Published online Jul 24, 2024. doi: 10.5306/wjco.v15.i7.848
Revised: May 14, 2024
Accepted: June 14, 2024
Published online: July 24, 2024
Processing time: 114 Days and 16.6 Hours
Poly (ADP-ribose) polymerase inhibitors (PARPis) are approved as first-line therapies for breast cancer gene (BRCA)-positive, human epidermal growth factor receptor 2-negative locally advanced or metastatic breast cancer. They are also effective for new and recurrent ovarian cancers that are BRCA- or homologous recombination deficiency (HRD)-positive. However, data on these mutations and PARPi use in the Middle East are limited.
To assess BRCA/HRD prevalence and PARPi use in patients in the Middle East with breast/ovarian cancer.
This was a single-center retrospective study of 57 of 472 breast cancer patients tested for BRCA mutations, and 25 of 65 ovarian cancer patients tested for HRD. These adult patients participated in at least four visits to the oncology service at our center between August 2021 and May 2023. Data were summarized using descriptive statistics and compared using counts and percentages. Response to treatment was assessed using Response Evaluation Criteria in Solid Tumors criteria.
Among the 472 breast cancer patients, 12.1% underwent BRCA testing, and 38.5% of 65 ovarian cancer patients received HRD testing. Pathogenic mutations were found in 25.6% of the tested patients: 26.3% breast cancers had germline BRCA (gBRCA) mutations and 24.0% ovarian cancers showed HRD. Notably, 40.0% of gBRCA-positive breast cancers and 66.0% of HRD-positive ovarian cancers were Middle Eastern and Asian patients, respectively. PARPi treatment was used in 5 (33.3%) gBRCA-positive breast cancer patients as first-line therapy (n = 1; 7-months progression-free), for maintenance (n = 2; > 15-months progression-free), or at later stages due to compliance issues (n = 2). Four patients (66.6%) with HRD-positive ovarian cancer received PARPi and all remained progression-free.
Lower testing rates but higher BRCA mutations in breast cancer were found. Ethnicity reflected United Arab Emirates demographics, with breast cancer in Middle Eastern and ovarian cancer in Asian patients.
Core Tip: Following National Comprehensive Cancer Network guidelines, breast cancer patients were tested for BRCA1/2 mutations, while ovarian cancer patients underwent homologous recombination defect testing. These mutations can be targeted with poly (ADP-ribose) polymerase inhibitor (PARPi) therapy, a form of precision medicine. In this single-center study, we analyzed the proportion of breast and ovarian cancer patients tested for mutations, demographics and disease characteristics of tested patients, and PARPi therapy outcomes. This first-of-its-kind study in the Middle East offers insights into targeted therapy use, contributing to knowledge on PARPis for breast and ovarian cancers.
- Citation: Syed N, Chintakuntlawar AV, Vilasini D, Al Salami AM, Al Hasan R, Afrooz I, Uttam Chandani K, Chandani AU, Chehal A. Low testing rates and high BRCA prevalence: Poly (ADP-ribose) polymerase inhibitor use in Middle East BRCA/homologous recombination deficiency-positive cancer patients. World J Clin Oncol 2024; 15(7): 848-858
- URL: https://www.wjgnet.com/2218-4333/full/v15/i7/848.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i7.848
Poly (ADP-ribose) polymerase (PARP) is a protein responsible for fixing single-stranded breaks. Proteins translated from other pathways, such as homologous recombination repair (HRR) genes, repair the double-strand breaks (DSBs) in DNA and help to maintain genomic stability[1]. BRCA1/2 are the most identifiable and actionable genes of the HRR pathway. There is an increased risk of breast (45%-72%) and ovarian (39%-44%) cancers in patients or carriers with BRCA1 and/or BRCA2 pathogenic variants compared to the general population[2]. PARP inhibition using drugs called PARP inhibitors (PARPis) leads single-stranded breaks to become DSBs. While normal cells can repair the DSBs because of a normal HRR pathway, cancer cells with these genes mutated cannot repair DSBs, leading to the accumulation of defects and selective cancer cell death.
The prevalence of BRCA mutations ranges widely from 1.8% to 36.9%, depending on whether the tested subjects were selected and the country from which they were reported[3,4]. Nearly 20% of BRCA mutated breast cancers are triple-negative breast cancer (TNBC) and have a bad prognosis[5,6]. TNBC with these mutations occur at a younger age, are high grade and highly aggressive, have a worse prognosis, and respond well to platinum-based chemotherapy with or without PARPi[7]. Patients with or carriers of these mutations have higher chances of a second cancer in the opposite breast, ovary, pancreas, and prostate[8]. Olaparib is a PARPi approved for locally advanced or metastatic human epidermal growth factor receptor 2 (HER2)-negative breast cancer[9,10].
The homologous recombination deficiency (HRD) test, which not only tests mutations of HRR pathway genes but also overall deficiency in this pathway, is preferred in ovarian cancer[11]. Ovarian cancers with HRD tend to occur at a young age, have a higher incidence of high-grade serous ovarian cancer (HGSOC), and respond well to standard platinum-based chemotherapy and PARPi[12-14]. Olaparib is approved as maintenance therapy after primary chemotherapy in germline BRCA (gBRCA) or somatic BRCA mutated patients who achieved complete response (CR) or stable disease (SD). Along with olaparib, niraparib and rucaparib are approved for recurrent ovarian cancer, but the specific indications depend on the BRCA or HRD status (SOLO-1, NOVA and ARIEL3) trials[14-16]. Moreover, the field of PARPi use in ovarian cancer is rapidly evolving, and new indications are emerging.
According to 2018 World Health Organization cancer statistics for the United Arab Emirates (UAE), breast cancer was the most common malignancy, accounting for 22.4% of diagnoses[17]. Studies in Gulf countries like UAE, Oman, and Kuwait have reported a 2%-8% prevalence of BRCA1/BRCA2 somatic variants in ovarian cancer[18]. Additionally, the prevalence of gBRCA mutations was found to be 10.2% in breast cancer and 30.7% in ovarian cancer in the region[19].
The aim of this study was to uncover the prevalence of gBRCA mutations in breast cancer and HRD in ovarian cancer. Beyond mere prevalence, the study compared the characteristics of patients with and without these pathogenic mutations within the tested breast and ovarian cancer groups. Finally, to assess the potential treatment implications of these findings, the study reported the proportion of patients who received PARPi therapy and their response to this targeted treatment. This study not only seeks to illuminate the selected DSB repair gene mutations in the region but also pave the way for understanding targeted treatment strategies.
This single-center, retrospective study delves into the genetics of breast and ovarian cancer patients tested from August 2021 to May 2023 (22 months).
Genetic susceptibility testing adhered to current clinical practice: BRCA1/2 analysis for breast cancer and full HRD assessment (including gene mutations and functional testing of HRR pathway) for ovarian cancer. We tested for these through ISO 15189-certified labs (Invitae, Genotypos, and Myriad) due to in-house limitations.
All patients were at least 18-years-old and participated in four or more clinic visits within the study period to ensure sufficient clinical workup for the included cancers.
Our analysis focused on the 82 patients who underwent BRCA or HRD testing. Resource limitations prevented us from definitively determining testing rates across the entire patient population (including the 455 patients who were not tested). Detailed National Comprehensive Cancer Network criteria were reported only in tested cancer patients (breast cancer: young age, family history of cancer, TNBC, advanced HER2-negative breast cancer and history of first primary cancer; ovarian cancer: all epithelial histological types and recurrent cancers).
Prior ethics approval was secured. Data on demographics, diagnoses, mutations, treatment response (Response Evaluation Criteria in Solid Tumors criteria), disease-free interval, progression-free interval, and other clinical details were sourced from electronic medical records and oncologist/radiology reports.
We employed descriptive statistics (e.g., central tendency, dispersion, frequency distributions) and compared percentages to identify patterns, trends, and group differences using Datatab software[20]. The sample size calculation was not determined, as the objective of this study was descriptive. Similarly, statistical methods for hypothesis testing were not employed due to the descriptive nature of the analysis.
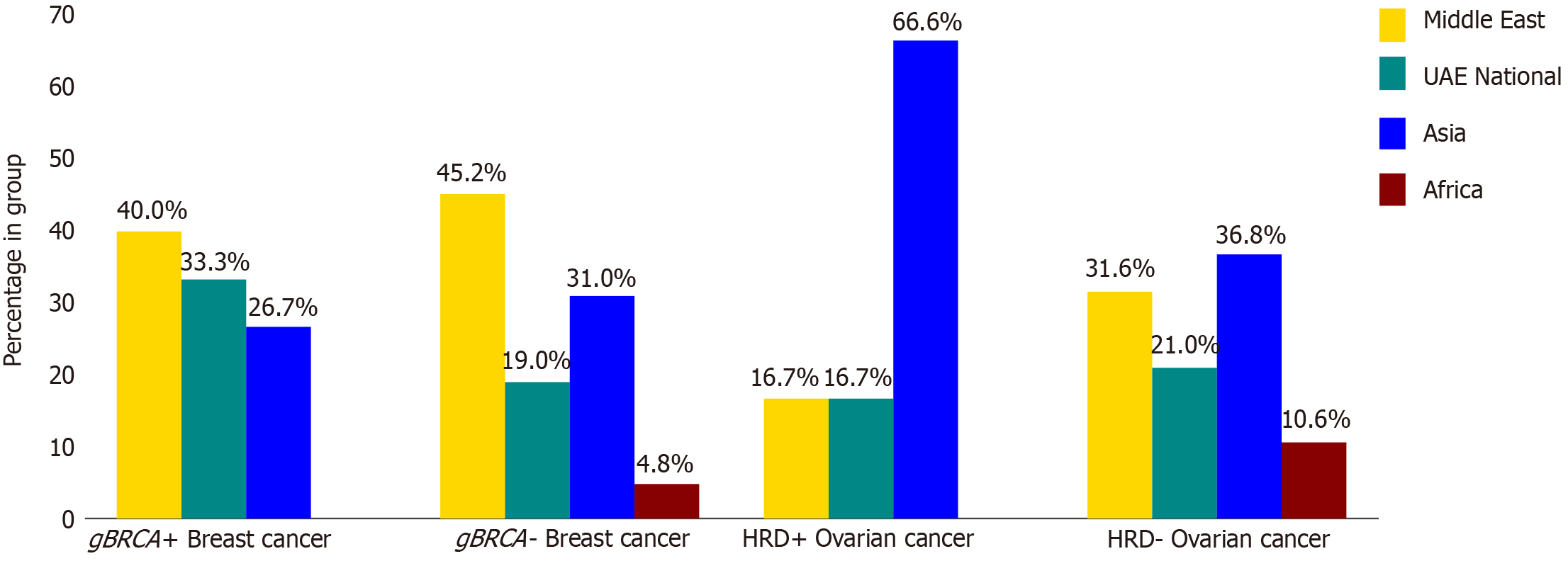
Of the 472 breast cancer patients, 12.1% (n = 57) received BRCA testing, and 38.5% (n = 25) of the 65 ovarian cancer patients underwent HRD testing. The distribution of ethnicity differed between the two cancer groups. UAE nationals were the largest ethnic group among breast cancer patients (45.3% or 214), followed by Middle Eastern (25.0%, 118/472), Asian (16.7%, 79/472), and African (10.4%, 49/472). Similarly, UAE nationals were the predominant group for ovarian cancer patients (33.8%, 22/65), followed by Asian (29.2%, 19/65), Middle Eastern (24.6%, 16/65), and African (7.6%, 5/65).
Among the 82 patients who underwent testing, 21 (25.6%) had pathogenic mutations identified. Among the tested breast cancer patients, testing criteria were as follows: 68.4% (39/57 patients) were young (age < 50 years), 15.8% (9/57 patients) had a family history of cancer, 7.0% (4/57 patients) had a history of recurrence or another primary cancer, and 8.8% (5/57 patients) had TNBC. All 25 tested ovarian cancer patients had single or mixed epithelial histology. PARPis were utilized in 42.8% of the patients with pathogenic mutations.
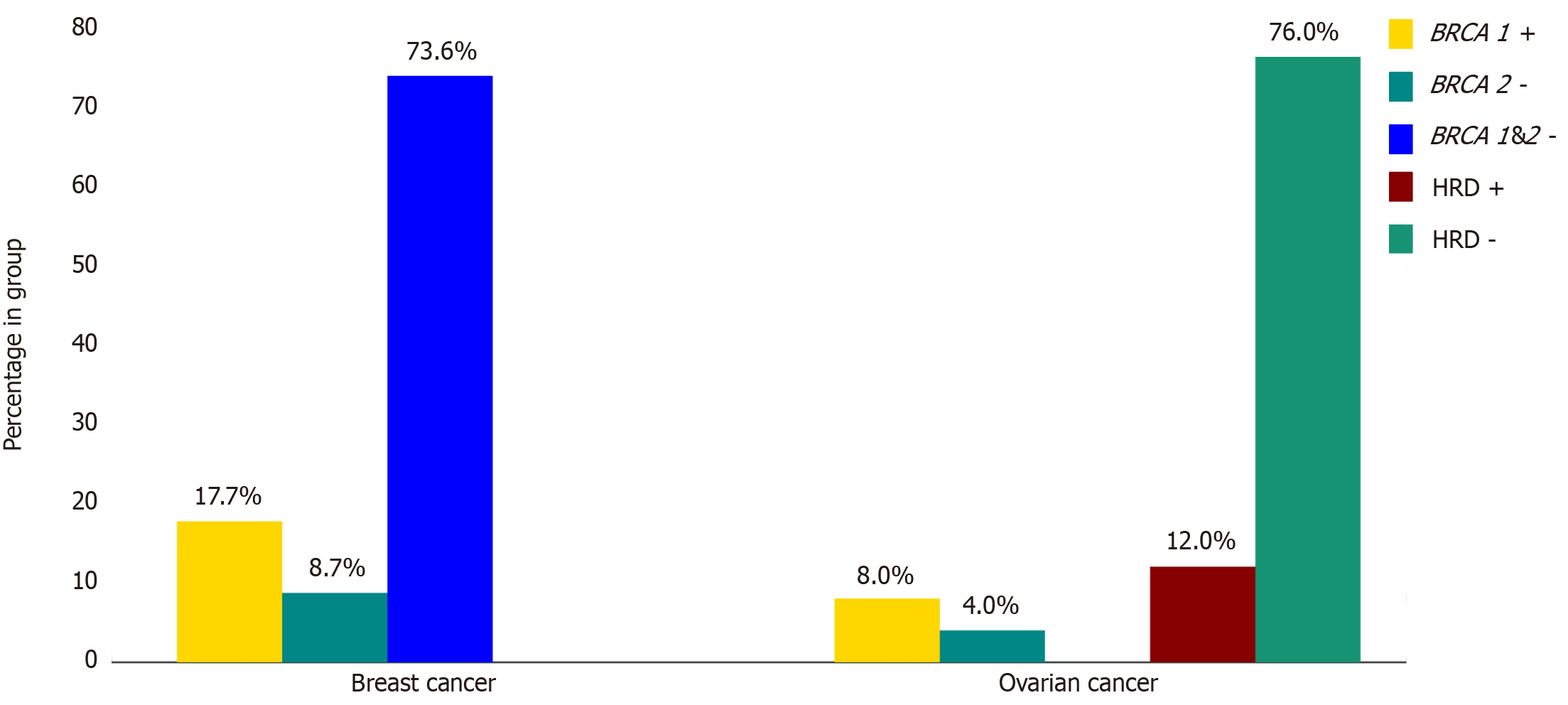
Among the 57 breast cancer cases tested for gBRCA mutations, 15 (26.3%) harbored pathogenic mutations. Of these, 10 (17.7%) involved gBRCA1 mutations, while the remaining 5 (8.7%) were associated with gBRCA2 as shown in Figure 1. Four cases had BRCA2 variants of unknown significance (VUS). The median age at time of diagnosis was 42.1 years in the BRCA mutated group vs 45.3 years in the non-mutated group. Patients from the Middle East, excluding UAE nationals, were the highest in both groups. UAE national patients were the second highest (33.3%) in the mutated group and the third (19%) in the non-mutated group, as shown in Figure 2. Family history of cancer (53.3% in the mutated group vs 38.1% in the non-mutated group) and a history of a first-degree relative having cancer (53.3% in the mutated group vs 21.3% in the non-mutated group) were more prevalent in the mutated group. Additionally, Eastern Cooperative Oncology Group performance status (ECOG PS) was distributed differently between the groups, with 86.7% of mutated patients and 95.2% of non-mutated patients having a score of 0, while 13.3% and 4.8% had a score of 1, respectively. The mutated group also showed a higher median grade of breast cancer (3 vs 2). Both groups were similar in cancer staging, wherein stage 2 was most common followed by stages 3 and 4. Both groups had invasive ductal carcinoma as the most common histological type (80% in the mutated group vs 83.3% in the non-mutated group), followed by invasive lobular carcinoma (6.6% vs 9.5%). The distribution of molecular subtypes also differed slightly, namely TNBC (20% vs 19%), hormone-positive (HR+)/HER2-negative (40% vs 35.7%), and HR+/HER2-positive (20% vs 14.3%), as detailed in Table 1.
| gBRCA1 | Positive | Negative |
| Total, n (%) | 15 (26.3) | 42 (73.7) |
| Age in yr at diagnosis, mean ± SD | 42.1 ± 11 | 45.3 ± 8.7 |
| Positive family history, n (%) | 8 (53.3) | 16 (38.1) |
| First-degree relative, n (%) | 8 (53.3) | 9 (21.3) |
| Non-first-degree relatives, n (%) | 4 (26.6) | 11 (26.1) |
| H/o IVF & Rx, n (%) | 0 | 0 |
| H/o hormone Rx, n (%) | 1 (6.6) | 4 (9.5) |
| ECOG PS at diagnosis2, n (%) | ||
| 0 | 13 (86.7) | 40 (95.2) |
| 1 | 2 (12.3) | 2 (4.8) |
| Histology at diagnosis, n (%) | ||
| Invasive ductal carcinoma | 12 (80) | 35 (83.3) |
| Invasive lobular carcinoma | 1 (6.6) | 4 (9.5) |
| Grade at diagnosis, mean ± SD | 3 ± 0.6 | 2 ± 0.7 |
| Stage at diagnosis, n (%) | ||
| 1 | 1 (6.6) | 0 |
| 2 | 7 (46.6) | 21 (50) |
| 3 | 4 (26.6) | 13 (31) |
| 4 | 3 (20) | 8 (19) |
| Categories3, n (%) | ||
| HER2-positive | 4 (26.6) | 12 (28.7) |
| Triple-negative | 3 (20) | 8 (19.0) |
| HR-positive and HER2-negative | 6 (40) | 15 (35.7) |
| HR-positive and HER2-positive | 3 (20) | 6 (14.3) |
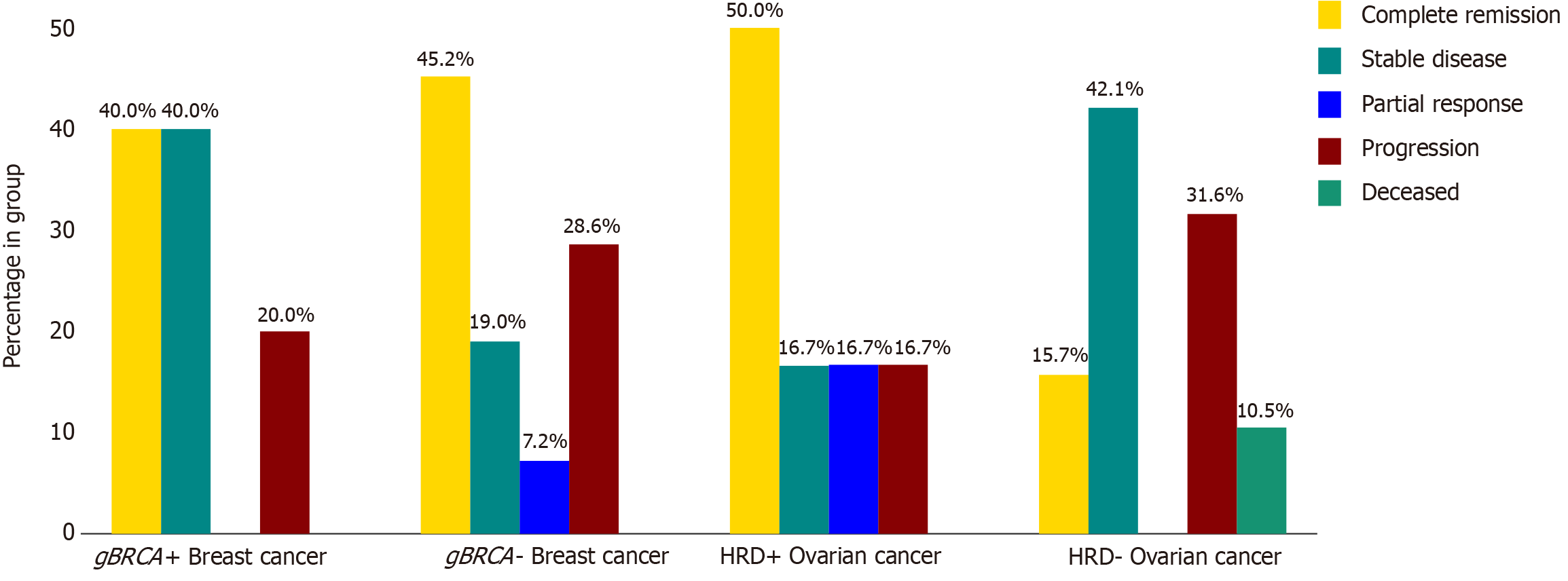
Notably, the response rates to chemotherapy were similar between the groups, with CR being 40% in the mutated group vs 45.2% in the non-mutated group, SD being 40% in the mutated group vs 19% in the non-mutated group, partial response (PR) being 0% in the mutated group vs 7.2% in the non-mutated group, and progressive disease being 20% in the mutated group vs 28.6% in the non-mutated group, as shown in Figure 3.
Within the mutated group (n = 15), 5 patients (33.3%) received PARPi. Of these, 1 patient with advanced disease received PARPi as first-line therapy and progressed after 7 months, the other patient with advanced disease presented to us after progression on ribociclib plus letrazole and could receive PARPi for 5 months only. The other 3 patients received PARPi after adjuvant or neoadjuvant chemotherapy and 2 achieved CR to chemotherapy and were disease-free for more than 15 months on olaparib. Unfortunately, 1 patient progressed on two lines of chemotherapy and could take PARPi for 40 days only. Detailed outcomes are presented in Table 2.
| Cancer type | Patient | Stage | Response change to PARPi | Duration of PARPi | PARPi status at end of study |
| BRCA+ breast cancer, 5/15 (33.3%) | 1 | II | CR–DF | 13 months+ | On Rx |
| 21 | II | PR–PR | 40 days | Stopped | |
| 3 | III | CR–DF | 17 months+ | On Rx | |
| 4 | IV | PR–PR | 5 months | Stopped | |
| 52 | IV | PR | 7 months | Stopped | |
| HRD+ ovarian cancer, 4/6 (66.6%) | 1 | III | PR–DF | 15 months+ | On Rx |
| 2 | IV | CR–DF | 13 months+ | On Rx | |
| 3 | IV | SD–PRF | 20 months+ | On Rx | |
| 4 | IV | CR–DF | 25 months+ | On Rx |
Among the 25 ovarian cancer cases, 6 (24.0%) harbored HRD, comprising 2 (8.0%) somatic BRCA1 and 1 (4.0%) somatic BRCA2. The remaining 19 (76.0%) cases were HRD-negative, as shown in Figure 1. The median age at time of diagnosis was slightly higher in the mutated group (56.5 years) compared to the non-mutated group (52.5 years). In terms of ethnicity, Asians formed the majority of the mutated group at 66.6%, followed by UAE nationals and patients from other Middle Eastern countries, both at 16.7% each. The non-mutated group displayed a different distribution, with Asians comprising 36.8%, followed by Middle Easterners at 31.6%, UAE nationals at 21%, and Africans at 10.6%, as detailed in Figure 2. The mutated group reported no such history, whereas 26.3% of the non-mutated group had a positive family history. Both groups were predominantly composed of patients with good PS (ECOG 0: 83.3% vs 89.4%). Serous adenocarcinoma was the most prevalent histological type in both groups (83.3% in the mutated and 47.3% in the non-mutated), followed by mixed type (0% and 26.3%, respectively). Notably, the majority in both groups presented with advanced-stage (stages 3 and 4) and high-grade (grade 4) ovarian cancer. Additionally, most patients in the mutated group underwent interval debulking surgery (66.7% and 26.4%, respectively), as detailed in Table 3.
| Characteristic | HRD-positive | HRD-negative |
| Total, n (%) | 6 (24) | 19 (76) |
| Age years at diagnosis, mean ± SD | 56.5 ± 11 | 52.5 ± 13 |
| Positive family history, n (%) | 0 | 5 (26.3) |
| ECOG PS at diagnosis, n (%) | ||
| 0 | 5 (83.3) | 17 (89.4) |
| 1 | 1 (16.7) | 1 (5.3) |
| 2 | 0 | 1 (5.3) |
| Histology, n (%) | ||
| Serous | 5 (83.3) | 9 (47.3) |
| Endometrioid/mucinous/clear cell | 1 (16.7) | 5 (26.3) |
| Mixed | 0 | 5 (26.3) |
| Grade of cancer, mean ± SD | 4 ± 0 | 4 ± 0.9 |
| Stage of cancer, n (%) | ||
| 1 | 1 (16.7) | 2 (10.5) |
| 2 | 0 | 0 |
| 3 | 2 (33.3) | 11 (58) |
| 4 | 3 (50) | 6 (31.5) |
| Surgical Rx, n (%) | 6 (100) | 17 (89.5) |
| Primary | 2 (33.3) | 12 (63.1) |
| Interval | 4 (66.7) | 5 (26.4) |
Overall treatment response to chemotherapy was more favorable in the mutated group, with a 50% CR rate compared to 15.7% in the non-mutated group. SD was also achieved by 16.7% of mutated patients vs 42.1% of non-mutated patients, while progressive disease rates were lower in the mutated group (16.7%) compared to the non-mutated group (31.6%) as shown in Figure 3. Among the patients with mutated ovarian cancer who received maintenance PARPi, 3 (75%) achieved disease-free status, while the remaining patient (25%) showed no evidence of disease progression. Notably, all patients remained on PARPi at study completion, as shown in Table 2. No deaths were reported in the mutated group, compared to 10.5% (2 patients) in the non-mutated group, as shown in Figure 3.
Among the 82 patients who underwent testing, 12 (14.6%) had a history of cancer. Notably, 4 (33.3%) of these patients harbored gBRCA- or HRD-positive mutations, while the remaining 8 (66.6%) had no identifiable mutations. Within the gBRCA group, 2 (16.7%) had recurrence of breast cancer, and in the HRD-positive group, 1 (16.7%) had recurrence of ovarian cancer. Additionally, 1 HRD-positive ovarian cancer patient had a history of another first primary, thyroid cancer. Among the unmutated group, 4 (50%) had recurrence of breast cancer, 1 (12.5%) had recurrent ovarian cancer, and 3 (37.5%) had different first primary cancer.
Our study, the first of its kind in the Middle East, investigated the genetic testing rates in cancer patients, prevalence of gBRCA mutations in tested breast cancer patients and HRD in tested ovarian cancer patients. We further compared the demographics, disease characteristics, and response to chemotherapy of these cancers with and without these mutations. Additionally, we analyzed the proportion of patients receiving PARPi therapy and their respective response outcomes. Notably, our cohort comprised 82 tested patients of 537 and 21 (25.6%) harbored pathogenic mutations, exceeding the previously reported rate[21]. Interestingly, 9 (11%) patients carried VUS, falling within the lower range of reported frequencies (0%-44%)[22]. These findings reflect the well-established role of BRCA and other HRR genes in these cancers[2].
BRCA/HRD testing rates were 12% (57 of 472 patients) for breast cancer and 38.4% (25 of 65 patients) for ovarian cancer. These rates are modestly lower than the figures of 14.2% and 43% reported elsewhere respectively[23,24]. However, it is important to acknowledge limitations in our data. Due to limited resources, we lacked information on the eligibility criteria for the untested patients (n = 455). This absence makes it challenging to determine the exact proportion of eligible patients who may not have received testing.
The prevalence of pathogenic gBRCA mutations in breast cancer patients tested in our study was 26.3%, more than two times the 12% reported in a large, multicenter study involving 2733 women[25]. BRCA testing is commonly reco
Response to chemotherapy, 80% of gBRCA-mutated patients and 64.2% in the non-mutated group achieved either complete or PR or SD to chemotherapy, as shown in Figure 3.
This negligible difference in outcomes in the mutated group compared to the non-mutated group aligns with findings from the previous reports that suggested no significant difference in response rates[9]. Of the 33% (n = 5) of gBRCA-positive patients who received PARPi therapy, 4 (80%) had TNBC, while the remaining 1 (20%) was HR+/HER2-negative, as shown in Table 2. Interestingly, the response to PARPi in 3 out of 5 patients was in line with that reported in the OlympiAD and OlympiA trials. These 3 patients received PARPi/olaparib after neoadjuvant or adjuvant chemotherapy; among them 2 patients remained progression free even after 15 months as in OlympiA trial[30]. Unfortunately, the other patient, who took olaparib for 40 days, presented in advanced stage after she had refused chemotherapy and had been lost to follow up. One patient received PARPi as first line for advanced disease progressed after 7 months similar to the duration that reported in the OlympiAD trial, whereas other advanced stage patients did receive PARPi for 5 months but as second line[9]. Our findings suggest that PARPi therapy is used in approximately one-third of patients with BRCA-positive cancer. Interestingly, patients who received PARPi treatment according to established guidelines from major clinical trials seemed to experience better outcomes compared to those who did not receive treatment as per the guidelines.
Our study revealed a 24% prevalence of HRD-positive ovarian cancers, which is in the 20%-25% range observed in similar studies from this region, and studies from North America and China[11,19,31]. The HRD-positive group had a higher median age (56.5 years) compared to the negative group (52.5 years), both of which fall within the 52-60 years range[11]. As shown in Figure 2, Asians were the majority among the tested ovarian cancer patients. This is consistent with the overall demographics of the UAE, where Asians comprise 71% of the population[27,32]. Additionally, Asians were the second-highest ethnicity among all ovarian cancer patients in our cohort, following UAE nationals. Both groups demonstrated similar proportions of patients with good ECOG performance scores, and neither group reported a positive history of family cancer. However, as shown in Table 3, the HRD-positive group displayed a significantly higher prevalence of HGSOC and advanced-stage cancer, reflecting a trend consistent with the reported literature. Most patients had advanced ovarian cancer, and their response to primary treatment was consistent with the expected course of the disease[13,14]. Overall, the HRD-positive and HRD-negative ovarian cancer groups were not similar and were in line with the earlier reports. The significance (P values) of these comparative values could not be calculated due to limitations in statistical analysis.
HRD-positive ovarian cancers have been shown to have better overall survival and platinum responsiveness, a trend observed in our cohort where 83.3% of patients with mutations achieved CR or SD, compared to only 57.8% of those without mutations. Notably, the proportion of patients experiencing progressive disease was 50% lower in the HRD-positive group compared to the non-mutated group, as shown in Figure 3[11,33]. We acknowledge that, due to the short duration of study, the majority of newly diagnosed patients may not have yet reached the point of relapse, which is typically 3 years.
Among HRD-positive patients, 66.6% received PARPi therapy as maintenance; among those, 3 patients (75%) remained disease-free and 1 patient (25%) remained progression-free. All of those who received olaparib remained progression-free at the end of study, in line with the trials that led to the approval of PARPi/olaparib alone or with bevacizumab[14,34]. Overall, our findings regarding prevalence, disease characteristics, and treatment response in HRD-positive ovarian cancer align with the existing literature.
Among the 15 gBRCA-positive breast cancer patients, 2 (13.3%) had a recurrence of breast cancer, while 2 (33.2%) of the 6 HRD-positive ovarian cancer patients had a recurrence of ovarian cancer. These recurrence rates or history of having first primary cancer in a mutation-positive group fall within the range reported in a large multicenter study[29].
Our study identified a higher-than-reported prevalence of BRCA mutations (26.3%) in the tested breast cancer patients. HRD positivity rates in ovarian cancer patients were at the upper end of the reported range. Testing rates for BRCA in overall breast cancer patients and HRD in ovarian cancer patients, however, remained low. While UAE nationals formed the majority of patients with both cancers in our cohort, the ethnic distribution of tested patients differed. Specifically, Middle Eastern patients dominated the positive BRCA mutation and testing group for breast cancer, while Asian patients formed the majority in the positive HRD and testing group for ovarian cancer. This pattern likely reflects the broader demographics of the UAE population, where Asians are the predominant ethnicity.
While most of our findings on disease characteristics (including triple-negative status, histological types, grades, and stages) and treatment responses to chemotherapy and PARPi aligned with published data, the limited sample size restricts definitive conclusions about statistical significance. Larger studies are necessary to confirm our observations and explore potentially unexpected trends, such as the observed ethnic distribution and the high prevalence of BRCA positivity in breast cancer patients.
We are grateful to Mohammed Saeid Abdoh for his invaluable assistance in tracking patients who underwent BRCA testing at our institute and for promptly providing the data. We would also like to acknowledge Laurette Lecharlois Bukasa for her invaluable contribution in reviewing the statistical methods and providing biostatistical support. During the preparation of this work the author(s) used Bard.ai to check grammar and rephrase sentences to increase the readability. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
| 1. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47165] [Article Influence: 3368.9] [Reference Citation Analysis (5)] |
| 2. | Petrucelli N, Daly MB, Pal T. BRCA1- and BRCA2-Associated Hereditary Breast and Ovarian Cancer. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2024. [PubMed] |
| 3. | Armstrong N, Ryder S, Forbes C, Ross J, Quek RG. A systematic review of the international prevalence of BRCA mutation in breast cancer. Clin Epidemiol. 2019;11:543-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 4. | Creeden JF, Nanavaty NS, Einloth KR, Gillman CE, Stanbery L, Hamouda DM, Dworkin L, Nemunaitis J. Homologous recombination proficiency in ovarian and breast cancer patients. BMC Cancer. 2021;21:1154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 5. | Peshkin BN, Alabek ML, Isaacs C. BRCA1/2 mutations and triple negative breast cancers. Breast Dis. 2010;32:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Fu X, Tan W, Song Q, Pei H, Li J. BRCA1 and Breast Cancer: Molecular Mechanisms and Therapeutic Strategies. Front Cell Dev Biol. 2022;10:813457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 7. | Wang CJ, Xu Y, Lin Y, Zhu HJ, Zhou YD, Mao F, Zhang XH, Huang X, Zhong Y, Sun Q, Li CG. Platinum-Based Neoadjuvant Chemotherapy for Breast Cancer With BRCA Mutations: A Meta-Analysis. Front Oncol. 2020;10:592998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Azim HA Jr, Partridge AH. Biology of breast cancer in young women. Breast Cancer Res. 2014;16:427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 320] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 9. | Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A, Wu W, Goessl C, Runswick S, Conte P. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med. 2017;377:523-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 2293] [Article Influence: 286.6] [Reference Citation Analysis (0)] |
| 10. | Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee KH, Fehrenbacher L, Yerushalmi R, Mina LA, Martin M, Roché H, Im YH, Quek RGW, Markova D, Tudor IC, Hannah AL, Eiermann W, Blum JL. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med. 2018;379:753-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1229] [Cited by in RCA: 1494] [Article Influence: 213.4] [Reference Citation Analysis (0)] |
| 11. | Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, Rendi MH, Thornton A, Norquist BM, Casadei S, Nord AS, Agnew KJ, Pritchard CC, Scroggins S, Garcia RL, King MC, Swisher EM. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 785] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 12. | Huang XZ, Jia H, Xiao Q, Li RZ, Wang XS, Yin HY, Zhou X. Efficacy and Prognostic Factors for PARP Inhibitors in Patients With Ovarian Cancer. Front Oncol. 2020;10:958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, Verheijen RH, van der Burg ME, Lacave AJ, Panici PB, Kenter GG, Casado A, Mendiola C, Coens C, Verleye L, Stuart GC, Pecorelli S, Reed NS; European Organization for Research and Treatment of Cancer-Gynaecological Cancer Group; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1666] [Cited by in RCA: 1772] [Article Influence: 118.1] [Reference Citation Analysis (0)] |
| 14. | Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke GS, Gourley C, Banerjee S, Oza A, González-Martín A, Aghajanian C, Bradley W, Mathews C, Liu J, Lowe ES, Bloomfield R, DiSilvestro P. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med. 2018;379:2495-2505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1408] [Cited by in RCA: 1956] [Article Influence: 279.4] [Reference Citation Analysis (0)] |
| 15. | Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I, Ben-Baruch NE, Marth C, Mądry R, Christensen RD, Berek JS, Dørum A, Tinker AV, du Bois A, González-Martín A, Follana P, Benigno B, Rosenberg P, Gilbert L, Rimel BJ, Buscema J, Balser JP, Agarwal S, Matulonis UA; ENGOT-OV16/NOVA Investigators. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med. 2016;375:2154-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1483] [Cited by in RCA: 1858] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 16. | Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, Colombo N, Weberpals JI, Clamp A, Scambia G, Leary A, Holloway RW, Gancedo MA, Fong PC, Goh JC, O'Malley DM, Armstrong DK, Garcia-Donas J, Swisher EM, Floquet A, Konecny GE, McNeish IA, Scott CL, Cameron T, Maloney L, Isaacson J, Goble S, Grace C, Harding TC, Raponi M, Sun J, Lin KK, Giordano H, Ledermann JA; ARIEL3 investigators. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949-1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1165] [Cited by in RCA: 1311] [Article Influence: 163.9] [Reference Citation Analysis (0)] |
| 17. | World Health Organization Team. Cancer United Arab Emirates 2020 country profile. 2020 Jan [cited 22 November 2023]. Available from: https://www.who.int/publications/m/item/cancer-are-2020. |
| 18. | Azribi F, Abdou E, Dawoud E, Ashour M, Kamal A, Al Sayed M, Burney I. Prevalence of BRCA1 and BRCA2 pathogenic sequence variants in ovarian cancer patients in the Gulf region: the PREDICT study. BMC Cancer. 2021;21:1350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Alhuqail AJ, Alzahrani A, Almubarak H, Al-Qadheeb S, Alghofaili L, Almoghrabi N, Alhussaini H, Park BH, Colak D, Karakas B. High prevalence of deleterious BRCA1 and BRCA2 germline mutations in arab breast and ovarian cancer patients. Breast Cancer Res Treat. 2018;168:695-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | DATAtab Team. DATAtab: Online Statistics Calculator. DATAtab e.U. Graz, Austria, 2023. [cited 24 November 2023]. Available from: https://datatab.net. |
| 21. | Heeke AL, Pishvaian MJ, Lynce F, Xiu J, Brody JR, Chen WJ, Baker TM, Marshall JL, Isaacs C. Prevalence of Homologous Recombination-Related Gene Mutations Across Multiple Cancer Types. JCO Precis Oncol. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 224] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 22. | Shao C, Wan J, Lam FC, Tang H, Marley AR, Song Y, Miller C, Brown M, Han J, Adeboyeje G. A comprehensive literature review and meta-analysis of the prevalence of pan-cancer BRCA mutations, homologous recombination repair gene mutations, and homologous recombination deficiencies. Environ Mol Mutagen. 2022;63:308-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Stenehjem DD, Telford C, Unni SK, Bauer H, Sainski A, Deka R, Schauerhamer MB, Ye X, Tak CR, Ma J, Dalvi TB, Gutierrez L, Kaye JA, Tyczynski JE, Brixner DI, Biskupiak JE. BRCA testing and outcomes in women with breast cancer. Breast Cancer Res Treat. 2021;186:839-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Neff RT, Senter L, Salani R. BRCA mutation in ovarian cancer: testing, implications and treatment considerations. Ther Adv Med Oncol. 2017;9:519-531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 25. | Copson ER, Maishman TC, Tapper WJ, Cutress RI, Greville-Heygate S, Altman DG, Eccles B, Gerty S, Durcan LT, Jones L, Evans DG, Thompson AM, Pharoah P, Easton DF, Dunning AM, Hanby A, Lakhani S, Eeles R, Gilbert FJ, Hamed H, Hodgson S, Simmonds P, Stanton L, Eccles DM. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19:169-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 313] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 26. | Al-Shamsi HO, Abdelwahed N, Al-Awadhi A, Albashir M, Abyad AM, Rafii S, Afrit M, Al Lababidi B, Abu-Gheida I, Sonawane YP, Nijhawan NA, Haq UU, Dreier N, Joshua TLA, Iqbal F, Yacoub T, Nawaz FA, Abdul Jabbar D, Tirmazy SH, El-Shourbagy DM, Hamza D, Omara M, Al Madhi SAS, Ghazal H, Darr H, Oner M, Vlamaki Z, El Kinge AR, Ramanathan D, Judah M, Almahmeed T, Ahmad M, Jonnada SB, Almansoori N, Razek AA, Al-Hamadi A, Balalaa N, Jamali F, Singarachari RA, Labban A, Das K, Luiten EJT, Abdelgawad T, Al-Khatib F, Alrawi S, Jaafar H. Breast Cancer in the United Arab Emirates. JCO Glob Oncol. 2023;9:e2200247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 27. | World Population Review Team. Dubai-population 2023. [cited 25 December 2023]. Available from: https://worldpopulationreview.com/world-cities/dubai-population. |
| 28. | Yoshida R. Hereditary breast and ovarian cancer (HBOC): review of its molecular characteristics, screening, treatment, and prognosis. Breast Cancer. 2021;28:1167-1180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 152] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 29. | Lambertini M, Ceppi M, Hamy AS, Caron O, Poorvu PD, Carrasco E, Grinshpun A, Punie K, Rousset-Jablonski C, Ferrari A, Paluch-Shimon S, Toss A, Senechal C, Puglisi F, Pogoda K, Pérez-Fidalgo JA, De Marchis L, Ponzone R, Livraghi L, Estevez-Diz MDP, Villarreal-Garza C, Dieci MV, Clatot F, Duhoux FP, Graffeo R, Teixeira L, Córdoba O, Sonnenblick A, Ferreira AR, Partridge AH, Di Meglio A, Saule C, Peccatori FA, Bruzzone M, t'Kint de Roodenbeke MD, Ameye L, Balmaña J, Del Mastro L, Azim HA Jr. Clinical behavior and outcomes of breast cancer in young women with germline BRCA pathogenic variants. NPJ Breast Cancer. 2021;7:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, Gelber RD, de Azambuja E, Fielding A, Balmaña J, Domchek SM, Gelmon KA, Hollingsworth SJ, Korde LA, Linderholm B, Bandos H, Senkus E, Suga JM, Shao Z, Pippas AW, Nowecki Z, Huzarski T, Ganz PA, Lucas PC, Baker N, Loibl S, McConnell R, Piccart M, Schmutzler R, Steger GG, Costantino JP, Arahmani A, Wolmark N, McFadden E, Karantza V, Lakhani SR, Yothers G, Campbell C, Geyer CE Jr; OlympiA Clinical Trial Steering Committee and Investigators. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N Engl J Med. 2021;384:2394-2405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 1031] [Article Influence: 257.8] [Reference Citation Analysis (0)] |
| 31. | Feng Z, Shao D, Cai Y, Bi R, Ju X, Chen D, Song C, Chen X, Li J, An N, Li Y, Zhou Q, Xiu Z, Zhu S, Wu X, Wen H. Homologous recombination deficiency status predicts response to platinum-based chemotherapy in Chinese patients with high-grade serous ovarian carcinoma. J Ovarian Res. 2023;16:53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 32. | Zhang Y, Luo G, Li M, Guo P, Xiao Y, Ji H, Hao Y. Global patterns and trends in ovarian cancer incidence: age, period and birth cohort analysis. BMC Cancer. 2019;19:984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 33. | Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, Dobrovic A, Birrer MJ, Webb PM, Stewart C, Friedlander M, Fox S, Bowtell D, Mitchell G. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654-2663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 952] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 34. | Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A, Berger R, Fujiwara K, Vergote I, Colombo N, Mäenpää J, Selle F, Sehouli J, Lorusso D, Guerra Alía EM, Reinthaller A, Nagao S, Lefeuvre-Plesse C, Canzler U, Scambia G, Lortholary A, Marmé F, Combe P, de Gregorio N, Rodrigues M, Buderath P, Dubot C, Burges A, You B, Pujade-Lauraine E, Harter P; PAOLA-1 Investigators. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N Engl J Med. 2019;381:2416-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 1347] [Article Influence: 224.5] [Reference Citation Analysis (0)] |