Published online Jun 24, 2024. doi: 10.5306/wjco.v15.i6.667
Revised: March 18, 2024
Accepted: April 24, 2024
Published online: June 24, 2024
Processing time: 198 Days and 0.7 Hours
Colorectal cancer (CRC) is the third most common cancer worldwide and the second most common cause of cancer death. Nanotherapies are able to selectively target the delivery of cancer therapeutics, thus improving overall antitumor eff
Core Tip: Chemotherapy is the main treatment strategy for cancer. Despite the rapid development of medicine, conventional chemotherapy has some limitations. Mesoporous silica nanoparticles (MSNs) which can increase solubility, prolong the circulation time and reduce toxicity are already in use today, and show great promise in clinical development. This is due to their favorable properties as a nanocarrier, such as high surface area, tailorable pore sizes, controllable particle sizes and shapes, and dual-functional surfaces (exterior and interior). MSNs based therapies have remarkable benefits in both in vitro and in vivo testing. These nanosystems may lead to major advances in individualized treatment and have great potential for clinical translation.
- Citation: Meng J, Wang ZG, Zhao X, Wang Y, Chen DY, Liu DL, Ji CC, Wang TF, Zhang LM, Bai HX, Li BY, Liu Y, Wang L, Yu WG, Yin ZT. Silica nanoparticle design for colorectal cancer treatment: Recent progress and clinical potential. World J Clin Oncol 2024; 15(6): 667-673
- URL: https://www.wjgnet.com/2218-4333/full/v15/i6/667.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i6.667
Cancer is currently the leading cause of death and the single most important barrier to increased life expectancy worldwide. Colorectal cancer (CRC) is the third most common cancer worldwide and the second most common cause of cancer death. There were over 1.8 million new CRC cases and 881000 deaths in 2018[1]. CRC is the second most common cause of cancer death in the United States[2]. In addition CRC was estimated to be the second most commonly diagnosed cancer and the second leading cause of cancer death in Europe in 2020, with nearly 520000 new cases and 245000 deaths[3]. The 5-year relative survival rate for CRC ranges from 90% to 14%, and as the tumor stage increases, the 5-year relative survival rate declines. The incidence of rectal cancer and colon cancer are 67% and 63%, respectively[4].
The current options for the standard treatment of CRC include surgery, chemotherapy, radiation therapy, immunotherapy and endocrine therapy. Chemotherapy is the main treatment strategy for cancer. Despite the rapid development of medicine, conventional chemotherapy has some limitations. First, many chemotherapeutics have a short half-life or low water solubility in the body, which prevents their clinical application. Second, chemotherapeutic drugs usually lack tumor specificity, which results in undifferentiated killing of healthy cells and highly toxic side effects, and even death in extreme cases.
Nanotherapies which can increase solubility, prolong the circulation time and reduce toxicity are already in use today, and show great promise in clinical development. Among these nanoparticle platforms, mesoporous silica nanoparticles (MSNs) are a class of materials which are a focus of research. This is due to their favorable properties as a nanocarrier, such as high surface area, tailorable pore sizes, controllable particle sizes and shapes, and dual-functional surfaces (exterior and interior). Therefore, MSNs have good drug encapsulation and delivery performance[5,6].
Kresge first reported the synthesis of MSNs in the 1990s and The United States Food and Drug Administration con
MSNs are characterized by particle sizes of 50–200 nm, pore sizes of 2–6 nm, bulk pore volume of 0.6–1 cm³/g and a large surface area of 700–1000 m²/g[8]. The presence of Si–OH groups on the surface allows MSNs to be functionalized using a wide variety of functional groups and allows targeted delivery to the required site of action[9].
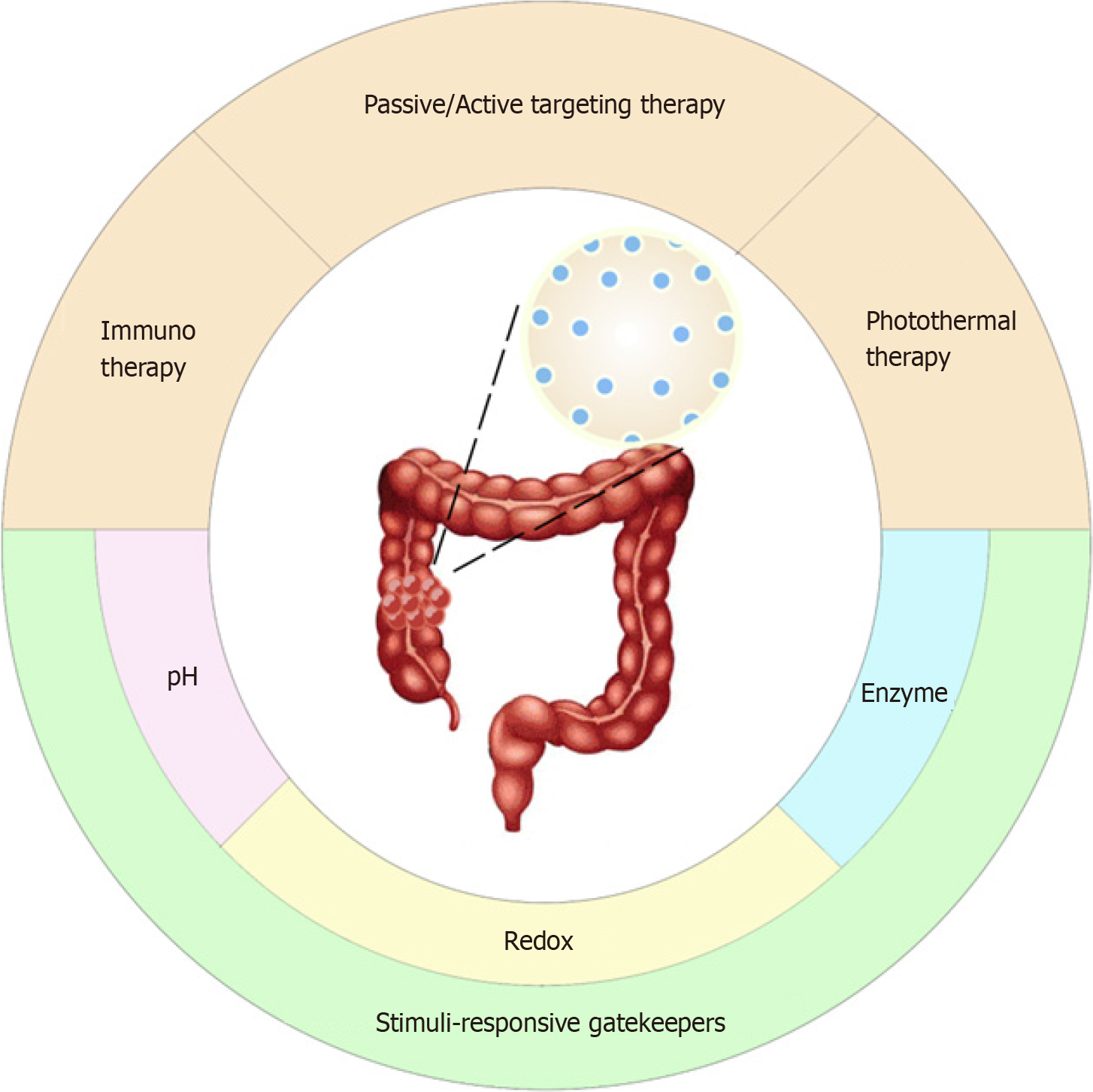
In this review, we assess the current field of CRC therapy with mesoporous silica and offer insights into the recent advances in MSN-based targeted nanosystems in CRC treatment and their potential clinical application value (Figure 1).
The prognosis of CRC patients depends on the stage of the tumor at diagnosis. The mortality rate widely differs by stage, being 8%–13% for stage I/II, 11%–47% for stage III, and almost 90% for stage IV. As the tumor stage increases, the overall survival rate declines[10].
Unfortunately, the primary diagnostic methods such as sigmoidoscopy, colonoscopy, computed tomography colonography can only detect polyps and cancers by shape/morphology. Smaller/flatter polyps are easily overlooked during routine colonoscopy, as well as nascent neoplasms with recurrent disease.
Diagnostic sensitivity can be improved to detect small/flat polyps at an early stage. Or take advantage of new mole
More than half of the cancer biomarkers are glycoproteins. Specifically, changes in the composition and quantity of cell surface glycosylation-associated molecules are common features of malignant transformation and progression. Fun
Chen et al[12] reported polyp-targeting, fluorescently-labeled MSNs for endoscopic detection of premalignant colonic lesions. FITC fluorescently-labeled MSNs enable fluorescence tracking both in vitro and in vivo. UEA1 (the α-L-fucose targeting lectin Ulex europaeus Agglutinin-1) has been shown to bind human colorectal adenocarcinomas, adenomas, and polyposis coli, but not normal epithelium. UEA1 was used for polyp-targeting, and demonstrated significant binding specificity of nanoconstructs to pathological lesions.
Nanoparticle targeting cancers mainly involves three approaches: One is passive tumor targeting by utilizing the enhanced permeability and retention (EPR) of the tumor region; the second is active targeting, in which nanoparticles are functionalized on the surface of overexpressed cancer-specific ligands, thereby guiding them to tumors and cancer cells; the third is stimuli-responsive gatekeeper tumor targeting[13].
The EPR effect has emerged as a promising therapeutic approach for cancer targeted treatment. There are already several cancer nanomedicines approved to treat solid and hematologic malignancies[14,15].
Irinotecan is a key chemotherapeutic agent for the treatment of CRC and pancreatic ductal adenocarcinoma. Despite its efficacy, irinotecan use is hindered by a high incidence of bone marrow and gastrointestinal (GI) toxicity. Liu et al[16] designed a PEGylation and lipid bilayer (LB) coated MSN carrier for encapsulated irinotecan delivery. LB-coated MSNs, are also known as “silicasomes”. Intravenous injection of silicasomes in a well-developed orthotopic colon cancer mouse model demonstrated improved efficacy, increased survival, and reduced bone marrow and GI toxicity.
The naturally occurring polyphenol resveratrol (RES) has anticancer activity. However, the promising potential of RES is hindered by its poor aqueous solubility, which limits its biological activity. Summerlin et al[17] encapsulated RES in MSNs, and found that solubility increased almost two-fold and mediated in vitro cell death was higher than that of pure RES.
The extent of EPR effect in tumor patients has been questioned. The EPR effect in human tumors is much more complicated than in animal models. The EPR effect varies depending on cancer patients' pathological features and shows intense heterogeneity in different cancer patients with solid tumors[18,19]. Active targeting can compensate for the deficiency of EPR to improve tumor accumulation and retention. Specific ligands are conjugated to MSNs via surface modification. Active targeting ligands can recognize receptors expressed on the membrane of cancerous cells via ligand–receptor interactions, thus improving the MSNs tumor targeting and therapeutic efficiency. The ligands could be peptides, aptamers (Apt), antibodies, proteins, saccharides, and folic acid[20]. Biomarkers on the CRC cell membrane, such as epidermal growth factor receptor (EGFR), vascular endothelial growth factors, epithelial cell adhesion molecule (EpCAM) and matrix metalloproteinases can be exploited as targets for ligand binding[21].
Since first synthesized by Heidelberger and his colleagues in 1957, fluorouracil (5-FU) has been used as a component in both first-line chemotherapy regimens especially for CRC treatment and has shown good therapeutic results[22].
Chen et al[23] and She et al[24] developed hollow MSNs functionalized with epidermal growth factor (EGF) which were used as nanocarriers for 5-FU delivery to CRC cells and CRC acquired drug resistance cells, and demonstrated efficient and high specificity, and excellent targeting performance via the EGF–EGFR interaction.
In preclinical oncology studies and clinical trials, mifepristone (MIF) had a potent anti-proliferative effect. Gao et al[25] constructed MSNs conjugated with the EpCAM antibody and loaded them with MIF. The functionalized nanoparticles targeted the circulating tumor cells in mouse blood long enough to deliver MIF and inhibit CRC lung metastasis.
Gene silencing by RNA interference is a personalized field of cancer treatment. Small interfering RNAs (siRNAs) can bind to the mRNA causing site-specific cleavage and results in the silencing of specific genes responsible for cancer or other pathological conditions. At present, the ideal delivery system should be able to target cancer cells with siRNAs and release them into the cytoplasm to prevent their degradation by nucleases without adverse effects[26].
Babaei et al[27] constructed targeted rod-shaped MSNs for the co-delivery of survivin short hairpin RNA and ca
In recent years, scientists have used the characteristics of the tumor microenvironment (TME) to design nanotargeted tumor systems. The characteristics of the TME, ATP, pH, redox reactants, hypoxia and excessive levels of certain enzyme secretions than normal tissues, are vital for tailoring stimuli-responsive targeting systems[28,29]. The pores of MSNs can be sealed with various gatekeepers and triggered by TME stimuli, which offers an opportunity to design stimuli-responsive targeting systems for controlled release.
Energetic metabolism and deregulated glycolysis in cancer cells result in a high level of lactic acid and the TME has a slightly lower pH (6.5-7.2). Thus, pH-responsive nanoparticles have attracted considerable attention due to the distinct acidic features of tumor tissues compared with normal tissues[30,31].
Polydopamine (PDA) can be stably coated on MSNs surface and acts as a pH-sensitive gatekeeper, controlling the sel
DM1 is a maytansine derivative and its application in clinical cancer therapy is limited by severe side effects. Xie et al[33] developed MSNs loaded with DM1,PDA and EpCAM Apt. This pH targeted system is used for the treatment of CRC.
MicroRNA-155 (miR-155) is one of the most salient oncogenic microRNAs, which has been shown to regulate several cancer-related pathways and is correlated with drug resistance and genome instability. Li et al[34] reported on anti-miR-155-loaded MSNs surface modified with pH-sensitive PDA and AS1411 APt for the pH-responsive targeted downregulation of miR-155 for cancer therapy.
Oral chemotherapy is the most preferred route of administration in cancer treatment. Unfortunately, poor oral bioavailability limits its applications. Oral delivery systems may improve drug bioavailability by enhancing drug solubility and avoiding premature release before reaching the GI tract targeted sites. The human body naturally exhibits different pH values from the stomach (pH: 1.0–3.0) to the colon (pH: 7.0–8.0). The high variation in GI tract pH provides challenges for nanostructures[35].
Nguyen et al[36] loaded prednisolone into 3-aminopropyl-functionalized MSNs and coated them with succinylated ε-polylysine (SPL). The pH-responsive SPL MSNs selectively released prednisolone in the pH conditions of the colon but not in the more acidic conditions of the stomach or small intestine.
Tian et al[37] used the polymer poly (acrylic acid) (PAA) to anchor onto the pore channels of MSNs, which acted as a pH-responsive gatekeeper. The PAA capped MSNs exhibited a high doxorubicin (DOX) loading capacity. The DOX was protected when passing through the gastric environment and achieved targeted release in the colon.
Zahiri et al[38] encapsulated DOX in MSNs coated with polycarboxylic acid dextran to provide pH-dependent drug release. Moreover, a CD133 RNA Apt was tagged to target CD133 overexpressed tumor cells. Narayan et al[39] used chitosan as a pH-sensitive gatekeeper for the controlled release of capecitabine.
The amount of glutathione in tumor cells is approximately 10 times greater than that in normal cells. Liu et al[40] used a new methodology by “weaving” polyethylenimine (PEI) on the MSN surface through disulfide bonds to achieve delivery of chemotherapy (DOX) and miRNA therapy (using miRNA-145). Furthermore, an active targeting WL8 peptide (WIFPWIQL) was used to target glucose-regulated protein-78. The reducing environment hydrolyzed the disulfide bond to remove the PEI gatekeeper, resulting in the release of DOX from the carrier. This redox-responsive co-delivery system realized synergistic antitumor efficacy in vitro and in vivo, and significant anti-metastatic activity in an orthotopic colorectal tumor model.
Kumar et al[41] utilized guar gum, a natural carbohydrate polymer as an enzyme-responsive gatekeeper to load 5-FU. The release of 5-FU from capped guar gum was specifically triggered via colonic enzyme biodegradation.
Kumar et al[42] also constructed a guar gum enzyme-responsive delivery system for drug release and near-infrared (NIR) triggered photodynamic therapy.
The small molecule inhibitor of the signaling hub kinase GSK3 can interfere in the PD-1/PD-L1 axis and provide effective cancer immunotherapy. Allen et al[43] loaded the GSK3 inhibitor AZD1080 into MSNs and coated them with a LB. Drug delivery reduced PD-1 expression and resulted in significant tumor shrinkage in mouse tumor models.
IR825 is a NIR photothermal fluorescent dye with excellent photostability and high photothermal conversion capa
Cancer progression is a collective result of numerous pathological events. During the disease course, cancers generally become more heterogeneous and have high mutation rates. As a result of this heterogeneity, the bulk tumor may include diverse cells harboring distinct molecular signatures with differential levels of sensitivity to treatment[45]. Thus, chemotherapy alone may not produce satisfactory therapeutic results. To overcome multidrug resistance and heterogeneity, combination therapies are given which are usually complementary to chemotherapy for better therapeutic effects. MSNs can be used not only as drug delivery systems, but also as immunotherapy and photothermal nanosystems. In this review, we have shown the potential of various MSN-based nanosystems and multimodal targeting MSNs, which have demonstrated their merit over conventional therapeutic agents. These MSNs can be used for diagnosis, targeted drug therapy, targeted gene therapy, immunotherapy and photothermal therapy of CRC. Although MSN chemotherapies have shown remarkable benefits in testing, only one clinical trial on MSNs has been conducted[46]. Long-term biosafety tests deserve further study at the preclinical and clinical levels. In addition, the optimal dosage ratio of nanotherapeutic drugs requires further investigation. MSN-based nanosystems may lead to major advances in individualized treatment and have great potential for clinical translation.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55774] [Article Influence: 7967.7] [Reference Citation Analysis (132)] |
| 2. | Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1548] [Reference Citation Analysis (3)] |
| 3. | Cardoso R, Guo F, Heisser T, Hackl M, Ihle P, De Schutter H, Van Damme N, Valerianova Z, Atanasov T, Májek O, Mužík J, Nilbert MC, Tybjerg AJ, Innos K, Mägi M, Malila N, Bouvier AM, Bouvier V, Launoy G, Woronoff AS, Cariou M, Robaszkiewicz M, Delafosse P, Poncet F, Katalinic A, Walsh PM, Senore C, Rosso S, Vincerževskienė I, Lemmens VEPP, Elferink MAG, Johannesen TB, Kørner H, Pfeffer F, Bento MJ, Rodrigues J, Alves da Costa F, Miranda A, Zadnik V, Žagar T, Lopez de Munain Marques A, Marcos-Gragera R, Puigdemont M, Galceran J, Carulla M, Chirlaque MD, Ballesta M, Sundquist K, Sundquist J, Weber M, Jordan A, Herrmann C, Mousavi M, Ryzhov A, Hoffmeister M, Brenner H. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: an international population-based study. Lancet Oncol. 2021;22:1002-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 257] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 4. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2268] [Cited by in RCA: 3263] [Article Influence: 652.6] [Reference Citation Analysis (2)] |
| 5. | Gao Y, Gao D, Shen J, Wang Q. A Review of Mesoporous Silica Nanoparticle Delivery Systems in Chemo-Based Combination Cancer Therapies. Front Chem. 2020;8:598722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 6. | Sábio RM, Meneguin AB, Ribeiro TC, Silva RR, Chorilli M. New insights towards mesoporous silica nanoparticles as a technological platform for chemotherapeutic drugs delivery. Int J Pharm. 2019;564:379-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 7. | Kresge CT, Leonowicz ME, Roth WJ, Vartuli J, Beck J. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature. 1992;359:710. |
| 8. | Castillo RR, Vallet-Regí M. Functional Mesoporous Silica Nanocomposites: Biomedical applications and Biosafety. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Hosseinpour S, Walsh LJ, Xu C. Biomedical application of mesoporous silica nanoparticles as delivery systems: a biological safety perspective. J Mater Chem B. 2020;8:9863-9876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 10. | Gallo G, Vescio G, De Paola G, Sammarco G. Therapeutic Targets and Tumor Microenvironment in Colorectal Cancer. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 11. | Miao W, Zhang C, Cai Y, Zhang Y, Lu H. Fast solid-phase extraction of N-linked glycopeptides by amine-functionalized mesoporous silica nanoparticles. Analyst. 2016;141:2435-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Chen NT, Souris JS, Cheng SH, Chu CH, Wang YC, Konda V, Dougherty U, Bissonnette M, Mou CY, Chen CT, Lo LW. Lectin-functionalized mesoporous silica nanoparticles for endoscopic detection of premalignant colonic lesions. Nanomedicine. 2017;13:1941-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Alshehri S, Imam SS, Rizwanullah M, Akhter S, Mahdi W, Kazi M, Ahmad J. Progress of Cancer Nanotechnology as Diagnostics, Therapeutics, and Theranostics Nanomedicine: Preclinical Promise and Translational Challenges. Pharmaceutics. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4951] [Cited by in RCA: 4719] [Article Influence: 188.8] [Reference Citation Analysis (0)] |
| 15. | Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387-6392. [PubMed] |
| 16. | Liu X, Jiang J, Chan R, Ji Y, Lu J, Liao YP, Okene M, Lin J, Lin P, Chang CH, Wang X, Tang I, Zheng E, Qiu W, Wainberg ZA, Nel AE, Meng H. Improved Efficacy and Reduced Toxicity Using a Custom-Designed Irinotecan-Delivering Silicasome for Orthotopic Colon Cancer. ACS Nano. 2019;13:38-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 17. | Summerlin N, Qu Z, Pujara N, Sheng Y, Jambhrunkar S, McGuckin M, Popat A. Colloidal mesoporous silica nanoparticles enhance the biological activity of resveratrol. Colloids Surf B Biointerfaces. 2016;144:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 18. | Shan X, Li S, Sun B, Chen Q, Sun J, He Z, Luo C. Ferroptosis-driven nanotherapeutics for cancer treatment. J Control Release. 2020;319:322-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 19. | Shi Y, van der Meel R, Chen X, Lammers T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics. 2020;10:7921-7924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 495] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 20. | Alyassin Y, Sayed EG, Mehta P, Ruparelia K, Arshad MS, Rasekh M, Shepherd J, Kucuk I, Wilson PB, Singh N, Chang MW, Fatouros DG, Ahmad Z. Application of mesoporous silica nanoparticles as drug delivery carriers for chemotherapeutic agents. Drug Discov Today. 2020;25:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 21. | Jabłońska-Trypuć A, Matejczyk M, Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhib Med Chem. 2016;31:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 692] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 22. | Hashimoto Y, Yoshida Y, Yamada T, Aisu N, Yoshimatsu G, Yoshimura F, Hasegawa S. Current Status of Therapeutic Drug Monitoring of 5-Fluorouracil Prodrugs. Anticancer Res. 2020;40:4655-4661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Chen L, She X, Wang T, He L, Shigdar S, Duan W, Kong L. Overcoming acquired drug resistance in colorectal cancer cells by targeted delivery of 5-FU with EGF grafted hollow mesoporous silica nanoparticles. Nanoscale. 2015;7:14080-14092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | She X, Chen L, Velleman L, Li C, Zhu H, He C, Wang T, Shigdar S, Duan W, Kong L. Fabrication of high specificity hollow mesoporous silica nanoparticles assisted by Eudragit for targeted drug delivery. J Colloid Interface Sci. 2015;445:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Gao Y, Gu S, Zhang Y, Xie X, Yu T, Lu Y, Zhu Y, Chen W, Zhang H, Dong H, Sinko PJ, Jia L. The Architecture and Function of Monoclonal Antibody-Functionalized Mesoporous Silica Nanoparticles Loaded with Mifepristone: Repurposing Abortifacient for Cancer Metastatic Chemoprevention. Small. 2016;12:2595-2608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Charbe NB, Amnerkar ND, Ramesh B, Tambuwala MM, Bakshi HA, Aljabali AAA, Khadse SC, Satheeshkumar R, Satija S, Metha M, Chellappan DK, Shrivastava G, Gupta G, Negi P, Dua K, Zacconi FC. Small interfering RNA for cancer treatment: overcoming hurdles in delivery. Acta Pharm Sin B. 2020;10:2075-2109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 27. | Babaei M, Abnous K, Taghdisi SM, Taghavi S, Sh Saljooghi A, Ramezani M, Alibolandi M. Targeted rod-shaped mesoporous silica nanoparticles for the co-delivery of camptothecin and survivin shRNA in to colon adenocarcinoma in vitro and in vivo. Eur J Pharm Biopharm. 2020;156:84-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 28. | Ovais M, Mukherjee S, Pramanik A, Das D, Mukherjee A, Raza A, Chen C. Designing Stimuli-Responsive Upconversion Nanoparticles that Exploit the Tumor Microenvironment. Adv Mater. 2020;32:e2000055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 29. | Wu J, Chen J, Feng Y, Tian H, Chen X. Tumor microenvironment as the "regulator" and "target" for gene therapy. J Gene Med. 2019;21:e3088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11:671-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1440] [Cited by in RCA: 1589] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 31. | Zhao T, Huang G, Li Y, Yang S, Ramezani S, Lin Z, Wang Y, Ma X, Zeng Z, Luo M, de Boer E, Xie XJ, Thibodeaux J, Brekken RA, Sun X, Sumer BD, Gao J. A Transistor-like pH Nanoprobe for Tumour Detection and Image-guided Surgery. Nat Biomed Eng. 2016;1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 32. | Cheng W, Zeng X, Chen H, Li Z, Zeng W, Mei L, Zhao Y. Versatile Polydopamine Platforms: Synthesis and Promising Applications for Surface Modification and Advanced Nanomedicine. ACS Nano. 2019;13:8537-8565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 552] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 33. | Xie X, Li F, Zhang H, Lu Y, Lian S, Lin H, Gao Y, Jia L. EpCAM aptamer-functionalized mesoporous silica nanoparticles for efficient colon cancer cell-targeted drug delivery. Eur J Pharm Sci. 2016;83:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 34. | Li Y, Duo Y, Bi J, Zeng X, Mei L, Bao S, He L, Shan A, Zhang Y, Yu X. Targeted delivery of anti-miR-155 by functionalized mesoporous silica nanoparticles for colorectal cancer therapy. Int J Nanomedicine. 2018;13:1241-1256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 35. | Wang J, Li Y, Nie G. Multifunctional biomolecule nanostructures for cancer therapy. Nat Rev Mater. 2021;6:766-783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 257] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 36. | Nguyen CT, Webb RI, Lambert LK, Strounina E, Lee EC, Parat MO, McGuckin MA, Popat A, Cabot PJ, Ross BP. Bifunctional Succinylated ε-Polylysine-Coated Mesoporous Silica Nanoparticles for pH-Responsive and Intracellular Drug Delivery Targeting the Colon. ACS Appl Mater Interfaces. 2017;9:9470-9483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 37. | Tian B, Liu S, Wu S, Lu W, Wang D, Jin L, Hu B, Li K, Wang Z, Quan Z. pH-responsive poly (acrylic acid)-gated mesoporous silica and its application in oral colon targeted drug delivery for doxorubicin. Colloids Surf B Biointerfaces. 2017;154:287-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 38. | Zahiri M, Babaei M, Abnous K, Taghdisi SM, Ramezani M, Alibolandi M. Hybrid nanoreservoirs based on dextran-capped dendritic mesoporous silica nanoparticles for CD133-targeted drug delivery. J Cell Physiol. 2020;235:1036-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 39. | Narayan R, Gadag S, Cheruku SP, Raichur AM, Day CM, Garg S, Manandhar S, Pai KSR, Suresh A, Mehta CH, Nayak Y, Kumar N, Nayak UY. Chitosan-glucuronic acid conjugate coated mesoporous silica nanoparticles: A smart pH-responsive and receptor-targeted system for colorectal cancer therapy. Carbohydr Polym. 2021;261:117893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 40. | Liu HJ, Luan X, Feng HY, Dong X, Yang SC, Chen ZJ, Cai QY, Lu Q, Zhang Y, Sun P. Integrated combination treatment using a “smart” chemotherapy and microrna delivery system improves outcomes in an orthotopic colorectal cancer model. Adv Funct Materi. 2018;28:1801118. |
| 41. | Kumar B, Kulanthaivel S, Mondal A, Mishra S, Banerjee B, Bhaumik A, Banerjee I, Giri S. Mesoporous silica nanoparticle based enzyme responsive system for colon specific drug delivery through guar gum capping. Colloids Surf B Biointerfaces. 2017;150:352-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 42. | Kumar B, Murali A, Bharath AB, Giri S. Guar gum modified upconversion nanocomposites for colorectal cancer treatment through enzyme-responsive drug release and NIR-triggered photodynamic therapy. Nanotechnology. 2019;30:315102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 43. | Allen SD, Liu X, Jiang J, Liao YP, Chang CH, Nel AE, Meng H. Immune checkpoint inhibition in syngeneic mouse cancer models by a silicasome nanocarrier delivering a GSK3 inhibitor. Biomaterials. 2021;269:120635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 44. | Wang ZH, Liu JM, Zhao N, Li CY, Lv SW, Hu Y, Lv H, Wang D, Wang S. Cancer Cell Macrophage Membrane Camouflaged Persistent Luminescent Nanoparticles for Imaging-Guided Photothermal Therapy of Colorectal Cancer. ACS Appl Nano Mater. 2020;3:7105. [DOI] [Full Text] |
| 45. | Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15:81-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1349] [Cited by in RCA: 2270] [Article Influence: 283.8] [Reference Citation Analysis (0)] |
| 46. | Lérida-Viso A, Estepa-Fernández A, García-Fernández A, Martí-Centelles V, Martínez-Máñez R. Biosafety of mesoporous silica nanoparticles; towards clinical translation. Adv Drug Deliv Rev. 2023;201:115049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 64] [Article Influence: 32.0] [Reference Citation Analysis (0)] |