Published online May 24, 2024. doi: 10.5306/wjco.v15.i5.635
Revised: February 8, 2024
Accepted: April 2, 2024
Published online: May 24, 2024
Processing time: 139 Days and 15.1 Hours
Although treatment options for gastric cancer (GC) continue to advance, the overall prognosis for patients with GC remains poor. At present, the predictors of treatment efficacy remain controversial except for high microsatellite instability.
To develop methods to identify groups of patients with GC who would benefit the most from receiving the combination of a programmed cell death protein 1 (PD-1) inhibitor and chemotherapy.
We acquired data from 63 patients with human epidermal growth factor receptor 2 (HER2)-negative GC with a histological diagnosis of GC at the Cancer Hospital, Chinese Academy of Medical Sciences between November 2020 and October 2022. All of the patients screened received a PD-1 inhibitor combined with chemo
As of July 1, 2023, the objective response rate was 61.9%, and the disease control rate was 96.8%. The median progression-free survival (mPFS) for all patients was 6.3 months. The median overall survival was not achieved. Survival analysis showed that patients with a combined positive score (CPS) ≥ 1 exhibited an extended trend in progression-free survival (PFS) when compared to patients with a CPS of 0 after receiving a PD-1 inhibitor combined with oxaliplatin and tegafur as the first-line treatment. PFS exhibited a trend for prolongation as the expression level of HER2 increased. Based on PFS, we divided patients into two groups: A treatment group with excellent efficacy and a treatment group with poor efficacy. The mPFS of the excellent efficacy group was 8 months, with a mPFS of 9.1 months after excluding a cohort of patients who received interrupted therapy due to surgery. The mPFS was 4.5 months in patients in the group with poor efficacy who did not receive surgery. Using good/poor efficacy as the endpoint of our study, univariate analysis revealed that both CPS score (P = 0.004) and HER2 expression level (P = 0.015) were both factors that exerted significant influence on the efficacy of treatment the combination of a PD-1 inhibitor and chemotherapy in patients with advanced GC (AGC). Finally, multivariate analysis confirmed that CPS score was a significant influencing factor.
CPS score and HER2 expression both impacted the efficacy of immunotherapy combined with chemotherapy in AGC patients who were non-positive for HER2.
Core Tip: For when considering non-positive human epidermal growth factor receptor 2 (HER2) advanced gastric cancer (AGC), combined positive score and HER2 expression are represent influencing factors for the treatment efficacy of AGC patients receiving immunotherapy combined with chemotherapy. Survival analysis with When using progression-free survival (PFS) as the end point, survival analysis suggested that the HER2 expression level was levels are suggestive of the efficacy of treatment featuring a programmed cell death protein 1 inhibitor combined with and chemotherapy. With the increase of HER2 expression, PFS also showed a tendency to prolong increase with an increase in HER2 expression.
- Citation: Ma XT, Ou K, Yang WW, Cao BY, Yang L. Human epidermal growth factor receptor 2 expression level and combined positive score can evaluate efficacy of advanced gastric cancer. World J Clin Oncol 2024; 15(5): 635-643
- URL: https://www.wjgnet.com/2218-4333/full/v15/i5/635.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i5.635
Gastric cancer (GC) is one of the most common forms of malignant tumor with the fifth highest incidence of cancer in the world; in addition, GC ranks third in terms of global fatalities from cancer, thus representing a serious threat to population health[1]. Surgical resection remains as the main radical treatment for GC at present. However, most patients with GC are in advanced stages of the disease at diagnosis; consequently, < 50% of patients can achieve R0 resection. A previous study reported that > 80% of GC patients are diagnosed at an advanced stage, and most are accompanied by extensive invasion and distant metastasis, thus missing the opportunity for radical surgery[2]. Despite the continuous progression of treatment options for GC, the overall prognosis for GC remains poor, traditional chemotherapy drugs have entered a bottleneck period, and the selection of targeted drugs is limited.
Over recent years, immune checkpoint inhibitors (ICIs), such as programmed cell death protein 1 (PD-1)/programmed cell death 1 Ligand 1 (PD-L1) inhibitors or cytotoxic T lymphocyte-associated antigen-4 inhibitors, have become the main treatment options for many types of cancer. For the first-line treatment of advanced GC (AGC), the combination of a PD-1 inhibitor and chemotherapy has gradually become the new standard mode of treatment. Multiple clinical studies have reported that approximately 85% of human epidermal growth factor receptor 2 (HER2)-negative (HER2 IHC 0 or 1+ or 2+/FISH-) patients achieved efficacious results following first-line treatment involving the combination of a PD-1 antibody and chemotherapy in the first-line treatment of AGC via the administration of ICIs and different chemotherapy regimens, when considering the comprehensive positive score (CPS) of PD-L1 expression on immunohistochemistry and different research endpoint indicators[3-6]. However, not all patients can benefit from treatment based on PD-1 inhibitors. In addition to the clear status of high microsatellite instability, the predictive value of the PD-L1 CPS score remains controversial, and other predictive factors, such as high tumor mutational burden, remain uncertain. Therefore, there is a clear need to develop a method to identify groups of patients with AGC who might benefit the most from the combination of a PD-1 inhibitor and chemotherapy.
In this study, we analyzed the efficacy of a first-line PD-1 inhibitor combined with chemotherapy in patients with AGC. We aimed to identify relevant risk factors that can predict treatment efficacy in order to provide a method to identify potential beneficiaries as early as possible and provide a basis for clinical practice.
We reviewed and collected data from 63 patients with HER2-negative patients with AGC who were diagnosed histologically at the Cancer Hospital, Chinese Academy of Medical Sciences from November 2020 to October 2022 (Table 1). All patients had proficient mismatch repair (pMMR). These patients were treated with oxaliplatin and tegafur (SOX) chemotherapy combined with tislelizumab (a PD-1 monoclonal antibody). All patients were at least 18 years of age and had measurable lesions according to the RECIST 1.1 criteria. The initial TNM staging was stage IV or recurrence and metastasis after radical surgery. Common distant metastatic sites included retroperitoneal lymph nodes, supraclavicular lymph nodes, peritoneum, liver, and ovaries. Patients with initial stage IV had not previously received anti-tumor therapy, and patients with postoperative recurrence had an interval of more than 6 months between adjuvant treatment after radical resection. The main exclusion criteria included previous chemotherapy or targeted therapy, ICI treatment history (excluding patients with recurrence and metastasis more than 6 months after adjuvant or neoadjuvant chem
| Variable | All (63) | Excellent efficacy group (42) | Poor efficacy group (21) |
| Age | 56 (51, 58) | 56 (50, 58) | 59 (50, 62) |
| Sex | |||
| Male | 34 (54.7) | 20 (47.6) | 14 (66.7) |
| Female | 29 (45.3) | 22 (52.4) | 7 (33.3) |
| ECOG-PS | |||
| 0 | 17 (27.0) | 12 (28.6) | 5 (23.8)) |
| 1 | 39 (61.9) | 26 (61.9) | 13 (61.9) |
| 2 | 7 (11.1) | 4 (9.5) | 3 (14.3) |
| Disease status | |||
| Unresectable | 55 (87.3) | 38 (90.5) | 17 (81.0) |
| Recurrent | 8 (12.7) | 4 (9.5) | 4 (19.0) |
| Number of organs involved | |||
| 1 | 40 (63.5) | 27 (64.3) | 13 (61.9) |
| ≥ 2 | 23 (36.5) | 15 (35.7) | 8 (38.1) |
| HER2 status | |||
| 0 | 31 (49.2) | 17 (40.5) | 14 (66.7) |
| 1 | 15 (23.8) | 10 (23.8) | 5 (23.8) |
| 2 | 11 (17.5) | 11 (26.2) | 0 (0) |
| Unmeasured | 6 (9.5) | 4 (9.5) | 2 (9.5) |
| CPS | |||
| 0 | 8 (12.7) | 2 (4.8) | 6 (28.6) |
| 1 ≤ CPS < 5 | 11 (17.5) | 7 (16.7) | 4 (19.0) |
| 5 ≤ CPS < 10 | 12 (19.0) | 9 (21.4) | 3 (14.3) |
| CPS ≥ 10 | 21 (33.3) | 18 (42.9) | 3 (14.3) |
| Unmeasured | 11 (17.5) | 6 (14.3) | 5 (23.8) |
| EBER | |||
| Positive | 7 (11.1) | 6 (14.3) | 1 (4.8) |
| Negative | 42 (66.7) | 26 (61.9) | 16 (76.2) |
| Unmeasured | 14 (22.2) | 10 (23.8) | 4 (19.0) |
This study was approved by the Ethics Committee of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College.
All of the selected patients received tislelizumab combined with chemotherapy every 3 wk. The specific dose of SOX regimen was 130 mg/m2 oxaliplatin (2 h intravenous infusion) on day 1, and tegafur was taken orally at doses of 80 mg, 100 mg, or 120 mg/d (< 1.25 m2, 1.25 to 1.5 m2, or > 1.5 m2) depending on body surface area, on days 1 to 14. Tislelizumab was given every 3 wk at a standard dose of 200 mg. If the combination treatment reached six cycles, we continued to maintain tegafur combined with tislelizumab after stopping oxaliplatin. The clinicians followed institutional guidelines for the use of premedication anti-emetics and growth factors. The dose of the chemotherapy regimen was reduced at the discretion of the clinicians. Some patients who were evaluated as operable by multi-disciplinary treatment after chemotherapy underwent radical gastrectomy.
We collected the following baseline demographic and clinical data: age, sex, performance status (PS) score, primary cancer site, metastatic organs, HER2 expression level, PD-L1 CPS score[7], Epstein-Barr virus-encoded small RNA (EBER) status, treatment plan and drug dosage adjustment, all of which were recorded in our medical record system.
All patients underwent tumor imaging examinations (computed tomography and/or magnetic resonance imaging) every two cycles, and were evaluated according to the response evaluation criteria in solid tumors 1.1 criteria. Objective response rate (ORR) was defined as the sum of the proportions of complete remission (CR) and partial remission (PR). Disease control rate (DCR) was defined as the proportion (%) of patients who achieved remission (PR + CR) and stable disease following treatment. Progression-free survival (PFS) was defined as the time from the beginning of chemotherapy to the date of imaging confirmation of disease progression or the date of radical surgical resection, or the time of death from any cause before disease progression (whichever came first). Overall survival (OS) was defined as the time from the beginning of chemotherapy to death due to any cause.
Data were statistically analyzed with R-language. PFS was estimated by Kaplan-Meir survival curve analysis. The Cox proportional risk model was used to estimate the risk ratio. All statistical tests were bidirectional, and P value of < 0.05 was considered significant. Logistic regression model analysis was performed to determine whether gender, age, PS score, PD-L1 CPS score, HER2 expression, EBER expression, and the number of distant metastasized organs were risk factors for efficacy. The Kruskal-Wallis test was used to analyze the variance of all patients after two cycles of treatment.
The clinical and pathological features of the patients included in this study are shown in Table 1. As of July 1, 2023, all patients had been followed-up for more than 6 months from enrollment to date. A total of 63 patients were included in this study, including 34 males and 29 females. The initial diagnosis was stage IV in 56 patients and postoperative recurrence occurred in seven patients. The included patients ranged in age from 27 to 76 years, with a mean and median age of 54 and 56 years, respectively. PS score ranged from 0 to 2. Distant sites of metastasis mainly included the liver, peritoneum, ovary and distant lymph nodes. pMMR status was determined by immunohistochemistry. HER2 was either not expressed or expressed at low levels (1+/2+ and FISH was not amplified). EBER status and PD-L1 CPS score were also determined by immunohistochemistry.
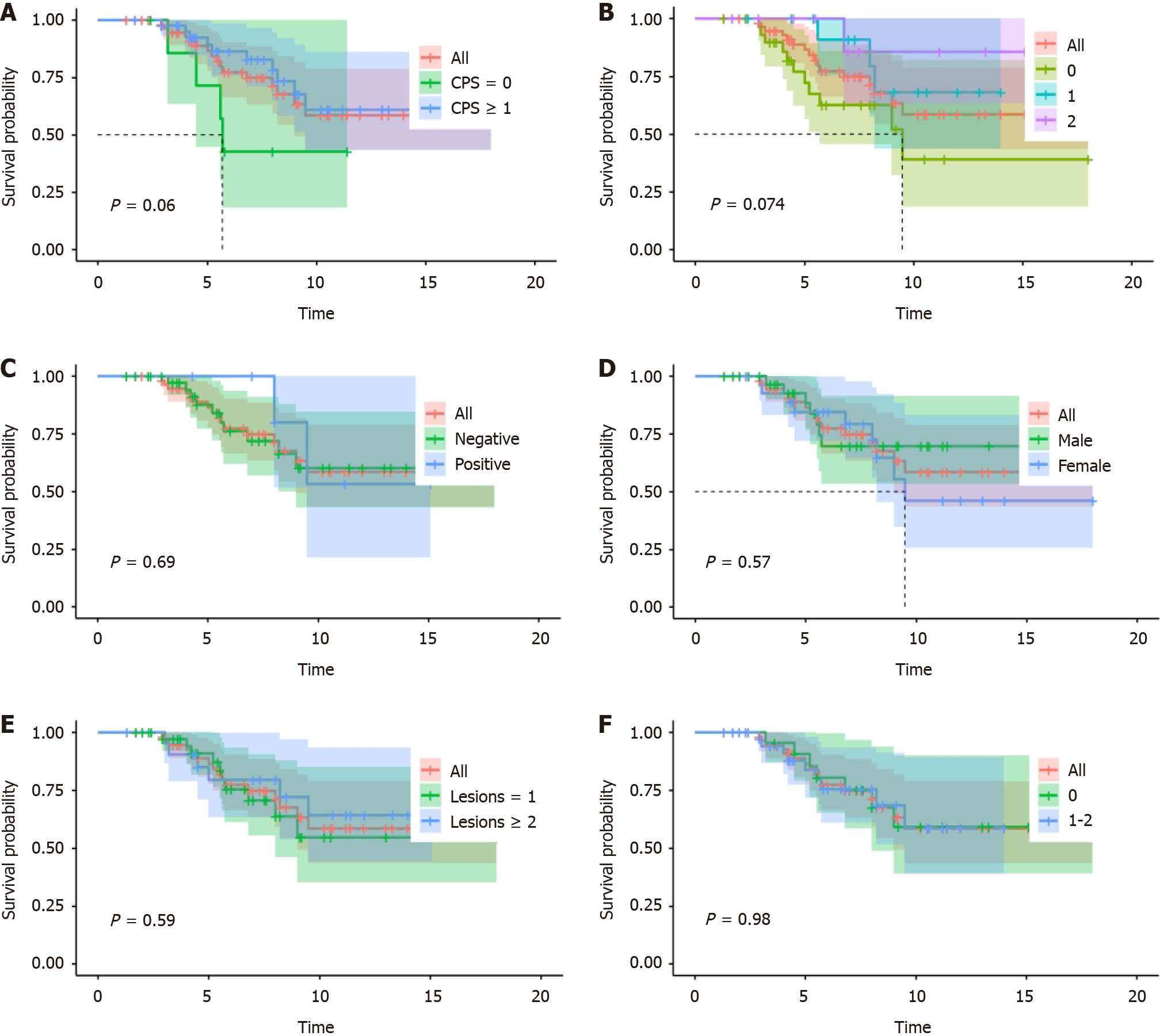
As of the July 1, 2023, the numbers of patients experiencing disease progression and death were 40 and 17, respectively. The ORR was 61.9% (range: 48.8%-73.4%) and the DCR was 96.8% (range: 89.0%-99.6%). The median PFS (mPFS) for the entire cohort was 6.3 months (range: 6.0-7.8 months). The median OS was not attained. Survival analysis was performed for patient age, sex, PS score, PD-L1 CPS score, HER2 expression, EBER status, and number of metastatic organs (Figure 1). Analysis showed that patients with a CPS ≥ 1 showed an extended trend in PFS when compared to the population of patients with a CPS score of 0 after receiving a PD-1 inhibitor combined with chemotherapy as the first-line treatment (P = 0.06). PFS exhibited a trend for prolongation as the expression level of HER2 increased (P = 0.074).
We divided patients into two groups based on their mPFS: A treatment group with excellent efficacy and a treatment group with poor efficacy. The excellent efficacy group included patients with a PFS > 6.3 months, or those who met surgical standards (the primary lesion was resectable and the metastases were indistinct) within 6.3 months and achieved radical resection. The poor efficacy group included patients with a PFS not exceeding 6.3 months or those who died from any cause within 6.3 months. The two sets of baseline characteristics are shown in Table 1.
The mPFS of the excellent efficacy group was 8 months (range: 6.9-9.2 months and 16 patients underwent surgery in this group. Excluding those who experienced an interruption of immunotherapy due to surgery, the remaining 26 patients had a mPFS of 9.1 months (range: 8.8-11.1 months). Patients in the group with poor efficacy who did not receive surgery had a mPFS of 4.5 months (range: 3.7-4.9 months).
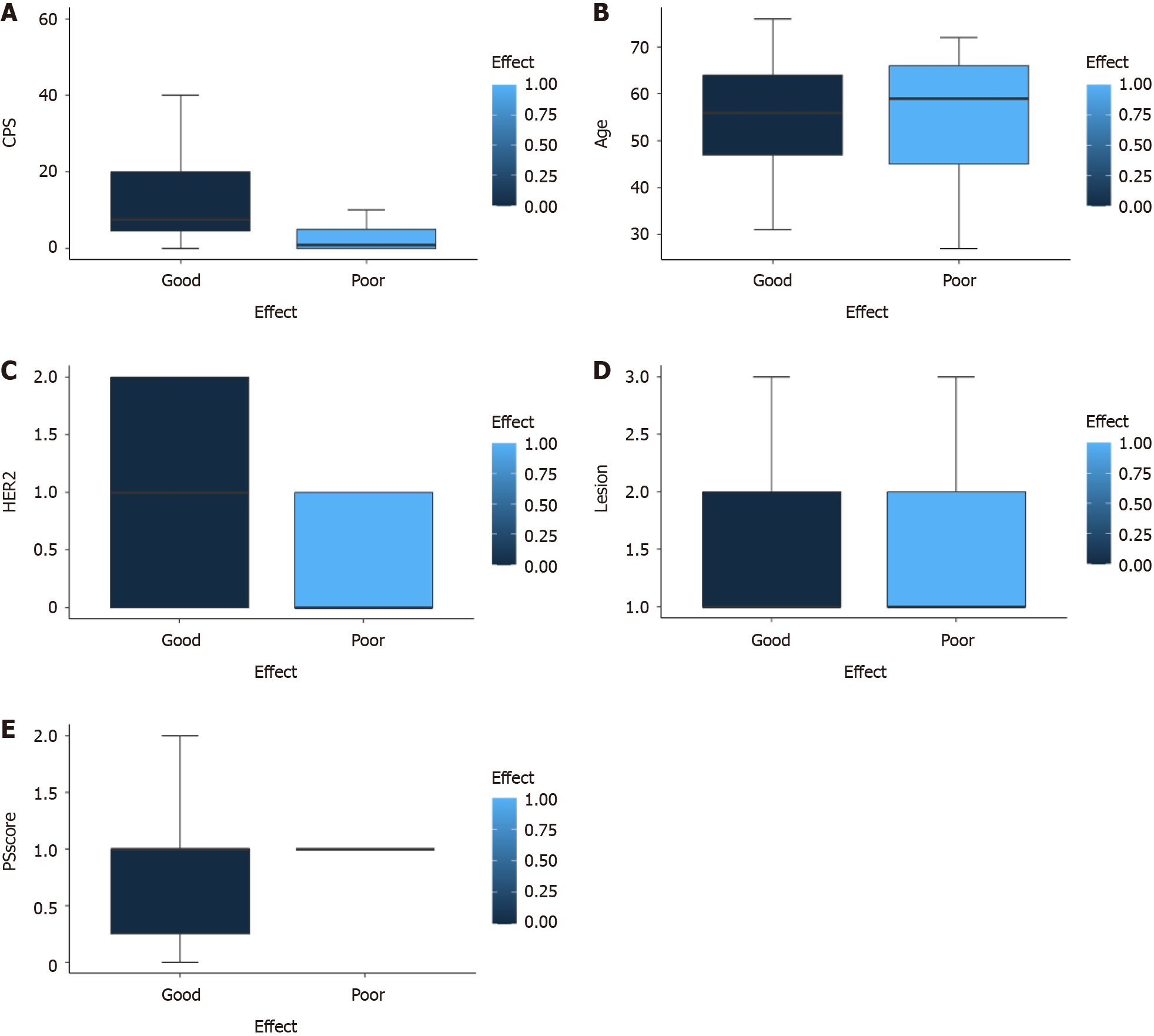
Next, we attempted to identify risk factors by analyzing patient age, sex, PS score, PD-L1 CPS score, HER2 expression, EBER status, and the number of metastatic organs. Univariate analysis revealed that age, sex, PS score, EBER status, and number of metastatic organs were not significant factors influencing the efficacy of AGC patients receiving a combination of a PD-1 inhibitor and chemotherapy. However, CPS score (P = 0.004) and HER2 expression (P = 0.015) were identified as factors influencing the efficacy of AGC patients receiving a combination of a PD-1 inhibitor and chemotherapy (Figure 2). The therapeutic effect of a CPS ≥ 1 was significantly better than that of a CPS = 0, and the therapeutic effect of a CPS ≥ 5 was significantly better than that of a CPS < 5. However, no significant difference in efficacy was detected between the two groups with a CPS ≥ 10 when compared with patients with a CPS < 10. The higher the expression of HER2, the better the therapeutic effect. Multivariate analysis further suggested that CPS score was a factor influencing the efficacy of AGC patients receiving a combination of a PD-1 inhibitor and chemotherapy, further confirming the role of CPS score in predicting the efficacy of immunotherapy in GC patients (Supplementary material).
Finally, we analyzed the objective efficacy of all patients after two cycles of treatment, with excellent/poor efficacy as the research endpoint. Of the 42 patients in the excellent efficacy group, 30 patients achieved PR after two cycles of treatment, with an ORR of 71.4% (range: 55.4%-84.3%). Of the 21 individuals in the poor efficacy group, nine achieved PR after two cycles of treatment, with an ORR of 42.9% (range: 21.8%-66.0%). There was a significant difference between the two groups in terms of ORR (P = 0.029) (Table 2). These findings suggested that patients who achieved PR in the first efficacy evaluation after two cycles of treatment were more likely to experience PFS for more than 6 months or have the opportunity for radical surgery.
| Group A (N = 42) | Group B (N = 21) | P value | |||
| n (%) | 95%CI | n (%) | 95%CI | ||
| Best overall response | |||||
| Complete response (CR) | 0 | - | 0 | - | - |
| Partial response (PR) | 30 (71.4) | - | 9 (42.9) | - | - |
| Stable disease (SD) | 12 (28.6) | - | 10 (47.6) | - | - |
| Progressive disease | 0 (0) | - | 2 (9.5) | - | - |
| Overall response rate (CR + PR) | 30 (71.4) | 55.4-84.3 | 9 (42.9) | 21.8-66.0 | 0.03 |
| Disease control rate (CR + PR + SD) | 42 (100) | 91.6-1.00 | 19 (90.5) | 69.6-98.8 | 2.527e-13 |
In this study, we found that HER2-negative, pMMR AGC, patients with a CPS ≥ 1 experienced significantly better treatment efficacy and a longer PFS when compared to those with a CPS of 0. Furthermore, PFS also exhibited a trend for prolongation as the expression level of HER2 increased.
HER2 is an important member of the ERBB family of receptors encoded by the ERBB2 gene, and can bind to other family members to form heterodimers and transmit proliferation and survival signals to cells by activating the downstream RAS-RAF-MEK-ERK or P13K-AKT-mTOR pathways. When cells overexpress HER2 or exert increased functionality, excessive cell proliferation signals can be transmitted downstream; this can lead to tumor formation[8]. At present, the prognostic value of HER2 status in GC remains controversial. Some studies have reported that HER2 positivity is a poor prognostic factor, while other studies have linked HER2 to better survival; other studies have reported that there is no association between HER2 and patient survival[9-11]. Continuous advancement in the field of precision therapy and the advent of ADC drugs has redefined HER2 expression into different classifications: HER2-negative (IHC 0), HER2-low expression (IHC 1+/2+ and FISH negative) and HER2-positive (IHC 3+ or 2+ and FISH positive). Zhang et al[12] analyzed the clinical characteristics and prognosis of these HER2 expression subtypes in breast cancer and found that when compared with patients who were HER2-negative, those with low levels of HER2 expression were more inclined to express low levels of Ki67 and have a better trend for long-term survival. Previous data analysis of 2310 non-HER2-amplified patients showed that breast cancer patients with low levels of HER2 expression were significantly different from those who were HER2-negative in terms of biological behavior, clinicopathological features, treatment response, and clinical outcomes[13]. According to previous studies, low levels of HER2 expression account for 40% to 60% of all GC patients[14]. In a study involving patients with stage III GC, Gao et al[15] reported that the prognosis of patients with an HER2 score of 1 or 2+ was significantly better than that of patients with an HER2 score of 0 or 3+. In addition, most previous studies focused on the relationship between HER2 and tumor cell growth and apoptosis; relatively few studies have been conducted on the role of HER2 with respect to the immune cycle of tumors and the role of immunotherapy.
The tumor immune microenvironment is composed of various immune cells and immune-active substances. Tumor infiltrating lymphocytes (TILs), macrophages, and neutrophils are the main immune cells that are found in the tumor microenvironment. PD-1/PD-L1 is considered an important pathway for immune escape in tumor cells. Changes in the immune microenvironment play an important role in the recurrence and metastasis of GC patients, and PD-L1 positivity has also been proven to be sensitive to immunotherapy. The immune response of the body to tumors mainly involves CD8+T cells, which infiltrate into tumor tissue and participate in the tumor immune cycle[16,17]. In the early stages of disease, the body can clear tumors by immune monitoring functionality; tumor cells can also suppress the immune system by immune editing functionality, and even evade immune system attacks via a range of different mechanisms[18]. No studies have directly analyzed the tumor-associated immune environment associated with low levels of HER2 expression and HER2-negative expression. However, a previous study involving breast ductal carcinoma in situ (DCIS), researchers found that the densities of intraepithelial lymphocytes, CD3+T cells, CD3+CD8 T cells, CD3+FOXP3+T cells and CD8+Ki67+T cells in HER2 positive DCIS were significantly higher than those of immune cell subsets in HER2- negative DCIS (P < 0.05)[19]. In another study, Mutka et al[20] reported that HER2-positive tumors had more TILs.
In the present study, the included population were all HER2-negative or HER2-low expression patients. We conducted subgroup analysis of our patient population. Multivariate analysis demonstrated that the expression levels of HER2 did not present an independent risk factor. However, survival analysis, using PFS as the endpoint, still indicated the predictive effect of HER2 expression on the efficacy of treatments involving a PD-1 inhibitor combined with chemotherapy. However, some previous reports contradicted our current findings. Some studies have found that HER2 bound to the carboxyl end tail of STING, and inhibited TBKL activity by phosphorylating specific positions of AKTl, thus preventing STING from binding to TBKL and inhibiting anti-tumor immune activity. These findings suggest that the level and activity of HER2 in tumor cells may also be a decisive factor in controlling immune perception ability[21-23]. Therefore, the relationship between HER2 expression level and the tumor immune environment needs to be further investigated by clinical research and basic scientific experiments with larger data sets.
Multiple large phase III randomized controlled studies have confirmed the efficacy of immunotherapy combined with chemotherapy for non-positive HER2 AGC; the most recent published data state that the mPFS is around 7 months[3-6]. Our study differed from other studies in that we directly used the PFS of the patient population as the cut-off point and the outcome endpoint as the binary variable to analyze efficacy. In addition, we also analyzed efficacy for all patients receiving two cycles of treatment to determine the predictive value of the first efficacy evaluation after treatment for subsequent efficacy.
There were some limitations to this study that need to be considered. First, the small sample size of patients and the lack of sufficient universality in a study based in a single center made it difficult to generalize our findings. Second, the small sample size caused by sub-grouping limited further subgroup analysis. In the future, we will conduct a multi-center study with a larger cohort to further validate our findings.
Analysis showed that CPS score and HER2 expression were both influencing factors for the efficacy of AGC patients who were non-positive for HER2 and receiving immunotherapy combined with chemotherapy. Multivariate analysis further confirmed the role of CPS score in predicting the efficacy of immunotherapy in patients with GC. Survival analysis, using PFS as the end point, suggested that the level of HER2 expression was suggestive of the efficacy of treatment involving the combination of a PD-1 inhibitor and chemotherapy. Finally, PFS showed a tendency to increase with an increased expression level of HER2.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Soldera J, Brazil S-Editor: Qu XL L-Editor: A P-Editor: Yuan YY
| 1. | de Mello RA, Amaral GA, Neves NM, Lippo EG, Parini F, Xu S, Tolia M, Charalampakis N, Tadokoro H, Castelo-Branco P, Zhu J. Current and potential biomarkers in gastric cancer: a critical review of the literature. Future Oncol. 2021;17:3383-3396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Cervantes A, Roda D, Tarazona N, Roselló S, Pérez-Fidalgo JA. Current questions for the treatment of advanced gastric cancer. Cancer Treat Rev. 2013;39:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 3. | Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J, Castro HR, Mansoor W, Braghiroli MI, Karaseva N, Caglevic C, Villanueva L, Goekkurt E, Satake H, Enzinger P, Alsina M, Benson A, Chao J, Ko AH, Wainberg ZA, Kher U, Shah S, Kang SP, Tabernero J. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6:1571-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 867] [Article Influence: 173.4] [Reference Citation Analysis (1)] |
| 4. | Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 1898] [Article Influence: 474.5] [Reference Citation Analysis (1)] |
| 5. | Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, Chung IJ, Yamaguchi K, Kato K, Sym SJ, Kadowaki S, Tsuji K, Chen JS, Bai LY, Oh SY, Choda Y, Yasui H, Takeuchi K, Hirashima Y, Hagihara S, Boku N. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23:234-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 487] [Article Influence: 162.3] [Reference Citation Analysis (0)] |
| 6. | Moehler MH, Kato K, Arkenau H, Oh D, Tabernero J, Cruz-Correa M, Wang H, Xu H, Li J, Yang S, Xu R. Rationale 305: phase 3 study of tislelizumab plus chemotherapy vs placebo plus chemotherapy as first-line treatment (1L) of advanced gastric or gastroesophageal junction adenocarcinoma (GC/GEJ). J Clin Oncol. 2023;41 Suppl 4:286. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Neuman T, London M, Kania-Almog J, Litvin A, Zohar Y, Fridel L, Sandbank J, Barshak I, Vainer GW. A Harmonization Study for the Use of 22C3 PD-L1 Immunohistochemical Staining on Ventana's Platform. J Thorac Oncol. 2016;11:1863-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 8. | Pollock NI, Grandis JR. HER2 as a therapeutic target in head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Oh N, Kim H, Kim KM, Cheong JH, Lee J, Noh SH, Sohn TS, Choi YY, An JY. Microsatellite Instability and Effectiveness of Adjuvant Treatment in pT1N1 Gastric Cancer: A Multicohort Study. Ann Surg Oncol. 2021;28:8908-8915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Janjigian YY, Werner D, Pauligk C, Steinmetz K, Kelsen DP, Jäger E, Altmannsberger HM, Robinson E, Tafe LJ, Tang LH, Shah MA, Al-Batran SE. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: a European and USA International collaborative analysis. Ann Oncol. 2012;23:2656-2662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 268] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 11. | Kataoka Y, Okabe H, Yoshizawa A, Minamiguchi S, Yoshimura K, Haga H, Sakai Y. HER2 expression and its clinicopathological features in resectable gastric cancer. Gastric Cancer. 2013;16:84-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Zhang G, Ren C, Li C, Wang Y, Chen B, Wen L, Jia M, Li K, Mok H, Cao L, Chen X, Lin J, Wei G, Li Y, Zhang Y, Balch CM, Liao N. Distinct clinical and somatic mutational features of breast tumors with high-, low-, or non-expressing human epidermal growth factor receptor 2 status. BMC Med. 2022;20:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 88] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 13. | Denkert C, Seither F, Schneeweiss A, Link T, Blohmer JU, Just M, Wimberger P, Forberger A, Tesch H, Jackisch C, Schmatloch S, Reinisch M, Solomayer EF, Schmitt WD, Hanusch C, Fasching PA, Lübbe K, Solbach C, Huober J, Rhiem K, Marmé F, Reimer T, Schmidt M, Sinn BV, Janni W, Stickeler E, Michel L, Stötzer O, Hahnen E, Furlanetto J, Seiler S, Nekljudova V, Untch M, Loibl S. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021;22:1151-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 332] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 14. | Moelans CB, Milne AN, Morsink FH, Offerhaus GJ, van Diest PJ. Low frequency of HER2 amplification and overexpression in early onset gastric cancer. Cell Oncol (Dordr). 2011;34:89-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Gao X, Zhao L, Zhang N, Han W, Liu K, Yan J, Chen L, Pan Y, Li R, Li W, Zhang H, Li H, Wang S, Gao X, Niu P, Wang W, Ji G, Zhao Q, Lu Y, Li Z, Shang L, Liang H, Wu K, Deng J, Chen Y, Nie Y; MAGIS Study Group. Impact of HER2 on prognosis and benefit from adjuvant chemotherapy in stage II/III gastric cancer patients: a multicenter observational study. Int J Surg. 2023;109:1330-1341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3297] [Cited by in RCA: 4772] [Article Influence: 397.7] [Reference Citation Analysis (1)] |
| 17. | Kim JM, Chen DS. Immune escape to PD-L1/PD-1 blockade: seven steps to success (or failure). Ann Oncol. 2016;27:1492-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 478] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 18. | Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1617] [Cited by in RCA: 1499] [Article Influence: 107.1] [Reference Citation Analysis (0)] |
| 19. | Almekinders MM, Bismeijer T, Kumar T, Yang F, Thijssen B, van der Linden R, van Rooijen C, Vonk S, Sun B, Parra Cuentas ER, Wistuba II, Krishnamurthy S, Visser LL, Seignette IM, Hofland I, Sanders J, Broeks A, Love JK, Menegaz B, Wessels L, Thompson AM, de Visser KE, Hooijberg E, Lips E, Futreal A, Wesseling J; Grand Challenge PRECISION Consortium. Comprehensive multiplexed immune profiling of the ductal carcinoma in situ immune microenvironment regarding subsequent ipsilateral invasive breast cancer risk. Br J Cancer. 2022;127:1201-1213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Mutka M, Joensuu K, Eray M, Heikkilä P. Quantities of CD3+, CD8+ and CD56+ lymphocytes decline in breast cancer recurrences while CD4+ remain similar. Diagn Pathol. 2023;18:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Wu S, Zhang Q, Zhang F, Meng F, Liu S, Zhou R, Wu Q, Li X, Shen L, Huang J, Qin J, Ouyang S, Xia Z, Song H, Feng XH, Zou J, Xu P. HER2 recruits AKT1 to disrupt STING signalling and suppress antiviral defence and antitumour immunity. Nat Cell Biol. 2019;21:1027-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 184] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 22. | Shu C, Sankaran B, Chaton CT, Herr AB, Mishra A, Peng J, Li P. Structural insights into the functions of TBK1 in innate antimicrobial immunity. Structure. 2013;21:1137-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Li S, Wang L, Berman M, Kong YY, Dorf ME. Mapping a dynamic innate immunity protein interaction network regulating type I interferon production. Immunity. 2011;35:426-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 282] [Article Influence: 20.1] [Reference Citation Analysis (0)] |