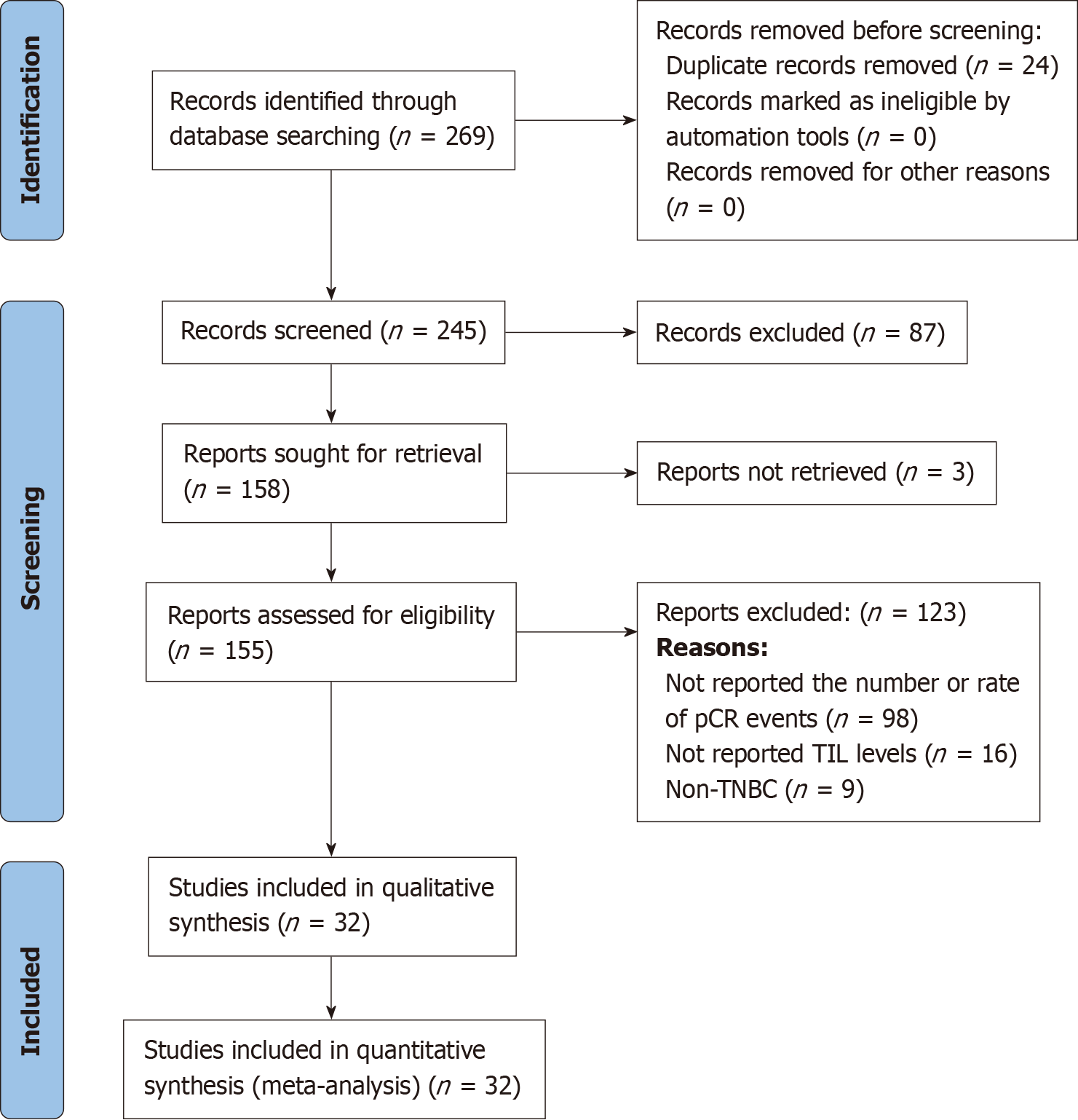
A preliminary literature search identified 269 articles, and after reviewing the titles and abstracts, we selected 158 articles for full-text reading. Subsequently, 125 articles were excluded because of the eligibility criteria. Finally, 32 eligible studies comprising 5406 TNBC patients were included in this systematic review and meta-analysis. The NOS quality scores of the eligible studies ranged from 6 to 9, with a median score of 8 (Figure 1).
Table 1 displays the characteristics of all the studies included in the analysis. Among the 32 included studies, 16 studies provided descriptions of TNBC before NAT based on T staging, including 4051 cases in T1/T2, 48 cases in T2/T3, 341 cases in T2-T4, and 1007 cases in T3/T4; fifteen (15) studies described N staging of pre-NAT TNBC, including 2937 cases in N0 and 2,704 cases in N1-N3; additionally, 11 studies described the clinical TNM staging of pre-NAT TNBC, including 91 cases in stage I, 923 cases in stage II, and 762 cases in stage III; and five studies did not report T or N stage or clinical TNM staging. Among the 27 included studies, TIL levels were assessed per the standardized method proposed by the International TILs Working Group in 2014[26], while five studies[27-30] did not report the specific method used for TIL assessment. The cutoff value for TIL level most commonly reported was 10% (n = 10)[18,29,31-38], followed by 50% (n = 8)[17,27,39-44], 20% (n = 4)[15,16,45,46], 30% (n = 3)[17,28,47], 40% (n = 3)[48-50], and 60% (n = 2)[51,52].
| Ref. | Data collection | Recruitment period | Sample size | Age in yr, median/mean (range) | TNM stage | Neoadjuvant regimen | Number of high/middle TILs as %, cut-off, and method | End point and pCR standard | Number of overall pCR as % | pCR rates as high TILs vs low TILs | OR or RR |
| Cerbelli et al[36], Germany | Retrospective consecutive cohort | 2011.6-2017.6 | 61 | 50 (28-74) | T1: 8; T2: 46; T3: 3; T4: 4; N0: 32; N1-N3: 29 | AC×4 (Q3W) →T×12 (QW) | 49 (17/32) (80.3), (50%) 10%, HE | pCR, ypT0 | 23 (37.7) | 18 (36.7) vs 5 (41.7) | OR: [U] 0.41 (0.17-0.95), 0.037; [M] 2.39 (0.96-5.96), 0.062 |
| Galvez et al[17], Peru | Retrospective cohort | 2003.1-2014.12 | 435 | 49 (24-84) | II: 72, III: 363; | AC×4 (Q3W) →T×12 (QW) | 181 (41.6), 50%, HE | pCR, ypT0 | 46 (11.0) | 26 (14.4) vs 20 (7.9) | NR |
| Abdelrahman et al[39], Egypt | Prospective cohort | 2017.1-2019.5 | 50 | 45 (22-72) | T1: 20; T2: 30; N0: 18; N1-N3: 32 | AC→T | 14 (28.0), 50%, HE | pCR, ypT0 | 20 (40.0) | 10 (71.4) vs 10 (27.8) | NR |
| Jung et al[53], Korea | Retrospective cohort | 2009.1-2014.12 | 143 | NR | T1-T2: 91; T3: 52; N0: 64; N1-N3: 79 | AC→T | 74 (51.7), 30%, HE | pCR, ypT0 | 66 (46.2) | 43 (58.1) vs 23 (33.3) | OR: [U] 2.774 (1.404-5.481), 0.003; [M] 3.484 (1.407-8.627), 0.007 |
| Russo et al[47], Venezuela | Retrospective cohort | 2008-2013 | 41 | NR | II: 80, III: 107; | AC→T | 14 (34.1), 30%, HE | pCR, ypT0 | 15 (36.6) | 11 (78.6) vs 4 (14.8) | OR: [U] 8.85 (3.62-21.66), 0.001 |
| Vicent et al[48], Spain | Retrospective cohort | 1998-2015 | 164 | 49 (29-81) | II: 63, III: 37 | AC×4 (Q3W) →T×12 (QW) | 58 (35.4), 40%, HE | pCR, ypT0/is, ypN0 | 61 (37.2) | 51 (88.0) vs 10 (9.0) | NR |
| Ochi et al[32], Japan | Retrospective consecutive cohort | 2001-2009 | 80 | 52 (27-75) | NR | AC→T | 55 (19/36) (68.8), (50%) 10%, HE | pCR, ypT0 | 25 (31.3) | 24 (43.6) vs 1 (4.0) | NR |
| Bockstal et al[49], Belgium | Retrospective consecutive cohort | 2015.1-2020.3 | 35 | 55.8 ± 13.3 | NR | AC→T | 10 (28.6), 40%, HE | pCR, ypT0 | 13 (37.1) | 8 (80.0) vs 5 (20.0) | NR |
| Rangan et al[43], India | NR | NR | 75 | NR | T1-T3: 49; T4: 26; N0: 36; N1-N3: 39 | NR | 57 (76.0), 50%, HE | pCR, ypT0 | 27 (36.0) | 25 (43.9) vs 2 (11.1) | OR: [U] 6.25 (1.312-29.763), 0.025 |
| Pang et al[18], ChiNR | Retrospective cohort | 2010.1-2018.12 | 310 | NR | T1-2: 298; T3-4: 97 | AC→T | 177 (85/92) (57.1), (20%) 10%, HE | pCR, ypT0 | 88 (28.4) | 53 (31.1) vs 33 (34.5) | NR |
| Zhang et al[52], America | Retrospective cohort | 2005-2016 | 58 | 46 (24-64) | T1: 7; T2-T4: 51; N0: 30; N1-N3: 28 | AC×4 (Q3W) →T×12 (QW) | 17 (29.3), 60%, HE | pCR, ypT0 | 26 (44.8) | 12 (70.6) vs 14 (34.1) | NR |
| Zhao et al[50], ChiNR | Retrospective cohort | 2017-2018 | 126 | 50.1 ± 11.2 | T1: 78; T2-T3: 48; N0: 74; N1-N3: 52 | AC→T | 42 (33.3), 40%, HE | pCR, ypT0 | 76 (60.3) | 38 (90.5) vs 38 (45.2) | NR |
| Cerbelli et al[40], Italy | Retrospective consecutive cohort | 2011.1-2016.12 | 54 | 50 (28-75) | T1: 7; T2-T4: 47; N0: 24; N1-N3: 30 | AC×4 (Q3W) →T×12 (QW) | 22 (40.7), 50%, HE | pCR, ypT0/is, N0 | 19 (35.2) | 11 (50.0) vs 8 (25.0) | OR: [U] 1.61 (0.40-6.52), 0.025 |
| Rao et al[30], ChiNR | Retrospective consecutive cohort | 2009.7-2014.6 | 52 | 46.9 (23-67) | II: 34, III: 16; | TAC | 21 (40.4), CD8: ≥ 0.15, HE | pCR, ypT0 DFS OS | 14 (26.9) | CD8: 10 (47.6) vs 4 (12.9) | CD8 OR: [U] 6.14 (1.6-23.8), 0.010 |
| Lusho et al[28], Japan | Retrospective consecutive cohort | 2008-2019 | 120 | 56 (28-86) | NR | TAC | 18 (15.0), 30%, HE | pCR, ypT0/Tis ypN0 | 34 (28.3) | 10 (55.6) vs 24 (23.5) | NR |
| Hida et al[37], Japan | Retrospective cohort | 2007-2014 | 48 | 56 (22-79) | T1: 93; T2: 59; T3: 2; N0: 98; N1-N3: 56 | AC×4 (Q3W) →T×12 (QW) | 31 (11/20) (64.6), (50%) 10%, HE | pCR, ypT0/is, ypN0 | 21 (43.8) | 18 (58.0) vs 3 (17.6) | NR |
| Hida et al[27], Japan | Retrospective consecutive cohort | 2007-2014 | 80 | NR | N0: 56; N1-N3: 24 | TAC | 23 (28.8), 50%, HE | pCR, ypT0/is, N0 | 28 (35.0) | 12 (52.2) vs 16 (28.1) | NR |
| Kolberg et al[51], Germany | Retrospective cohort | NR | 311 | NR | NR | AC→T | 59 (19.0), 60%, HE | pCR, ypT0 | 110 (35.4) | 35 (59.3) vs 75 (29.8) | OR: [U] 3.44 (1.92-6.18), 0.001 |
| Foldi et al[38], America | II RCT | 2015.12-2018.11 | 54 | NR | I: 12, II: 33, III: 14; | T→ddAC- Durvalumab (3 and 10 mg/kg) | 26 (16/10) (48.1), (30%) 10%, HE | pCR, ypT0/Tis ypN0 | 23 (42.6) | 15 (57.7) vs 8 (28.6) | NR |
| Abuhadra et al[16], America | Prospective cohort | 2015.10-2019.11 | 318 | 52.5 (24-77) | I: 38, II: 210, III: 70; | ddAC→T+ (Atezolizumab/ Panitumumab/ Bevacizumab) | 106 (33.3), 20%, HE | pCR, ypT0 | 130 (40.9) | 68 (64.2) vs 62 (29.2) | NR |
| Denkert et al[33], Germany | RCT IPD pooled analysis | 2010.1-2016.12 | 906 | NR | NR | T+ Bevacizumab | 646 (273/373) (71.3), (60%) 10%, HE | pCR, ypT0 | 333 (36.8) | 253 (39.2) vs 80 (30.8) | NR |
| Yuan et al[34], America | II RCT | 2012.1-2018.8 | 63 | 52 (28-79) | II: 55, III: 12; | TCb | 28 (6/22) (45.9), (60%) 10%, HE | pCR, ypT0 | 30 (47.6) | 17 (60.7) vs 13 (39.3) | Medium vs low1: OR: [U] 2.23 (0.74- 6.69), 0.16; high vs low1: OR: [U] 3.06 (0.49-9.30), 0.23 |
| Sharma et al[46], America | II RCT | 2015.7-2018.5 | 100 | 51 (29–70) | T1: 19; T2: 70; T3-T4: 11; N0: 70; N1-N3: 30 | Arm-A: CbP + AC; Arm-B: CbD | 39 (43.3), 20%, HE | pCR ypT0/is, ypN0 | 51 (56.7) | 26 (66.7) vs 25 (49.0) | OR: [U] 2.08 (0.88-4.93), 0.096 |
| Pons et al[45], Spain | NR | 2016-2022 | 67 | NR | T1-T2: 59; T3: 10; N0: 43; N1-N3: 26 | TCb + ddAC | 24 (35.8), 20%, HE | pCR, ypT0/is, ypN0 | 36 (53.7) | 14 (58.3) vs 22 (51.2) | NR |
| Abuhadra et al[15], America | NR | 2015.10-2020.10 | 408 | 51 (23–77) | I: 41, II: 284, III: 83 | AC→TCb | 143 (35.0), 20%, HE | pCR, ypT0/is, N0 | 166 (40.7) | 85 (59.4) vs 81 (30.6) | NR |
| Asano et al[31], Japan | Retrospective cohort | 2007-2013 | 61 | NR | T1: 24; T2-T4: 153; N0: 41; N1-N3: 136 | FEC→T | 48 (78.7), 10%, HE | pCR, ypT0 | 28 (45.9) | 26 (54.2) vs 2 (15.4) | NR |
| Ono et al[54], Japan | NR | 1999-2007 | 92 | 52 (23-76) | II: 23, III: 36; | AC→T CEF | 67 (72.8)1, high: (3-5), HE | pCR, ypT0 | 29 (31.5) | 25 (37.3) vs 4 (16.0) | NR |
| Wang et al[35], America | NR | 2007-2014 | 72 | NR | T1: 5; T2: 48; T3: 15; T4: 5; N0: 38; N1-N3: 34 | NR | 53 (1/52) (73.6), (50%) 10%, HE | pCR, ypT0 | 38 (52.8) | 35 (66.0) vs 3 (15.8) | NR |
| Dong et al[29], ChiNR | Retrospective cohort | 2010.1-2014.12 | 170 | NR | T1-2: 110; T3-4: 60 | TAC | 122 (74/48) (71.8), (20%) 10%, HE | pCR, ypT0 DFS OS | 48 (28.2) | 38 (31.1) vs 10 (24.8) | NR |
| Würfel et al [44], Germany | NR | 2015.5-2017.4 | 146 | NR | T1: 59; T2-T4: 90 | NR | 24 (16.4), 50%, HE | pCR ypT0 ypN0 | 56 (38.4) | 16 (66.7) vs 40 (32.8) | NR |
| Hamy et al[42], France | NR | 2015.1-2017.3 | 717 | NR | T1-T2: 529; T3: 189; N0: 282; N1-N3: 435 | NR | 81 (11.3), 50%, HE | pCR, ypT0 | 202 (28.2) | 48 (59.2) vs 154 (24.2) | OR: [U] 5.02 (4.27-5.77), 0.001 |
| Cerbelli et al[41], Italy | Retrospective consecutive cohort | NR | 59 | 49 (28-74) | II: 36, III: 24 | NR | 17 (28.8), 50%, HE | pCR, ypT0 | 22 (37.3) | 13 (76.5) vs 9 (21.4) | NR |
Overall meta-analysis: A meta-analysis of 32 studies revealed that the patients with high TIL levels had a high pro
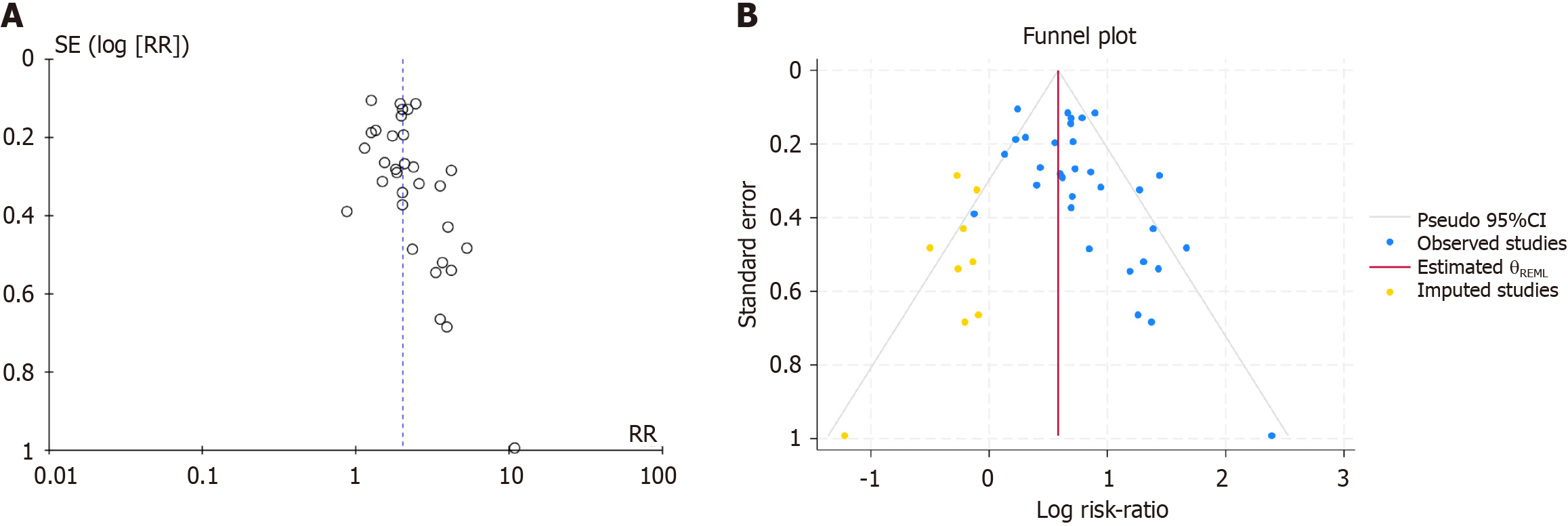
Publication bias analysis: An asymmetric funnel plot and Egger’s test P value (P = 0.001) less than 0.05 suggested poten
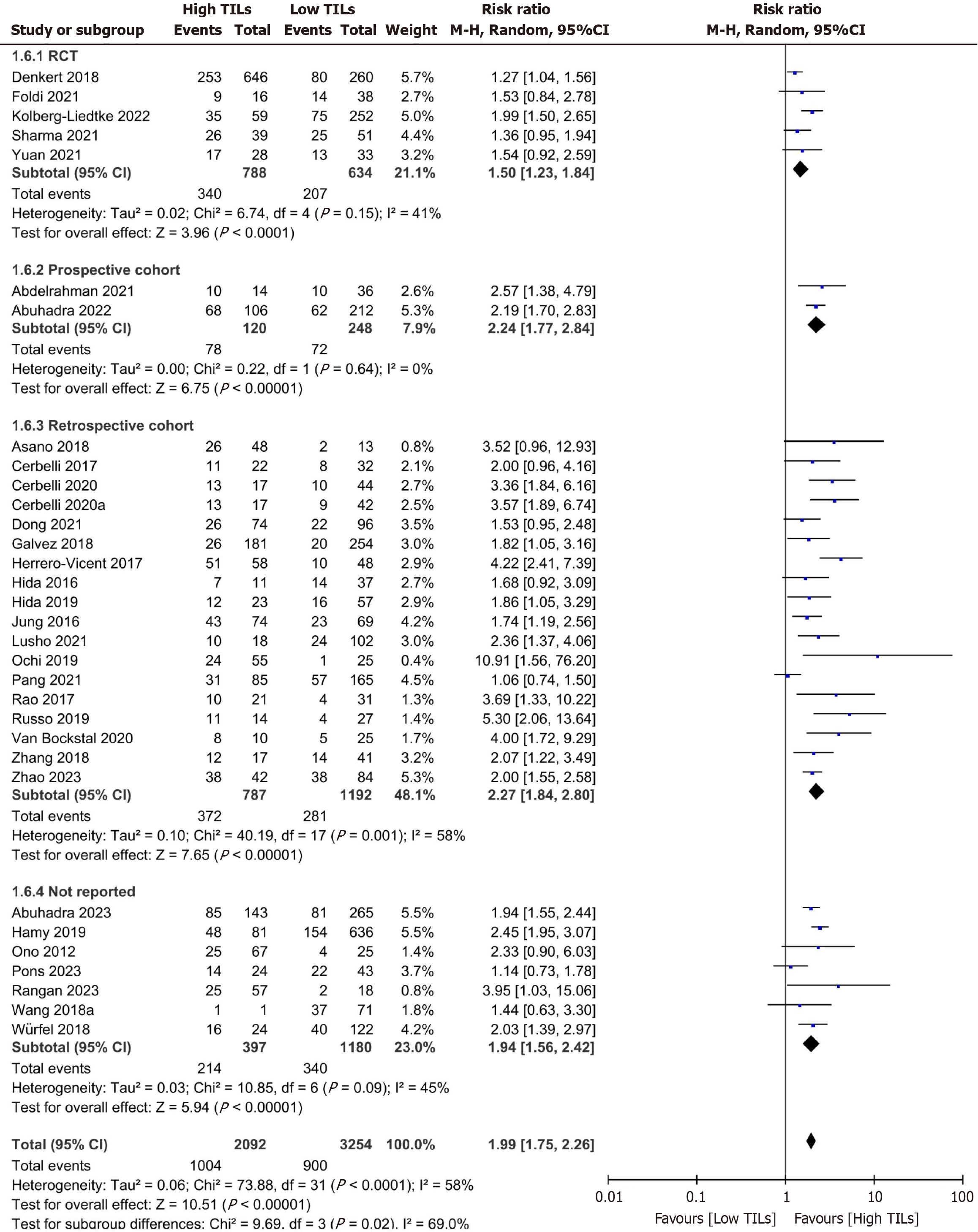
Subgroup analysis: Due to significant heterogeneity among the included studies in the overall meta-analysis, subgroup analysis was conducted based on important variables, including study design, TIL cutoff value, sample size, and geo
| Analysis | No. of studies | Risk ratio (95%CI) | I2 statistic (%) | P value for heterogeneity | Analytical model | P value for subgroup differences |
| Study design | ||||||
| RCT | 5 | 1.42 (1.23-1.64) | 41 | 0.15 | FEM | |
| Prospective cohort | 2 | 2.24 (1.77-2.83) | 0 | 0.64 | FEM | |
| Retrospective cohort | 18 | 2.27 (1.84-2.80) | 58 | 0.01 | REM | |
| Not reported | 7 | 2.05 (1.77-2.36) | 45 | 0.09 | FEM | 0.02 |
| Cut-off | ||||||
| 60% | 2 | 2.01 (1.57-2.58) | 0 | 0.90 | FEM | |
| 50% | 8 | 2.31 (1.95-2.74) | 0 | 0.71 | FEM | |
| 40% | 3 | 3.06 (1.60-5.84) | 78 | 0.01 | REM | |
| 30% | 3 | 2.33 (1.61-3.37) | 46 | 0.16 | FEM | |
| 20% | 4 | 1.68 (1.29-2.20) | 67 | 0.03 | REM | |
| 10% | 10 | 1.63 (1.24-2.15) | 49 | 0.04 | REM | |
| Locations | ||||||
| Asia | 12 | 1.90 (1.62-2.24) | 46 | 0.04 | FEM | |
| Europe | 11 | 2.07 (1.58-2.71) | 77 | 0.01 | REM | |
| Americas | 9 | 2.01 (1.76-2.30) | 34 | 0.14 | FEM | 0.35 |
| Sample size | ||||||
| n ≤ 80 | 16 | 2.62 (2.14-3.20) | 35 | 0.08 | FEM | |
| n > 80 | 16 | 1.82 (1.56-2.12) | 69 | 0.01 | REM | 0.04 |
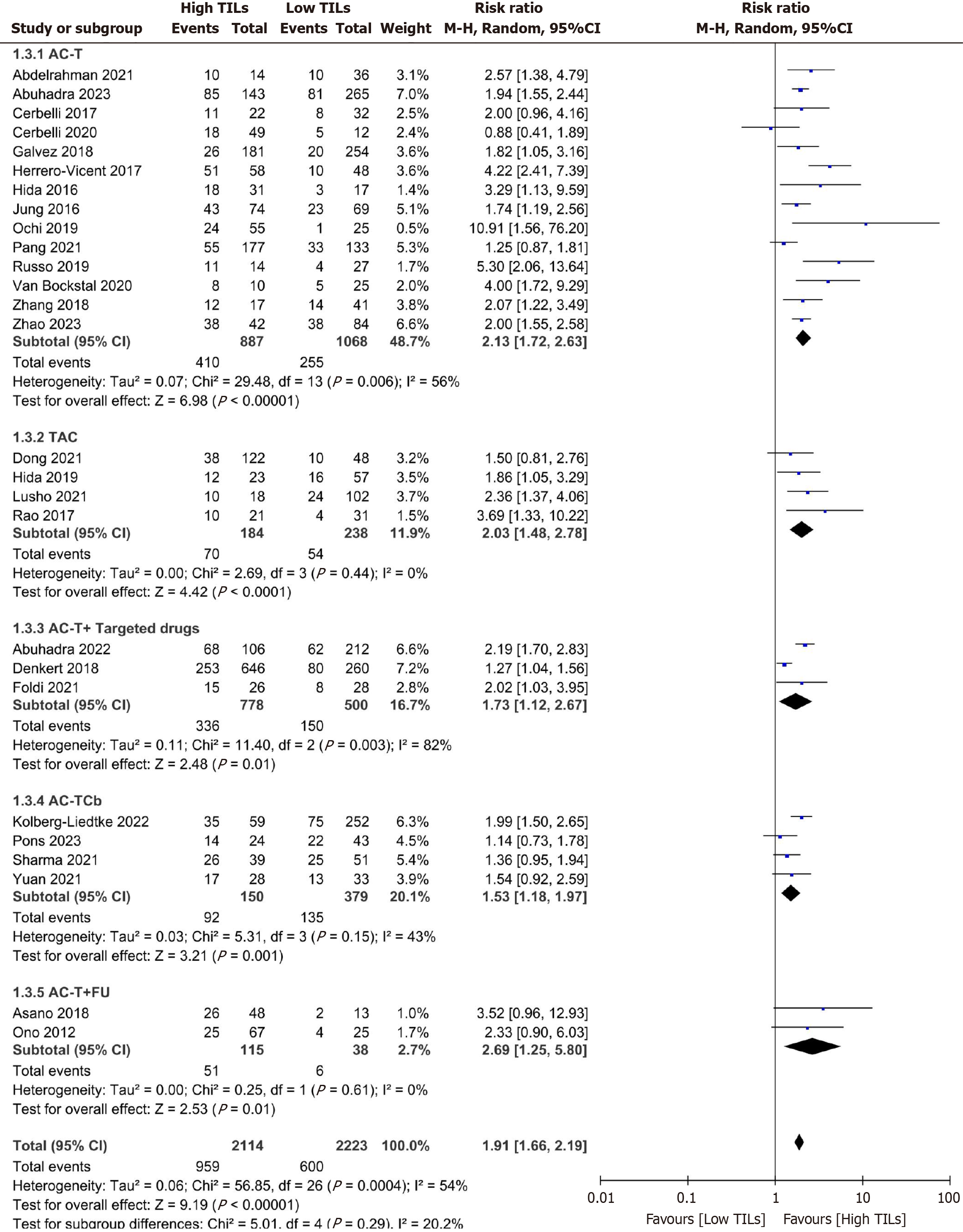
| NAT regimens | ||||||
| AC-T | 14 | 2.13 (1.72-2.63) | 56 | 0.01 | REM | |
| TAC | 4 | 1.99 (1.43-2.75) | 0 | 0.44 | FEM | |
| AC-T + targeted therapy | 3 | 1.73 (1.12-2.67) | 82 | 0.01 | REM | |
| AC-TCb | 4 | 1.57 (1.31-1.90) | 43 | 0.15 | FEM | |
| AC-T + Fu | 2 | 2.75 (1.28-5.92) | 0 | 0.61 | FEM | 0.02 |
Among the 32 studies, except for five studies[35,41-44] without a description of the NAT regimen, the reported NAT regimens in 27 included 14 studies with anthracycline combined with cyclophosphamide (AC) followed by sequential paclitaxel (T) (AC-T) [15,17,18,32,36,37,39,40,47-50,52,53], three studies with AC followed by sequential T in combination with anti-HER2 targeted therapy (AC-T + targeted therapy)[16,33,38], four studies with AC followed by sequential T in combination with platinum (Cb) agents (AC-TCb)[34,45,46,51], two studies with AC followed by sequential T in com
The included studies were analyzed according to the NAT regimens, and the results revealed that patients with high TIL levels in different NAT regimens, such as AC-T, AC-TCb, AC-T + targeted therapy, AC-T + FU, and TAC, had 1.57 to 2.75 times greater rates of pCR events than those with low TIL levels. Moreover, there was no significant difference in the statistics among the various NAT regimens (P = 0.29). The detailed meta-analysis data of TILs associated with treatment response to different NAT regimens in TNBC patients are presented in Figure 5 and Table 2.
Tumor immunity plays a crucial role in the body’s defense against tumors and in mediating the response to anti-cancer treatments. The presence of TILs in breast tumors has been associated with improved clinical outcomes[55]. The role of TILs in the NAT response in TNBC patients has been extensively studied. Based on the existing studies evaluating the correlation between TIL assessment and NAT treatment outcomes in TNBC patients, we conducted a systematic review and meta-analysis of the relationship between TIL status and the response to NAT in TNBC patients. The results showed that TNBC patients with high levels of TILs had greater NAT pCR rates than did those with low TIL levels. Furthermore, analysis based on different NAT regimens revealed that TIL levels were significantly associated with treatment response in all NAT regimens incorporating anthracycline combined with taxane drugs. This suggests that TILs have predictive value for treatment response in these NAT regimens. To our knowledge, this is the first comprehensive and specific evaluation of the ability of TILs to predict the response of TNBC patients to NAT, which offers important insights into predicting treatment response based on pretreatment tumor immune status in TNBC patients.
TILs play a vital role in the surveillance and defense against tumors within the tumor immune microenvironment. The positioning, clustering, interaction, and costimulation of TIL subgroups are crucial for effective antitumor immune res
The systematic assessment and meta-analysis conducted herein provide substantial evidence that TNBC patients exhibiting high TIL levels exhibit superior treatment responses regardless of the specific NAT scheme employed, particularly in terms of higher pCR rates. Moreover, an increase in the TIL level following NAT treatment is associated with improved therapeutic outcomes in BC patients. The study findings indicate that the administration of anthracycline-based chemotherapy drugs along with cyclophosphamide augments TIL levels in BC patients receiving NAT, and this increase in TIL levels is positively correlated with an improved pCR rate[33,59]. A study that stratified TNBC cohorts into lymphocyte-predominant BC (LPBC) and non-LPBC based on stromal TIL levels revealed that higher levels of stromal TILs in TNBC patients not only correlated with a greater pCR rate but also supported a greater pCR rate in LPBC patients than in non-LPBC patients. Additionally, even within the LPBC subgroup, the inclusion of platinum-based drugs in anthracycline-based chemotherapy followed by sequential paclitaxel yielded more significant benefits than in non-LPBC patients[60]. These clinical findings have been validated in various established experimental models of carcinogen-induced BC. In these animal models, the administration of doxorubicin amplifies the tumor antigen-specific proliferation of CD8+ T cells in tumor-draining lymph nodes in a homologous antigen-specific manner. Furthermore, it augments the ratio of CD8+ T cells infiltrating the tumor tissue and elicits tumor antigen-specific interferon-gamma production by these CD8+ TILs. Ultimately, the therapeutic effects of doxorubicin are mediated through these two mechanisms[61].
Due to the substantial heterogeneity observed in the meta-analysis of the 32 eligible studies, we performed subgroup analysis to investigate the sources of heterogeneity. The subgroup analysis showed that TNBC patients with high preo
Despite our comprehensive evaluation of the association between TIL levels in preoperative BC tissue treated with NATs and pCR in TNBC patients, our systematic review and meta-analysis has several limitations. First, the assessment of TILs is subjective, and there may be substantial variations in determining TIL levels among different studies due to the subjective judgments of various pathology experts. This subjectivity may impact the true relationship between TIL levels and treatment response and introduce heterogeneity across studies. Additionally, the analysis was limited by the paucity of studies that examined the correlation between TIL levels and NAT treatment response according to different molecular marker types of TILs. Consequently, it was not possible to more comprehensively conduct a subgroup analysis based on TIL molecular subtypes to explore the relationship between TIL levels and NAT treatment response. Finally, the res
In summary, this systematic review and meta-analysis indicated that TNBC patients with elevated TILs exhibited sig
The authors wish to acknowledge Dr. Ping ZG, Professor of College of Public Health, Zhengzhou University, for his help in reviewing and guiding the statistical methods of this study.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64681] [Article Influence: 16170.3] [Reference Citation Analysis (177)] |
| 2. | Garrido-Castro AC, Lin NU, Polyak K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov. 2019;9:176-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 922] [Article Influence: 153.7] [Reference Citation Analysis (0)] |
| 3. | Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429-4434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2803] [Cited by in RCA: 3582] [Article Influence: 199.0] [Reference Citation Analysis (0)] |
| 4. | Bianchini G, De Angelis C, Licata L, Gianni L. Treatment landscape of triple-negative breast cancer - expanded options, evolving needs. Nat Rev Clin Oncol. 2022;19:91-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 718] [Article Influence: 179.5] [Reference Citation Analysis (0)] |
| 5. | Denkert C, Liedtke C, Tutt A, von Minckwitz G. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies. Lancet. 2017;389:2430-2442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 621] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 6. | Leon-Ferre RA, Goetz MP. Advances in systemic therapies for triple negative breast cancer. BMJ. 2023;381:e071674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 141] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 7. | Vaidya JS, Massarut S, Vaidya HJ, Alexander EC, Richards T, Caris JA, Sirohi B, Tobias JS. Rethinking neoadjuvant chemotherapy for breast cancer. BMJ. 2018;360:j5913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 8. | Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE Jr, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3169] [Cited by in RCA: 3205] [Article Influence: 291.4] [Reference Citation Analysis (2)] |
| 9. | Savas P, Salgado R, Denkert C, Sotiriou C, Darcy PK, Smyth MJ, Loi S. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol. 2016;13:228-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 646] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 10. | Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, von Törne C, Weichert W, Engels K, Solbach C, Schrader I, Dietel M, von Minckwitz G. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1344] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 11. | Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, Takahashi M, Foukakis T, Fasching PA, Cardoso F, Untch M, Jia L, Karantza V, Zhao J, Aktan G, Dent R, O'Shaughnessy J; KEYNOTE-522 Investigators. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med. 2020;382:810-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1006] [Cited by in RCA: 1903] [Article Influence: 380.6] [Reference Citation Analysis (0)] |
| 12. | Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, Takahashi M, Untch M, Fasching PA, Cardoso F, Andersen J, Patt D, Danso M, Ferreira M, Mouret-Reynier MA, Im SA, Ahn JH, Gion M, Baron-Hay S, Boileau JF, Ding Y, Tryfonidis K, Aktan G, Karantza V, O'Shaughnessy J; KEYNOTE-522 Investigators. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N Engl J Med. 2022;386:556-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 711] [Article Influence: 237.0] [Reference Citation Analysis (0)] |
| 13. | Paijens ST, Vledder A, de Bruyn M, Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol Immunol. 2021;18:842-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 573] [Article Influence: 143.3] [Reference Citation Analysis (0)] |
| 14. | Loi S, Michiels S, Adams S, Loibl S, Budczies J, Denkert C, Salgado R. The journey of tumor-infiltrating lymphocytes as a biomarker in breast cancer: clinical utility in an era of checkpoint inhibition. Ann Oncol. 2021;32:1236-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 139] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 15. | Abuhadra N, Sun R, Yam C, Rauch GM, Ding Q, Lim B, Thompson AM, Mittendorf EA, Adrada BE, Damodaran S, Virani K, White J, Ravenberg E, Sun J, Choi J, Candelaria R, Arun B, Ueno NT, Santiago L, Saleem S, Abouharb S, Murthy RK, Ibrahim N, Sahin A, Valero V, Symmans WF, Litton JK, Tripathy D, Moulder S, Huo L. Predictive Roles of Baseline Stromal Tumor-Infiltrating Lymphocytes and Ki-67 in Pathologic Complete Response in an Early-Stage Triple-Negative Breast Cancer Prospective Trial. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 16. | Abuhadra N, Sun R, Litton JK, Rauch GM, Yam C, Chang JT, Seth S, Bassett R Jr, Lim B, Thompson AM, Mittendorf E, Adrada BE, Damodaran S, White J, Ravenberg E, Candelaria R, Arun B, Ueno NT, Santiago L, Saleem S, Abouharb S, Murthy RK, Ibrahim N, Sahin AA, Valero V, Symmans WF, Tripathy D, Moulder S, Huo L. Prognostic Impact of High Baseline Stromal Tumor-Infiltrating Lymphocytes in the Absence of Pathologic Complete Response in Early-Stage Triple-Negative Breast Cancer. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 17. | Galvez M, Castaneda CA, Sanchez J, Castillo M, Rebaza LP, Calderon G, Cruz M, Cotrina JM, Abugattas J, Dunstan J, Guerra H, Mejia O, Gomez HL. Clinicopathological predictors of long-term benefit in breast cancer treated with neoadjuvant chemotherapy. World J Clin Oncol. 2018;9:33-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Pang J, Zhou H, Dong X, Wang S, Xiao Z. Relationship Between the Neutrophil to Lymphocyte Ratio, Stromal Tumor-infiltrating Lymphocytes, and the Prognosis and Response to Neoadjuvant Chemotherapy in Triple-negative Breast Cancer. Clin Breast Cancer. 2021;21:e681-e687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Loi S, Drubay D, Adams S, Pruneri G, Francis PA, Lacroix-Triki M, Joensuu H, Dieci MV, Badve S, Demaria S, Gray R, Munzone E, Lemonnier J, Sotiriou C, Piccart MJ, Kellokumpu-Lehtinen PL, Vingiani A, Gray K, Andre F, Denkert C, Salgado R, Michiels S. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J Clin Oncol. 2019;37:559-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 591] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 20. | Telli ML, Chu C, Badve SS, Vinayak S, Silver DP, Isakoff SJ, Kaklamani V, Gradishar W, Stearns V, Connolly RM, Ford JM, Gruber JJ, Adams S, Garber J, Tung N, Neff C, Bernhisel R, Timms KM, Richardson AL. Association of Tumor-Infiltrating Lymphocytes with Homologous Recombination Deficiency and BRCA1/2 Status in Patients with Early Triple-Negative Breast Cancer: A Pooled Analysis. Clin Cancer Res. 2020;26:2704-2710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Mao Y, Qu Q, Zhang Y, Liu J, Chen X, Shen K. The value of tumor infiltrating lymphocytes (TILs) for predicting response to neoadjuvant chemotherapy in breast cancer: a systematic review and meta-analysis. PLoS One. 2014;9:e115103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 22. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40601] [Article Influence: 10150.3] [Reference Citation Analysis (2)] |
| 23. | Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. [cited 30 August 2023]. Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 24. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40566] [Article Influence: 1448.8] [Reference Citation Analysis (2)] |
| 25. | Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7948] [Cited by in RCA: 9100] [Article Influence: 364.0] [Reference Citation Analysis (0)] |
| 26. | Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, Perez EA, Thompson EA, Symmans WF, Richardson AL, Brock J, Criscitiello C, Bailey H, Ignatiadis M, Floris G, Sparano J, Kos Z, Nielsen T, Rimm DL, Allison KH, Reis-Filho JS, Loibl S, Sotiriou C, Viale G, Badve S, Adams S, Willard-Gallo K, Loi S; International TILs Working Group 2014. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1473] [Cited by in RCA: 2220] [Article Influence: 201.8] [Reference Citation Analysis (0)] |
| 27. | Hida AI, Watanabe T, Sagara Y, Kashiwaba M, Sagara Y, Aogi K, Ohi Y, Tanimoto A. Diffuse distribution of tumor-infiltrating lymphocytes is a marker for better prognosis and chemotherapeutic effect in triple-negative breast cancer. Breast Cancer Res Treat. 2019;178:283-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Lusho S, Durando X, Mouret-Reynier MA, Kossai M, Lacrampe N, Molnar I, Penault-Llorca F, Radosevic-Robin N, Abrial C. Platelet-to-Lymphocyte Ratio Is Associated With Favorable Response to Neoadjuvant Chemotherapy in Triple Negative Breast Cancer: A Study on 120 Patients. Front Oncol. 2021;11:678315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Dong X, Liu C, Yuan J, Wang S, Ding N, Li Y, Wu Y, Xiao Z. Prognostic Roles of Neutrophil-to-Lymphocyte Ratio and Stromal Tumor-Infiltrating Lymphocytes and Their Relationship in Locally Advanced Triple-Negative Breast Cancer Treated with Neoadjuvant Chemotherapy. Breast Care (Basel). 2021;16:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Rao N, Qiu J, Wu J, Zeng H, Su F, Qiu K, Wu J, Yao H. Significance of Tumor-Infiltrating Lymphocytes and the Expression of Topoisomerase IIα in the Prediction of the Clinical Outcome of Patients with Triple-Negative Breast Cancer after Taxane-Anthracycline-Based Neoadjuvant Chemotherapy. Chemotherapy. 2017;62:246-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Asano Y, Kashiwagi S, Goto W, Takada K, Takahashi K, Hatano T, Takashima T, Tomita S, Motomura H, Ohsawa M, Hirakawa K, Ohira M. Prediction of Treatment Response to Neoadjuvant Chemotherapy in Breast Cancer by Subtype Using Tumor-infiltrating Lymphocytes. Anticancer Res. 2018;38:2311-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Ochi T, Bianchini G, Ando M, Nozaki F, Kobayashi D, Criscitiello C, Curigliano G, Iwamoto T, Niikura N, Takei H, Yoshida A, Takei J, Suzuki K, Yamauchi H, Hayashi N. Predictive and prognostic value of stromal tumour-infiltrating lymphocytes before and after neoadjuvant therapy in triple negative and HER2-positive breast cancer. Eur J Cancer. 2019;118:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 33. | Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen F, Furlanetto J, Schmitt WD, Blohmer JU, Karn T, Pfitzner BM, Kümmel S, Engels K, Schneeweiss A, Hartmann A, Noske A, Fasching PA, Jackisch C, van Mackelenbergh M, Sinn P, Schem C, Hanusch C, Untch M, Loibl S. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 1438] [Article Influence: 179.8] [Reference Citation Analysis (0)] |
| 34. | Yuan Y, Lee JS, Yost SE, Li SM, Frankel PH, Ruel C, Schmolze D, Robinson K, Tang A, Martinez N, Stewart D, Waisman J, Kruper L, Jones V, Menicucci A, Uygun S, Yoder E, van der Baan B, Yim JH, Yeon C, Somlo G, Mortimer J. Phase II Trial of Neoadjuvant Carboplatin and Nab-Paclitaxel in Patients with Triple-Negative Breast Cancer. Oncologist. 2021;26:e382-e393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Wang Y, Brodsky AS, Xiong J, Lopresti ML, Yang D, Resnick MB. Stromal Clusterin Expression Predicts Therapeutic Response to Neoadjuvant Chemotherapy in Triple Negative Breast Cancer. Clin Breast Cancer. 2018;18:e373-e379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Cerbelli B, Botticelli A, Pisano A, Pernazza A, Campagna D, De Luca A, Ascierto PA, Pignataro MG, Pelullo M, Rocca CD, Marchetti P, Fortunato L, Costarelli L, d'Amati G. CD73 expression and pathologic response to neoadjuvant chemotherapy in triple negative breast cancer. Virchows Arch. 2020;476:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Hida AI, Sagara Y, Yotsumoto D, Kanemitsu S, Kawano J, Baba S, Rai Y, Oshiro Y, Aogi K, Sagara Y, Ohi Y. Prognostic and predictive impacts of tumor-infiltrating lymphocytes differ between Triple-negative and HER2-positive breast cancers treated with standard systemic therapies. Breast Cancer Res Treat. 2016;158:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 38. | Foldi J, Silber A, Reisenbichler E, Singh K, Fischbach N, Persico J, Adelson K, Katoch A, Horowitz N, Lannin D, Chagpar A, Park T, Marczyk M, Frederick C, Burrello T, Ibrahim E, Qing T, Bai Y, Blenman K, Rimm DL, Pusztai L. Neoadjuvant durvalumab plus weekly nab-paclitaxel and dose-dense doxorubicin/cyclophosphamide in triple-negative breast cancer. NPJ Breast Cancer. 2021;7:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 39. | Abdelrahman AE, Rashed HE, MostafaToam, Omar A, Abdelhamid MI, Matar I. Clinicopathological significance of the immunologic signature (PDL1, FOXP3+ Tregs, TILs) in early stage triple-negative breast cancer treated with neoadjuvant chemotherapy. Ann Diagn Pathol. 2021;51:151676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Cerbelli B, Pernazza A, Botticelli A, Fortunato L, Monti M, Sciattella P, Campagna D, Mazzuca F, Mauri M, Naso G, Marchetti P, d'Amati G, Costarelli L. PD-L1 Expression in TNBC: A Predictive Biomarker of Response to Neoadjuvant Chemotherapy? Biomed Res Int. 2017;2017:1750925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Cerbelli B, Scagnoli S, Mezi S, De Luca A, Pisegna S, Amabile MI, Roberto M, Fortunato L, Costarelli L, Pernazza A, Strigari L, Della Rocca C, Marchetti P, d'Amati G, Botticelli A. Tissue Immune Profile: A Tool to Predict Response to Neoadjuvant Therapy in Triple Negative Breast Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Hamy AS, Bonsang-Kitzis H, De Croze D, Laas E, Darrigues L, Topciu L, Menet E, Vincent-Salomon A, Lerebours F, Pierga JY, Brain E, Feron JG, Benchimol G, Lam GT, Laé M, Reyal F. Interaction between Molecular Subtypes and Stromal Immune Infiltration before and after Treatment in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy. Clin Cancer Res. 2019;25:6731-6741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 43. | Rangan R, Kanetkar SR, Bhosale SJ, Mane DA, Patil NJ, Gudur RA. Assessment of Intratumoural and Stromal Infiltrating Lymphocytes In The Various Subtypes of Breast Carcinoma Patients who have Received Neoadjuvant Chemotherapy. Asian Pac J Cancer Prev. 2023;24:2347-2352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 44. | Würfel F, Erber R, Huebner H, Hein A, Lux MP, Jud S, Kremer A, Kranich H, Mackensen A, Häberle L, Hack CC, Rauh C, Wunderle M, Gaß P, Rabizadeh S, Brandl AL, Langemann H, Volz B, Nabieva N, Schulz-Wendtland R, Dudziak D, Beckmann MW, Hartmann A, Fasching PA, Rübner M. TILGen: A Program to Investigate Immune Targets in Breast Cancer Patients - First Results on the Influence of Tumor-Infiltrating Lymphocytes. Breast Care (Basel). 2018;13:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 45. | Pons L, Hernández L, Urbizu A, Osorio P, Rodríguez-Martínez P, Castella E, Muñoz A, Sanz C, Arnaldo L, Felip E, Quiroga V, Tapia G, Margelí M, Fernandez PL. Pre- and Post-Neoadjuvant Clinicopathological Parameters Can Help in the Prognosis and the Prediction of Response in HER2+ and Triple Negative Breast Cancer. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 46. | Sharma P, Kimler BF, O'Dea A, Nye L, Wang YY, Yoder R, Staley JM, Prochaska L, Wagner J, Amin AL, Larson K, Balanoff C, Elia M, Crane G, Madhusudhana S, Hoffmann M, Sheehan M, Rodriguez R, Finke K, Shah R, Satelli D, Shrestha A, Beck L, McKittrick R, Pluenneke R, Raja V, Beeki V, Corum L, Heldstab J, LaFaver S, Prager M, Phadnis M, Mudaranthakam DP, Jensen RA, Godwin AK, Salgado R, Mehta K, Khan Q. Randomized Phase II Trial of Anthracycline-free and Anthracycline-containing Neoadjuvant Carboplatin Chemotherapy Regimens in Stage I-III Triple-negative Breast Cancer (NeoSTOP). Clin Cancer Res. 2021;27:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 47. | Russo L, Maltese A, Betancourt L, Romero G, Cialoni D, De la Fuente L, Gutierrez M, Ruiz A, Agüero E, Hernández S. Locally advanced breast cancer: Tumor-infiltrating lymphocytes as a predictive factor of response to neoadjuvant chemotherapy. Eur J Surg Oncol. 2019;45:963-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Herrero-Vicent C, Guerrero A, Gavilá J, Gozalbo F, Hernández A, Sandiego S, Algarra MA, Calatrava A, Guillem-Porta V, Ruiz-Simón A. Predictive and prognostic impact of tumour-infiltrating lymphocytes in triple-negative breast cancer treated with neoadjuvant chemotherapy. Ecancermedicalscience. 2017;11:759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Van Bockstal MR, Noel F, Guiot Y, Duhoux FP, Mazzeo F, Van Marcke C, Fellah L, Ledoux B, Berlière M, Galant C. Predictive markers for pathological complete response after neo-adjuvant chemotherapy in triple-negative breast cancer. Ann Diagn Pathol. 2020;49:151634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Zhao M, Xing H, He J, Wang X, Liu Y. Tumor infiltrating lymphocytes and neutrophil-to-lymphocyte ratio in relation to pathological complete remission to neoadjuvant therapy and prognosis in triple negative breast cancer. Pathol Res Pract. 2023;248:154687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 51. | Kolberg-Liedtke C, Feuerhake F, Garke M, Christgen M, Kates R, Grischke EM, Forstbauer H, Braun M, Warm M, Hackmann J, Uleer C, Aktas B, Schumacher C, Kuemmel S, Wuerstlein R, Graeser M, Nitz U, Kreipe H, Gluz O, Harbeck N. Impact of stromal tumor-infiltrating lymphocytes (sTILs) on response to neoadjuvant chemotherapy in triple-negative early breast cancer in the WSG-ADAPT TN trial. Breast Cancer Res. 2022;24:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 52. | Zhang L, Wang XI, Zhang S. Tumor-infiltrating lymphocyte volume is a better predictor of neoadjuvant therapy response and overall survival in triple-negative invasive breast cancer. Hum Pathol. 2018;80:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Jung YY, Hyun CL, Jin MS, Park IA, Chung YR, Shim B, Lee KH, Ryu HS. Histomorphological Factors Predicting the Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer. J Breast Cancer. 2016;19:261-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Ono M, Tsuda H, Shimizu C, Yamamoto S, Shibata T, Yamamoto H, Hirata T, Yonemori K, Ando M, Tamura K, Katsumata N, Kinoshita T, Takiguchi Y, Tanzawa H, Fujiwara Y. Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat. 2012;132:793-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 237] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 55. | Cao B, Zhang Z, Wang C, Lv X. Prognostic relevance of tumorinfiltrating lymphocytes in residual tumor tissue from patients with triplenegative breast cancer following neoadjuvant chemotherapy: A systematic review and metaanalysis. Oncol Lett. 2023;26:441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 56. | Heckler M, Ali LR, Clancy-Thompson E, Qiang L, Ventre KS, Lenehan P, Roehle K, Luoma A, Boelaars K, Peters V, McCreary J, Boschert T, Wang ES, Suo S, Marangoni F, Mempel TR, Long HW, Wucherpfennig KW, Dougan M, Gray NS, Yuan GC, Goel S, Tolaney SM, Dougan SK. Inhibition of CDK4/6 Promotes CD8 T-cell Memory Formation. Cancer Discov. 2021;11:2564-2581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 57. | Vennin C, Cattaneo CM, Bosch L, Vegna S, Ma X, Damstra HGJ, Martinovic M, Tsouri E, Ilic M, Azarang L, van Weering JRT, Pulver E, Zeeman AL, Schelfhorst T, Lohuis JO, Rios AC, Dekkers JF, Akkari L, Menezes R, Medema R, Baglio SR, Akhmanova A, Linn SC, Lemeer S, Pegtel DM, Voest EE, van Rheenen J. Taxanes trigger cancer cell killing in vivo by inducing non-canonical T cell cytotoxicity. Cancer Cell. 2023;41:1170-1185.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 58. | Kester L, Seinstra D, van Rossum AGJ, Vennin C, Hoogstraat M, van der Velden D, Opdam M, van Werkhoven E, Hahn K, Nederlof I, Lips EH, Mandjes IAM, van Leeuwen-Stok AE, Canisius S, van Tinteren H, Imholz ALT, Portielje JEA, Bos MEMM, Bakker SD, Rutgers EJ, Horlings HM, Wesseling J, Voest EE, Wessels LFA, Kok M, Oosterkamp HM, van Oudenaarden A, Linn SC, van Rheenen J. Differential Survival and Therapy Benefit of Patients with Breast Cancer Are Characterized by Distinct Epithelial and Immune Cell Microenvironments. Clin Cancer Res. 2022;28:960-971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 59. | Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, de Azambuja E, Quinaux E, Di Leo A, Michiels S, Piccart MJ, Sotiriou C. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1020] [Cited by in RCA: 1261] [Article Influence: 105.1] [Reference Citation Analysis (0)] |
| 60. | Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, Pfitzner BM, Salat C, Loi S, Schmitt WD, Schem C, Fisch K, Darb-Esfahani S, Mehta K, Sotiriou C, Wienert S, Klare P, André F, Klauschen F, Blohmer JU, Krappmann K, Schmidt M, Tesch H, Kümmel S, Sinn P, Jackisch C, Dietel M, Reimer T, Untch M, Loibl S. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33:983-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 803] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 61. | Mattarollo SR, Loi S, Duret H, Ma Y, Zitvogel L, Smyth MJ. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res. 2011;71:4809-4820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 262] [Article Influence: 18.7] [Reference Citation Analysis (1)] |