Published online Apr 24, 2024. doi: 10.5306/wjco.v15.i4.540
Peer-review started: December 28, 2023
First decision: January 19, 2024
Revised: March 19, 2024
Accepted: March 21, 2024
Article in press: March 21, 2024
Published online: April 24, 2024
Processing time: 115 Days and 12.9 Hours
Immunotherapy have demonstrated promising outcomes in patients with high microsatellite instability (MSI) (MSI-H) metastatic colorectal cancer. However, the comparative effectiveness of Immunotherapy and chemotherapy for patients with low MSI (MSI-L), and microsatellite stable (MSS) metastatic colorectal cancer remains unclear.
To investigate immunotherapy vs chemotherapy for treatment of MSI-L/MSS metastatic colorectal cancer, and to evaluate the success of immunotherapy against chemotherapy in managing MSI-H metastatic colorectal cancer during a follow-up of 50 months.
We conducted a retrospective cohort study using the National Cancer Database (NCDB) to evaluate the overall survival (OS) of patients with metastatic colorectal cancer treated with immunotherapy or chemotherapy. The study population was stratified by MSI status (MSI-H, MSI-L, and MSS). Multivariable Cox proportional hazard models were used to assess the association between treatment modality and OS, adjusting for potential confounders.
A total of 21951 patients with metastatic colorectal cancer were included in the analysis, of which 2358 were MSI-H, and 19593 were MSI-L/MSS. In the MSI-H cohort, immunotherapy treatment (n = 142) was associated with a sig
In this population-based study using the NCDB, immunotherapy treatment was associated with significantly improved OS compared to chemotherapy in patients with MSI-H metastatic colorectal cancer, but not in those with MSI-L/MSS metastatic colorectal cancer. Further studies are warranted to determine the optimal therapeutic approach for patients with MSI-L/MSS metastatic colorectal cancer.
Core Tip: Our population-based study demonstrates that immunotherapy treatment is associated with significantly improved overall survival in patients with high microsatellite instability (MSI-H) metastatic colorectal cancer. However, immunotherapy does not significantly benefit patients with microsatellite stable (MSS) metastatic colorectal cancer. The lower response rates to immunotherapy in MSS tumors can be attributed to the lower tumor mutational burden and reduced immunogenicity compared to MSI-H tumors. These findings indicate that while immunotherapy is a promising treatment for MSI-H colorectal cancer, its efficacy in MSS cases remains uncertain, warranting further investigation to develop targeted therapies for these patients.
- Citation: Niu CG, Zhang J, Rao AV, Joshi U, Okolo P. Comparative effectiveness of immunotherapy and chemotherapy in patients with metastatic colorectal cancer stratified by microsatellite instability status. World J Clin Oncol 2024; 15(4): 540-547
- URL: https://www.wjgnet.com/2218-4333/full/v15/i4/540.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i4.540
Colorectal cancer is globally recognized as the third most widespread form of cancer and the second leading cause of death due to cancer[1,2]. The 2023 statistics from the American Cancer Society predict that there will be 153020 new cases of colorectal cancer in the United States, with an estimated death count of 52550[3]. The treatment of metastatic colorectal cancer poses a significant difficulty in clinical practice, with an overall 5-year survival rate of just 14%[4]. Conventional frontline therapies for this condition often consist of Fluoropyrimidine-based chemotherapy, complemented by targeted treatments including anti-vascular endothelial growth factor and anti-epidermal growth factor receptor agents[5-8]. A mounting body of evidence suggests that tumors with high microsatellite instability (MSI) (MSI-H) may not be ideally suited to standard chemotherapy treatments[9-11]. MSI-H colorectal cancers, known for their high mutation rate, generate neoantigens that activate the immune system[11]. The KEYNOTE-177 and CheckMate-142 trials have demonstrated that immunotherapy offers significant clinical benefit in the treatment of MSI-H/dMMR metastatic colorectal cancer[12,13]. While immunotherapy has shown enhanced effectiveness in treating metastatic colorectal cancers characterized by MSI-H, it demonstrates limited success in microsatellite stable (MSS) variants, which account for the majority (95%) of these cases[14].
A thorough literature review highlights a significant data gap in immunotherapy application for MSS patients. Consequently, the majority of those with MSS metastatic colorectal cancer have yet to see the benefits of current immunotherapy methods[14]. Meanwhile, large-scale data evaluating the relationship between MSI-H metastatic colorectal cancer and immunotherapy is scarce. Hence, leveraging the National Cancer Data Base (NCDB)—which captures over 70% of new cancer diagnoses in the United States [15]—this research intends to: (1) Investigate immunotherapy vs chemotherapy for treatment of MSS colorectal cancer; and (2) Evaluate the success of immunotherapy against chemotherapy in managing MSI-H metastatic colorectal cancer during a follow-up of 50 months.
Our research involved a retrospective cohort analysis utilizing the NCDB, a collaborative initiative between the American College of Surgeons and the American Cancer Society, encompassing over 70% of new cancer diagnoses in the United States[16]. Our research entailed a detailed retrospective analysis utilizing the NCDB, focusing on a cohort of adult patients diagnosed with stage IV colorectal adenocarcinoma on 2020. This study encompassed patients identified by primary tumor site codes C18 (malignant neoplasm of the colon) and C20 (malignant neoplasm of the rectum), which are ICD-10 codes. The analysis concentrated on key variables, including gender, age at diagnosis, and tumor size. Tumor size was categorized into two clinically relevant groups: ≤ 20 mm and > 20 mm. Furthermore, patient MSI status was a crucial variable, alongside the initial treatment strategy, categorized into immunotherapy and chemotherapy. Vital status was utilized to determine whether each patient in the study was deceased or alive. The present study was a database analysis using de-identified data; therefore, institutional review board approval was not required for this type of study.
In profiling the study population, we gathered demographic information and clinical characteristics. This included age at diagnosis, gender, race, socioeconomic background, and types of healthcare facilities where treatment was administered. The Charlson-Deyo Comorbidity score was employed to evaluate comorbid conditions, with scores truncated to 0, 1, 2, or 3 (for scores ≥ 3). Data regarding treatment modalities, immunotherapy, chemotherapy, and additional supportive treatments, were analyzed with a primary focus on the initial course of therapy.
The focus of our research was on the initial systemic therapy administered to patients, divided into two categories: Immunotherapy and chemotherapy, including both single-agent and combination therapies. The primary outcome for evaluation was overall survival (OS), which we defined as the period from the diagnosis of metastatic colorectal cancer until death from any cause or the most recent follow-up. We tracked OS from the point of cancer diagnosis, monitoring up to the occurrence of death or the last recorded follow-up, and calculated both one-year, three-year, and 50 months survival rates. Our methodology and data analysis conformed to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
All analyses were conducted using Stata version 17.0 (StataCorp, College Station, Texas 77845, United States). We calculated the median follow-up duration, with survival time measured from the date of diagnosis to either death or the last known contact. Descriptive statistics were employed to summarize the baseline characteristics of the patient cohort. The Kaplan-Meier method was used to estimate survival probabilities, and the log-rank test was applied to compare differences between prognostic factors. To assess the impact of various factors on five-year OS, Cox proportional hazards models were utilized. These models generated hazard ratios (HR) along with their 95%CI. Additionally, multivariate analysis was conducted to calculate the adjusted HR (aHR), accounting for variables like race, gender, and age. The proportional hazards assumptions of our models were graphically verified. Furthermore, the accuracy of the American Joint Committee on Cancer sixth edition staging system was evaluated by calculating a concordance index, complete with 95%CIs. All statistical tests were two-sided, with a significance threshold set at P < 0.05.
Our comprehensive study analyzed 21951 patients diagnosed with stage IV colorectal cancer, categorized based on MSI status. Within this cohort, 2358 patients were identified as MSI-H, and 19593 as MSS. The treatment breakdown revealed that in the MSI-H group, 142 patients opted for the novel approach of immunotherapy, while a significant portion, 860 patients, underwent conventional chemotherapy. Similarly, in the MSS group, 88 patients received immunotherapy, compared to 8085 who chose chemotherapy. This distinction in treatment choices underscores the evolving landscape of cancer therapeutics. The average follow-up duration for patients receiving immunotherapy in the MSI-H group was 21.91 ± 12.23 months, and 19.83 ± 12.89 months for those receiving chemotherapy. The MSS group had a slightly longer mean follow-up of 18.48 ± 11.37 months for immunotherapy and 20.61 ± 11.71 months for chemotherapy. The median ages in these groups varied, with 77 years and 63 years for MSI-H patients on immunotherapy and chemotherapy, respectively, and 67.5 and 62 years for the MSS cohort, reflecting the demographic diversity of the study population (Table 1).
| Microsatellite instability-high, n = 2358 | Microsatellite stable, n = 19593 | |||
| Immunotherapy, n = 142 | Chemotherapy, n = 860 | Immunotherapy, n = 88 | Chemotherapy, n = 8085 | |
| Follow up duration (month) | ||||
| mean ± SD | 21.91 ± 12.23 | 19.83 ± 12.89 | 18.48 ± 11.37 | 20.61 ± 11.71 |
| Median (Range) | 22.46 (0.53-48.76) | 18.58 (0.26-48.69) | 18.88 (0.79-47.31) | 30.52 (0-49.97) |
| Age (yr) | ||||
| mean ± SD | 72.32 ± 14.70 | 62.43 ± 14.42 | 66.10 ± 15.41 | 61.53 ± 13.38 |
| Median (Range) | 77 (27-90) | 63 (21-90) | 67.5 (27-90) | 62 (19-90) |
| < 65, n (%) | 34 (23.94) | 465 (54.07) | 39 (44.32) | 4661 (57.65) |
| ≥ 65, n (%) | 108 (76.06) | 395 (45.93) | 49 (55.68) | 3424 (42.35) |
| Sex, n (%) | ||||
| Male | 52 (36.62) | 437 (50.81) | 48 (54.55) | 4466 (55.24) |
| Female | 90 (63.38) | 423 (49.19) | 40 (45.45) | 3619 (44.76) |
| Race, n (%) | ||||
| White | 9 (6.34) | 47 (5.47) | 76 (6.36) | 6356 (78.61) |
| Black | 123 (86.62) | 679 (78.95) | 8 (9.09) | 1123 (13.89) |
| Other | 9 (6.34) | 125 (14.53) | 0 | 8 (0.10) |
| Unknown | 1 (0.70) | 9 (1.05) | 4 (4.41) | 598 (7.4) |
| Charlson-Deyo Score, n (%) | ||||
| 0 | 9 (6.34) | 644 (74.88) | 65 (73.86) | 6039 (74.69) |
| 1 | 123 (86.62) | 132 (15.35) | 16 (18.18) | 1260 (15.58) |
| 2 | 9 (6.34) | 43 (5.00) | 6 (6.82) | 400 (4.95) |
| ≥ 3 | 1 (0.70) | 41 (4.77) | 1 (1.14) | 386 (4.77) |
| Tumor size, n (%) | ||||
| ≤ 20 mm | 107 (75.35) | 623 (72.44) | 61 (69.32) | 5621 (69.52) |
| > 20 mm | 35 (24.65) | 237 (27.56) | 27 (30.68) | 2464 (30.48) |
| Tumor grade, n (%) | ||||
| Well differentiated | 0 | 0 | 0 | 0 |
| Moderate differentiated | 0 | 0 | 0 | 0 |
| Poorly differentiated | 0 | 0 | 0 | 0 |
| Unknown | 142 (100.00) | 860 (100.00) | 88 (100.00) | 8085 (100.00) |
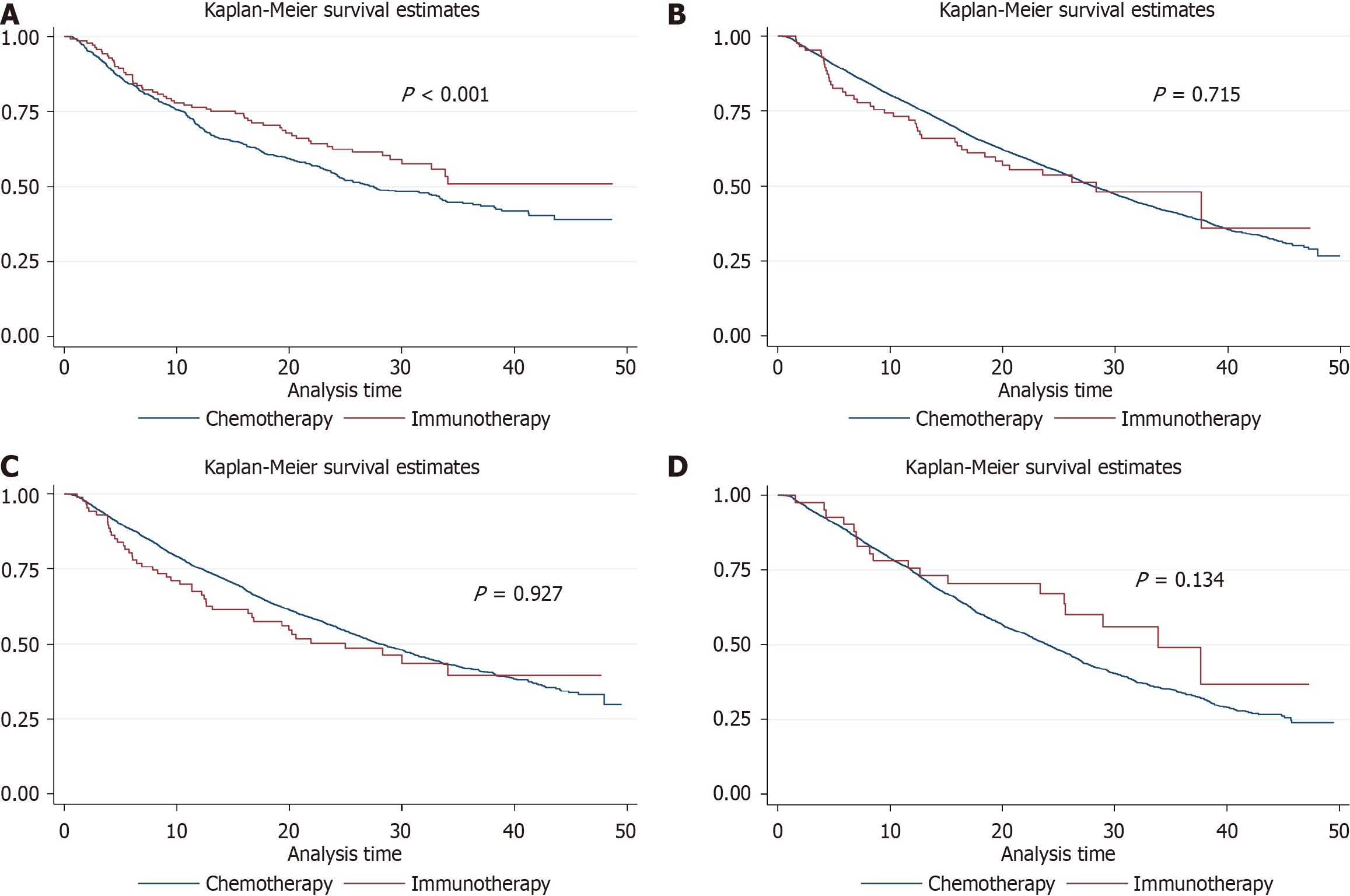
Analyzing the survival outcomes, MSI-H patients who received immunotherapy experienced a pronounced survival benefit with an aHR of 0.57 (95%CI: 0.43-0.77), suggesting a robust response to this treatment modality. This benefit contrasts with the MSS group, where immunotherapy did not provide a significant survival advantage (aHR = 0.94; 95%CI: 0.69-1.29). The one-year survival rates further illustrate this difference: 71.96% for MSS patients on immunotherapy and 76.78% for those on chemotherapy, compared to 76.55% and 69.91% for MSI-H patients, respectively. A similar pattern was observed at the three-year follow-up, with survival rates of 48.06% for immunotherapy and 40.38% for chemotherapy in the MSS group, and 50.96% and 44.35% in the MSI-H group, indicating a more pronounced long-term benefit for immunotherapy in the MSI-H category (Tables 2 and 3). The Kaplan-Meier survival curves for these groups are depicted in Figure 1A (MSS) and Figure 1B (MSI-H).
| Survival analysis | Microsatellite instability-high | Microsatellite stable | ||
| Immunotherapy vs chemotherapy | Hazard ratio (95%CI) | Adjusted hazard ratio (95%CI) | Hazard ratio (95%CI) | Adjusted hazard ratio (95%CI) |
| Overall | 0.75 (0.57-0.99) | 0.57 (0.43-0.77) | 1.05 (0.77-1.43) | 0.94 (0.69-1.29) |
| One year | 1.32 (0.92-1.92) | 1.23 (0.84-1.81) | 1.43 (0.95-2.14) | 1.37 (0.91-2.06) |
| Three year | 0.74 (0.56-0.98) | 0.62 (0.46-0.82) | 0.98 (0.72-1.34) | 0.88 (0.65-1.21) |
| Survival rate | Microsatellite stable | Microsatellite instability-high | ||
| Immunotherapy | Chemotherapy | Immunotherapy | Chemotherapy | |
| 1 yr (%) | 71.96 (61.14-80.25) | 76.78 (75.83-77.70) | 76.55 (68.64-82.72) | 69.91 (66.65-72.91) |
| 3 yr (%) | 48.06 (35.30-58.70) | 40.38 (39.01-41.74) | 50.96 (39.83-61.04) | 44.35 (40.38-48.24) |
The study also delved into the impact of KRAS mutation status on treatment outcomes. For KRAS wild-type patients, no significant difference in survival was observed between immunotherapy and chemotherapy (HR = 1.16; 95%CI: 0.86-1.56). However, in KRAS mutated patients, a trend toward improved survival was noted with immunotherapy (HR = 0.67; 95%CI: 0.42-1.07), hinting at the potential effectiveness of personalized treatment based on genetic profiles. This trend, though not statistically significant, signals a possible avenue for enhancing patient-specific treatment strategies in the future (Table 4). The corresponding survival curves are shown in Figure 1C (KRAS wild type) and Figure 1D (KRAS mutated type).
| Survival analysis | KRAS wild type | KRAS mutated type | ||
| Immunotherapy vs chemotherapy | Hazard ratio (95%CI) | Adjusted hazard ratio (95%CI) | Hazard ratio (95%CI) | Adjusted hazard ratio (95%CI) |
| Overall | 1.16 (0.86-1.56) | 1.01 (0.75-1.37) | 0.67 (0.42-1.07) | 0.70 (0.44-1.12) |
| One year | 1.28 (0.88-1.87) | 1.14 (0.78-1.68) | 1.33 (0.71-2.49) | 1.33 (0.71-2.50) |
| Three year | 1.17 (0.87-1.58) | 1.02 (0.76-1.37) | 0.66 (0.41-1.07) | 0.68 (0.42-1.09) |
In our study, utilizing data from the NCDB, we observed that in patients with MSI-H metastatic colorectal cancer, immu
Our findings echo those of Le et al[12] and Overman et al[13], underscoring the divergent responses to immunotherapy in MSI-H vs MSI-L/MSS metastatic colorectal cancers. Le et al’s research delves into the efficacy of programmed death-1 (PD-1) blockade in mismatch repair-deficient tumors, showing significant positive responses in colorectal and other cancers with MSI-H – a notable advancement in immunotherapy for these patients[12]. Similarly, Overman et al’s study focuses on the use of Nivolumab, a PD-1 inhibitor, in treating metastatic colorectal cancer patients with mismatch repair deficiencies or MSI-H, adding to the growing body of evidence in this field[13]. Boland and colleagues highlighted the significant influence of MSI on colorectal cancer, particularly emphasizing the unique tumor characteristics and varied treatment responses associated with it[17]. These findings collectively underline the intricacies of tumor biology and the critical need to incorporate MSI status in devising treatment strategies.
Our research indicates that immunotherapy does not significantly benefit patients with MSI-L/MSS metastatic colorectal cancer, a finding that contrasts sharply with the substantial efficacy observed in MSI-H metastatic colorectal cancer. This notable difference may imply a potential resistance to immunotherapeutic strategies within the MSI-L/MSS subtype, hinting at a complex, yet unexplored aspect of its molecular profile. The lower response rates to immunotherapy in MSI-L/MSS tumors can be attributed to the lower tumor mutational burden and reduced immunogenicity compared to MSI-H tumors[17]. Nonetheless, several ongoing clinical trials are investigating combination strategies, such as the use of immunotherapy with chemotherapy, targeted therapies, to enhance the efficacy of immunotherapy in MSI-L/MSS metastatic colorectal cancer[18-21].
While at first glance these results in MSI-L/MSS metastatic colorectal cancer patients may seem like a setback, they actually represent a significant advancement in our understanding of metastatic colorectal cancer. They highlight the necessity of re-evaluating our current therapeutic approaches and underscore the importance of further investigation into the distinct molecular features of the MSI-L/MSS subtype. Our findings serve as a catalyst for this critical research, driving the development of more targeted and effective treatment strategies for metastatic colorectal cancer. Echoing the sentiments of Mármol et al[22], our study supports the push towards personalized medicine in the treatment of metastatic colorectal cancer. Tailoring treatments based on genetic markers such as MSI can potentially lead to more effective and targeted therapies.
In our study, the evaluation of OS benefits associated with immunotherapy, in comparison to chemotherapy, revealed no significant differences in both KRAS mutated and wild-type colorectal cancer populations. This outcome highlights the complex interplay between genetic profiles and tumor response to immunotherapeutic agents. Existing literature has consistently shown that KRAS mutations are a common feature in colorectal cancers, often correlating with a challenging prognosis and reduced responsiveness to certain treatments, such as anti-EGFR therapies. The lack of a distinct OS advantage in either KRAS cohort within our study may suggest a broader pattern of resistance or insensitivity to immunotherapy across these genetic variations. This observation emphasizes the critical need for developing more refined and individualized treatment strategies, especially for KRAS-mutated colorectal cancer, a substantial subset of the patient population.
Our study underscores the necessity of integrating genetic profiling into therapeutic decision-making, potentially improving patient outcomes in metastatic colorectal cancer. Such an approach aligns with the evolving paradigm of personalized medicine. However, this endeavor requires careful consideration of the metastatic colorectal cancer’s genetic heterogeneity, the development of sophisticated genomic analysis techniques, and a thorough understanding of the practicalities and challenges in implementing personalized treatment regimens, including economic and logistical factors.
This study encountered several limitations that are important to acknowledge. Firstly, the retrospective nature of the study may have introduced selection bias, as the choice of treatment might have been influenced by unmeasured factors. Additionally, the NCDB lacks detailed information on treatment regimens, duration, and response to therapy, which precludes further exploration of the impact of different agents, combinations, or lines of therapy. Information on potential predictive biomarkers, such as tumor mutational burden and PD-L1 expression, was not available. Another significant limitation is the variability in data due to incomplete information on specific molecular characteristics of the colorectal tumors in some patients, which may impact the study's conclusions. Lastly, our study population included patients diagnosed till 2020, which may not reflect the most recent advances in metastatic colorectal cancer treatment. Given these limitations, it is crucial to undertake further research in this field to enhance our understanding of MSS metastatic colorectal cancer and to develop more effective treatment strategies.
Our population-based study demonstrates that immunotherapy treatment is associated with significantly improved OS in patients with MSI-H metastatic colorectal cancer, but not in those with MSI-L/MSS metastatic colorectal cancer. These findings suggest that immunotherapy treatment should be considered for patients with MSI-H metastatic colorectal cancer, while further studies are warranted to determine the optimal therapeutic approach for patients with MSI-L/MSS metastatic colorectal cancer.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Moldovan CA, Romania S-Editor: Zhang L L-Editor: A P-Editor: Zhao S
| 1. | Granados-Romero JJ, Valderrama-Treviño AI, Contreras-Flores EH, Barrera-Mera B, Herrera Enríquez M, Uriarte-Ruíz K, Ceballos-Villalba JC, Estrada-Mata AG, Alvarado Rodríguez C, Arauz-Peña G. Colorectal cancer: a review. Int J Res Med Sci. 2017;5:4667. Available from: https://doi.org/10.18203/2320-6012.ijrms20174914. |
| 2. | Simon K. Colorectal cancer development and advances in screening. Clin Interv Aging. 2016;11:967-976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 428] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 3. | Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 9940] [Article Influence: 4970.0] [Reference Citation Analysis (2)] |
| 4. | National Cancer Institute Surveillance, Epidemiology, and End Results Program. 2021 [cited 2021 Jan 28]. Database: Cancer stat facts: colorectal cancer [Internet]. Available from: https://seer.cancer.gov/statfacts/html/colorect.html. |
| 5. | Guo Y, Xiong BH, Zhang T, Cheng Y, Ma L. XELOX vs. FOLFOX in metastatic colorectal cancer: An updated meta-analysis. Cancer Invest. 2016;34:94-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Baraniskin A, Buchberger B, Pox C, Graeven U, Holch JW, Schmiegel W, Heinemann V. Efficacy of bevacizumab in first-line treatment of metastatic colorectal cancer: A systematic review and meta-analysis. Eur J Cancer. 2019;106:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Oliner KS, Tian Y, Xu F, Sidhu R. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 423] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 8. | Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Hielscher J, Scholz M, Müller S, Link H, Niederle N, Rost A, Höffkes HG, Moehler M, Lindig RU, Modest DP, Rossius L, Kirchner T, Jung A, Stintzing S. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1331] [Article Influence: 121.0] [Reference Citation Analysis (0)] |
| 9. | Tougeron D, Sueur B, Zaanan A, de la Fouchardiére C, Sefrioui D, Lecomte T, Aparicio T, Des Guetz G, Artru P, Hautefeuille V, Coriat R, Moulin V, Locher C, Touchefeu Y, Lecaille C, Goujon G, Ferru A, Evrard C, Chautard R, Gentilhomme L, Vernerey D, Taieb J, André T, Henriques J, Cohen R; Association des Gastro-entérologues Oncologues (AGEO). Prognosis and chemosensitivity of deficient MMR phenotype in patients with metastatic colorectal cancer: An AGEO retrospective multicenter study. Int J Cancer. 2020;147:285-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Shulman K, Barnett-Griness O, Friedman V, Greenson JK, Gruber SB, Lejbkowicz F, Rennert G. Outcomes of Chemotherapy for Microsatellite Instable-High Metastatic Colorectal Cancers. JCO Precis Oncol. 2018;2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Bendell J, Le DT, Yoshino T, Van Cutsem E, Yang P, Farooqui MZH, Marinello P, Diaz LA Jr; KEYNOTE-177 Investigators. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med. 2020;383:2207-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 1808] [Article Influence: 361.6] [Reference Citation Analysis (0)] |
| 12. | Le DT, Kim TW, Van Cutsem E, Geva R, Jäger D, Hara H, Burge M, O'Neil B, Kavan P, Yoshino T, Guimbaud R, Taniguchi H, Elez E, Al-Batran SE, Boland PM, Crocenzi T, Atreya CE, Cui Y, Dai T, Marinello P, Diaz LA Jr, André T. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J Clin Oncol. 2020;38:11-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 696] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 13. | Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, Goldberg MV, Cao ZA, Ledeine JM, Maglinte GA, Kopetz S, André T. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1775] [Cited by in RCA: 2089] [Article Influence: 261.1] [Reference Citation Analysis (0)] |
| 14. | Eng C, Kim TW, Bendell J, Argilés G, Tebbutt NC, Di Bartolomeo M, Falcone A, Fakih M, Kozloff M, Segal NH, Sobrero A, Yan Y, Chang I, Uyei A, Roberts L, Ciardiello F; IMblaze370 Investigators. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019;20:849-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 409] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 15. | Mohanty S, Bilimoria KY. Comparing national cancer registries: The National Cancer Data Base (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) program. J Surg Oncol. 2014;109:629-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 16. | Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, Lamas GA; MOde Selection Trial Investigators. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107:2932-2937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1195] [Cited by in RCA: 1217] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 17. | Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073-2087.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1677] [Cited by in RCA: 1544] [Article Influence: 102.9] [Reference Citation Analysis (0)] |
| 18. | Mettu NB, Twohy E, Ou F-S, Halfdanarson TR, Lenz HJ, Breakstone R, Boland PM, Crysler O, Wu C, Grothey A, Nixon AB, Bolch E, Niedzwiecki D, Fruth B, Schweitzer B, Elsing A, Hurwitz H, Fakih MG, Bekaii-Saab T. BACCI: A phase II randomized, double-blind, multicenter, placebo-controlled study of capecitabine (C) bevacizumab (B) plus atezolizumab (A) or placebo (P) in refractory metastatic colorectal cancer (mCRC): An ACCRU network study. Ann Oncol. 2019;30:v203. |
| 19. | Fang XF, Zhong CH, Zhu N, Weng SS, Hu HG, Wang J, Xiao Q, Wang JW, Song YM, Sun LF, Xu D, Liao XJ, Dong CX, Zhang SZ, Li J, Ding KF, Yuan Y. A phase 2 trial of sintilimab (IBI 308) in combination with CAPEOX and bevacizumab (BBCAPX) as first-line treatment in patients with RAS-mutant, microsatellite stable, unresectable metastatic colorectal cancer. J Clin Oncol. 2022;40:3563-3563. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Fumet JD, Chibaudel B, Bennouna J, Borg C, Martin-Babau J, Cohen R, Fonck M, Taieb J, Thibaudin M, Limagne E, Blanc J, Bertaut A, Ghiringhelli F. 433P Durvalumab and tremelimumab in combination with FOLFOX in patients with previously untreated RAS-mutated metastatic colorectal cancer: First results of efficacy at one year for phase II MEDITREME trial. Ann Oncol. 2021;32:S551. [RCA] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 21. | Damato A, Iachetta F, Normanno N, Bergamo F, Maiello E, Zaniboni A, Antonuzzo L, Nasti G, Tonini G, Bordonaro R, Fabio DF, Romagnani A, Berselli A, Pinto C. NIVACOR: Phase II study of nivolumab in combination with FOLFOXIRI/bevacizumab in first-line chemotherapy for advanced colorectal cancer RASm/BRAFm patients. J Clin Oncol. 2020;38:TPS4118-TPS4118. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 520] [Cited by in RCA: 907] [Article Influence: 113.4] [Reference Citation Analysis (2)] |