Published online Mar 24, 2024. doi: 10.5306/wjco.v15.i3.456
Peer-review started: January 2, 2024
First decision: January 10, 2024
Revised: January 23, 2024
Accepted: February 21, 2024
Article in press: February 21, 2024
Published online: March 24, 2024
Processing time: 79 Days and 21.6 Hours
SMARCA4 is a component of chromatin remodeling of SWItch/sucrose-nonfermenting (SWI/SNF) complexes and plays an essential role in oncogenesis. SMARCA4-deficient malignancies arising from the gastrointestinal tract are rare and have a poor prognosis. There is no standard treatment for advanced and undifferentiated SMARCA4-deficient duodenal malignancies. Programmed death 1 (PD-1) antibodies, known as immune checkpoint inhibitor antibodies, potentially play a role in treating gastrointestinal tract malignancies.
We present two patients with SMARCA4 deficiency and TP53 gene mutation in advanced undifferentiated carcinomas of the duodenum. For both patients, SMARCA4 deficiency was confirmed by immunohistochemical staining for the BRG1 protein, while TP53 gene mutations were observed via next-generation sequencing. Both patients were administered chemotherapy in combination with an anti-PD-1 antibody. The two patients exhibited completely different responses to treatment and had different prognoses. Case 1 experienced rapid progression after PD-1 infusion and chemotherapy, case 2 experienced a remarkable response after treatment, and the progression-free survival was more than 6 months.
This study described our clinical and pathological observations of SMARCA4-deficient advanced undifferentiated carcinoma of the duodenum. PD-1 combined with chemotherapy showed a certain efficacy in select patients, providing options for treating these highly malignant tumors. Patients with liver metastases had a worse prognosis than did those with only lymph node metastasis.
Core Tip: SMARCA4-deficient malignancies arising from the gastrointestinal tract are rare and have a poor prognosis. We present two patients diagnosed with advanced duodenal undifferentiated carcinoma by immunohistochemical staining for SMARCA4 deficiency and TP53 gene mutations. Patients with high tumor mutational burden responded well to programmed death 1 antibodies in combination with chemotherapy, and those with liver metastases had a worse prognosis.
- Citation: Shi YN, Zhang XR, Ma WY, Lian J, Liu YF, Li YF, Yang WH. PD-1 antibody in combination with chemotherapy for the treatment of SMARCA4-deficient advanced undifferentiated carcinoma of the duodenum: Two case reports. World J Clin Oncol 2024; 15(3): 456-463
- URL: https://www.wjgnet.com/2218-4333/full/v15/i3/456.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i3.456
The small intestine accounts for only 1%-1.4% of all gastrointestinal (GI) malignancies. The most common primary malignancy in the small bowel is adenocarcinoma, with the duodenum being the most frequently affected site. Various histological subtypes of duodenal malignancies, including adenocarcinoma, sarcoma, lymphoma, and neuroendocrine tumors, have been identified. Undifferentiated carcinoma of the duodenum, characterized by rhabdoid features, is a rare form of malignancy. A previous study revealed that, in most cases, patients with undifferentiated/rhabdoid carcinoma in the gastrointestinal tract lacked expression of at least one of the four SWItch/sucrose-nonfermenting (SWI/SNF) complex subunits, namely, SMARCB1, SMARCA2, SMARCA4, and ARID1A[1,2]. The SWI/SNF complex is an ATP-consuming multisubunit cellular machine that modulates chromatin compaction, thereby regulating DNA-related processes such as transcription, replication, and DNA repair and oncogenesis[3,4]. The absence of the key proteins INI1 and BRG1, which are encoded by SMARCB1 and SMARCA4, respectively, is commonly observed in various malignancies, including atypical teratoid/rhabdoid tumors, epithelioid sarcoma, and ovarian small-cell carcinomas of the hypercalcemic type[3,5].
Recently, investigations into SMARCA4 inactivation as a driver event in malignancies have been performed. Mutations leading to the loss of expression of these protein components have also been identified in a subset of poorly differentiated or undifferentiated carcinomas at many sites throughout the body. These include the sinonasal tract[4], lung[5], gastrointestinal tract[6,7], and uterus[8].
In this case report, we documented two patients diagnosed with duodenal SMARCA4-deficient undifferentiated carcinoma who underwent immunohistopathological tests combined with next-generation sequencing and multiplex immunofluorescence analysis. There is no current standard treatment for advanced and undifferentiated SMARCA4-deficient duodenal malignancies. Both patients were administered chemotherapy in combination with programmed death 1 (PD-1) antibody, known as immune checkpoint inhibitor (ICI). Patients with high tumor mutational burden (TMB) responded well to PD-1 antibodies in combination with chemotherapy, and those with liver metastases had a worse prognosis.
Case 1: A 51-year-old male patient presented to our hospital with increasing upper abdominal pain for more than one month.
Case 2: A 43-year-old female complained of intermittent upper abdominal pain for 4 months and enlargement of the left supraclavicular lymph nodes.
Case 1: The patient’s premorbidity was good, with an Eastern Cooperative Oncology Group performance status score of 0. Gastroscopy revealed an ulcer in the descending part of the duodenum, approximately 3 cm × 3.5 cm, with irregular protrusions around the perimeter. A biopsy from the descending part of the duodenum was performed by endoscopy, and an ulcerative mass was found.
Case 2: Gastroenteroscopy was also conducted, and an ulcer in the duodenal papilla was found. Biopsy revealed undifferentiated carcinoma of the duodenum, and contrast-enhanced computed tomography (CT) indicated multiple lymph node metastases involving the retroperitoneal lymph nodes.
Case 1: He had no history of smoking or drinking or a significant medical history.
Case 2: She had no history of smoking or drinking or a significant medical history.
Case 1: The patient had no personal or family history.
Case 2: The patient had no personal or family history.
Case 1: The patient had mild upper abdominal tenderness.
Case 2: A mass in the left neck was palpated.
Case 1: All of the tumor marker results in the blood sample were negative.
Case 2: An elevated CEA level of 5.14 μg/L was observed. CA199, CA242, and CA724 were all negative.
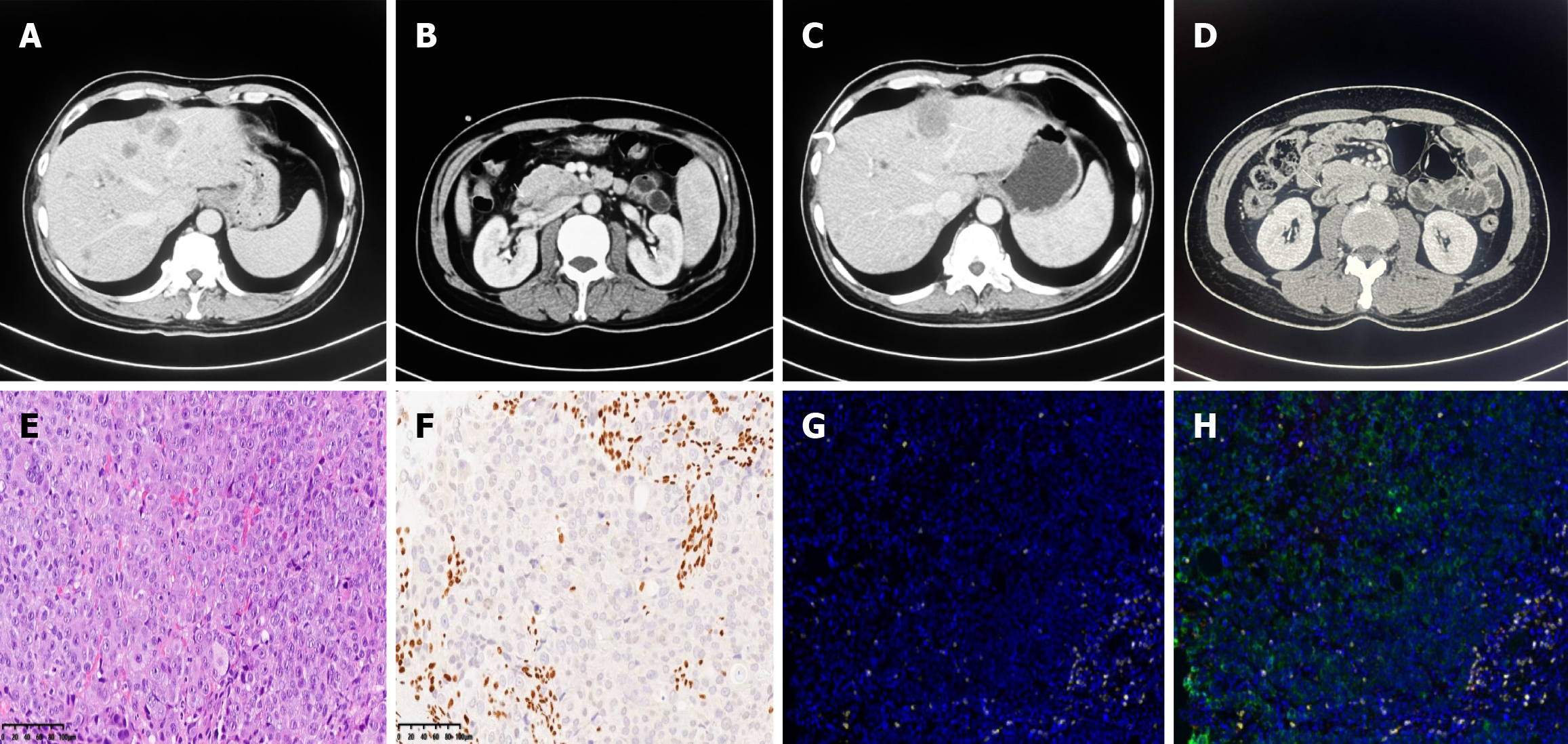
Case 1: An initial CT scan and magnetic resonance imaging (MRI) with enhanced contrast agent injection showed abnormal wall thickening in the duodenal ampulla and multiple liver nodular masses revealing malignant metastases. Retroperitoneal lymphadenopathy was also considered (Figure 1A and B).
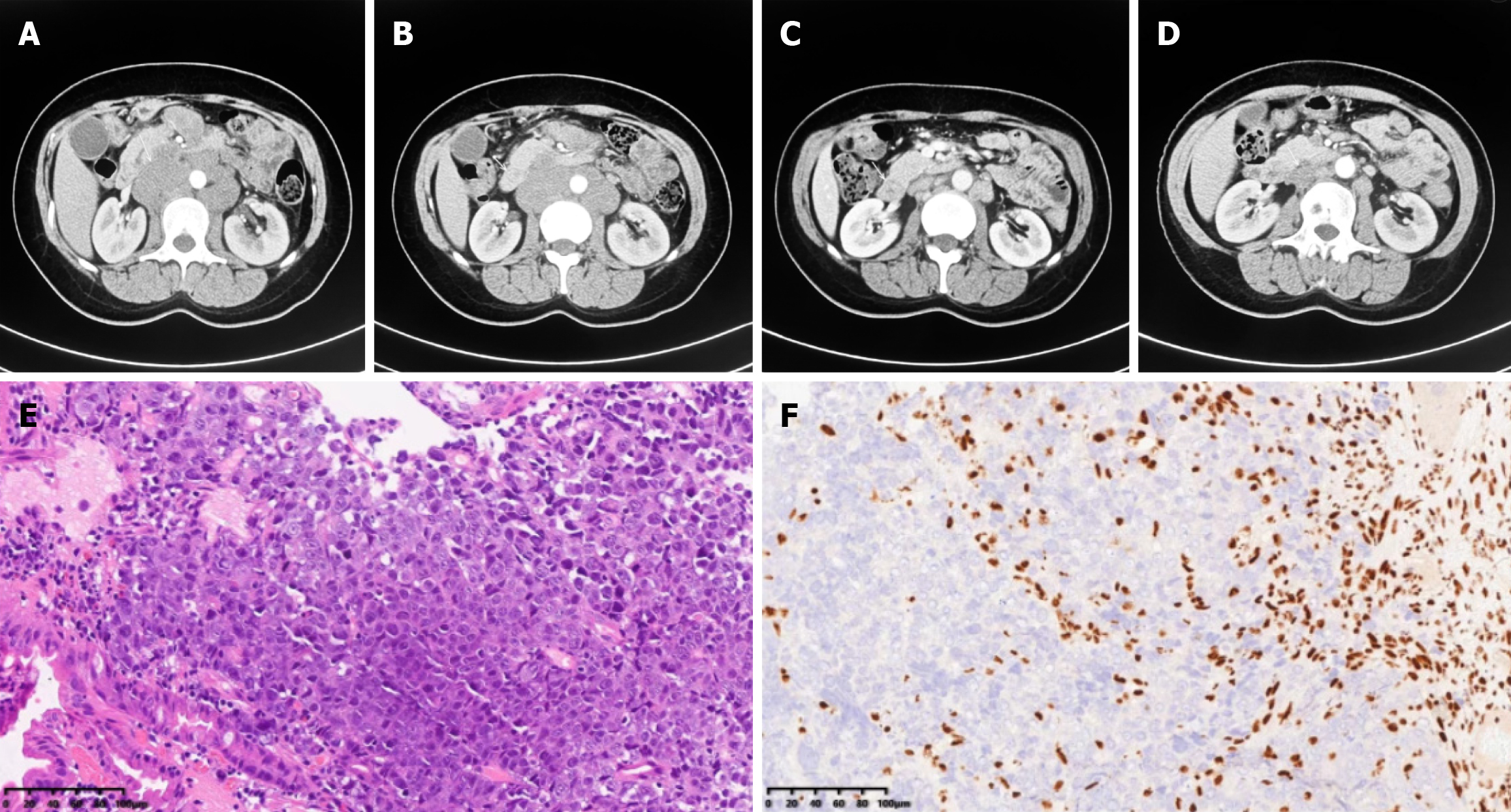
Case 2: Gastroenteroscopy was also conducted, and an ulcer in the duodenal papilla was found. Biopsy revealed undifferentiated carcinoma of the duodenum. Contrast-enhanced CT indicated multiple lymph node metastases involving the retroperitoneal lymph nodes (Figure 2A and B).
Case 1: Histology was performed, and the epithelioid or undifferentiated tumor cells grew in cords or nests. hematoxylin-eosin staining is shown in Figure 1E. Extensive areas of necrosis were observed within the tumor. The immunohistological analysis revealed the following results: Ki-67 (approximately more than 90% positive), CK7 (-), SMARCA4 (-), and INSM1 (-) (Figure 1F). Multiplex immunofluorescence staining was also analyzed. PD-L1 expression was positive, with a CPS of 5, and microsatellite stabilization (MSS) was observed. The tumor immune microenvironment was classified as an acquired immune tolerance type according to the presence of both CD8+ tumor-infiltrated lymphocytes TILs and PD-L1-expressing cells. Notably, tertiary lymphoid structures were not found in the tumor area (Figure 1G and H). Next-generation sequencing of biopsy tissue was subsequently performed. The results revealed TP53 and CTNNB1 mutations (Table 1). However, the SMARCA4 mutation variant was not observed in this patient.
| Gene | Transcript description | Mutant allele frequency | |
| Case 1 | TP53 | NM_000546.5:c.742C>T(p.Arg 248Trp) | 20.9% |
| CTNNB1 | NM_001098209.1:c.134C>T(p.Ser45Phe) | 23.4% | |
| Case 2 | TP53 | NM_000546.5: c.1024C>T(p.R342) | 23.2% |
| NCOR1 | NM_006311.3 :c.5689T>G (p.S1897A) | 18.7% | |
| MYH9 | NM_002473.4 : c.1560C[6>7] (p.I524Hfs*44) | 15.9% | |
| ERBB3 | NM_001982.3 : c.850G>C (p.G284R) | 15.0% | |
| RAD52 | NM_134424.2 : c.779G>A (p.R260Q) | 14.8% | |
| CTNNB1 | NM_001904.3 :c.133T>C (p.S45P) | 14.2% |
The final pathological diagnosis was duodenal SMARCA4-deficient undifferentiated carcinoma, clinical stage IV with multiple liver metastases and retroperitoneal lymph node metastases.
Case 2: Immunohistopathological staining revealed the following results: Ki-67 (90%+) and SMARCA4 (-) (Figure 2E and F). Next-generation sequencing revealed TP53, NCOR1, MYH9, ERBB3, RAD52 and CTNNB1 mutations (Table 1), and the TMB increased to 10.56/Mb, with a score of 6 for PD-L1 expression. MSS was observed. However, the SMARCA4 mutation variant was not observed in this patient.
The final pathological diagnosis was duodenal SMARCA4-deficient undifferentiated carcinoma, clinical stage IV with multiple lymph node metastases.
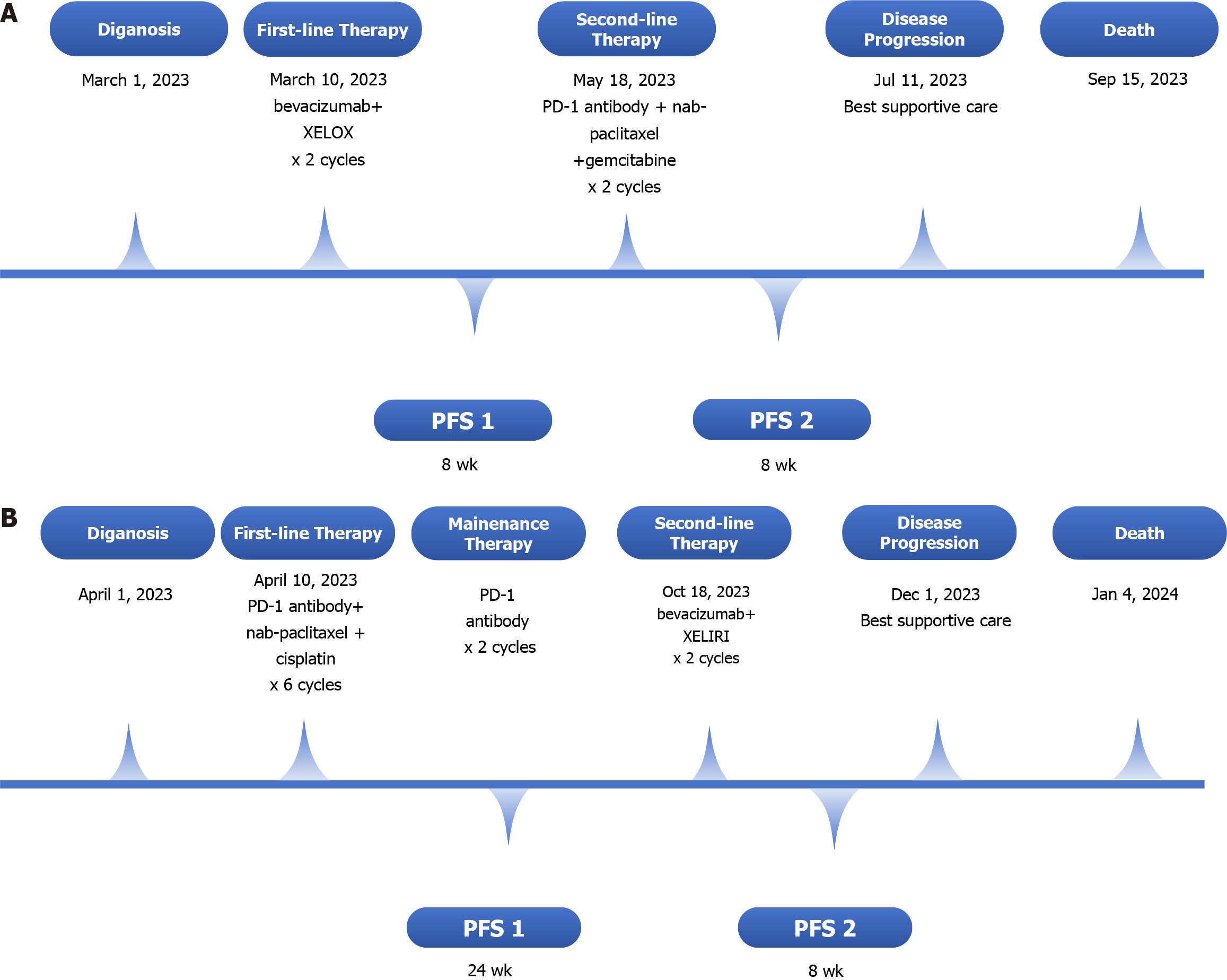
First, two cycles of bevacizumab in combination with the XELOX regimen were initiated on March 10, 2023 (bevacizumab, 7.5 mg/kg D1 + oxaliplatin, 130 mg/m2 D1 + capecitabine, 1250 mg/m2 D1-14; Q21D). However, an enlargement of liver metastases was detected, and disease progression was considered. Given the positive expression of PD-L1 and the acquired immune tolerance environment, we introduced a second-line combination therapy comprising a PD-1 antibody, nab-paclitaxel and gemcitabine (nab-paclitaxel 220 mg/m2 D1 + gemcitabine 1000 mg/m2 D1 and D8; Q21D), and the patient initially exhibited good tolerance. After two cycles of second-line immunotherapy-chemotherapy combination therapy, progressive disease was observed as liver-targeted lesions increased significantly after infusion (Figure 1C and D). Disease progression was considered. However, the patient refused subsequent immunochemotherapy. The timeline of the summarizing events is shown in Figure 3A.
This patient received PD-1 antibody in combination with chemotherapy comprising nab-paclitaxel and cisplatin for six cycles (nab-paclitaxel 220 mg/m2 D1 + cisplatin 80 mg/m2 D1; Q21D). The patient responded positively to this combination therapy. Disease regression with extensively shrunken retroperitoneal lymph nodes was observed (Figure 2C and D), with an improvement in the general condition of the patient. After six cycles of combination therapy, the patient started PD-1 antibody maintenance treatment, and the disease was in remission. Patients showed disease progression with liver metastases at 6 months after diagnosis, and second-line therapy was administered. The second-line therapy consisted of the XELIRI regimen and bevacizumab (bevacizumab, 7.5 mg/kg D1 + irinotecan, 180 mg/m2 D1 + capecitabine, 1250 mg/m2 D1-14; Q21D). The patient was administered two cycles of second-line treatment and started receiving the best supportive care for cachexia and obvious weight loss. The timeline of the summarizing events is shown in Figure 3B.
The patient suffered from significant mixed jaundice and showed no improvement after supportive care. Finally, the patient died from liver failure on September 15, 2023, with an overall survival period of 6 months. The progression-free survival (PFS) times for first-line and second-line therapy were both 8 wk.
The patient died from this malignancy on January 4, 2024.
Duodenal malignancies are uncommon but highly fatal, and undifferentiated duodenal malignancies are extremely rare. As extensive immunohistochemical profiling has been performed for malignancies such as atypical rhabdoid tumors or epithelioid sarcomas, SMARCA4-deficient malignancies are frequently observed and can be identified[1,9]. Undifferentiated carcinomas with rhabdoid cells are characteristic diagnostic clues, and SWI/SNF complex deficiencies are complex molecular events of undifferentiated carcinoma. It is highly malignant and has a short survival time, with no standard treatment at present. Several case reports and retrospective studies have analyzed SMARCA4-deficient malignancies arising from the gastrointestinal tract[1,6,7,9]. A previous study demonstrated that a small proportion of gastroesophageal carcinomas exhibit loss of SMARCA4 expression separately or with coinactivation of other subunits of SWI/SNF complexes[1]. Among the two patients, not all SMARCA4-deficient tumors harbored SMARCA4 pathological genomic variants. Although these tumors exhibit poorly differentiated and undifferentiated morphologies, they exhibit a broad range of genomic variant features[7,9,10]. First, compared with gastroesophageal adenocarcinomas, SMARCA4-deficient gastroesophageal carcinomas exhibit a similar range of somatic mutations, including enrichment of TP53, KRAS, ARID1A, and APC mutations. The most common cooccurring mutations in pathogenic SMARCA4 were identified in TP53, APC, ARID1A, CDKN2A, and CTNNB1[7]. On the one hand, this difference may be attributed to the limitations of NGS in accurately detecting large deletions. Additionally, epigenetic modifications, including DNA methylation, histone modifications, and noncoding RNAs, may also contribute to deficiencies in gene expression. Therefore, some investigators have proposed that most SMARCA4-deficient gastroesophageal carcinomas are considered undifferentiated or dedifferentiated gastroesophageal adenocarcinomas rather than distinct biological entities[9,10].
Several factors, such as PD-L1 expression, tumor mutational burden, body mass index, and laboratory parameters (including the neutrophil-to-lymphocyte ratio), may predict the response to immunotherapy as well as immune chemotherapy.
As somatic mutations generate neoantigens, a high TMB is expected to induce a positive antitumor response. However, it can serve as a biomarker for predicting favorable responses to ICIs. The ability of high TMB to predict the efficacy of immunotherapy was not affected by the expression of PD-L1. Hence, some patients benefit from immunotherapy and have prolonged survival[10,11]. The infiltration of CD8+ lymphocytes and positive PD-L1 expression are also considered potential predictive biomarkers of immunotherapy response[12].
In previous studies, immunochemotherapy has been shown to have various anticancer effects, including immunogenic cell death and a reduction in the number of tumor cells that are engaged in the production of immunosuppressive substances[13,14]. Furthermore, the combination of chemotherapy and immunotherapy results in the depletion of myeloid-derived suppressor cells and regulatory T cells. Building upon these principles, the use of chemotherapy combined with immune checkpoint inhibitors (ICIs) has demonstrated synergistic effects. Over the past few years, several trials investigating combination strategies of chemotherapy and ICIs have been presented and published, leading to their approval for diverse solid tumors. Examples of these approved combinations can be found in non-small cell lung cancer, gastric cancer and urothelial carcinoma[15]. As mentioned above, in our second patient, a high TMB was associated with a major partial response to PD-1 antibodies during first-line therapy.
The clinical significance of recognizing SWI/SNF complex-deficient undifferentiated carcinoma/rhabdoid carcinoma lies in its aggressive clinical behavior and poor response to traditional chemotherapy. SMARCA4-deficient undifferentiated tumors originating from the gastrointestinal tract might have a worse prognosis than those originating from the thorax[11,16]. Adding immunotherapy to conventional chemotherapy may improve the treatment efficacy and increase the response rate. This study was the first to describe the response of SMARCA4-deficient undifferentiated duodenal tumors to immunochemotherapy. According to our observations, case 1 did not benefit from immunotherapy as a second-line chemotherapy, while case 2 benefited from immunochemotherapy combination therapy. The differences in the response of the two patients were partially due to differences in metastatic target organs, differences in mutation genes and differences in the tumor microenvironment, which resulted in differences in tumor mutation burdens. A study reported a median progression-free survival time of 7.2 months in patients who initially presented with metastatic disease, whereas the median overall survival was 13.6 months in patients with malignancies involving the esophagogastric junction and stomach[9].
In brief, this case report presented the histopathological and clinical responses of two patients with SMARCA4-deficient advanced undifferentiated carcinoma of the duodenum. PD-1 combined with chemotherapy showed a certain efficacy in select patients, providing options for treating these highly malignant tumors. Patients with liver metastases had a worse prognosis than did those with only lymph node metastasis. Potential molecular mechanisms need to be further studied to elucidate this phenomenon.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Rizzo A, Italy S-Editor: Gong ZM L-Editor: A P-Editor: Zhao S
| 1. | Agaimy A, Daum O, Märkl B, Lichtmannegger I, Michal M, Hartmann A. SWI/SNF Complex-deficient Undifferentiated/Rhabdoid Carcinomas of the Gastrointestinal Tract: A Series of 13 Cases Highlighting Mutually Exclusive Loss of SMARCA4 and SMARCA2 and Frequent Co-inactivation of SMARCB1 and SMARCA2. Am J Surg Pathol. 2016;40:544-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 2. | Mardinian K, Adashek JJ, Botta GP, Kato S, Kurzrock R. SMARCA4: Implications of an Altered Chromatin-Remodeling Gene for Cancer Development and Therapy. Mol Cancer Ther. 2021;20:2341-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 3. | Jelinic P, Mueller JJ, Olvera N, Dao F, Scott SN, Shah R, Gao J, Schultz N, Gonen M, Soslow RA, Berger MF, Levine DA. Recurrent SMARCA4 mutations in small cell carcinoma of the ovary. Nat Genet. 2014;46:424-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 286] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 4. | Rooper LM, Uddin N, Gagan J, Brosens LAA, Magliocca KR, Edgar MA, Thompson LDR, Agaimy A, Bishop JA. Recurrent Loss of SMARCA4 in Sinonasal Teratocarcinosarcoma. Am J Surg Pathol. 2020;44:1331-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | Sauter JL, Graham RP, Larsen BT, Jenkins SM, Roden AC, Boland JM. SMARCA4-deficient thoracic sarcoma: a distinctive clinicopathological entity with undifferentiated rhabdoid morphology and aggressive behavior. Mod Pathol. 2017;30:1422-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 137] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 6. | Gupta S, Noona SW, Pambuccian SE, Robinson B, Martin LW, Williams E, Stelow EB, Raghavan SS. Malignant undifferentiated and rhabdoid tumors of the gastroesophageal junction and esophagus with SMARCA4 loss: a case series. Hum Pathol. 2023;134:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Neil AJ, Zhao L, Isidro RA, Srivastava A, Cleary JM, Dong F. SMARCA4 Mutations in Carcinomas of the Esophagus, Esophagogastric Junction, and Stomach. Mod Pathol. 2023;36:100183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Kolin DL, Dong F, Baltay M, Lindeman N, MacConaill L, Nucci MR, Crum CP, Howitt BE. SMARCA4-deficient undifferentiated uterine sarcoma (malignant rhabdoid tumor of the uterus): a clinicopathologic entity distinct from undifferentiated carcinoma. Mod Pathol. 2018;31:1442-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 9. | Chang B, Sheng W, Wang L, Zhu X, Tan C, Ni S, Weng W, Huang D, Wang J. SWI/SNF Complex-deficient Undifferentiated Carcinoma of the Gastrointestinal Tract: Clinicopathologic Study of 30 Cases With an Emphasis on Variable Morphology, Immune Features, and the Prognostic Significance of Different SMARCA4 and SMARCA2 Subunit Deficiencies. Am J Surg Pathol. 2022;46:889-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Zhu PP, Li XX, Liu JH, Du XL, Su HY, Wang J. [SMARCA4-deficient undifferentiated carcinoma of the gastrointestinal tract: a clinicopathological and immunohistochemical study of nine cases]. Zhonghua Bing Li Xue Za Zhi. 2022;51:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Schoenfeld AJ, Bandlamudi C, Lavery JA, Montecalvo J, Namakydoust A, Rizvi H, Egger J, Concepcion CP, Paul S, Arcila ME, Daneshbod Y, Chang J, Sauter JL, Beras A, Ladanyi M, Jacks T, Rudin CM, Taylor BS, Donoghue MTA, Heller G, Hellmann MD, Rekhtman N, Riely GJ. The Genomic Landscape of SMARCA4 Alterations and Associations with Outcomes in Patients with Lung Cancer. Clin Cancer Res. 2020;26:5701-5708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 186] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 12. | Iijima Y, Sakakibara R, Ishizuka M, Honda T, Shirai T, Okamoto T, Tateishi T, Sakashita H, Tamaoka M, Takemoto A, Kumaki Y, Ikeda S, Miyazaki Y. Notable response to nivolumab during the treatment of SMARCA4-deficient thoracic sarcoma: a case report. Immunotherapy. 2020;12:563-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Rizzo A, Ricci AD, Lanotte L, Lombardi L, Di Federico A, Brandi G, Gadaleta-Caldarola G. Immune-based combinations for metastatic triple negative breast cancer in clinical trials: current knowledge and therapeutic prospects. Expert Opin Investig Drugs. 2022;31:557-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 14. | Mollica V, Rizzo A, Marchetti A, Tateo V, Tassinari E, Rosellini M, Massafra R, Santoni M, Massari F. The impact of ECOG performance status on efficacy of immunotherapy and immune-based combinations in cancer patients: the MOUSEION-06 study. Clin Exp Med. 2023;23:5039-5049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 108] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 15. | Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, Rodríguez-Cid J, Wilson J, Sugawara S, Kato T, Lee KH, Cheng Y, Novello S, Halmos B, Li X, Lubiniecki GM, Piperdi B, Kowalski DM; KEYNOTE-407 Investigators. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med. 2018;379:2040-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1941] [Cited by in RCA: 2666] [Article Influence: 380.9] [Reference Citation Analysis (0)] |
| 16. | Kim TK, Herbst RS, Chen L. Defining and Understanding Adaptive Resistance in Cancer Immunotherapy. Trends Immunol. 2018;39:624-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |