Published online May 24, 2024. doi: 10.5306/wjco.v15.i5.614
Peer-review started: December 27, 2023
First decision: January 17, 2024
Revised: February 12, 2024
Accepted: March 28, 2024
Article in press: March 28, 2024
Published online: May 24, 2024
Processing time: 145 Days and 19.3 Hours
Lung cancer (LC) is the leading cause of morbidity and mortality among mali
To evaluate the morphological features and clinical significance of tumor MVs in lung squamous cell carcinoma (LUSC).
A single-center retrospective cohort study examined medical records and archival paraffin blocks of 62 and 180 patients with stage I-IIIA LUSC in the training and main cohorts, respectively. All patients underwent radical surgery (R0) at the Orenburg Regional Cancer Clinic from May/20/2009 to December/14/2021. Tumor sections were routinely processed, and routine Mayer's hematoxylin and eosin staining and immunohistochemical staining for cluster of differentiation 34 (CD34), podoplanin, Snail and hypoxia-inducible factor-1 alpha were performed. The morphological features of different types of tumor MVs, tumor parenchyma and stroma were studied according to clinicopathological characteristics and LUSC prognosis. Statistical analysis was performed using Statistica 10.0 software. Univariate and multivariate logistic regression analyses were performed to identify potential risk factors for LUSC metastasis to regional lymph nodes (RLNs) and disease recurrence. Receiver operating characteristic curves were constructed to discriminate between patients with and without metastases in RLNs and those with and without disease recurrence. The effectiveness of the predictive models was assessed by the area under the curve. Survival was analyzed using the Kaplan-Meier method. The log-rank test was used to compare survival curves between patient subgroups. A value of P < 0.05 was considered to indicate statistical significance.
Depending on the morphology, we classified tumor vessels into the following types: normal MVs, dilated capillaries (DCs), atypical DCs, DCs with weak expression of CD34, "contact-type" DCs, structures with partial endothelial linings, capillaries in the tumor solid component and lymphatic vessels in lymphoid and polymorphocellular infiltrates. We also evaluated the presence of loose, fine fibrous connective tissue (LFFCT) and retraction clefts in the tumor stroma, tumor spread into the alveolar air spaces (AASs) and fragmentation of the tumor solid component. According to multivariate analysis, the independent predictors of LUSC metastasis in RLNs were central tumor location (P < 0.00001), the presence of retraction clefts (P = 0.003), capillaries in the tumor solid component (P = 0.023) and fragmentation in the tumor solid component (P = 0.009), whereas the independent predictors of LUSC recurrence were tumor grade 3 (G3) (P = 0.001), stage N2 (P = 0.016), the presence of LFFCT in the tumor stroma (P < 0.00001), fragmentation of the tumor solid component (P = 0.0001), and the absence of tumor spread through the AASs (P = 0.0083).
The results obtained confirm the correctness of our previously proposed classification of different types of tumor vessels and may contribute to improving the diagnosis and treatment of LUSC.
Core Tip: In this retrospective study, we examined the morphology of different types of tumor microvessels, tumor parenchyma, and tumor stroma and their associations with the risk of regional metastasis and disease recurrence in lung squamous cell carcinoma (LUSC) patients. Independent predictors of LUSC metastases in regional lymph nodes were the central location of the tumor (P < 0.00001), the presence of retraction clefts (P = 0.003), capillaries in the solid component of the tumor (P = 0.023) and fragmentation of the solid component of the tumor (P = 0.009), while independent predictors of LUSC recurrence were tumor grade 3 (P = 0.001), N2 stage (P = 0.016), the presence of loose fine fibrous connective tissue in the tumor stroma (P < 0.00001), fragmentation of the tumor solid component (P = 0.0001) and the absence of tumor spread through the alveolar air spaces (P = 0.0083). These findings may help improve the diagnosis and treatment of LUSC.
- Citation: Senchukova MA, Kalinin EA, Volchenko NN. Different types of tumor microvessels in stage I-IIIA squamous cell lung cancer and their clinical significance. World J Clin Oncol 2024; 15(5): 614-634
- URL: https://www.wjgnet.com/2218-4333/full/v15/i5/614.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i5.614
Lung cancer (LC) is the leading cause of morbidity and mortality among malignant neoplasms[1,2]. There are two main histological forms of LC: Non-small cell LC (NSCLC) and small cell LC (SCLC). NSCLC accounts for up to 80% of all LC cases[3], 20% to 67.5% of which are lung squamous cell carcinomas (LUSC)[4-6]. Despite a decrease in the incidence of NSCLC, mortality rates remain very high and are associated with late diagnosis, an aggressive course of the disease even in the early stages, and the presence of severe comorbidities in patients older than 60 years; these conditions prevail among patients with NSCLC, and radical treatment is often impossible for these patients.
Although both LUSC and lung adenocarcinoma are classified as NSCLC, increasing evidence suggests that these cancers have different origins, different mutational profiles, different biological behaviors, different sensitivities to drugs and radiation therapy (RT), and different disease prognoses[4,7,8]. The development of LUSC is preceded by squamous metaplasia of the bronchial epithelium, which is closely associated with smoking. In addition, alcohol consumption; infections such as human papillomavirus and Epstein–Barr virus[4,7]; and environmental pollution also increase the risk of LUSC[9]. LUSC can have both central and peripheral localization and is characterized by a high rate of genetic mutations, chromosomal instability, and a high degree of cellular heterogeneity[7,10]. Late diagnosis and the lack of treatment methods specific for this disease determine the high mortality rate from this pathology, which is almost 30% greater than the mortality rate from lung adenocarcinoma[4,8].
The choice of optimal treatment for LUSC, determining the indications for neoadjuvant and adjuvant therapy, is based on an assessment of the risk of disease relapse[11,12]. Currently, this assessment is based on disease stage, histopathological features and genetic alterations. However, for LUSC patients with the same clinicopathological and molecular genetic characteristics, the response to treatment and survival rates can differ significantly[11,13,14]. Thus, the search for new prognostic and predictive markers of LUSC has not lost relevance.
Angiogenesis is one of the key factors in tumor progression[15,16]. Its activation is associated with hypoxia, inflammatory processes, epithelial–mesenchymal transition (EMT), the formation of the phenotype of stem cells, and other factors[16-18]. Assessment of angiogenesis activity is currently considered the most important factor associated with disease prognosis and therapeutic effectiveness[16,19,20].
Previously, in gastric cancer, breast cancer, and squamous cell carcinoma of the cervix, we proposed a classification of tumor microvessels (MVs) based on their morphology and correlations with the clinical and pathological characteristics and prognosis of these diseases[21-23]. The aim of this study was to evaluate the morphological features and clinical significance of various types of tumor MVs, as well as tumor parenchymal and stromal components, in LUSC.
This single-center retrospective cohort study of the "case–control" type was conducted in accordance with the Helsinki Declaration and internationally recognized guidelines after receiving study approval from the Ethics Committee of Orenburg State Medical University (No. 281, dated 30 September 2021). One hundred eighty archived paraffin blocks of patients with LUSC stages I-IIIA were retrieved from the tumor bank of the Orenburg Regional Cancer Clinic. All patients underwent radical surgery (R0) at this clinic from May/20/2009 to December/14/2021. None of the patients included in the study underwent preoperative chemotherapy (ChT) or RT. The patients did not receive steroids, nonsteroidal anti-inflammatory drugs, or antihistamines and had no significant comorbid pathologies in the deco
The age of the patients was 61.6 ± 6.7 years (median 62 years). There were 174 men and 6 women. The right lung was affected in 93 (51.7%) patients, and the left lung was affected in 87 patients (48.3%). The tumor was localized in the upper lobe in 102 (56.7%) patients, in the middle lobe in 5 (2.8%), in the lower lobe in 49 (27.2%), and in the main bronchus in 24 (13.3%).
To study the features of the tumor MV morphology and their correlations with clinical and morphological characteristics and disease prognosis, 20 archived paraffin blocks of patients with stage I LUSC, 22 with stage II LUSC and 20 with stage IIIA LUSC were randomly selected. The patients composed the training cohort. There were no differences in age, type, comorbidity status, histology or tumor grade between the main and training groups of patients, while differences in the volume of surgery and adjuvant therapy were because, in the main cohort, there was a greater percentage of patients with locally advanced LUSC. In addition, it should be noted that in the training cohort, a greater percentage of patients received postoperative radiotherapy as an adjuvant treatment. This is because patients in this group received treatment between 2009 and 2013. During this period, radiotherapy was most commonly used as an adjuvant treatment for LUSC. However, over the past 10 years, ChT or chemoradiotherapy has been used most often as an adjuvant treatment for LC. The baseline patient clinicopathological and treatment information is shown in Table 1.
| Clinical and pathological data | Patient groups | Significance level (P value) | |||
| Main cohort (n = 180) | Training cohort (n = 62) | ||||
| n | % | n | % | ||
| Age (yr) | |||||
| < 60 | 60 | 33.3 | 20 | 32.3 | 0.897 |
| 60-69 | 98 | 54.5 | 33 | 53.2 | |
| ≥ 70 | 22 | 12.2 | 9 | 14.5 | |
| Tumor location | |||||
| Central | 119 | 66.1 | 38 | 61.3 | 0.493 |
| Peripheral | 61 | 33.9 | 24 | 38.7 | |
| Comorbidity | |||||
| Absence | 22 | 12.2 | 8 | 12.3 | 0.888 |
| Presence | 158 | 87.8 | 54 | 87.7 | |
| Histology | |||||
| KSCC | 56 | 31.1 | 21 | 33.9 | 0.687 |
| NKSCC | 124 | 68.9 | 41 | 66.1 | |
| Tumor grade | |||||
| G1 | 57 | 31.7 | 23 | 37.1 | 0.325 |
| G2 | 88 | 48.9 | 32 | 51.6 | |
| G3 | 35 | 19.4 | 7 | 11.3 | |
| T stage | |||||
| T1a | 9 | 5.0 | 8 | 12.9 | 0.039a |
| T1b | 15 | 8.3 | 6 | 9.7 | |
| T2a | 82 | 45.6 | 34 | 54.8 | |
| T2b | 17 | 9.4 | 5 | 8.1 | |
| T3 | 57 | 31.7 | 9 | 14.5 | |
| N stage | |||||
| N0 | 48 | 26.7 | 22 | 35.5 | 0.411 |
| N1 | 88 | 48.9 | 26 | 41.9 | |
| N2 | 44 | 24.4 | 14 | 22.6 | |
| Stage | |||||
| I | 30 | 16.7 | 20 | 32.3 | 0.03a |
| II | 73 | 40.6 | 22 | 32.4 | |
| III | 77 | 42.8 | 20 | 32.3 | |
| Type of surgery | |||||
| Lobectomy | 84 | 46.7 | 40 | 64.4 | 0.001a |
| Bilobectomy | 6 | 3.3 | 5 | 8.1 | |
| Pneumonectomy | 86 | 47.8 | 13 | 21.0 | |
| Segmentectomy | 4 | 2.2 | 4 | 6.5 | |
| Adjuvant therapy | |||||
| Absent | 51 | 28.3 | 27 | 43.5 | 0.002a |
| Chemotherapy | 68 | 37.8 | 10 | 16.1 | |
| Radiation therapy | 38 | 21.1 | 21 | 33.9 | |
| Chemo- and radiotherapy | 23 | 12.8 | 4 | 6.5 | |
During the observation period, 27 (15.0%) patients were diagnosed with cancer in other locations: Prostate cancer in 12 (6.7%), nasal mucosa cancer in 4 (2.2%), skin cancer in 7 (3.9%), bladder cancer in 1 (0.6%), laryngeal cancer in 1 (0.6%), breast cancer in 1 (0.6%), and colon cancer in 1 (0.6%).
As of January/31/2023, 84 (46.7%) patients were alive. LUSC recurrence was diagnosed in 95 (52.8%) patients, 83 of whom died from disease progression; 13 (7.2%) patients with LC died from nononcological pathology. LUSC recurrence was local in 44 (24.4%) patients, systemic in 41 (22.7%), and local and systemic in 7 (3.9%). In particular, metastases to the lungs and pleura were detected in 30 (16.7%) patients; to the mediastinum, 15 (8.3%); to the liver, 10 (5.6%); to the brain, 5 (2.8%); to the cervical lymph nodes, 3 (1.7%); and to the bones, 6 (3.3%). Multiple metastases were detected in 24 (13.3%) patients. Forty-four patients received 1 to 4 courses of mono- or polychemotherapy due to LC recurrence. Eight patients with LC relapse continued to receive monochemotherapy.
In 2020-2022, six patients were treated for COVID-19, and one patient died from a new coronavirus infection. The median follow-up period was 66 months for patients in the main cohort and 72 months for patients in the training cohort.
Sections (4 μm) were cut on a microtome and transferred to glass slides (SuperFrost® Plus, Menzel, Thermo Scientific, United States). Sections were stained with Mayer's hematoxylin and eosin (H&E) and studied via light microscopy (Levenhuk D740T digital microscope connected to a 5.1 MP camera, Russia). At 200 × magnification, the presence or absence of loose, fine fibrous connective tissue (LFFCT) in the tumor stroma, fragmentation in the tumor solid com
The morphology of the tumor MVs was studied via immunohistochemical staining of histological sections with antibodies against cluster of differentiation 34 (CD34) and podoplanin (PDPN). For immunohistochemistry (IHC), 4-µm sections were stained with the following antibodies: Monoclonal antibody against CD34, 1:100 dilution; polyclonal antibody against PDPN, 1:50 dilution; polyclonal antibody against Snail homolog 1 (SNAI1), 1:100 dilution; and polyclonal antibody against hypoxia-inducible factor-1 alpha (HIF-1a), 1:200 dilution (Cloud-Clone Corp®, Texas, United States). Staining with antibodies against SNAI1 and HIF-1a was performed when fragmentation in the solid component of the tumor was detected in the test samples. The staining procedure was performed according to the manufacturers' protocols as described previously[23]. For the negative control sections, the primary antibodies were replaced with phosphate-buffered saline, and the sections were processed in the same manner. All sections were carefully and completely scanned by two of the authors (Evgeniy A Kalinin and Marina A Senchukova) without knowledge of the clinical and pathological data. The MV density (MVD) was assessed by counting the number of CD34-positive and PDPN-positive normal MVs in accordance with the international consensus on the methodology and criteria for the quantitative evaluation of angi
The density of dilated capillaries (DCs), atypical DCs (ADCs) and structures with a partial endothelial lining (SPELs) was estimated at low magnification (× 200) in the three fields of view in the selected "hot spots". The presence or absence of DCs with weak expression of CD34, contact-type DCs, lymphatic vessels (LVs) in lymphoid and polymorphic cell infiltrates and tumor emboli in CD34-positive and PDPN-positive vessels was also assessed in the tumor samples.
Statistical analysis was performed using Statistica 10.0 software. Quantitative data are presented as the mean ± SD, while categorical variables are presented as numbers and percentages (n, %). Correlations between different data were evaluated using the nonparametric Spearman's rank correlation or gamma correlation. Chi-square tests were carried out to analyze the differences in distribution among the categorized data. The Mann–Whitney U nonparametric test was used to compare the values of the quantitative data. Both univariate and multivariate logistic regression analyses were performed to identify potential risk factors for LUSC metastasis to regional lymph nodes (RLNs) and disease recurrence. Receiver operating characteristic (ROC) curves were constructed to discriminate between patients with and without LUSC metastases in the RLNs and those with and without LUSC recurrence. The best threshold (cutoff) values were determined by the largest Youden's index (J = sensitivity + specificity - 1). The effectiveness of the predictive models was assessed by the area under the curve (AUC). Survival was analyzed using the Kaplan–Meier method. The log-rank test was used to compare survival curves between patient subgroups. A value of P < 0.05 was considered to indicate statistical significance.
Previously, in squamous cell carcinoma of the cervix, we proposed classifying tumor MVs into eight types according to their size, shape, clarity of contours, localization, characteristics of the endothelial lining, intensity of CD34 and PDPN staining, and content of the MV lumen[23]. The types of MVs described included normal MVs, DCs, ADCs, DCs with weak CD34 expression, contact-type DCs, SPELs, capillaries in the tumor solid component, and LVs in lymphoid and polymorphocellular infiltrates. These tumor MVs not only differed in morphology but were also associated with different clinical and morphological characteristics of squamous cell carcinoma of the cervix and long-term treatment results.
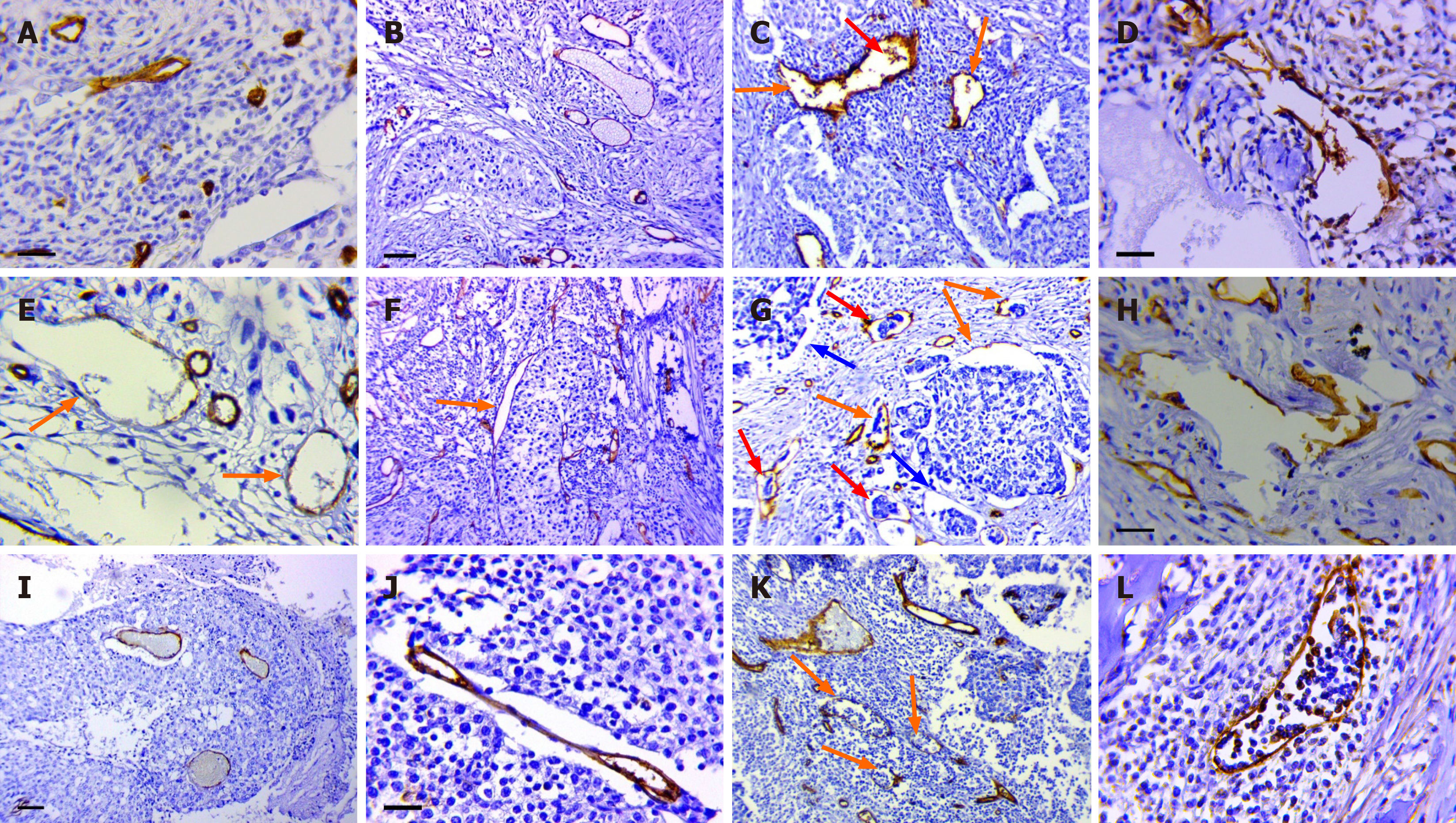
In the first phase of this study, we identified eight previously described tumor MVs and endothelial-lined structures in 62 LUSC specimens (from the training patient cohort) via immunohistochemical staining with antibodies against CD34 and PDPN (Figure 1). Here, is a brief description of them:
Normal MVs: The normal MVs were capillaries with a diameter of 5-40 microns (Figure 1А). The MVD was 14.0 ± 4.7 per conventional unit area (range 2.4–28.8, median 13.3). The cytoplasm of some vessels of this type was positive when stained with antibodies against PDPN.
DCs: DCs were defined as MVs larger than 40 microns in size that were regular in shape with clear, even contours (Figure 1B). The DC density was 4.4 ± 3.3 per conventional unit area (range 0–12.4, median 4.1), and it was significantly greater along the invasive edge of the tumor than in the central region (P = 0.002). The cytoplasm of the endothelial cells in these vessels was negative when stained with antibodies against PDPN.
ADCs: The ADCs were MVs larger than 40 microns in size and irregular in shape with a chaotic arrangement of endothelial lining cells (Figure 1C). Tumor emboli and CD34-positive cells were often observed in the lumen of these vessels. The endothelial lining of dilated lymphatic capillaries had similar characteristics, so we believe that some ADCs were LVs (Figure 1D). The density of ADCs (CD34) was 7.7 ± 3.9 per conventional unit area (range 1.0–19.4, median 7.5), and the density of dilated LVs was 2.1 ± 1.2 per conventional unit area (range 0.3–5.0, median 1.9).
DCs with weak expression of CD34: These vessels were defined as capillaries with a regular shape and smooth contours and very weak, sometimes barely detectable, expression of CD34 in the endothelial cell cytoplasm. These cells had large, pale nuclei with weakly condensed chromatin (Figure 1E). The average diameter of vessels of this type was 68.7 ± 29.5 µm. The described vessels were observed only in LFFCT and were not stained with antibodies against PDPN; that is, they were blood vessels. These vessels were identified in 26 (41.9%) of the LUSC samples.
The contact-type DCs: The described vessels were MVs, the walls of which were in direct contact with tumor cells. The described MVs had a regular shape and clear, even contours (Figure 1F). The average diameter was 68.7 ± 29.5 µm. Some vessels of this type were stained with PDPN; that is, they were LVs. Contact-type DCs were found in 36 (58.1%) of the studied samples.
Structures with partial endothelial linings: Structures with partial endothelial linings were first described in gastric cancer[21]. We observed two types of SPELs in both LUSC and squamous cell carcinoma of the cervix. The first type was associated with retraction clefts. In tumor samples with type 1 SPELs, structures without an endothelial lining (retraction clefts) and vessels with complexes of tumor cells in their lumen were often observed (Figure 1G). The described SPELs were identified in 28 (45.2%) of the samples. In most cases, these structures were positive when stained with antibodies against PDPN.
Type 2 SPELs were observed predominantly in the peritumoral stroma; they were often linear or irregular in shape and were positive when stained with antibodies against CD34 and PDPN (Figure 1H). The density of CD34-positive SPELs was 3.2 ± 1.8 per arbitrary unit area (range 0.3-7.6, median 2.8), and the density of PDPN-positive SPELs was 1.2 ± 1.0 (range 0–4.4, median 1.1).
Capillaries in the solid component of the tumor: We observed 2 types of such vessels. The first type of vessel consisted of capillaries, the walls of which were in direct contact with tumor cells (Figure 1I). The average diameter of these vessels was 34 ± 32.1 µm. There was no content in the lumen of the vessels, or it was represented by erythrocytes. Type 1 capillaries were found in 29.1% of the LUSC samples. The second type of vessel was linear capillaries whose walls were retracted from tumor cells (Figure 1J). Vessels of the second type were found in 32.3% of the samples. In 35% of the samples, capillaries of both types were observed in the solid component of the tumor. Both types of vessels were negative when stained with antibodies against PDPN.
LVs in lymphoid and polymorphic cell infiltrates: LVs in lymphoid and polymorphic cell infiltrates had very thin, sometimes slightly noticeable endothelial linings when stained with antibodies against CD34 and were not visible when stained with Mayer's H&E (Figure 1K). Lymphocytes or other leukocytes were found in large numbers in the lumen. All vessels of this type were positive when stained with antibodies against PDPN (Figure 1L). The described vessels were found in 32.3% of the LUSC samples.
Considering the published data and our earlier results, we included the following features of the parenchymal and stromal components of the tumor in the analysis:
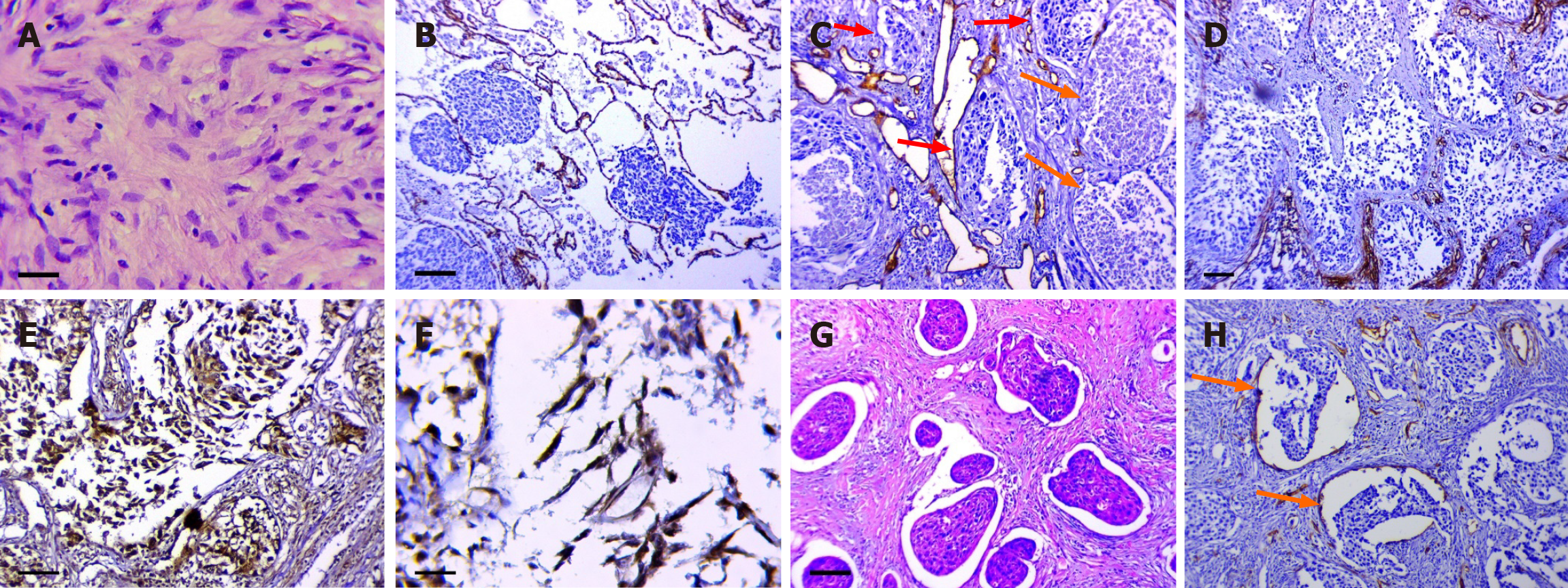
The presence of LFFCT in the tumor stroma: LFFCT was rich in cells with large pale nuclei and weakly condensed chromatin (Figure 2A). LFFCT was most often detected peritumorally and was noted in 57.8% of the LUSC samples.
The tumor spread through the AASs: In our study, tumor spread through the AASs was observed in 41 (66.1%) samples. Only in 6 samples did tumor cells spread through relatively unchanged AASs (Figure 2B). In other samples, the walls of the alveoli were thickened and contained DCs, sometimes merging with each other (Figure 2C). In the AASs, both unchanged clusters of tumor cells and completely necrotic tumor masses were observed. In a number of samples, frag
The fragmentation of the tumor solid component: This phenomenon was defined as the appearance of individual fibroblast-like tumor cells with nuclear expression of HIF-1a and Snail in the tumor solid component or in the tumor masses spreading through the AASs (Figure 2E and F). This phenomenon was noted in 46 (68%) of the LUSC samples.
The presence of retraction clefts: This phenomenon has been described in some tumor types and involves the presence of empty spaces around tumor nests[25,26]. Several studies have demonstrated that the presence of retraction clefts may be associated with a poor prognosis in patients with various malignant tumors[27-29] or more aggressive tumors[30]. In LUSC, retraction clefts were detected in 77% of the samples (Figure 2G). In 28 (45.2%) samples, the described structures had partial endothelial linings (Figure 2H).
It should be noted that in some samples, the described phenomenon had to be differentiated from tumor spread through AASs. The retraction of tumor nests from a pronounced stroma, presented by fibrous connective tissue, was regarded as a retraction cleft.
The density of normal MVs (MVD), DCs, ADCs and SPELs according to the clinical and pathological characteristics and prognosis of patients with LUSC are presented in Supplementary Table 1.
According to the obtained results, neither the density of the described vessels nor the SPELs were associated with the presence of metastases in RLNs or the prognosis of LC. MVD was greater in patients younger than 60 years, in stage T2a and T2b, in stage N0 and N1, in nonkeratinizing squamous cell carcinoma (NKSCC), in the presence of LVI in the tumor samples, and in patients with a high or moderate degree of tumor malignancy (G3 and G2); however, the differences in the groups were not statistically significant (P > 0.05). The DC density was significantly lower in G3 than in G1 and G2 (P = 0.04) and somewhat lower in the presence of LVI than in the absence of LVI; however, these differences were not statistically significant (P > 0.05). Correlations between the density of ADCs or SPELs (CD34) and clinical and pathological characteristics and LUSC prognosis have not been established; however, the LV density and density of SPELs (PDPN) were significantly greater in peripheral LCs than in central LCs (P = 0.003 and P = 0.007, respectively). In addition, the density of SPELs (PDPN) was greater in the presence of LVI than in the absence of LVI (P = 0.04).
Similarly, according to the ROC analysis data, neither the density of the described vessels nor the SPELs were associated with the presence of RLN metastases (RLNM) or the prognosis of LC. The ROC analysis data are presented in Supplementary Figure 1.
The data on the frequency of DCs with weak expression of CD34, DCs of contact type, capillaries in the solid com
As shown in the data, DCs with weak expression of CD34 were detected significantly more often in patients with LUSC recurrence than in patients without LUSC recurrence and somewhat more often in patients with keratinizing squamous cell carcinoma (KSCC) than in patients with NKSCC (P > 0.05). In patients with and without disease relapse, these vessels were detected in 61.5% and 27.8%, respectively (P = 0.008).
The analysis showed that contact-type DCs were detected significantly more often in stage I and IIIA LUSC than in stage II (P = 0.04) and somewhat more often in peripheral LUSC than in central LUSC (P > 0.05) and in stage N0 and N2 than in stage N1 (P > 0.05). However, in patients with stage II and IIIA LUSC, contact-type DCs were significantly more often detected at stage IIIA than at stage II (70.0% vs 36.4%, respectively, P = 0.03); moreover, contact-type DCs were more common in the presence of disease recurrence than in its absence (61.9% vs 33.3%, respectively, P = 0.06). It can be cautiously assumed that for small tumors, vessels of this type can adequately provide tumor cells with oxygen, while for large tumors, they can contribute to tumor progression, for example, by activating epithelial–mesenchymal tran
There were no significant differences in the frequency of LVs in lymphoid and polymorphic cell infiltrates according to clinical and morphological characteristics or the LUSC prognosis. These vessels were somewhat more common in stage T1a and T3 than in stage T1b-T2b (P = 0.054), in stage I and IIIA than in stage II, in KSCC than in NKSCC, and in the presence of LVI in the tumor samples than in the absence of LVI; however, the differences among the groups were not statistically significant (P > 0.05).
We also investigated the correlations between tumor parenchymal and stromal component features and LUSC clinical and pathological characteristics and disease prognosis. The data are presented in Supplementary Table 3.
The analysis revealed that LFFCT in the tumor stroma was significantly more common in the tumor samples from patients with LUSC recurrence than in those from patients without LUSC recurrence (76.9% vs 44.4%, P = 0.01) and was somewhat more common in the tumor samples from patients older than 70 years; in patients younger than 60 years, aged 60 to 70 years and older than 70 years, LFFCT was detected in 45.0%, 57.6% and 88.9% of the tumor samples, respectively (P = 0.16).
In turn, fragmentation in the tumor solid component was observed in 79.5%, 81.3% and 42.9% of the tumor samples from patients with G1, G2 and G3, respectively (P = 0.09); in 70.0%, 72.7% and 100.0% of the tumor samples from patients younger than 60 years, aged 60 to 70 years and older than 70 years, respectively (P = 0.18); in 50%, 50%, 79.4%, 100% and 88.9% of the tumor samples from patients in stages T1a, T1b, T2a, T2b and T3, respectively (P = 0.13); in 63.6%, 81.8% and 85.0% of the tumor samples from patients in stages N0, N1 and N2, respectively (P = 0.24); in 60.0%, 81.8% and 85.0% of the tumor samples from patients in stages I, II and IIIA, respectively (P = 0.13); and in 84.0% and 69.4% of the tumor samples from patients with and without disease recurrence, respectively (P = 0.17).
Retraction clefts were detected in 54.5%, 61.5% and 78.6% of the tumor samples from patients with stage N0, N1 and N2 LUSC, respectively (P = 0.049).
Tumor spread through the AASs was significantly more often detected in patients with peripheral LUSC than in those with central LUSC (78.9% vs 41.7%, P = 0.003); in the N0 stage than in the N1 and N2 stages (90.9% vs 50.0% and 50.0%, P = 0.006); and in patients with stage I LUSC than in those with stage II or IIIA LUSC (90.0% vs 54.5% and 50.0%, resp
Considering that tumor MVs form in the altered stroma, we analyzed the correlations of different types of tumor MVs with the features of the stromal and solid components of the tumor. The results are shown in Table 2.
| Fragmentation in the tumor solid component | The presence of LFFCT in the tumor stroma | Retraction clefts | Tumor spread in the AASs | |||||
| Gamma | P value | Gamma | P value | Gamma | P value | Gamma | P value | |
| MVD | 0.240 | 0.24 | 0.034 | 0.84 | 0.042 | 0.79 | -0.537 | 0.002a |
| DCs | 0.064 | 0.66 | 0.014 | 0.90 | 0.211 | 0.06 | -0.251 | 0.04a |
| ADCs | 0.555 | 0.0006a | -0.537 | 0.0005a | 0.115 | 0.45 | 0.248 | 0.15 |
| SPELs | 0.071 | 0.65 | 0.124 | 0.43 | 0.517 | 0.0006a | 0.435 | 0.007a |
| DCs with weak expression of CD34 | 0.297 | 0.14 | 0.943 | 0.0000a | 0.193 | 0.21 | -0.210 | 0.22 |
| Contact-type DCs | 0.344 | 0.03a | 0.502 | 0.001a | 0.666 | 0.0000a | -0.077 | 0.66 |
| The capillaries in the tumor solid component | 0.040 | 0.82 | 0.009 | 0.96 | 0.231 | 0.16 | -0.069 | 0.72 |
| LVs in lymphoid and polymorphic cell infiltrates | -0.538 | 0.001a | 0.054 | 0.75 | 0.284 | 0.07 | 0.302 | 0.08 |
According to the data obtained, the most significant correlations (P < 0.01) were: (1) A negative correlation of MVD density with tumor spread in the AASs; (2) positive correlations of ADC density with the presence of tumor destruction and fragmentation in the tumor solid component and a negative correlation with the presence of LFFCT in the tumor stroma; (3) positive correlations of SPEL density with retraction clefts and tumor spread in the AASs; (4) positive correlations of DCs with weak expression of CD34 with LFFC in the tumor stroma; and (5) positive correlations of contact-type DCs with the presence of LFFC in the tumor stroma and the presence of retraction clefts.
Considering the obtained results, to evaluate the independent predictors associated with the risk of LUSC metastasis in RLNs and the risk of disease recurrence in the main group of patients, the following predictors were included in the univariate and multivariate analyses: Patient age, tumor location, histology, tumor grade, T stage, N stage, TNM stage, DCs with weak expression of CD34, contact-type DCs, capillaries in the tumor solid component, LFFCT in the tumor stroma, fragmentation in the tumor solid component, retraction clefts and tumor spread through the AASs.
The results of univariate and multifactorial logistic regression analyses of risk factors for RLNM in patients with LUSC are presented in Table 3.
| Characteristic | Univariate analysis, OR (95%CI) | P value | Multivariate analysis, OR (95%CI) | P value |
| Age | ||||
| < 60 | 1 | - | ||
| 60-69 | 1.48 (0.71-3.07) | 0.291 | ||
| ≥ 70 | 0.75 (0.27-2.10) | 0.584 | ||
| Tumor location | ||||
| Peripheral | 1 | - | 1 | - |
| Central | 4.77 (2.36-9.63) | 0.0000a | 7.80 (3.33-18.26) | 0.0000a |
| Histology | ||||
| KSCC | 1 | - | ||
| NKSCC | 0.77 (0.37-1.60) | 0.482 | ||
| Tumor grade | ||||
| G1 | 1 | - | ||
| G2 | 1.07 (0.50-2.29) | 0.858 | ||
| G3 | 0.78 (0.31-1.97) | 0.597 | ||
| T stage | ||||
| T1a | 1 | - | 1 | - |
| T1b | 5.50 (0.91-33.18) | 0.063 | 4.93 (0.76-31.78) | 0.093 |
| T2a | 6.20 (1.42-27.09) | 0.015a | 2.82 (0.59-13.53) | 0.195 |
| T2b | 9.33 (1.45-60.21) | 0.019a | 5.27 (0.75-37.28) | 0.096 |
| T3 | 5.60 (1.24-25.25) | 0.026a | 1.66 (0.31-8.80) | 0.551 |
| DCs with weak expression of CD34 | ||||
| Absence | 1 | - | ||
| Presence | 1.10 (0.56-2.15) | 0.783 | ||
| DCs of "contact type" | ||||
| Presence | 1 | - | ||
| Absence | 1.67 (0.86-3.25) | 0.13 | ||
| The capillaries in the tumor solid component | ||||
| Absence | 1 | - | 1 | - |
| Presence | 2.89 (1.33-6.28) | 0.008a | 2.72 (1.14-6.48) | 0.023a |
| LFFCT in the tumor stroma | ||||
| Absence | 1 | - | ||
| Presence | 0.70 (0.32-1.55) | 0.383 | ||
| Fragmentation in the tumor solid component | ||||
| Absence | 1 | - | 1 | - |
| Presence | 2.55 (1.28-5.06) | 0.008a | 3.03 (1.33-6.94) | 0.009a |
| Retraction clefts | ||||
| Absence | 1 | - | 1 | - |
| Presence | 2.62 (1.33-5.16) | 0.005a | 3.52 (1.56-7.96) | 0.003a |
| Tumor spread through the AAS | ||||
| Absence | 1 | - | ||
| Presence | 0.72 (0.37-1.42) | 0.342 | ||
According to the univariate logistic regression analysis, we identified 5 prognostic factors associated with the risk of RLNM, namely, tumor location, T stage, capillaries in the tumor solid component, fragmentation in the solid component of the tumor and retraction clefts. All of these factors, with the exception of T stage, were found to be independent predictors of RLNM. The risk of RLNM was significantly greater in patients with capillaries in the tumor solid component, fragmentation in the tumor solid component and retraction clefts in the tumor tissue and in patients with central LUSC.
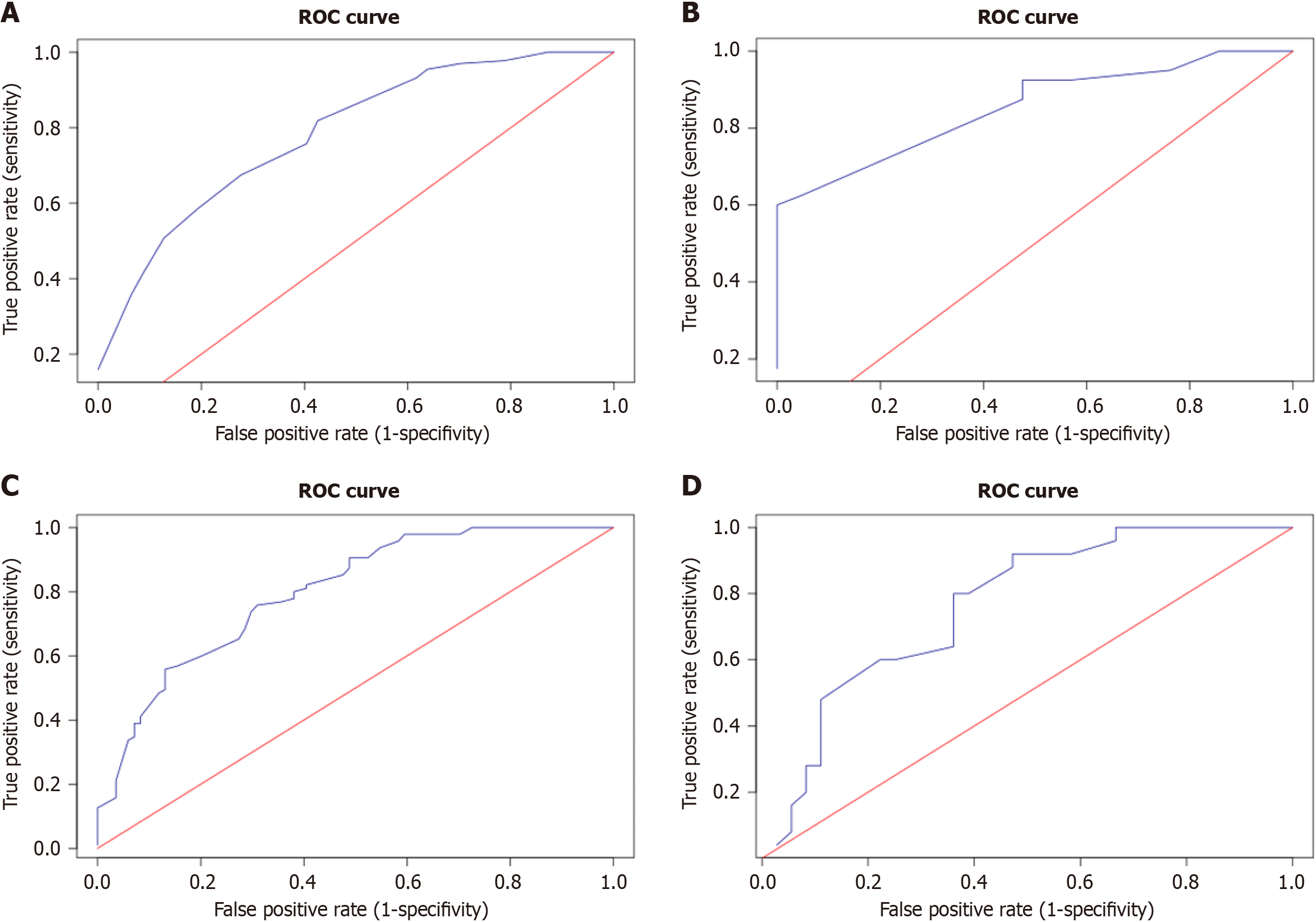
We summarized the ORs of the independent predictors for each patient in the main and training groups. For example, for a patient with peripheral LUSC [odds ratio (OR) = 1], capillaries in the solid component of the tumor (OR = 2.72) and retraction clefts (OR = 3.53), without fragmentation in the solid component of the tumor (OR = 1), this number was 8.25 (1 + 2.72 + 3.53 + 1). On the basis of these results, ROC curves were constructed to discriminate between patients with and without RLNM (Figure 3A and B).
For patients in the main cohort, the AUC was 0.784 (DI = 0.71 - 0.915, P < 0.0001), and for the training cohort, the AUC was 0.853 (DI = 0.766 - 0.94, P < 0.0001). When the OR sum (ORS) was greater than or equal to 12.52 (cutoff), 89 (87.25%) of the 102 patients in the main groups and 23 (100%) of the 23 patients in the training group had metastases in the RLNs. The sensitivity, accuracy and specificity of the method for the main cohort were 66.9%, 68.9% and 72.9%, respectively; for the training cohort, they were 60%, 74.2% and 100%, respectively. Interestingly, in 8 of the 13 patients in the main group with an ORS of 12.52 or more without metastases in RLNs, local (4 patients) or systemic (4 patients) recurrence of the disease was noted within 3 years.
The results of univariate and multivariate analyses evaluating the independent factors associated with the risk of LUSC recurrence are presented in Table 4.
| Characteristic | Univariate analysis, OR (95%CI) | P value | Multivariate analysis, OR (95%CI) | P value |
| Age | ||||
| < 60 | 1 | - | ||
| 60-69 | 1.87 (0.97-3.58) | 0.06 | ||
| ≥ 70 | 2.45 (0.89-6.72) | 0.082 | ||
| Tumor location | ||||
| Peripheral | 1 | - | 1 | - |
| Central | 1.86 (0.99-3.47) | 0.052a | 1.04 | 0.92 |
| Histology | ||||
| KSCC | 1 | - | ||
| NKSCC | 1.79 (0.94-3.38) | 0.075 | ||
| Tumor grade | ||||
| G1 | 1 | - | 1 | - |
| G2 | 1.51 (0.76-2.95) | 0.232 | 1.36 (0.59-3.01) | 0.466 |
| G3 | 3.44 (1.39-8.48) | 0.007a | 4.24 (1.37-13.14) | 0.001a |
| T stage | ||||
| T1a | 1 | - | ||
| T1b | 7.00 (1.04-46.95) | 0.045a | ||
| T2a | 4.26 (0.83-21.74) | 0.082 | ||
| T2b | 5.00 (0.79-31.62) | 0.087 | ||
| T3 | 3.38 (0.65-17.69) | 0.149 | ||
| N stage | ||||
| N0 | 1 | - | 1 | - |
| N1 | 2.00 (0.97-4.11) | 0.059 | 1.89 (0.81-4.39) | 0.137 |
| N2 | 3.22 (1.37-7.57) | 0.007a | 3.43 (1.26-9.34) | 0.016a |
| Stage | ||||
| I | 1 | - | 1 | - |
| II | 2.17 (0.89-5.27) | 0.088 | 1.87 (0.45-7.69) | 0.385 |
| III | 3.13 (1.29-7.60) | 0.012a | 3.44 (0.50-23.49) | 0.207 |
| Adjuvant therapy | ||||
| Radiation therapy | 1 | - | 1 | - |
| Chemo- and radiotherapy | 1.41 (0.50-3.97) | 0.513 | 0.57 (0.16-1.98) | 0.376 |
| Chemotherapy | 3.02 | 0.008 | 2.07 (0.78-5.53) | 0.146 |
| DCs with weak expression of CD34 | ||||
| Absence | 1 | - | 1 | - |
| Presence | 4.26 (2.25-8.05) | 0.0000a | 1.18 (0.43-3.23) | 0.749 |
| Contact-type DCs | ||||
| Absence | 1 | - | ||
| Presence | 0.97 (0.54-1.75) | 0.934 | ||
| The capillaries in the tumor solid component | ||||
| Absence | 1 | - | 1 | - |
| Presence | 2.32 (1.24-4.35) | 0.008a | 1.20 (0.56-2.59) | 0.638 |
| LFFCT in the tumor stroma | ||||
| Absence | 1 | - | 1 | - |
| Presence | 6.24 (2.85-13.67) | 0.0000a | 7.34 (3.03-17.76) | 0.0000a |
| Fragmentation of the tumor solid component | ||||
| Absence | 1 | - | 1 | - |
| Presence | 3.03 (1.58-5.82) | 0.0009a | 5.23 (2.27-12.07) | 0.0001a |
| Retraction clefts | ||||
| Absence | 1 | - | ||
| Presence | 0.71 (0.38-1.29) | 0.262 | ||
| Tumor spread through the AASs | ||||
| Presence | 1 | - | 1 | - |
| Absence | 2.06 (1.13-3.76) | 0.019a | 3.37 (1.22-5.66) | 0.0083a |
According to the univariate logistic regression analysis, we identified 8 prognostic factors associated with the risk of LUSC recurrence, namely, G3, N2 stage, stage IIIA, the presence of adjuvant ChT (A-ChT), the presence of DCs with weak expression of CD34, capillaries in the tumor solid component, LFFCT in the tumor stroma and fragmentation in the tumor solid component, as well as the absence of tumor spread through the AASs. However, only tumor grade 3, N2 stage, the presence of LFFCT in the tumor stroma, fragmentation of the tumor solid component, and the absence of tumor spread through the AASs were found to be independent predictors of a high risk of LUSC recurrence.
Similarly, we summarized the ORs of independent predictors of the risk of disease recurrence in patients in the main and training groups, and based on the results obtained, we constructed ROC curves that distinguish between patients with and without disease recurrence (Figure 3C and D).
For patients in the main cohort, the AUC was 0.799 (DI = 0.735 - 0.863, P < 0.0001), and for the training cohort, the AUC was 0.767 (DI = 0.648 - 0.885, P < 0.0001). When the ORS was less than or equal to 12.11 (cutoff), 35 (94.59%) of the 37 patients in the main cohort and 20 (90.91%) of the 22 patients in the training cohort had no LUSC recurrence. The sensitivity, accuracy and specificity of the method for the main cohort were 41.18%, 71.10% and 97.89%, respectively, and those for the training cohort were 54.05%, 69.35% and 92.00%, respectively.
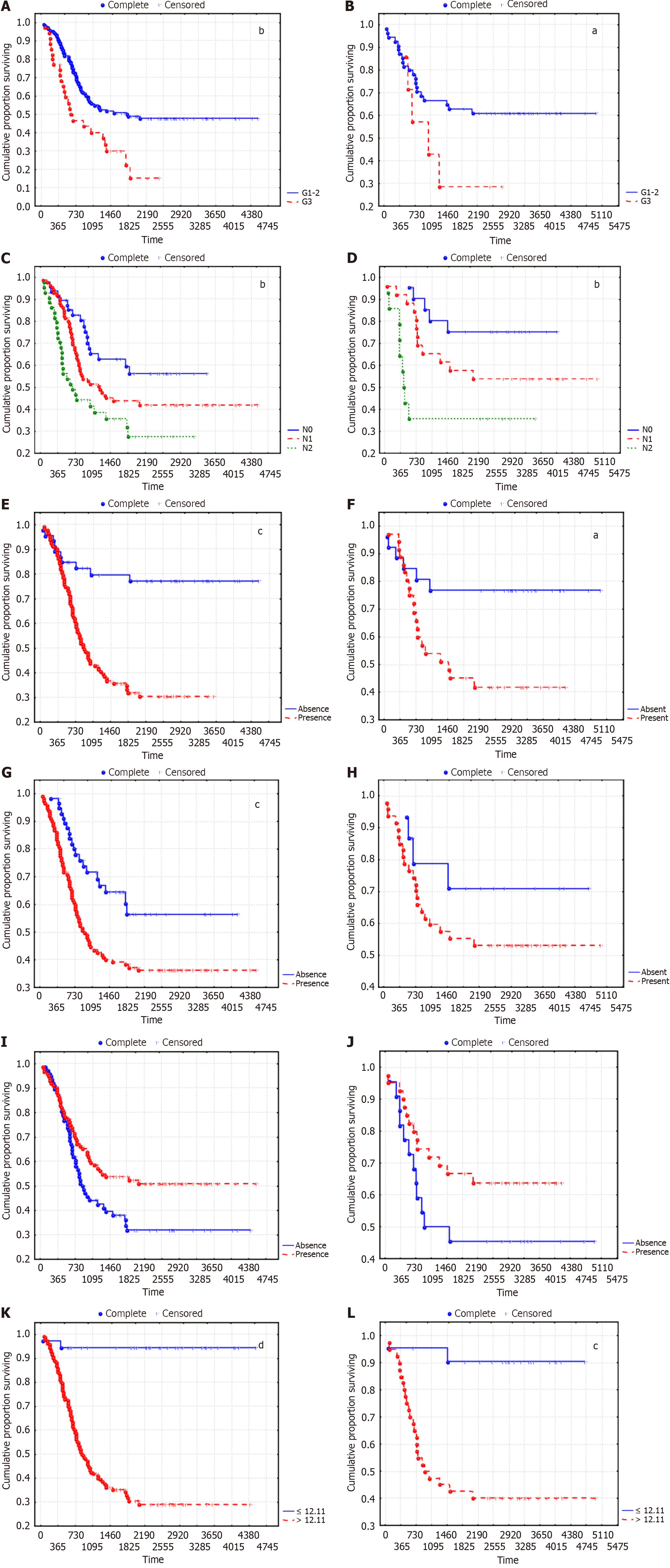
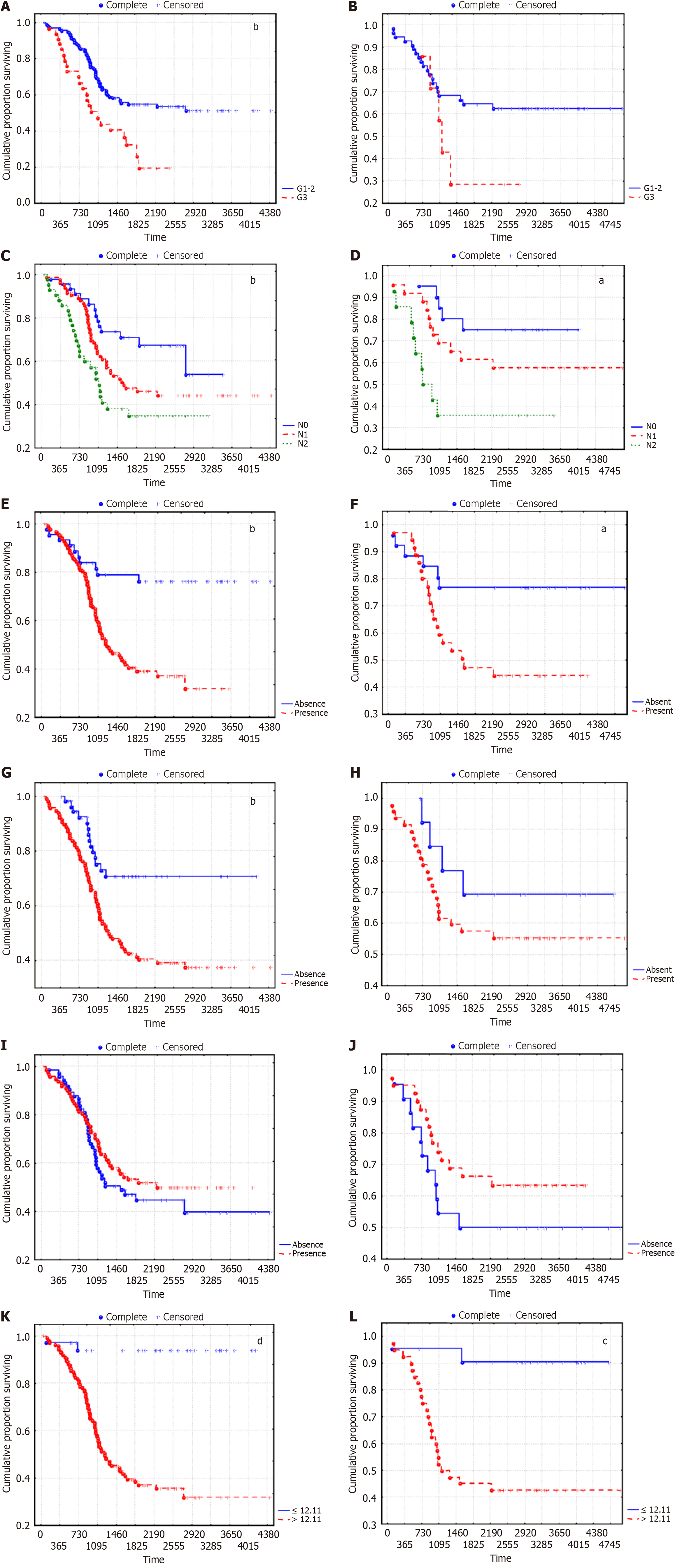
We analyzed the survival of LUSC patients according to the established predictors of disease recurrence risk. The recurrence free survival (RFS) curves are shown in Figure 4.
The overall survival (OS) curves according to the predictors of a high risk of LC recurrence are shown in Figure 5.
Survival analysis indicated that the OS and disease-free survival (DFS) of patients with LUSC were significantly lower in G3 than in G1 and G2, in N2 than in N0 and N1, and in the presence of LFFCT in the tumor stroma and fragmentation in the tumor solid component than in the absence of LFFCT. The best survival rates were observed for patients with an ORS ≤ 12.11. Moreover, the distribution of patients according to LUSC stage did not significantly differ between the groups of patients in whom the ORS was ≤ 12.11 and those in whom the ORS was > 12.11 (P = 0.483). In particular, 29.7%, 37.8% and 32.4% of patients in the first group had stage I, II and IIIA LUSC, respectively, and 13.3%, 41.3% and 45.5%, respectively, of patients in the second group. Tumor spread through the AASs had the least effect on DFS and was not associated with OS; moreover, the nonsignificant difference in survival in the training cohort, depending on tumor grade and fragmentation in the tumor solid component, seems to be due to the small number of patients in individual sub
According to multivariate analysis, although the type of adjuvant therapy was not an independent prognostic factor, the 5-year RFS in the absence of adjuvant therapy, with A-ChT, with adjuvant RT (A-RT) and with A-chemoradiotherapy were 64.6%, 30.4%, 57.9% and 47.8%, respectively (Р = 0.0027). In these groups, 60.4%, 14.6%, 0% and 0% of patients had stage I LUSC; 25.0%, 47.8%, 60.5% and 21.7% had stage II LUSC; and 14.6%, 50.7%, 39.5% and 78.3% had stage IIIA LUSC (P < 0.0001).
LC is an important medical, social and economic problem in most countries worldwide[1,2,31]. Unsatisfactory long-term treatment results for this pathology are associated with late diagnosis, either due to a lack of symptoms in early-stage disease or due to presentation with nonspecific symptoms common with a broad range of alternative diagnoses. According to a large meta-analysis of 78979 R0 resections for NSCLC, the 5-year survival rates ranged from 40% to 74% for stage IA, 38% to 68% for IB, 28% to 53% for stage II, and 18% to 39% for stage IIIA[12]. Thus, early detection of this pathology is critical for reducing mortality in patients with NSCLC.
NSCLC is a very heterogeneous disease. The most common subtypes of NSCLC are adenocarcinoma and LUSC, the incidence of which is 19%–60% and 20%–67.5%, respectively, of the total number of NSCLC cases[4-6]. Despite major breakthroughs in the treatment of lung adenocarcinoma, there are no treatment methods specific for LUSC; as a result, the prognosis of this disease has been poor, especially in the late stages[4,7,8]. The treatment efficacy of LUSC will be largely determined by the choice of optimal treatment regimens and will depend on the sensitivity of the tumor to radiation, ChT and targeted therapy. Thus, determining the risk of disease relapse, including assessing the status of RLNs, is of key importance for choosing the optimal treatment for LUSC. In addition, research in this direction can contribute to understanding the mechanisms of LUSC progression and, consequently, to new approaches for the trea
Currently, angiogenesis is regarded as both an important factor in disease prognosis[15,16] and in the effectiveness of angiogenesis blockers, ChT and targeted therapy[16,20,32-34].
Methodological approaches for assessing angiogenesis in NSCLC vary widely. In most studies, the authors limit themselves to a quantitative assessment of MVD or the level of vascular endothelial growth factor (VEGF) expression. In addition, when calculating the MVD, researchers often consider only normal MVs, i.e., vessels whose lumen does not exceed the diameter of erythrocytes; other types of tumor MVs are excluded from the assessment. As a result of these studies, correlations between MVD and various factors associated with tumor progression have been established; for example, correlations between MVD and the expression of markers such as TFIIB-related factor 2[3], ASK1-interacting protein-1[13] and chitinase 3-like 1[35] in tumor tissue, as well as between MVD and the levels of CXC chemokine ligand 4 in plasma[36]. Several studies have noted a correlation between the prognosis of NSCLC and MVD[37] and between the prognosis of NSCLC and the expression of VEGF-A and angiopoietin-2 (Ang-2)[38]. However, this dependence was observed predominantly for lung adenocarcinoma and not for LUSC. The serum levels of VEGF, Ang-2, and Interleukin-8 were also not associated with survival in patients with NSCLC[39]. Notably, the assessment of MVD in patients with advanced NSCLC (stage IIIA) using an antibody against CD31 showed that a high MVD is associated with a decrease in the 2-year survival of patients but is not correlated with VEGF-A expression[40].
We believe that the ambiguity of the results obtained may be associated with the heterogeneity of tumor MVs, which differ not only in origin and morphology but also in clinical significance[41,42]. Paulsen et al[43], investigating tumor MVs in NSCLC in accordance with the classification proposed by Pezzella et al[44], identified three angiogenic subtypes of tumor blood supply, namely, the basal, diffuse and papillary subtypes and the nonangiogenic alveolar subtype[43]. The authors revealed correlations of different vascular patterns with the immune microenvironment of the tumor, the severity of hypoxia and EMF markers. Moreover, the relationships of angiogenic subtypes with clinical and pathological characteristics and the prognosis of NSCLC have not been established. In lung adenocarcinomas, when a nonangiogenic alveolar pattern was detected, there was a significant decrease in patient survival, but in LUSC, such a dependence was not noted[43].
Over the past few years, we have been actively studying the different types of tumor MVs in gastric cancer, breast cancer and cervical cancer[21-23]. In this study, we investigated the features of different types of tumor MVs in LUSC and confirmed the acceptability of the previously proposed classification of tumor MVs. In this study, we also characterized the features of the tumor stromal and parenchymal components as factors that, on the one hand, can influence the formation of certain types of vessels, while on the other hand, different types of tumor vessels can provide the tumor tissue with oxygen and nutrients to different degrees; consequently, they can influence the behavior of the tumor, as well as its tendency to invade and metastasize[45].
The present study established a number of independent predictors associated with the risk of LUSC metastasis in RLNs and the risk of disease recurrence. The first group of predictors included tumor location, the presence of capillaries in the tumor solid component, the presence of fragmentation in the tumor solid component, and the presence of retraction clefts. We summarized the ORs of the independent predictors for each patient and, on the basis of these results, constructed ROC curves that discriminate between patients with and without RLNM. When the ORS was greater than or equal to 12.52 (cutoff), 89 (87.25%) of the 102 patients in the main cohort and 23 (100%) of the 23 patients in the training cohort had metastases in the RLNs. Furthermore, in 8 out of the 13 patients in the main group with an ORS of 12.52 or more without metastases in RLNs, local (4 patients) or systemic (4 patients) disease recurrence was noted within 3 years.
In turn, tumor grade 3, N2 stage, the presence of LFFCT in the tumor stroma, fragmentation in the tumor solid component and tumor spread through the AASs were found to be independent predictors of a high risk of LUSC recurrence. Similarly, the constructed ROC curves made it possible to identify a group of patients with a low risk of LUSC recurrence (less than 90%). When the ORS was less than or equal to 12.11 (cutoff), 35 (94.59%) of the 37 patients in the main cohort and 20 (90.91%) of the 22 patients in the training cohort had no LUSC recurrence.
The main cause of death in cancer patients is metastasis. A number of studies have shown that circulating clusters of tumor cells are associated with a greater risk of metastasis and relapse in NSCLC patients than single circulating tumor cells[46-48]. Previously, we proposed a possible mechanism for the formation of tumor cell clusters in tumor MVs[21]. This mechanism is associated with the retraction of tumor cells from the underlying stroma and the formation of hollow structures with tumor cells in the lumen, which corresponds to the previously described phenomenon of retraction clefts[27,28,29]. Subsequently, the inner surface of the described hollow structures may be partially or completely lined with endothelium. Characteristic features of this stage include the presence of SPELs and vascular invasion with clusters of tumor cells in the lumen. When the formed cavity structures merge with blood or LVs, clusters of tumor cells may enter the blood or lymphatic bed. In gastric cancer, breast cancer and cervical cancer, the presence of retraction clefts and SPELs was correlated with the presence of metastases in RLNs and was associated with a high risk of disease recurrence[21-23]. In a study by Hisakane et al[49], a vascular invasion size greater than 425 μm was the most significant factor associated with poor prognosis in patients with stage I LUSC. Tumor cells within larger areas of vascular invasion expressed higher levels of PDPN and cancer stem cell markers; a greater MVD; and greater numbers of CD204 (+) macrophages and α-SMA (+) myofibroblasts than did those within smaller vascular invasion areas[49].
Importantly, increasing evidence suggests that intravasation of single, apoptotic or clusters of tumor cells can occur via leaky neoangiogenetic vessels in the tumor core and not by crossing the adjacent tumor stroma after EMT[48]. Hamilton et al[48] emphasized that some tumor emboli may be located in blind nonfunctioning tumor vessels, which may limit their entry into the bloodstream. We believe that these data indirectly confirm the possibility of the formation of cir
In our study, we also observed fragmentation of the solid component of the tumor, which was manifested by the appearance of fibroblast-like cells expressing HIF-1α and Snail, indicating its connection with the mechanisms of EMT. According to multivariate analysis, the described phenomenon was an independent predictor of a high risk of LUSC metastasis and disease relapse. Similar results were obtained for squamous cell carcinoma of the cervix[23]. Thus, frag
Of particular interest is also the association between LFFCT and a high risk of disease relapse. A number of studies have shown that tumor tissue contains several types of noncancerous regenerative cells, including embryonic stem cells, mesenchymal stem cells and adult stem cells. These cells are the source of cancer-associated fibroblasts (CAFs), tumor endothelial cells, and tumor-associated macrophages. These cells play key roles not only in cancer progression but also in the development of drug resistance[50]. It can be assumed that the cells with large pale nuclei and weakly condensed chromatin observed in LFFCT may belong to one of these cell types. In our study, LFFCT was detected predominantly at the invasive margin of the tumor. Several studies have shown that tumor-promoting stromal cells, including PDPN-positive CAFs, CD204-positive tumor-associated macrophages, and CD34+ microvascular cells, are also more frequently recruited to invasive tumor margins[51].
Notably, the majority of the LUSC specimens studied (68%) exhibited tumor spread through AASs. Currently, tumor spread through AASs is regarded as a factor associated with an unfavorable prognosis in patients with NSCLC[52-54]. A decrease in patient survival is likely associated with co-option of the interalveolar septum vessels by the tumor, which leads to a decrease in the effectiveness of systemic therapy due to the development of resistance to antiangiogenic agents and chemotherapy[55,56]. However, in our study, tumor spread through AASs had no significant effect on the survival of patients with LUSC. Moreover, according to multivariate analysis, the absence of tumor spread through the AASs was an independent predictor associated with the risk of LUSC recurrence. It can be assumed that tumor spread through AASs leads to co-option of the vessels of the interalveolar septa by tumors, which, on the one hand, reduces hypoxia in the tumor and the need for the formation of new vessels and, on the other hand, can increase the sensitivity of the LUSC to radiation and chemotherapy. This assumption is supported by the negative correlations of the MVD and density of DCs with tumor spread through AASs. However, this assumption requires verification.
We would also like to note that according to the clinical guidelines for the treatment of NSCLC, A-RT is considered to be a factor associated with worse survival in patients[57]. However, in our study, the five-year survival rate of patients who received A-RT was even greater than that of patients who received A-ChT. We believe that this may be because most studies of NSCLC have not taken tumor histology into account when analyzing the effect of treatment modalities on long-term outcomes. For example, in a meta-analysis by Smeltzer et al[12], more than 50% of patients with NSCLC were diagnosed with adenocarcinoma, which is known to be less sensitive to RT than is squamous cell carcinoma. Moreover, it can be assumed that in patients with LUSC, the spread of tumors along AASs can even increase the effectiveness of RT by reducing hypoxia in tumor tissue, which was reflected in the treatment results of patients with LUSC. However, these assumptions require confirmation in clinical studies.
Thus, according to the results of this study, the independent predictors associated with a high risk of LUSC metastasis to RLNs are the central location of the tumor, the presence of capillaries in the solid component of the tumor, fragmentation of the tumor solid component, and the presence of peritumoral retraction clefts. In turn, tumor grade 3, N2 stage, the presence of LFFCT in the tumor stroma, fragmentation of the tumor solid component, and absence of tumor spread through the AASs are independent predictors of a high risk of LUSC recurrence. Notably, these results are similar to those obtained when studying different types of tumor vessels in gastric, breast and squamous cell carcinomas of the cervix. This study has several limitations. First, this was a single-center retrospective study; therefore, the results obtained need to be confirmed in other prospective clinical studies. Second, there is a need to standardize the quantitative indicators of the studied markers for their correct use in clinical practice. Third, the small sample size (180 patients) may have led to selection bias despite the use of a fairly homogeneous group of LUSC patients. Thus, future prospective multicenter studies with larger cohorts are needed to further explore the prognostic and predictive significance of different types of tumor MVs in patients with LUSC. Considering the data on the relationship of a certain type of vessel with the characteristics of the stromal and parenchymal components of the tumor, further research in this direction may also be of great scientific and practical interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang X, China S-Editor: Li L L-Editor: A P-Editor: Zhao YQ
| 1. | Deng Y, Peng L, Li N, Zhai Z, Xiang D, Ye X, Hu J, Zheng Y, Yao J, Wang S, Wei B, Xu P, Zhang D, Chen T, Dai Z. Tracheal, bronchus, and lung cancer burden and related risk factors in the United States and China. Am J Transl Res. 2021;13:1928-1951. [PubMed] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64637] [Article Influence: 16159.3] [Reference Citation Analysis (176)] |
| 3. | Lu M, Tian H, Yue W, Li L, Li S, Qi L, Hu W, Gao C, Si L. TFIIB-related factor 2 over expression is a prognosis marker for early-stage non-small cell lung cancer correlated with tumor angiogenesis. PLoS One. 2014;9:e88032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Wang W, Liu H, Li G. What's the difference between lung adenocarcinoma and lung squamous cell carcinoma? Evidence from a retrospective analysis in a cohort of Chinese patients. Front Endocrinol (Lausanne). 2022;13:947443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Tembo MJ, Kayamba V, Zulu E. Histopathological characterization of lung tumours at the University Teaching Hospital, Lusaka, Zambia: a pilot study. Afr Health Sci. 2022;22:31-36. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Mir MH, Siraj F, Mehfooz N, Sofi MA, Syed NA, Dar NA, Choh NA, Qadri SK, Bhat GM. Clinicopathological Profile of Non-small Cell Lung Cancer and the Changing Trends in Its Histopathology: Experience From a Tertiary Care Cancer Center in Kashmir, India. Cureus. 2023;15:e34120. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Lau SCM, Pan Y, Velcheti V, Wong KK. Squamous cell lung cancer: Current landscape and future therapeutic options. Cancer Cell. 2022;40:1279-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 8. | Wu R, Ma R, Duan X, Zhang J, Li K, Yu L, Zhang M, Liu P, Wang C. Identification of specific prognostic markers for lung squamous cell carcinoma based on tumor progression, immune infiltration, and stem index. Front Immunol. 2023;14:1236444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 9. | Gawełko J, Cierpiał-Wolan M, Bwanakare S, Czarnota M. Association between Air Pollution and Squamous Cell Lung Cancer in South-Eastern Poland. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Ding Y, Zhang L, Guo L, Wu C, Zhou J, Zhou Y, Ma J, Li X, Ji P, Wang M, Zhu W, Shi C, Li S, Wu W, Xiao D, Fu C, He Q, Sun R, Mao X, Lizaso A, Li B, Han-Zhang H, Zhang Z. Comparative study on the mutational profile of adenocarcinoma and squamous cell carcinoma predominant histologic subtypes in Chinese non-small cell lung cancer patients. Thorac Cancer. 2020;11:103-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Kris MG, Gaspar LE, Chaft JE, Kennedy EB, Azzoli CG, Ellis PM, Lin SH, Pass HI, Seth R, Shepherd FA, Spigel DR, Strawn JR, Ung YC, Weyant M. Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I to IIIA Completely Resected Non-Small-Cell Lung Cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J Clin Oncol. 2017;35:2960-2974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 269] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 12. | Smeltzer MP, Lin CC, Kong FS, Jemal A, Osarogiagbon RU. Survival impact of postoperative therapy modalities according to margin status in non-small cell lung cancer patients in the United States. J Thorac Cardiovasc Surg. 2017;154:661-672.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Chen C, Geng Q, Sun D, Hu W, Zhong C, Fan L, Song X. Low Expression of ASK1-Interacting Protein-1 Is Significantly Correlated with Tumor Angiogenesis and Poor Survival in Patients with Early Stage Non-Small Cell Lung Cancer. Onco Targets Ther. 2019;12:10739-10747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Brascia D, De Iaco G, Schiavone M, Panza T, Signore F, Geronimo A, Sampietro D, Montrone M, Galetta D, Marulli G. Resectable IIIA-N2 Non-Small-Cell Lung Cancer (NSCLC): In Search for the Proper Treatment. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Teleanu RI, Chircov C, Grumezescu AM, Teleanu DM. Tumor Angiogenesis and Anti-Angiogenic Strategies for Cancer Treatment. J Clin Med. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 326] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 16. | Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77:1745-1770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 422] [Cited by in RCA: 1179] [Article Influence: 196.5] [Reference Citation Analysis (0)] |
| 17. | Jiang X, Wang J, Deng X, Xiong F, Zhang S, Gong Z, Li X, Cao K, Deng H, He Y, Liao Q, Xiang B, Zhou M, Guo C, Zeng Z, Li G, Xiong W. The role of microenvironment in tumor angiogenesis. J Exp Clin Cancer Res. 2020;39:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 457] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 18. | Zhou W, Yang L, Nie L, Lin H. Unraveling the molecular mechanisms between inflammation and tumor angiogenesis. Am J Cancer Res. 2021;11:301-317. [PubMed] |
| 19. | Hwang I, Kim JW, Ylaya K, Chung EJ, Kitano H, Perry C, Hanaoka J, Fukuoka J, Chung JY, Hewitt SM. Tumor-associated macrophage, angiogenesis and lymphangiogenesis markers predict prognosis of non-small cell lung cancer patients. J Transl Med. 2020;18:443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 20. | Chen Y, Mathy NW, Lu H. The role of VEGF in the diagnosis and treatment of malignant pleural effusion in patients with nonsmall cell lung cancer (Review). Mol Med Rep. 2018;17:8019-8030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Senchukova M, Kiselevsky MV. The "cavitary" type of angiogenesis by gastric cancer. Morphological characteristics and prognostic value. J Cancer. 2014;5:311-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Senchukova MA, Nikitenko NV, Tomchuk ON, Zaitsev NV, Stadnikov AA. Different types of tumor vessels in breast cancer: morphology and clinical value. Springerplus. 2015;4:512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Senchukova MA, Makarova EV, Shurygina EI, Volchenko NN. Morphological Characteristics and Clinical Significance of Different Types of Tumor Vessels in Patients with Stages I-IIA of Squamous Cervical Cancer. J Oncol. 2020;2020:3818051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M, Beliën JA, de Waal RM, Van Marck E, Magnani E, Weidner N, Harris AL, Dirix LY. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer. 2002;38:1564-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 347] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 25. | Acs G, Dumoff KL, Solin LJ, Pasha T, Xu X, Zhang PJ. Extensive retraction artifact correlates with lymphatic invasion and nodal metastasis and predicts poor outcome in early stage breast carcinoma. Am J Surg Pathol. 2007;31:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Huang L, Li Y, Du J, Li H, Lu M, Wang Y, Zhou W, Wang W, Wu H. The Prognostic Value of Retraction Clefts in Chinese Invasive Breast Cancer Patients. Pathol Oncol Res. 2021;27:1609743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 27. | Jain D, Tikku G, Bhadana P, Dravid C, Grover RK. The Impact of Peritumoral Retraction Clefting & Intratumoral Eosinophils on Overall Survival in Oral Squamous Carcinoma Patients. Pathol Oncol Res. 2019;25:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Acs G, Khakpour N, Kiluk J, Lee MC, Laronga C. The presence of extensive retraction clefts in invasive breast carcinomas correlates with lymphatic invasion and nodal metastasis and predicts poor outcome: a prospective validation study of 2742 consecutive cases. Am J Surg Pathol. 2015;39:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Li W, Jia H, Wang S, Guo X, Zhang X, Zhang L, Wen HY, Fu L. The presence of retraction clefts correlates with lymphovascular invasion and lymph node metastasis and predicts poor outcome: Analysis of 2497 breast cancer patients. Ann Diagn Pathol. 2022;61:152047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Džombeta T, Krušlin B. High Grade T1 Papillary Urothelial Bladder Cancer Shows Prominent Peritumoral Retraction Clefting. Pathol Oncol Res. 2018;24:567-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Liu C, Shi J, Wang H, Yan X, Wang L, Ren J, Parascandola M, Chen W, Dai M. Population-level economic burden of lung cancer in China: Provisional prevalence-based estimations, 2017-2030. Chin J Cancer Res. 2021;33:79-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 32. | Crinò L, Metro G. Therapeutic options targeting angiogenesis in nonsmall cell lung cancer. Eur Respir Rev. 2014;23:79-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Janning M, Loges S. Anti-Angiogenics: Their Value in Lung Cancer Therapy. Oncol Res Treat. 2018;41:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Itatani Y, Kawada K, Yamamoto T, Sakai Y. Resistance to Anti-Angiogenic Therapy in Cancer-Alterations to Anti-VEGF Pathway. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 249] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 35. | Wang XW, Cai CL, Xu JM, Jin H, Xu ZY. Increased expression of chitinase 3-like 1 is a prognosis marker for non-small cell lung cancer correlated with tumor angiogenesis. Tumour Biol. 2015;36:901-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Spaks A, Svirina D, Spaka I, Jaunalksne I, Breiva D, Tracums I, Krievins D. CXC chemokine ligand 4 (CXCL4) is predictor of tumour angiogenic activity and prognostic biomarker in non-small cell lung cancer (NSCLC) patients undergoing surgical treatment. Biomarkers. 2016;21:474-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Sikora J, Słodkowska J, Radomyski A, Giedronowicz D, Kobos J, Kupis W, Rudzinski P. Immunohistochemical evaluation of tumour angiogenesis in adenocarcinoma and squamous cell carcinoma of lung. Rocz Akad Med Bialymst. 1997;42:271-279. [PubMed] |
| 38. | Qin S, Yi M, Jiao D, Li A, Wu K. Distinct Roles of VEGFA and ANGPT2 in Lung Adenocarcinoma and Squamous Cell Carcinoma. J Cancer. 2020;11:153-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Keskin S, Kutluk AC, Tas F. Prognostic and Predictive Role of Angiogenic Markers in Non- Small Cell Lung Cancer. Asian Pac J Cancer Prev. 2019;20:733-736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Bačić I, Karlo R, Zadro AŠ, Zadro Z, Skitarelić N, Antabak A. Tumor angiogenesis as an important prognostic factor in advanced non-small cell lung cancer (Stage IIIA). Oncol Lett. 2018;15:2335-2339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Nagy JA, Dvorak HF. Heterogeneity of the tumor vasculature: the need for new tumor blood vessel type-specific targets. Clin Exp Metastasis. 2012;29:657-662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 42. | Fleischer JR, Jodszuweit CA, Ghadimi M, De Oliveira T, Conradi LC. Vascular Heterogeneity With a Special Focus on the Hepatic Microenvironment. Front Physiol. 2020;11:591901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Paulsen EE, Andersen S, Rakaee M, Pedersen MI, Lombardi AP, Pøhl M, Kilvaer T, Busund LT, Pezzella F, Donnem T. Impact of microvessel patterns and immune status in NSCLC: a non-angiogenic vasculature is an independent negative prognostic factor in lung adenocarcinoma. Front Oncol. 2023;13:1157461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 44. | Pezzella F, Pastorino U, Tagliabue E, Andreola S, Sozzi G, Gasparini G, Menard S, Gatter KC, Harris AL, Fox S, Buyse M, Pilotti S, Pierotti M, Rilke F. Non-small-cell lung carcinoma tumor growth without morphological evidence of neo-angiogenesis. Am J Pathol. 1997;151:1417-1423. [PubMed] |
| 45. | Treps L, Ager A, Hida K. Editorial: Tumor Vessels as Directors of the Tumor Microenvironment: New Findings, Current Challenges & Perspectives. Front Cell Dev Biol. 2022;10:885670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 46. | Sawabata N, Susaki Y, Nakamura T, Kawaguchi T, Yasukawa M, Taniguchi S. Cluster circulating tumor cells in surgical cases of lung cancer. Gen Thorac Cardiovasc Surg. 2020;68:975-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Sawabata N, Kawaguchi T, Watanabe T, Yohikawa D, Ouji-Sageshima N, Ito T. Pure Solid Pattern of Non-Small Cell Lung Cancer and Clustered Circulating Tumor Cells. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 48. | Hamilton G, Rath B, Stickler S. Significance of circulating tumor cells in lung cancer: a narrative review. Transl Lung Cancer Res. 2023;12:877-894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 49. | Hisakane K, Saruwatari K, Fujii S, Kirita K, Umemura S, Matsumoto S, Yoh K, Niho S, Ohmatsu H, Kuwata T, Ochiai A, Gemma A, Tsuboi M, Goto K, Ishii G. Unique intravascular tumor microenvironment predicting recurrence of lung squamous cell carcinoma. J Cancer Res Clin Oncol. 2016;142:593-600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Arora L, Pal D. Remodeling of Stromal Cells and Immune Landscape in Microenvironment During Tumor Progression. Front Oncol. 2021;11:596798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 51. | Naito M, Aokage K, Saruwatari K, Hisakane K, Miyoshi T, Hishida T, Yoshida J, Masato S, Kojima M, Kuwata T, Fujii S, Ochiai A, Sato Y, Tsuboi M, Ishii G. Microenvironmental changes in the progression from adenocarcinoma in situ to minimally invasive adenocarcinoma and invasive lepidic predominant adenocarcinoma of the lung. Lung Cancer. 2016;100:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Xie H, Su H, Zhu E, Gu C, Zhao S, She Y, Ren Y, Xie D, Zheng H, Wu C, Dai C, Chen C. Morphological Subtypes of Tumor Spread Through Air Spaces in Non-Small Cell Lung Cancer: Prognostic Heterogeneity and Its Underlying Mechanism. Front Oncol. 2021;11:608353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Yagi Y, Aly RG, Tabata K, Barlas A, Rekhtman N, Eguchi T, Montecalvo J, Hameed M, Manova-Todorova K, Adusumilli PS, Travis WD. Three-Dimensional Histologic, Immunohistochemical, and Multiplex Immunofluorescence Analyses of Dynamic Vessel Co-Option of Spread Through Air Spaces in Lung Adenocarcinoma. J Thorac Oncol. 2020;15:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 54. | Mino-Kenudson M. Significance of tumor spread through air spaces (STAS) in lung cancer from the pathologist perspective. Transl Lung Cancer Res. 2020;9:847-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 55. | Bridgeman VL, Vermeulen PB, Foo S, Bilecz A, Daley F, Kostaras E, Nathan MR, Wan E, Frentzas S, Schweiger T, Hegedus B, Hoetzenecker K, Renyi-Vamos F, Kuczynski EA, Vasudev NS, Larkin J, Gore M, Dvorak HF, Paku S, Kerbel RS, Dome B, Reynolds AR. Vessel co-option is common in human lung metastases and mediates resistance to anti-angiogenic therapy in preclinical lung metastasis models. J Pathol. 2017;241:362-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 56. | Coelho AL, Gomes MP, Catarino RJ, Rolfo C, Lopes AM, Medeiros RM, Araújo AM. Angiogenesis in NSCLC: is vessel co-option the trunk that sustains the branches? Oncotarget. 2017;8:39795-39804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 57. | Majem M, Juan O, Insa A, Reguart N, Trigo JM, Carcereny E, García-Campelo R, García Y, Guirado M, Provencio M. SEOM clinical guidelines for the treatment of non-small cell lung cancer (2018). Clin Transl Oncol. 2019;21:3-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |