Published online Apr 24, 2024. doi: 10.5306/wjco.v15.i4.554
Peer-review started: January 2, 2024
First decision: January 20, 2024
Revised: February 1, 2024
Accepted: March 20, 2024
Article in press: March 20, 2024
Published online: April 24, 2024
Processing time: 111 Days and 7.8 Hours
Esophageal squamous cell carcinoma (ESCC) is a prevalent malignancy with a high morbidity and mortality rate. TMEM100 has been shown to be suppressor gene in a variety of tumors, but there are no reports on the role of TMEM100 in e
To investigate epigenetic regulation of TMEM100 expression in ESCC and the effect of TMEM100 on ESCC proliferation and invasion.
Firstly, we found the expression of TMEM100 in EC through The Cancer Genome Atlas database. The correlation between TMEM100 gene expression and the survival of patients with EC was further confirmed through Kaplan-Meier analysis. We then added the demethylating agent 5-AZA to ESCC cell lines to explore the regulation of TMEM100 expression by epigenetic modification. To observe the effect of TMEM100 expression on tumor proliferation and invasion by overexpressing TMEM100. Finally, we performed gene set enrichment analysis using the Kyoto Encyclopaedia of Genes and Genomes Orthology-Based Anno
In the present study, by bioinformatic analysis we found that TMEM100 was lowly expressed in EC patients compared to normal subjects. Kaplan-meier survival analysis showed that low expression of TMEM100 was associated with poor prognosis in patients with EC. Then, we found that the demethylating agent 5-AZA resulted in increased expression of TMEM100 in ESCC cells [quantitative real-time PCR (qRT-PCR) and western blotting]. Subsequently, we confirmed that overexpression of TMEM100 leads to its increased expression in ESCC cells (qRT-PCR and western blotting). Overexpression of TMEM100 also inhibited proliferation, invasion and migration of ESCC cells (cell counting kit-8 and clone formation assays). Next, by enrichment analysis, we found that the gene set was significantly enriched in the MAPK signaling pathway. The involvement of TMEM100 in the regulation of MAPK signaling pathway in ESCC cell was subsequently verified by western blotting.
TMEM100 is a suppressor gene in ESCC, and its low expression may lead to aberrant activation of the MAPK path
Core Tip:TMEM100 has been shown to be an oncogene in a variety of tumors, but there are no reports on the role of TMEM100 in esophageal cancer. In the present study, we found that TMEM100 was lowly expressed in esophageal squamous cell carcinoma (ESCC). Methylation may play a key role in regulating TMEM100 protein low expression. Overexpression of TMEM100 resulted in its increased expression in ESCC cells. Overexpression of TMEM100 also inhibited proliferation, invasion and migration of ESCC cells. Low expression of TMEM100 in ESCC may lead to aberrant activation of the mitogen-activated protein kinases pathway.
- Citation: Xu YF, Dang Y, Kong WB, Wang HL, Chen X, Yao L, Zhao Y, Zhang RQ. Regulation of TMEM100 expression by epigenetic modification, effects on proliferation and invasion of esophageal squamous carcinoma. World J Clin Oncol 2024; 15(4): 554-565
- URL: https://www.wjgnet.com/2218-4333/full/v15/i4/554.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i4.554
Esophageal cancer (EC) is a common malignant tumour of the digestive tract and is recognised for its high incidence and mortality rate[1,2]. The disease primarily manifests in two forms, namely squamous carcinoma and adenocarcinoma[2]. Esophageal squamous cell carcinoma (ESCC) represents the predominant subtype of EC and is particularly prevalent in Asia, while esophageal adenocarcinoma is more commonly observed in Europe[3]. China bears a significant burden, accounting for nearly 50% of ESCC cases worldwide and over 90% within Asia[4]. The predominant treatment approach for ESCC primarily involves surgical procedures. While outcomes are relatively favourable for early-stage patients with EC, those with intermediate to advanced disease face a more challenging prognosis, with a 5-year overall survival rate ranging from 10%–30%[5]. The emergence of immunotherapy brings a promising dimension to EC treatment[6]. However, the efficacy and safety of immunotherapy for patients with tumours require further validation. Anticipated advancements in identifying more clinical targets hold the potential to improve the effectiveness of immunotherapy.
TMEM100 is a gene that encodes a 134-amino-acid protein located at locus 17q32. This gene possesses two hypothetical transmembrane structural domains (amino acids 53–75 and 85–107)[7]. Initially identified as a transcription factor in the murine gene, TMEM100 is highly conserved and exhibits a structure dissimilar to any known protein family across various species[8]. In the context of TMEM100’s involvement with tumours, research findings indicate its association with a variety of malignancies. A study by Han et al[9] revealed a correlation between TMEM100 and the proliferation of lung cancer cells. Similarly, a study by Ou et al[10] suggested that TMEM100 exhibits low expression in hepatocellular carcinoma and is closely related to both its proliferation and invasion. A study by Ye et al[11] revealed that TMEM100 exhibits low expression in patients with prostate cancer and is associated with tumour stage and metastasis. In a study conducted by Li et al[12], TMEM100 demonstrated significantly low expression in colorectal cancer, and the overexpression of TMEM100 inhibited the malignant progression of tumours through the regulation of the transforming growth factor β pathway.
Epigenetic modifications are heritable alterations in gene expression that do not stem from primary DNA sequence changes, playing a pivotal role in the development of tumours such as leukaemia. These modifications primarily encompass three regulatory mechanisms: DNA methylation, non-coding RNA regulation, and histone modification[13]. DNA methylation involves the transfer of a methyl to the 5' position of cytosine through the action of DNA methyltransferase. This process utilises S-adenosylmethionine as the methyl donor, resulting in the formation of 5'-methylcytosine[14]. In the context of EC, multiple oncogenes, including EPB41L3/GPX3/TMEM176A, exhibit methylation in their promoter regions[15-17]. Despite the critical role of epigenetics in gene regulation, the literature on the mechanisms governing the expression of TMEM100 in EC is limited. Nevertheless, the significance of epigenetic regulation cannot be overlooked. The impact of DNA methylation on TMEM100 expression in tumours remains unexplored.
In this study, our objective was to elucidate the function of TMEM100 in malignant growth and invasion in vitro within ESCC cells. We sought to investigate the expression of TMEM100 and its impact on the activation of the mitogen-activated protein kinases (MAPK) signalling pathway in ESCC cells. Additionally, we aimed to explore the epigenetic regulation of TMEM100 expression in ESCC to provide a theoretical foundation for considering TMEM100 as a potential new therapeutic target for ESCC.
Hieff Trans Liposomal Transfection Reagent and PAGE Gel Quick Preparation Kit (12.5%) were purchased from Yeasen (Shanghai, China). Penicillin-streptomycin solution (100 ×), RIPA lysis buffer, and crystal violet were sourced from Beyotime (Shanghai, China). Fetal bovine serum (FBS) and RPMI-1640 medium were obtained from Bio-Channel (Nanjing, China). TRIzol reagent and dimethyl sulfoxide were purchased from Biosharp (Hefei, China). 5-Azacytidine was acquired from Selleck (Houston, United States of America). Paraformaldehyde was obtained from Servicebio (Wuhan, China). Cell counting kit-8 (CCK-8) was sourced from topscience (Shanghai, China). Nitrocellulose filter (NC) membranes were purchased from PALL (New York, United States of America). TMEM100 and β-actin primers were procured from Tsingke (Beijing, China). TMEM100 monoclonal antibodies were purchased from Proteintech (Wuhan, China). Human monoclonal antibodies against extracellular regulated kinase 1/2 (ERK1/2), phosphorylated (p-) ERK1/2, the c-Jun N-terminal kinase (JNK), phosphorylated (p-)JNK, p38, phosphorylated (p-) p38, goat anti-rabbit horse radish peroxidase (HRP) IgG, goat anti-mouse HRP IgG, and GAPDH were purchased from Zen Bioscience (Chengdu, China).
Human ESCC cell lines KYSE-450 (Cobioer Biosciences, Nanjing, China) and KYSE-150 (Typical Culture Preservation Committee Cell Bank, Chinese Academy of Sciences, Shanghai, China) were used in this study. Both cell lines were cultured in RPMI-1640 medium supplemented with 10% FBS and 1% penicillin-streptomycin solution (100 ×). The culture conditions were maintained at 37 °C with 5% CO2.
The recombinant plasmid overexpressing TMEM100 was designed by General Biol (Chuzhou, China). Cells cultured at 70% density in 6-well plates were transfected with recombinant plasmids using Hieff Trans Liposomal Transfection Reagent, following the manufacturer's protocol. After 24 h, cells were collected for quantitative real-time PCR (qRT-PCR), CCK-8 assay, colony formation assay, and western blotting.
Total RNA was isolated from K-150 and K-450 cells using TRIzol reagent, following the manufacturer's instructions. Subsequently, the RNA was reverse transcribed using a cDNA synthesis kit (Promega, Fitchburg, United States of America). The resulting cDNA was amplified through 42 cycles, and the initial reaction volume was 20 μL, comprising 1 μL of reverse transcription product and 0.8 μL of primers. The housekeeping gene β-actin was used as a standardized internal control. Table 1 provides details on the gene-specific primers utilised in PCR amplification.
| Gene | Primer pair |
| TMEM100 | F: 5-ACAGTCCCTCTGGTCAGTGAGA-3 R: 5-GGCGATGAAGACAACCACAGCA-3 |
| β-actin | F: 5-CACCATTGGCAATGAGCGGTTC-3 R: 5-AGGTCTTTGCGGATGTCCACGT-3 |
ESCC cells were lysed using RIPA lysis buffer. The resulting total cell lysates were then separated on a 12.5% sodium dodecyl sulfate polyacrylamide gel and transferred to NC membranes. After blocking in phosphate buffered saline with tween-20 containing 5% non-fat milk, membranes were incubated overnight at 4 °C with specific primary antibodies, followed by a 2 h incubation at 27 °C with HRP-conjugated specific secondary antibodies. Detection was achieved using the enhanced chemiluminescence western blotting detection system (Tanon, Shanghai, China). GAPDH was utilized to ensure equal protein loading on the gel.
For colony formation studies, ESCC cells were harvested following a 24-h treatment with transient transfection. These cells were then seeded at a density of 300 cells per 35 mm plate in RPMI-1640 medium with 10% FBS and cultured at 37 °C for two weeks. Thereafter, the cells were treated with 4% paraformaldehyde for 20 min and dyed with 1 mL of 0.1% crystal violet for 30 min. Photographs were captured after the stain was removed.
During the exponential growth phase, three thousand cells treated with transient transfection were seeded into each well of a 96-well plate (100 μL/well). At specified time points (day 1, day 2, day 3), 10 μL of CCK-8 solution was added to each well, and the optical density (450 nm) values were measured using a microplate reader after 1 h of incubation.
The efficient channel attention transcriptional data, sourced from The Cancer Genome Atlas (TCGA) database, enco
Statistical analysis and data visualization were performed using R software and GraphPad Prism 9.0. A P value < 0.05 was considered statistically significant unless otherwise specified. R software, comprising several packages, was employed for various analyses. When assessing differences between groups, statistical comparisons were conducted in GraphPad Prism 9.0 using the Student's t-test.
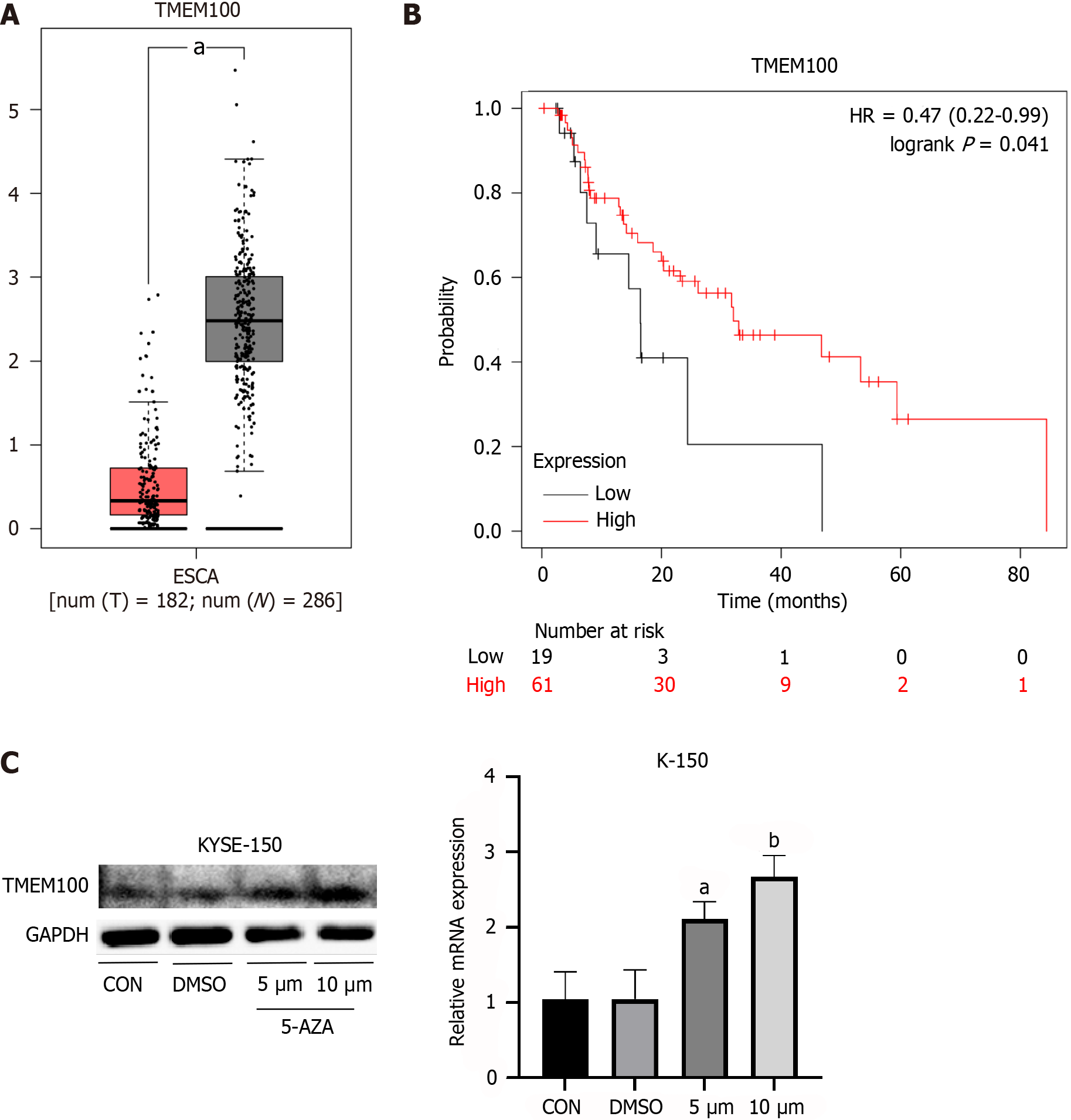
Analysis of TCGA data extracted from GEPIA revealed that the TMEM100 gene exhibited underexpression in EC specimens compared to adjacent normal tissue (Figure 1A). The correlation between TMEM100 gene expression and the survival of patients with EC was further confirmed through Kaplan-Meier analysis. Patients with high TMEM100 expression demonstrated a significantly higher overall survival rate compared to those with low expression of this gene (Figure 1B).
To validate the impact of decreased DNA methylation on TMEM100 expression, ESCC cell lines were treated with 5-AZA. Both qRT-PCR and western blotting analyses revealed upregulation of TMEM100 at both mRNA and protein levels (Figure 1C). These findings suggest that changes in DNA methylation levels affect the expression levels of TMEM100.
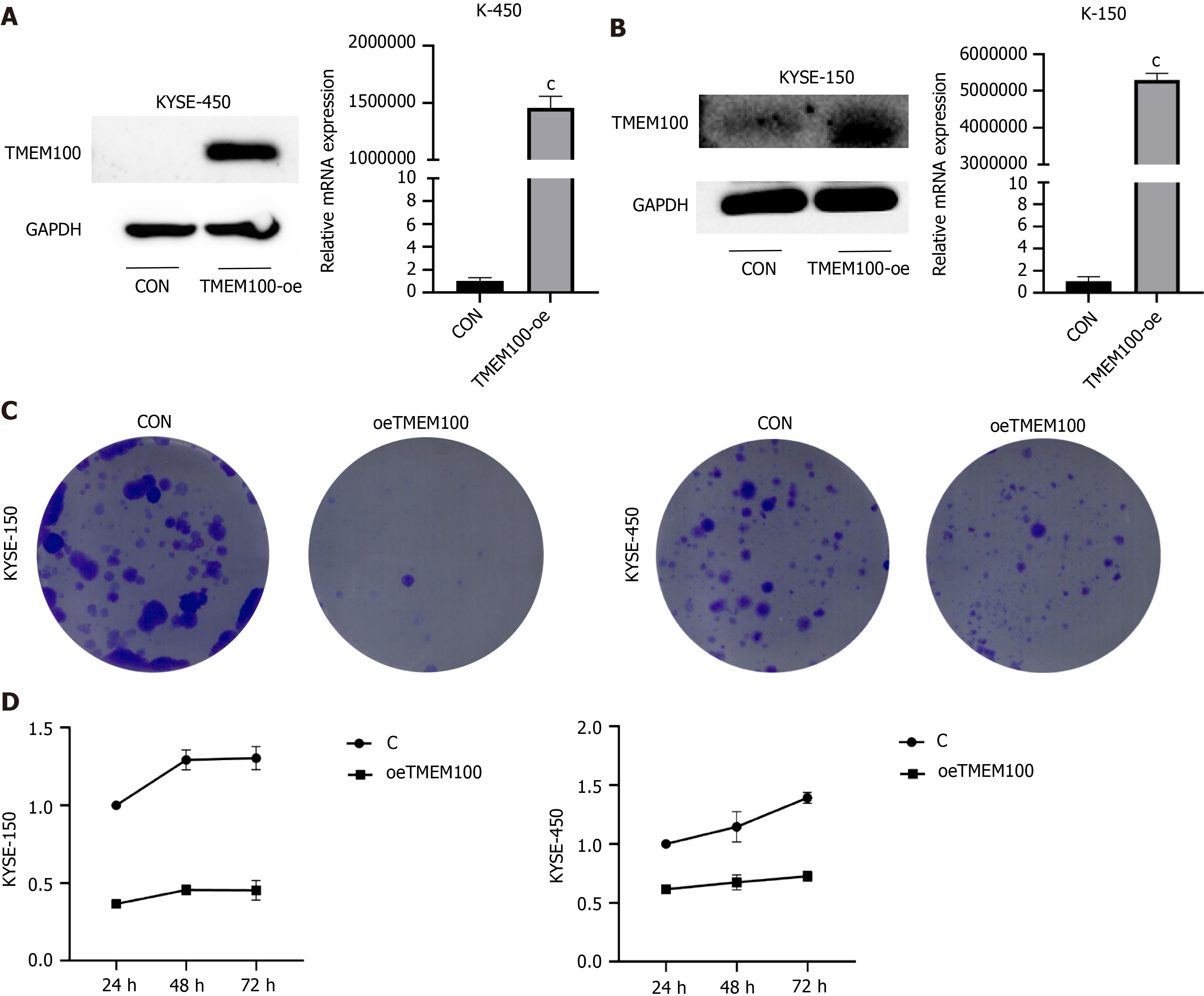
To ascertain the impact of TMEM100 overexpression, recombinant plasmids were transfected into K-150 and K-450 cell lines using Hieff Trans Liposomal Transfection Reagent. Examination of TMEM100 expression through qRT-PCR and western blotting analyses revealed a significant increase in both mRNA and protein levels upon transfection with the recombinant plasmid (Figure 2A and B).
In order to explore the long-term effects of TMEM100 on cancer cell growth, the colony-forming capacity was evaluated. TMEM100 overexpression was observed to significantly inhibit the colony-forming ability of both K-150 and K-450 cells (Figure 2C). Additionally, the impact of altered TMEM100 expression on the proliferation of K-150 and K-450 cells was examined using the CCK-8 assay (Figure 2D). These results indicate that the overexpression of TMEM100 exerts inhibitory effects on the proliferation and invasive ability of ESCC.
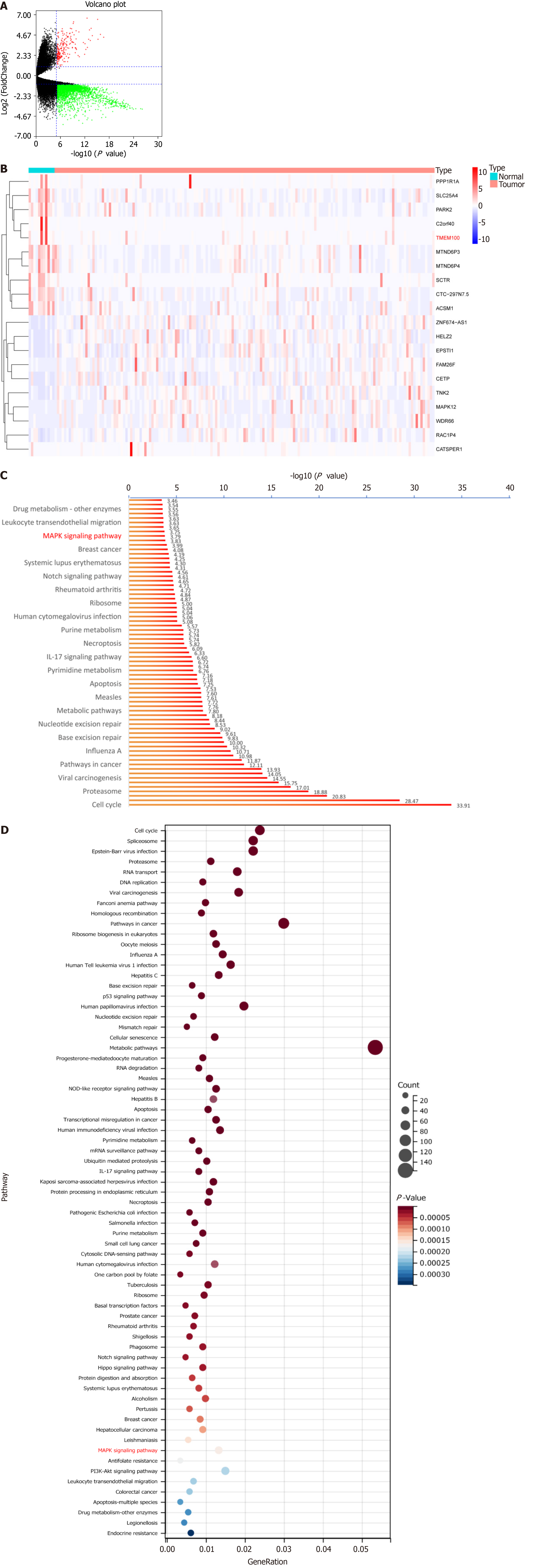
An analysis of the TCGA database resulted in the identification of a total of 50940 differential genes between EC tissue and normal tissue. Further screening narrowed down the list to 3720 differential genes containing TMEM100 (Figure 3A and B). Subsequently, the KEGG pathway enrichment analyses were conducted (Figure 3C and D), revealing a significant enrichment in the MAPK signalling pathway (P < 0.0005).
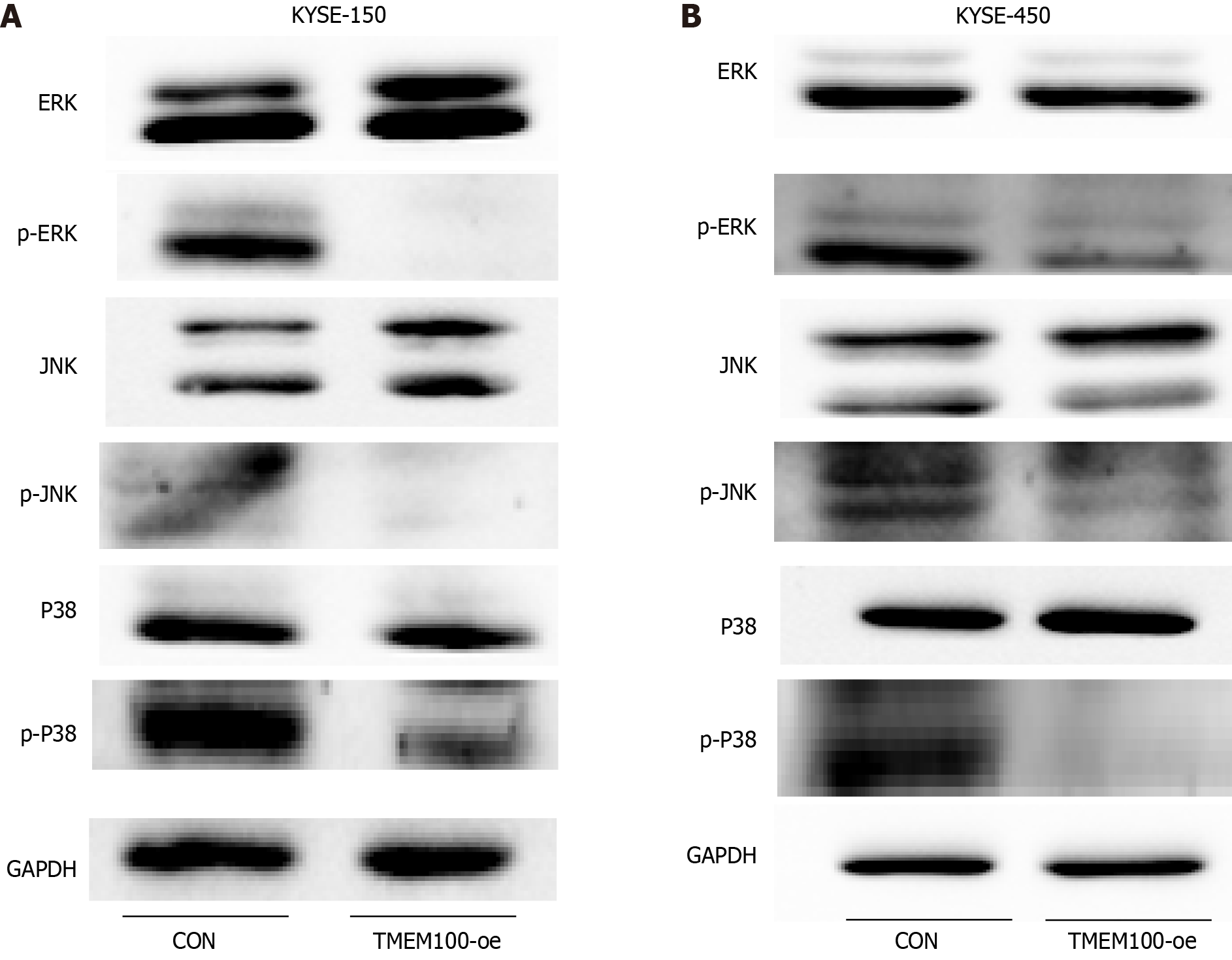
The MAPK signalling pathway plays a pivotal role in various cellular physiological activities, including cell growth, development, differentiation, and apoptosis. Given its significant involvement in tumourigenesis, we investigated whether TMEM100 mediated the cascade of the classical MAPK pathway. Western blotting results demonstrated a significant reduction in the expression of phosphorylated ERK, phosphorylated JNK, and phosphorylated p38 following transfection with the TMEM100 overexpression plasmid (Figure 4). These findings suggest that the impact of TMEM100 on ESCC cell proliferation may be regulated through the ERK/MAPK, JNK/MAPK, and p38/MAPK signalling pa
The prognosis for ESCC remains challenging, partially due to the absence of prognostic biomarkers capable of identifying high-risk patients and facilitating the assignment of risk-appropriate monitoring and treatment regimens. TMEM100 is well established as an oncogene, as demonstrated by its inhibitory role in colorectal cancer progression through the promotion of ubiquitin/proteasome degradation of hypoxia-inducible factor-1 alpha[22]. The downregulation of TMEM100, mediated by histone deacetylase 6, expedites the development and progression of non-small cell lung cancer[23]. However, the expression and function of TMEM100 in ESCC have yet to be elucidated.
In our study, we initially identified TMEM100 as a DEG between patients with EC and individuals without the con
To explore the underlying mechanisms of ESCC, we performed a KEGG enrichment analysis to identify potential pathways. The analysis revealed that TMEM100 may be involved in signalling pathways, including p53, interleukin-17, and MAPK. We chose to focus on the MAPK signalling pathway in our research, as it has been extensively shown to be associated with tumour cell proliferation, differentiation, apoptosis, and stress response compared to other pathways[26-29]. This choice aligns with the results of our CCK-8 and clone formation experiments. Subsequent investigations revealed that the phosphorylation levels of ERK, p38, and JNK were significantly inhibited in ESCC cells overexpressing TMEM100. These results suggest that TMEM100 exerts an inhibitory effect on ESCC proliferation and invasion by negatively regulating the ERK, p38, and JNK pathways.
This study has several limitations. First, the robustness of TMEM100 as a prognostic indicator for ESCC requires further validation in large or prospective cohort studies. Second, the in vivo effects of TMEM100 overexpression on ESCC proliferation need additional clarification. Third, the regulation of DNA methylation for TMEM100 expression in ESCC requires further investigation. Nevertheless, this study provides initial insights into the role of TMEM100 in the development of ESCC and its specific mechanism of action. These findings lay the foundation for further understanding the mechanism of action of TMEM100 in other malignant tumours, carrying important theoretical and clinical significance.
TMEM100 functions as a suppressor gene in ESCC cells, and its low expression in ESCC may contribute to aberrant activation of the MAPK pathway. Promoter methylation likely plays a crucial role in regulating the low expression of TMEM100.
TMEM100 is associated with multiple malignancies but its role in esophageal squamous cell carcinoma (ESCC) remains unknown.
This study aimed to investigate the regulatory mechanism of TMEM100 expression in ESCC and its effect on ESCC cell growth and proliferation.
This study hopes to clarify the role of TMEM100 in ESCC as well as to preliminarily investigate the epigenetic regulation of TMEM100 expression.
We used R software and online analysis databases to analyze the expression, prognosis and pathway of TMEM100 in esophageal cancer (EC). Utilization of real-time PCR and western blotting to probe the expression of TMEM100 and pathway proteins in ESCC. In addition, the effects of TMEM100 overexpression on the proliferation, invasion and migration of ESCC cells were assessed by CCK-8 and clone formation assays.
Kaplan-meier survival analysis revealed that low expression of TMEM100 correlated with poor prognosis in patients with EC. Further, treatment with the demethylating agent 5-AZA resulted in increased TMEM100 expression in ESCC cells. Additionally, TMEM100 overexpression exhibited inhibitory effects on the proliferation, invasion, and migration of ESCC cells. Enrichment analysis highlighted significant enrichment in the mitogen-activated protein kinases (MAPK) signalling pathway, which was validated using western blotting, confirming TMEM100’s involvement in the regulation of the MAPK signalling pathway in ESCC cells.
TMEM100 is highly expressed in normal subjects and lowly expressed in EC patients, and patients with high TMEM100 expression in EC patients have a better prognosis. The expression of TMEM100 was increased in ESCC cells treated with the methylation inhibitor 5-AZA. Overexpression of TMEM100 gene inhibited the growth and proliferation of ESCC cells and negatively regulated the MAPK signaling pathway.
The robustness of TMEM100 as a prognostic indicator for ESCC needs to be further validated. Further clarification of the in vivo effects of overexpression of TMEM100 on the proliferation of esophageal squamous carcinoma is needed.
We thank Xiu Zhu and Kai-Ming Wu for their contributions to the experiment preparation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Brown J, South Africa S-Editor: Luo ML L-Editor: A P-Editor: Zhao S
| 1. | Li Q, Dai Z, Xia C, Jin L, Chen X. Suppression of long non-coding RNA MALAT1 inhibits survival and metastasis of esophagus cancer cells by sponging miR-1-3p/CORO1C/TPM3 axis. Mol Cell Biochem. 2020;470:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Zhang XM, Wang J, Liu ZL, Liu H, Cheng YF, Wang T. LINC00657/miR-26a-5p/CKS2 ceRNA network promotes the growth of esophageal cancer cells via the MDM2/p53/Bcl2/Bax pathway. Biosci Rep. 2020;40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Wu Y, Yang X, Chen Z, Tian L, Jiang G, Chen F, Li J, An P, Lu L, Luo N, Du J, Shan H, Liu H, Wang H. m(6)A-induced lncRNA RP11 triggers the dissemination of colorectal cancer cells via upregulation of Zeb1. Mol Cancer. 2019;18:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 302] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 4. | Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology. 2018;154:360-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1155] [Article Influence: 165.0] [Reference Citation Analysis (1)] |
| 5. | Paul S, Altorki N. Outcomes in the management of esophageal cancer. J Surg Oncol. 2014;110:599-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Kono K, Mimura K, Yamada R, Ujiie D, Hayase S, Tada T, Hanayama H, Min AKT, Shibata M, Momma T, Saze Z, Ohki S. Current status of cancer immunotherapy for esophageal squamous cell carcinoma. Esophagus. 2018;15:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Kawai J, Shinagawa A, Shibata K, Yoshino M, Itoh M, Ishii Y, Arakawa T, Hara A, Fukunishi Y, Konno H, Adachi J, Fukuda S, Aizawa K, Izawa M, Nishi K, Kiyosawa H, Kondo S, Yamanaka I, Saito T, Okazaki Y, Gojobori T, Bono H, Kasukawa T, Saito R, Kadota K, Matsuda H, Ashburner M, Batalov S, Casavant T, Fleischmann W, Gaasterland T, Gissi C, King B, Kochiwa H, Kuehl P, Lewis S, Matsuo Y, Nikaido I, Pesole G, Quackenbush J, Schriml LM, Staubli F, Suzuki R, Tomita M, Wagner L, Washio T, Sakai K, Okido T, Furuno M, Aono H, Baldarelli R, Barsh G, Blake J, Boffelli D, Bojunga N, Carninci P, de Bonaldo MF, Brownstein MJ, Bult C, Fletcher C, Fujita M, Gariboldi M, Gustincich S, Hill D, Hofmann M, Hume DA, Kamiya M, Lee NH, Lyons P, Marchionni L, Mashima J, Mazzarelli J, Mombaerts P, Nordone P, Ring B, Ringwald M, Rodriguez I, Sakamoto N, Sasaki H, Sato K, Schönbach C, Seya T, Shibata Y, Storch KF, Suzuki H, Toyo-oka K, Wang KH, Weitz C, Whittaker C, Wilming L, Wynshaw-Boris A, Yoshida K, Hasegawa Y, Kawaji H, Kohtsuki S, Hayashizaki Y; RIKEN Genome Exploration Research Group Phase II Team and the FANTOM Consortium. Functional annotation of a full-length mouse cDNA collection. Nature. 2001;409:685-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 494] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 8. | Moon EH, Kim MJ, Ko KS, Kim YS, Seo J, Oh SP, Lee YJ. Generation of mice with a conditional and reporter allele for Tmem100. Genesis. 2010;48:673-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Han Z, Wang T, Han S, Chen Y, Chen T, Jia Q, Li B, Li B, Wang J, Chen G, Liu G, Gong H, Wei H, Zhou W, Liu T, Xiao J. Low-expression of TMEM100 is associated with poor prognosis in non-small-cell lung cancer. Am J Transl Res. 2017;9:2567-2578. [PubMed] |
| 10. | Ou D, Yang H, Hua D, Xiao S, Yang L. Novel roles of TMEM100: inhibition metastasis and proliferation of hepatocellular carcinoma. Oncotarget. 2015;6:17379-17390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Ye Z, Xia Y, Li L, Li B, Chen W, Han S, Zhou X, Chen L, Yu W, Ruan Y, Cheng F. Effect of transmembrane protein 100 on prostate cancer progression by regulating SCNN1D through the FAK/PI3K/AKT pathway. Transl Oncol. 2023;27:101578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 12. | Li H, Cheng C, You W, Zheng J, Xu J, Gao P, Wang J. TMEM100 Modulates TGF-β Signaling Pathway to Inhibit Colorectal Cancer Progression. Gastroenterol Res Pract. 2021;2021:5552324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Ntziachristos P, Abdel-Wahab O, Aifantis I. Emerging concepts of epigenetic dysregulation in hematological malignancies. Nat Immunol. 2016;17:1016-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Bird A. Perceptions of epigenetics. Nature. 2007;447:396-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1909] [Cited by in RCA: 1832] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 15. | Wang Y, Zhang Y, Herman JG, Linghu E, Guo M. Epigenetic silencing of TMEM176A promotes esophageal squamous cell cancer development. Oncotarget. 2017;8:70035-70048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | He Y, Wang Y, Li P, Zhu S, Wang J, Zhang S. Identification of GPX3 epigenetically silenced by CpG methylation in human esophageal squamous cell carcinoma. Dig Dis Sci. 2011;56:681-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Zeng R, Liu Y, Jiang ZJ, Huang JP, Wang Y, Li XF, Xiong WB, Wu XC, Zhang JR, Wang QE, Zheng YF. EPB41L3 is a potential tumor suppressor gene and prognostic indicator in esophageal squamous cell carcinoma. Int J Oncol. 2018;52:1443-1454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Cancer Genome Atlas Research Network; Analysis Working Group: Asan University; BC Cancer Agency; Brigham and Women’s Hospital; Broad Institute; Brown University; Case Western Reserve University; Dana-Farber Cancer Institute; Duke University; Greater Poland Cancer Centre; Harvard Medical School; Institute for Systems Biology; KU Leuven; Mayo Clinic; Memorial Sloan Kettering Cancer Center; National Cancer Institute; Nationwide Children’s Hospital; Stanford University; University of Alabama; University of Michigan; University of North Carolina; University of Pittsburgh; University of Rochester; University of Southern California; University of Texas MD Anderson Cancer Center; University of Washington; Van Andel Research Institute; Vanderbilt University; Washington University; Genome Sequencing Center: Broad Institute; Washington University in St. Louis; Genome Characterization Centers: BC Cancer Agency; Broad Institute; Harvard Medical School; Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University; University of North Carolina; University of Southern California Epigenome Center; University of Texas MD Anderson Cancer Center; Van Andel Research Institute; Genome Data Analysis Centers: Broad Institute; Brown University:; Harvard Medical School; Institute for Systems Biology; Memorial Sloan Kettering Cancer Center; University of California Santa Cruz; University of Texas MD Anderson Cancer Center; Biospecimen Core Resource: International Genomics Consortium; Research Institute at Nationwide Children’s Hospital; Tissue Source Sites: Analytic Biologic Services; Asan Medical Center; Asterand Bioscience; Barretos Cancer Hospital; BioreclamationIVT; Botkin Municipal Clinic; Chonnam National University Medical School; Christiana Care Health System; Cureline; Duke University; Emory University; Erasmus University; Indiana University School of Medicine; Institute of Oncology of Moldova; International Genomics Consortium; Invidumed; Israelitisches Krankenhaus Hamburg; Keimyung University School of Medicine; Memorial Sloan Kettering Cancer Center; National Cancer Center Goyang; Ontario Tumour Bank; Peter MacCallum Cancer Centre; Pusan National University Medical School; Ribeirão Preto Medical School; St. Joseph’s Hospital &Medical Center; St. Petersburg Academic University; Tayside Tissue Bank; University of Dundee; University of Kansas Medical Center; University of Michigan; University of North Carolina at Chapel Hill; University of Pittsburgh School of Medicine; University of Texas MD Anderson Cancer Center; Disease Working Group: Duke University; Memorial Sloan Kettering Cancer Center; National Cancer Institute; University of Texas MD Anderson Cancer Center; Yonsei University College of Medicine; Data Coordination Center: CSRA Inc; Project Team: National Institutes of Health. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1458] [Cited by in RCA: 1343] [Article Influence: 167.9] [Reference Citation Analysis (0)] |
| 19. | Bu D, Luo H, Huo P, Wang Z, Zhang S, He Z, Wu Y, Zhao L, Liu J, Guo J, Fang S, Cao W, Yi L, Zhao Y, Kong L. KOBAS-i: intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021;49:W317-W325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 1079] [Article Influence: 269.8] [Reference Citation Analysis (0)] |
| 20. | Li L, Lei Q, Zhang S, Kong L, Qin B. Screening and identification of key biomarkers in hepatocellular carcinoma: Evidence from bioinformatic analysis. Oncol Rep. 2017;38:2607-2618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 21. | Sun C, Yuan Q, Wu D, Meng X, Wang B. Identification of core genes and outcome in gastric cancer using bioinformatics analysis. Oncotarget. 2017;8:70271-70280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 22. | Zheng Y, Zhao Y, Jiang J, Zou B, Dong L. Transmembrane Protein 100 Inhibits the Progression of Colorectal Cancer by Promoting the Ubiquitin/Proteasome Degradation of HIF-1α. Front Oncol. 2022;12:899385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 23. | Wang Y, Ha M, Li M, Zhang L, Chen Y. Histone deacetylase 6-mediated downregulation of TMEM100 expedites the development and progression of non-small cell lung cancer. Hum Cell. 2022;35:271-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Xu Y, Yu X, Chen Q, Mao W. Neoadjuvant versus adjuvant treatment: which one is better for resectable esophageal squamous cell carcinoma? World J Surg Oncol. 2012;10:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Liang H, Fan JH, Qiao YL. Epidemiology, etiology, and prevention of esophageal squamous cell carcinoma in China. Cancer Biol Med. 2017;14:33-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 249] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 26. | Cohen JV, Sullivan RJ. Developments in the Space of New MAPK Pathway Inhibitors for BRAF-Mutant Melanoma. Clin Cancer Res. 2019;25:5735-5742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Ryan MB, Finn AJ, Pedone KH, Thomas NE, Der CJ, Cox AD. ERK/MAPK Signaling Drives Overexpression of the Rac-GEF, PREX1, in BRAF- and NRAS-Mutant Melanoma. Mol Cancer Res. 2016;14:1009-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Tesio M, Tang Y, Müdder K, Saini M, von Paleske L, Macintyre E, Pasparakis M, Waisman A, Trumpp A. Hematopoietic stem cell quiescence and function are controlled by the CYLD-TRAF2-p38MAPK pathway. J Exp Med. 2015;212:525-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Vasilevskaya IA, Selvakumaran M, Hierro LC, Goldstein SR, Winkler JD, O'Dwyer PJ. Inhibition of JNK Sensitizes Hypoxic Colon Cancer Cells to DNA-Damaging Agents. Clin Cancer Res. 2015;21:4143-4152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |