Published online Feb 24, 2024. doi: 10.5306/wjco.v15.i2.178
Peer-review started: October 28, 2023
First decision: December 29, 2023
Revised: January 6, 2024
Accepted: January 25, 2024
Article in press: January 25, 2024
Published online: February 24, 2024
Processing time: 114 Days and 16.9 Hours
Gliomas are primary brain tumors derived from glial cells of the central nervous system, afflicting both adults and children with distinct characteristics and therapeutic challenges. Recent developments have ushered in novel clinical and molecular prognostic factors, reshaping treatment paradigms based on classification and grading, determined by histological attributes and cellular lineage. This review article delves into the diverse treatment modalities tailored to the specific grades and molecular classifications of gliomas that are currently being discussed and used clinically in the year 2023. For adults, the therapeutic triad typically consists of surgical resection, chemotherapy, and radiotherapy. In contrast, pediatric gliomas, due to their diversity, require a more tailored approach. Although complete tumor excision can be curative based on the location and grade of the glioma, certain non-resectable cases demand a chemotherapy approach usually involving, vincristine and carboplatin. Addi
Core Tip: Gliomas are primary brain tumors derived from glial cells of the central nervous system, afflicting both adults and children. This review article explores the therapeutic modalities employed for both adult and pediatric gliomas in the context of their molecular classification. Gliomas are classified by World Health Organization using a grading system grade 1 to 4 differentiating the grades by the gliomas attributes such as progression. The current trident approach to glioma treatment is surgery, radiotherapy, and chemotherapy. Pediatric cases are more nuanced, creating a more challenging approach to treating pediatric gliomas compared to their adult counterpart.
- Citation: Mohamed AA, Alshaibi R, Faragalla S, Mohamed Y, Lucke-Wold B. Updates on management of gliomas in the molecular age. World J Clin Oncol 2024; 15(2): 178-194
- URL: https://www.wjgnet.com/2218-4333/full/v15/i2/178.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i2.178
Gliomas, a diverse group of primary brain tumors arising from glial cells, continue to pose formidable challenges in the field of oncology. They account for the overarching majority of adult brain tumors, making it critical to achieve proper management and better prognoses. The World Health Organization (WHO) classifies gliomas based on histology, grade, and molecular information, with the primary focus being molecular markers that govern treatment plans and expected outcomes[1,2]. The broad spectrum encapsulated by gliomas range from low-grade tumors with relatively favorable prognoses to highly aggressive and invariably fatal high-grade glioblastomas. In previous decades, limited therapeutic options existed, disposing patients to a bleak prognosis. However, recent years have seen a surge in academic efforts, leading to a deeper understanding of the molecular and genetic underpinnings of gliomas.
The current trident approach to glioma treatment of surgery, radiotherapy, and chemotherapy has significantly benefited from molecular diagnostic tools that provide providers with advanced tools for both diagnosis and targeted treatment of patients[3]. Since recurrent gliomas can occur due to neural stem-cell regeneration of tumors after surgical resection, treatments have become geared towards the molecular suppression of such cells[4]. This is increasingly important as prognosis is often highly dependent on the molecular make-up of the tumor. For instance, isocitrate dehydrogenase type (IDH) mutations and 1p/19q codeletions have been associated with lower-grade gliomas, while CDKN2A/B deletions suggest a more disadvantageous prognosis[5-7]. The specific significance of molecular markers will change as a patient ages, as the aforementioned factors become more sparse in an advanced age population. Other valuable measures, such as the positive prognostic factor of O6-methylguanine-DNA methyltransferase (MGMT), are noted in this older population[8].
With an increasing ability to further identify and classify tumors, advancements in molecular genetics offer a path to better prognosis for many patients. This review article addresses the current state of management for gliomas, focusing on tailored treatment modalities which aim to maximize lifespan and quality of life.
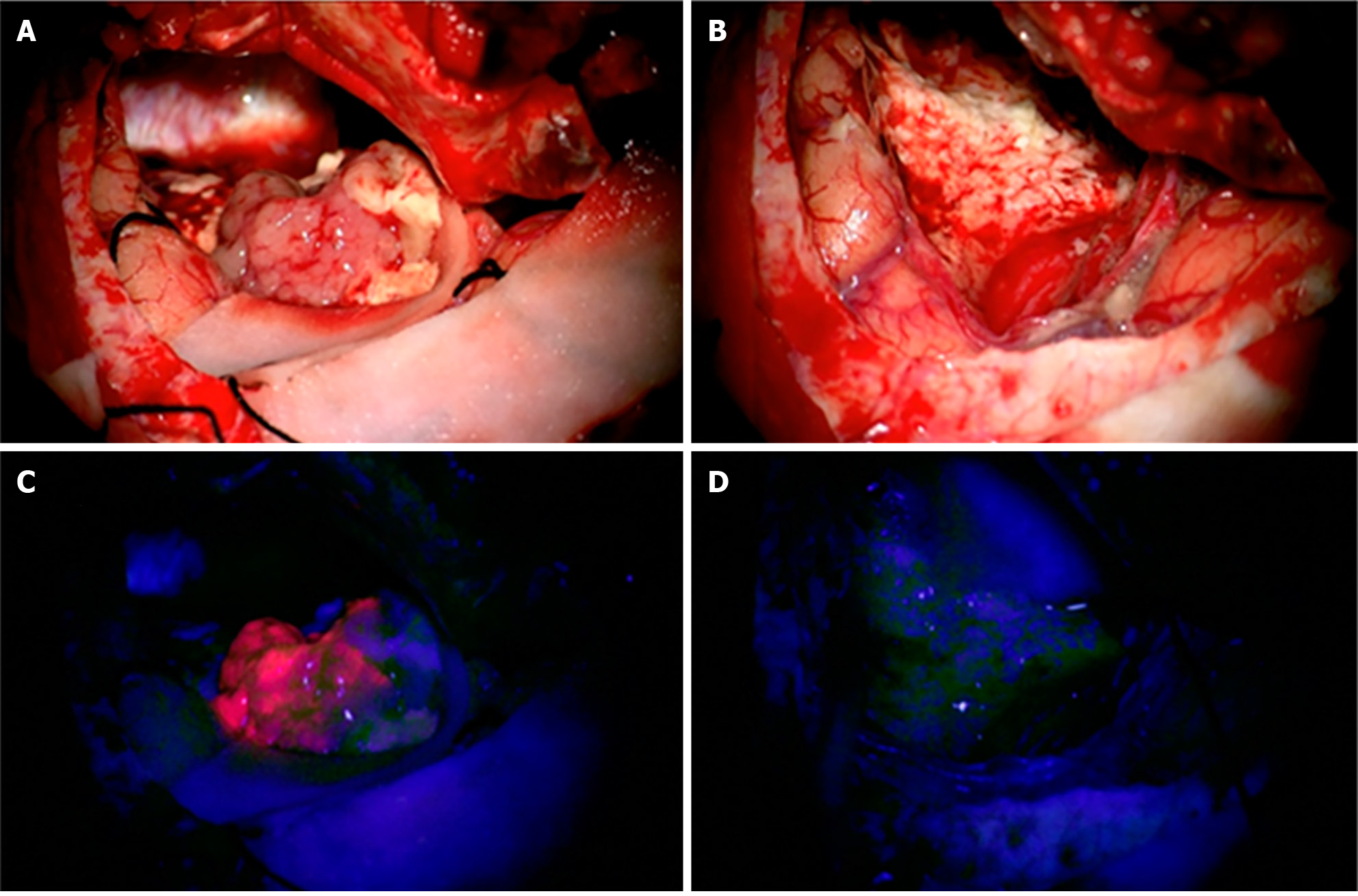
In resectable tumors, surgical advancements have established a maximal safe resection model, removing more non-enhancing residual volume of gliomas rather than previous models of gross resection of contrast-enhancing areas (Figure 1)[9,10]. Intra-operative ultrasound, in combination with neuronavigation, has provided better residual tumor margin control[11]. The management of recurrent malignancies near the resection site has seen improvement with the approval of the surgically targeted radiation therapy GammaTile, utilizing it in combination with standard-of-care external beam radiation therapy to decrease the likelihood of recurrence[12,13]. These measures, with the help of molecular identification of each tumor, have increased patient survival and developed a better algorithm for resection strategy. Not merely addressing the quantity of survival years, the quality of patient survival is at the forefront of studies using real-time brain stimulation mapping to preserve cognitive functions intra-operatively. This is done through the association of intra-operative tasks with specific brain functions. With awake craniotomies, decreased neural deficits have been observed, yielding higher preservation of motor and language functions[14,15]. More advanced stimulation mapping techniques aim to maintain higher order functions post-operatively. Administration of the Stroop color-word task, related to executive function, during resections has been associated with a reduction in post-operative executive function deficits[16].
The overall surgical management has shifted to rely more heavily on the molecular biomarkers of each glioma. Recommendations seeing the best outcomes suggest gross total resection with aggressive margin control of low-grade diffuse gliomas with wild-type IDH, with only subtotal resection for diffuse gliomas with mutated IDH[17]. In anaplastic gliomas, wild-type IDH tumors are conservatively treated with enhancing tissue resection, while those with mutant IDH can either be treated by total gross resection if they do not possess a 1p/19q codeletion or a subtotal resection if a 1p/19q codeletion exists in a functional area[17,18,19].
Following neurosurgical intervention, radiotherapy and chemotherapy are commonly employed. These postoperative therapies are administered as appropriate based on tumor type, WHO grade, and molecular features.
WHO classified grade 2 adult gliomas are defined as slow growing tumors that are locally infiltrative in middle-aged and young adults[20,21]. Referred commonly as low-grade gliomas (LGG), these tumors typically progress to more aggressive subtypes and present a poor prognosis, especially in older patients[22]. The two tumors in this class include IDH-mutant astrocytomas, and IDH-mutant, 1p/19q-codeleted oligodendrogliomas.
IDH-mutant astrocytoma: Following gross total resection by neurosurgical intervention, patients without a history of seizures and young patients can be cautiously managed with a watch-and-wait policy. Patients with a history or propensity to develop seizures and patients older than 40 years may benefit from early radiotherapy. Although early radiotherapy does not seem to improve overall survival in patients, it has been shown to improve seizure control and progression-free survival[23]. Typical dose ranging of radiotherapy is 45 to 50 Gy. No observable improvements have been demonstrated with high doses of 60 Gy and there is a concern of radiation-induced neurological implications in patients with long-term survival expectations[24,25]. The addition of chemotherapy with radiotherpay demonstrates superior progression-free survival when compared to chemotherapy alone[26]. However, patients intolerant to radiotherapy can benefit from the sole administration of chemotheraoy with temozolomide (TMZ). Younger patients between the ages of 13 and 39, and older patients over the age of 40 demonstrated a 4 to 5 year increase in overall survival when treated with postoperative radiotherapy and a chemotherapy regimen consistent of procarbazine, lomustine, and vincristine (PCV) for high risk gliomas[27].
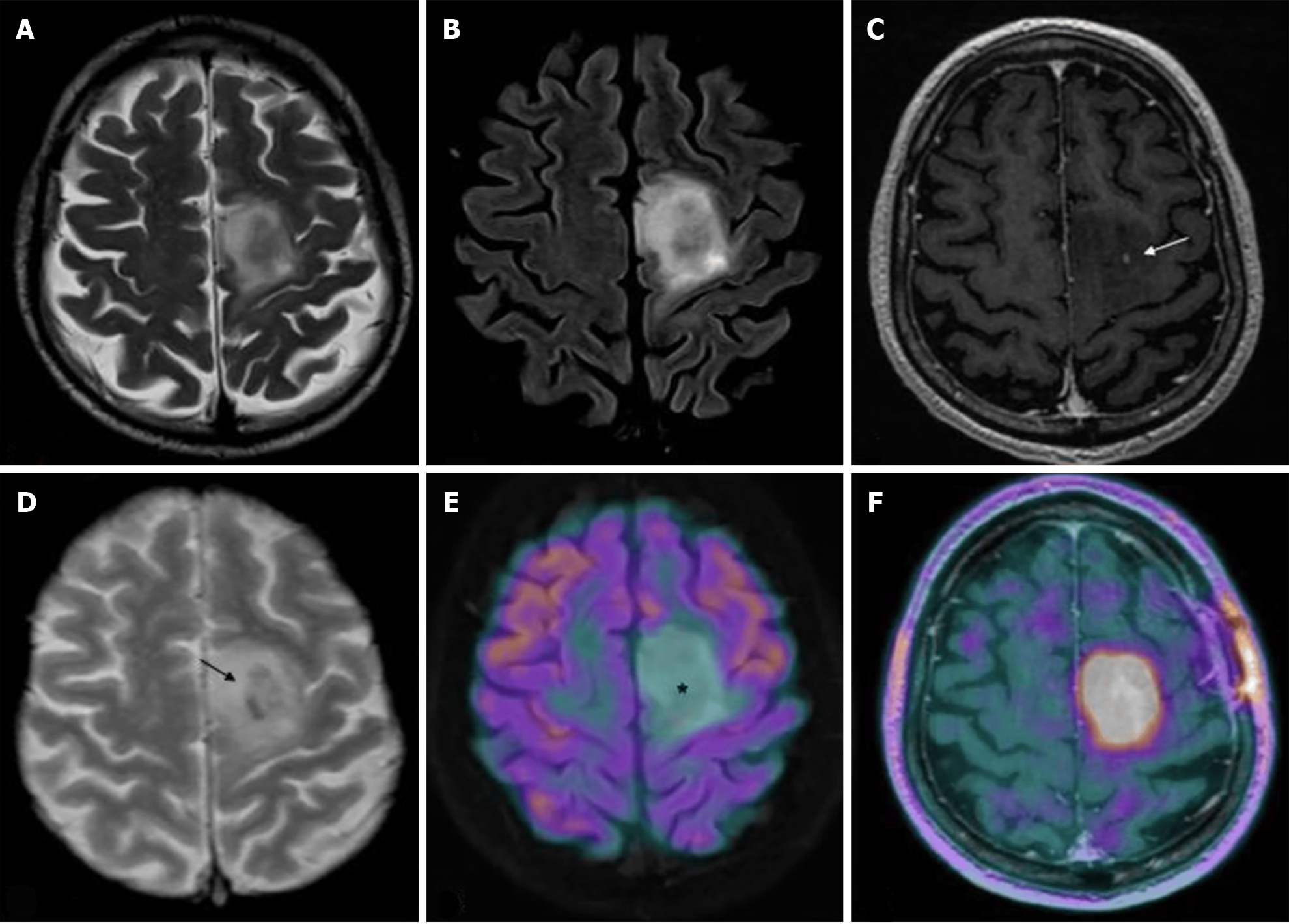
IDH-mutant, 1p/19q-codeleted oligodendroglioma: Management of IDH-mutant, 1p/19q-codeleted oligodendrogliomas (Figure 2) is consistent with what has been described for IDH-mutant astrocytomas with few exceptions. It is unclear if progression-free survival is impacted in patients who receive chemotherapy alone when compared to patients who receive chemotherapy in addition to radiotherapy[28]. Although typical practice is to incorporate radiotherapy with chemotherapy treatment, further investigation is required to determine their independent effects on patient outcomes.
WHO classified grade 3 adult gliomas, also known as anaplastic gliomas, are malignant tumors that have a median age of diagnosis ranging from 35 to 55.5 years based on the displayed mutations[29]. Tumors in this class include IDH-mutant astrocytomas, and IDH-mutant, 1p/19q-codeleted oligodendrogliomas.
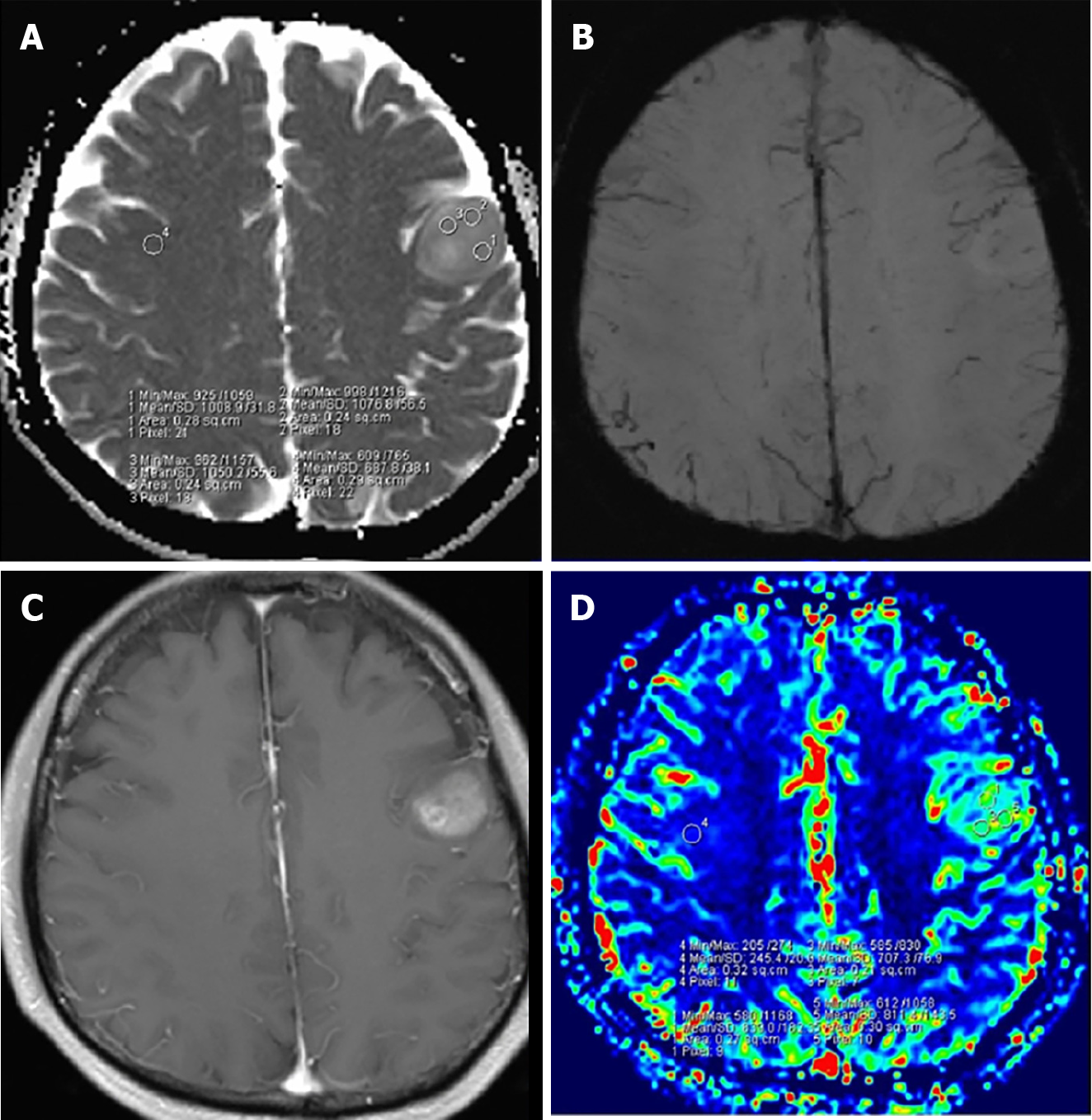
IDH-mutant astrocytoma: As described in WHO classified grade 2 gliomas, radiotherapy is a typical recommendation following gross total resection of grade 3 gliomas as well. Patients with IDH-mutant astrocytoma (Figure 3) receiving radiotherapy with adjuvant TMZ have demonstrated improved survival when compared to patients receiving concurrent TMZ with radiotherapy or radiotherapy alone[30,31]. Appropriate radiotherapy dose for these tumors is 60 Gy.
IDH-mutant, 1p/19q-codeleted oligodendroglioma: Following the response to treatment trend seen in the discussed tumors, radiotherapy remains to be highly indicated following surgery. Patients with IDH-mutant, 1p/19q-codeleted oligodendrogliomas have not demonstrated an improvement in survival with the administration of PCV or TMZ without prior radiotherapy[32]. However, an absolute survival benefit of 5 to 6 years has been demonstrated by postoperative radiotherapy and a chemotherapy regimen consistent of PCV[33,34]. This combined therapeutic approach also demonstrated improved overall survival when compared to postoperative radiotherapy alone. As with IDH-mutant astrocytomas, a radiotherapy dose of 60 Gy is appropriate.
The response demonstrated following the administration of these different protocols suggest that the best management is achieved with gross total resection followed by radiotherapy and a chemotherapy regimen of PCV. A current trial is underway to determine if TMZ can be used to replace a chemotherapy regimen of PCV as it has demonstrated mild myelosuppression and thus, a superior safety profile[35,36].
WHO classified grade 4 adult gliomas constitue both the most aggressive and most common primary malignancies of the brain. Grade 4 gliomas are recognized as highly heterogenous because of the variability in tumor size, histopathology, and molecular characteristics[37]. Tumors of this class have a 5-year survival rate of 5 to 10% and include IDH-mutant astrocytomas and IDH-wildtype glioblastoma.
IDH-mutant astrocytoma (previously: IDH-mutant glioblastoma): IDH-mutant astrocytoma constitutes a newly defined type of grade 4 glioma by the WHO in 2021[38,39]. This type of glioma has a favorable diagnosis compared to IDH-wildtype glioblastoma and is related to young age and dedifferentiation of a prior low grade gliomas (WHO grade 1 and 2)[40]. Treatment recommendation is consistent with previous management guidelines prior to its establishment as its own type of grade 4 glioma because of a lack of independent research on treatment efficacy specific to IDH-mutant astrocytoma. Landmark studies identifying treatment options for IDH-wildtype glioblastoma included IDH-mutant astrocytoma within their investigations[36]. Thus, further studies assessing independent efficacy of treatment on this newly classified type of grade 4 glioma is required to reasonably substantiate deintensification of treatment.
IDH-wildtype glioblastoma (IDH-wildtype astrocytoma): IDH-wildtype Glioblastoma is the most common and aggressive type of primary central nervous system (CNS) malignancy. This type of glioma constitutes almost half of all CNS tumors with an overall survival of 5 to 10 percent in 5 years[41-43]. A widely adopted standard of treatment for IDH-wildtype glioblastoma is the Stupp regimen[36]. This regimen constitutes radiotherapy with concurrent and adjuvant TMZ.
Radiotherapy dosage is 60 Gy in 30 fractions, with 1.8 to 2 Gy per fraction, for patients less than 70 years and demonstrating good performance[36]. The use of radiosurgery, brachytherapy, or doses greater than 60 Gy did not demonstrate any survival benefits[44,45]. In patients 70 and greater, or patients with poor performance, a hypofractionated radiotherapy dosage of 40 or 34 Gy in 15 and 10 fractions respectively can be utilized[46,47]. Employing an ultra-short radiotherapy course with 5 by 5 Gy doses did not improve overall survival for these patients[48].
Concurrent TMZ with the discussed radiotherapy approach in younger patients with good performance is admi
The literature suggest that the best treatment approach is the utilization of radiotherapy with concurrent and adjuvant TMZ at the discussed dosages for younger patients or patients with good performance. The treatment approach for elder patients or patients with poor performance is hypofractionated radiotherapy with the option of TMZ based on clinical intuition and potential consequences of increased toxicity[51]. Future trials are needed to definitively determine the role of concurrent TMZ with radiotherapy for these patients when treating this grade 4 glioma.
With variability in the clinical presentations of gliomas in the pediatric population, an array of treatment modalities follow. The International Consortium on Low Grade Glioma and the European Society for Paediatric Oncology Brain Tumor Group have established protocols for treatment and therapy[52]. Gross total resection is commonly curative and results in high overall and progression-free survival rates[53]. Following complete resection, patients should be monitored clinically for neuro-deficits and radiologically for tumor relapse. Absence of radiological tumor growth and post-operative symptoms is an indication for continued follow-up and no additional chemotherapy or radiotherapy.
In some cases, complete tumor resection is not achievable due to the neural-anatomic site and potential for permanent neurological effects[53]. Unresectable or incompletely resected pediatric low-grade gliomas (pLGG) tend to progress and warrant close observation and additional treatment. The optimal timing for follow-up chemo- and radiotherapy is controversial. International pLGG trials have studied whether prognostic differences exist among immediate and delayed post-operative chemo- or radiotherapy. Subjects with early post-operative radiotherapy demonstrated longer duration to tumor progression than those with delayed radiotherapy, but no significant differences in survival[54]. Indications for immediate therapy include visual and neurological symptoms or the presence of tumor growth more than 25% on imaging[55-57].
Majority of pediatric pLGG fall under the category of grade 1 and grade 2 lesions as recognized by the 2021 WHO Classification of Tumors of the Central Nervous System[38]. WHO CNS5 describes the current approach for dividing brain tumors into 6 main categories, one of which includes pediatric type diffuse low-grade glioma[38]. Common tumors in this class include pilocytic (Figure 4) and diffuse astrocytoma, ganglioglioma, and dysembryolastic neuroepithelial tumors. Additionally, 15%-20% of children with pLGG have neurofibromatosis type 1 (NF-1), affecting treatment options and prognosis. This class of gliomas is the most frequently diagnosed solid tumor in the pediatric population and generally has a good prognosis, usually presenting as slow growing tumors[38,55]. However, factors including age of diagnosis, histological findings, and genetic mutation status distinguish pLGG and individualize the clinical impact.
First-line chemotherapy: Children with pLGG who meet the criteria for non-surgical intervention or who show tumor progression following surgical intervention are subsequently treated with chemotherapy. Chemotherapy was first introduced in the 1980s to avoid or delay pediatric exposure to therapeutic doses of radiation. The first line treatment in Europe and the United States for pLGG remains a combination of vincristine and carboplatin (V/C) as standard induction[58-62]. The standard dosage of V/C may vary considering characteristics of the patient, tumor, and specific protocol. A standard induction consists of ten weekly doses of 1.5 mg/m2 vincristine intravenous bolus and four total doses of 550 mg/m2 carboplatin intravenous infusion at 3-wk intervals followed by 3 more cycles of V/C simultaneous doses at 4-wk intervals. Total duration of induction is 24 wk. A standard consolidation consists of ten doses of V/C at 6-wk intervals from week 25-85[55,59].
In an expanded follow-up study assessing outcomes of V/C treatment, a 56% objective response rate and 68% ± 7% progression free survival rate at 3 years post-diagnosis was seen among 78 pediatric patients diagnosed with pLGG[62]. Further studies have been conducted to assess effects of additions to the standard treatment on survival and response outcomes. In a randomized trial involving 497 newly diagnosed pediatric patients across 11 countries, the addition of etoposide to vincristine and carboplatin (V/C/E) showed no difference as compared to V/C treatment arms. Overall survival for V/C and V/C/E was 46% and 45%, and progression-free survival 89% and 89%, respectively[59]. It is worth mentioning that among children diagnosed with pLGG who were treated with V/C, event-free survival in children with NF-1 was significantly higher than non-NF1 patients[60].
Alternative chemotherapeutic options: Second and third-line chemotherapy strategies exist in the treatment of pLGG and may be employed in certain scenarios such as progressive tumor growth, recurrence, first-line intolerance, transformation to higher-grade, or patient choice. Patients who experience late recurrence of tumor growth following V/C regime may be re-treated with the same therapy if a positive response was demonstrated early on. Following a Common Terminology Criteria for Adverse Events Grade ≥ 2 due to a hypersensitivity reaction to carboplatin, second-line therapy is a replacement of carboplatin with cycles of 30 mg/m2 cisplatin and 1500 mg/m2 cyclophosphamide in combination with vincristine[55,59,63].
Alternatively, second line treatment following ineffectiveness of primary V/C therapy includes monotherapy with vinblastine. Vinblastine has shown promise in refractory pLGG and its low toxicity makes it a good option for carboplatin hypersensitivity[64,65]. Treatment includes one intravenous bolus of 6 mg/m2 vinblastine weekly for 70 wk[55,65-67]. Among 50 children previously receiving first-line chemotherapy for pLGG, most commonly pilocytic astrocytoma and ganglioglioma, the five-year overall survival and five-year progression-free survival was 93.2% ± 3.8% and 42.3% ± 7.2%, respectively[64]. In another study of 54 children with treatment naive pLGG, most commonly pilocytic astrocytoma, five-year overall survival and progression-free survival was 94.4% and 53.2%, respectively[65].
Additionally trialed chemotherapeutic options that have demonstrated promise with low toxicity profiles include a combination of 10 mg/kg bevacizumab and 10 mg/kg irinotecan, every 2-wk for a maximum of 2 years[68,69]. Alternatively, a regimen of tioguanine, procarbazine, lomustine, and vincristine (TPCV) has been compared to standard treatment with V/C, with no significant differences in 5-year overall and progression-free survival identified[55,70]. However, TPCV demonstrates a more toxic profile when compared to V/C, excluding carboplatin hypersensitivity reactions, and presents with increased risk secondary malignancy and infertility, especially in NF-1 patient groups[55,70,71].
Radiotherapy: While chemotherapy remains the primary non-surgical treatment, radiotherapy can be employed alone or as an adjuvant to chemotherapy in LGGs that cannot be resected[71]. However, traditional photon radiotherapy possesses dangerous long-term cognitive, endocrine, and growth defects, most prominent in young children. Modern radiotherapy techniques such as stereotactic and proton therapy are capable of more precision and are effective in minimizing the long-term neurocognitive and endocrine sequalae associated with brain radiation in children.
No prospective studies comparing optimal radiotherapy dose-fractionation have been conducted in pediatric cohorts. In a prospective multicenter study of LGG treatment modalities, 147 patients received radiotherapy with three-dimensional conformal planning secondary to severe or progressive symptoms. Doses were equal or below 54 Gy in 1.8 Gy increments, and equal or below 45.2 Gy in 1.6 Gy increments for children less than five-years of age. The 10-year progression free survival was 62% with 9.2% of tumors progressing and no differences between grade 1 and grade 2 identified[72]. In a retrospective study of children diagnosed with LGG treated with three-dimensional conformal radiotherapy, median doses of 54 Gy in 1.8 Gy, increments were associated with an overall survival of 94% and seven-year progression free survival of 67%[73].
Stereotactic radiotherapy has proven effective in the management of progressive and inoperable pLGG. A mean total dose of 52.2 Gy in 1.8 Gy fractions employing a 2-mm margin to the target has shown a five-year progression-free survival rate of 82.5% and an overall survival rate of 97.8% at 5 years[74]. Additionally, the use of fractionated stereotactic radiotherapy for reirradiation has been evaluated in recurrent gliomas among adult patients, but its use among the pediatric population warrants further study[75].
Proton therapy, with its precision-targeted approach, offers a promising avenue for the treatment of pLGGs, potentially minimizing radiation exposure to the surrounding healthy brain tissue. Treatment of progressive pLGG with proton therapy included radiation of gross tumor volume with a 5-mm margin and a clinical target volume dose of 54 Gy. Outcomes demonstrate a 3-year overall survival of 95% and a 3-year progression-free survival of 87%[76]. In another pilot study of magnetic resonance imaging (MRI)-guided adaptive planning for pLGG treated with proton therapy, it was found that mid-treatment imaging adjustments, based on a standard dose of 52.2 Gy in 1.9 Gy fractions, led to notable reductions in doses to key organs, potentially minimizing brainstem and optic nerve toxicities[77].
Biologics: Though the use of adjuvant chemo- and radiotherapy have been historically used in the treatment of progressive LGG, these therapies are associated with toxicity and long-term sequalae. Such considerations are magnified by a good prognosis for pLGG despite a low progression-free survival. As a result, discovery of specific molecular pathways in pLGG have led to the development of effective targeted therapies with lower toxicity profiles.
First-line therapy remains a combination of BRAF and MEK inhibitors vs BRAF inhibitor monotherapy due to combined treatment demonstrating improved progression-free survival and reduced toxicity in adults in separate studies[78,79]. A multi-center Phase II clinical trial is underway in investigating the activity of dabrafenib in combination with trametinib in adolescents with BRAF V600 mutation positive LGG[80]. The treatment regime under investigation in pediatric patients includes 0.5-5.25 mg/kg/d dabrafenib orally, twice daily, and 0.025-0.032 mg/kg/d trametinib, orally, once daily[80].
There is significant evidence of BRAF-targeted therapy in brain tumors harboring the BRAF gene[81,82]. BRAF mutations have been identified in 5%-15% of pLGG, most prominently in pleomorphic xanthoastrocytoma and ganglioglioma[83]. Approved therapies include combination therapies of BRAF inhibitors such as vemurafenib and dabrafenib, and MEK inhibitors such as selumetinib and trametinib[84].
A series of case reports have shown promising results with the use of first-generation BRAF inhibitor monotherapy in recurrent pLGGs such as pilocytic astrocytoma, hypothalamic glioma, ganglioglioma, and other low-grade brain tumors[85-90]. A 2-part clinical trial explored the safety of dabrafenib treatment in pediatric patients with BRAF V600 positive refractory LGG, including pilocytic astrocytoma, ganglioglioma, pilomyxoid astrocytoma, and pleomorphic xanthoastrocytoma[91]. Dabrafenib administration was started at 3.0 mg/kg, twice daily, with increments of 0.75 mg/kg, not exceeding the adult recommended dose of 300 mg/d[91,92]. Results extrapolated from part I and IIa of the study demonstrated this drug was well tolerated by pediatric patients at doses that induced pharmacokinetics reported in adult trials and an overall response rate of 44% with a one-year progression free survival of 85%[91,92].
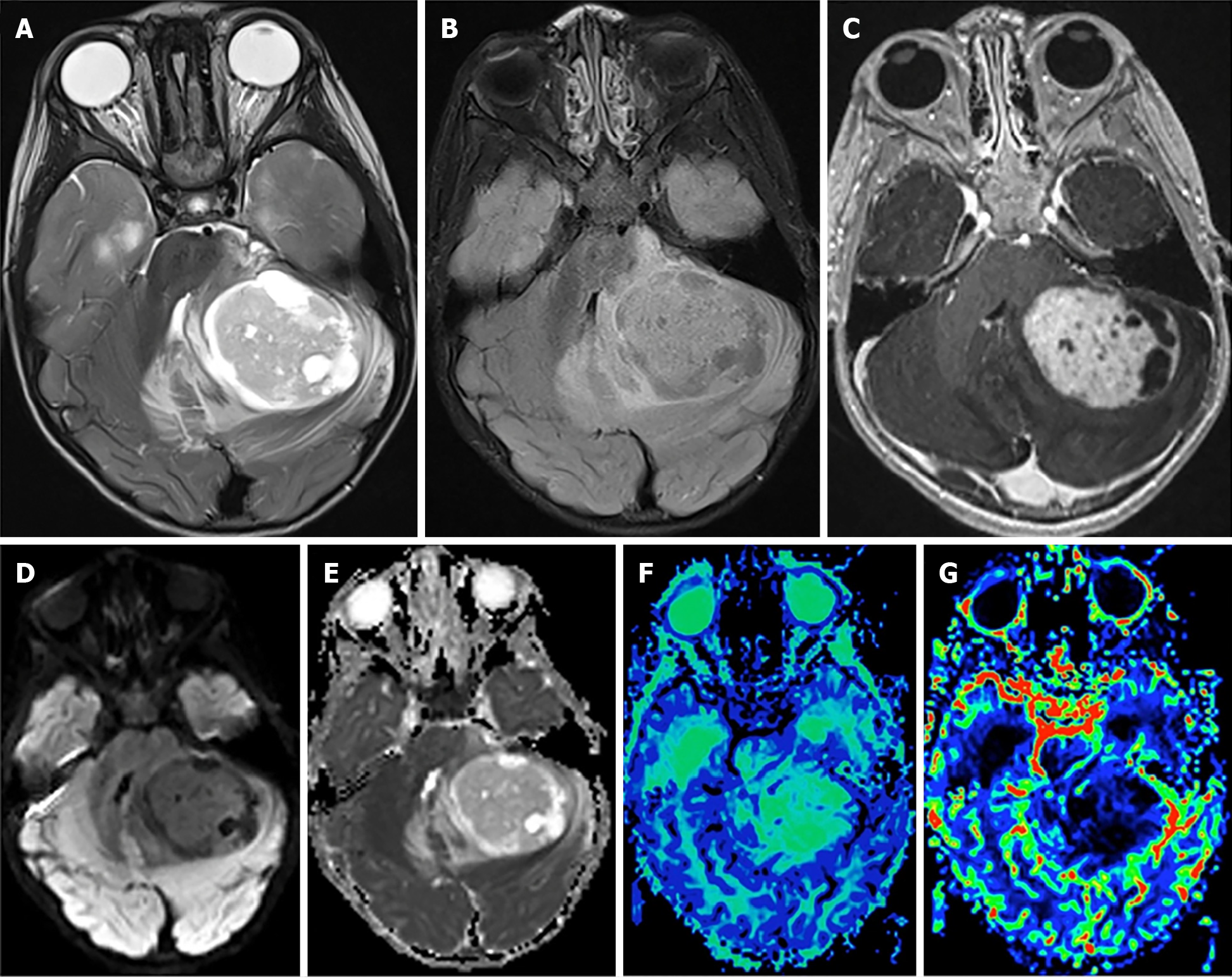
Close to 20% of all childhood gliomas are categorized as pediatric high-grade glioma (pHGG) (Figure 5)[93]. The latest WHO CNS5 classification introduces a new category for high grade gliomas, pediatric type diffuse high-grade gliomas, which includes both grade 3 and grade 4 gliomas[38]. In this updated classification, the high-grade glioma (HGG) family is also comprised of four tumor types with molecular nomenclature: Diffuse midline glioma, H3 K27-altered; Diffuse hemispheric glioma, H3 G34-mutant; Diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype; Infant-type hemispheric glioma[38]. Overlap of grade among tumor types is dependent on multiple histological and clinical factors, and grade progression of tumors is common in the pediatric population. At presentation, anaplastic astrocytoma, including IDH-wildtype and IDH-mutant astrocytoma, and IDH-mutant anaplastic oligodendroglioma are typical grade 3 pHGG. Glioblastoma, both IDH-wildtype and IDH-mutant, and diffuse hemispheric glioma, H3 G34-mutant, commonly fall under grade 4 classification[38].
The overall prognosis of pHGG is poor despite intensive treatment protocol, with five-year survival rates near 20% and prognosis most influenced by the extent of tumor resection[94,95]. Treatment for HGG commonly includes surgical resection followed by adjuvant chemo- and radiotherapy, however more targeted molecular approaches are of interest to reduce harmful side effects of systemic therapies.
Current recommendations for postoperative treatment of anaplastic astrocytoma are dependent on the extent of excision and tumor involvement in eloquent brain regions. According to the 2023 National Comprehensive Cancer Network guidelines on HGG, a multidisciplinary approach precedes surgical intervention to assess feasibility and safety in maximal tumor resection[96]. Maximal safe resection of tumor is the preferred initial approach in adults, with post-operative imaging for verification of complete resection, however no trials have been conducted in pediatric populations[97,98]. Integrating positron emission tomography scanning data with MRI during navigational planning can improve tumor volume definition and surgical precision in brain tumor resections, leading to more complete removal of metabolically active tumor regions[99].
IDH-mutant anaplastic astrocytoma; WHO grade 3: The treatment of pediatric patients with IDH-mutant astrocytoma is less well-defined than the treatment protocols for adults. The rarity of IDH-mutant astrocytoma in pediatric populations compared to adults contributes to this lack of specific guidelines. General approaches to these tumors in children are informed by a combination of pHGG guidelines and the adult IDH-mutant astrocytoma literature. Anaplastic astrocytoma generally present with poor prognosis with 5 and 10-year survival rates of 23.6% and 15.1% respectively[100,101].
Following maximal safe resection and integration of histologic and molecular features, recommended treatment of anaplastic astrocytoma is standard radiotherapy by conformal techniques including three-dimensional conformal radiation therapy and intensity-modulated radiation therapy. For radiation treatment planning, pre- and post-surgery MRI can accurately determine tumor dimensions, with the suggested treatment volume being the detected tumor size plus an additional 1-2 cm, typically receiving between 45-54 Gy in fractions of 2.0 Gy, followed by a boost plan of 14 Gy in 2 Gy fractions[23,24]. In the event of recurrence, reirradiation with advanced techniques such as sterotactic radiosurgery are employed for smaller tumors and proton therapy aids in protecting previously irradiated brain tissue[96,102].
In addition to standard radiotherapy, adjuvant or concurrent chemotherapy is recommended in the treatment of grade 3 IDH-mutant astrocytoma. TMZ is the primary systemic therapy recommended administered, followed by PCV[96]. When administered as an adjuvant following radiotherapy, TMZ is typically started at 150 mg/m2 per day for five days during the first cycle. If no hematologic toxicity is observed, the dose may be increased to 200 mg/m2 per day for five days in subsequent cycles. Adjuvant TMZ is given in 12 cycles, each lasting 4 wk[50,103]. When administered concurrently with standard radiotherapy, TMZ is administered 75 mg/m2 per day, 12 cycles each lasting 4 wk[103]. No significant survival differences were identified between adjuvant TMZ and PCV, although TMZ has shown greater tolerability[104].
Recurrent high-grade astrocytoma common within 3 years of diagnosis, necessitates a thorough evaluation, potentially including biopsy or resection[105]. Recommended regimens of systemic therapy still include TMZ without standard radiotherapy at 180-200 mg/m2 as monthly 5-d courses for up to 12 cycles[106]. Additional options include lomustine/carmustine high-dose chemotherapy and targeted therapy including VEGF inhibitor bevacizumab[107-109]. Similar recommendations are in place for recurrence of anaplastic oligodendroglioma[96].
IDH-mutant, 1p/19q-codeleted oligodendroglioma; WHO grade 3: Oligodendroglioma is a rare tumor subtype of diffuse glioma in the pediatric population, contributing to less than 1% of brain tumor diagnoses[110,111]. Similar to anaplastic astrocytoma, the rarity of this tumor in children has led to gaps in the literature. Initial treatment is surgery, with chemotherapy and radiotherapy typically administered as adjuvant treatment[96,110,111]. Prognosis for grade 3 anaplastic oligodendroglioma is generally poor, with estimated 5-year overall survival to be 53%[110]. For the diagnosis of anaplastic oligodendroglioma, precise molecular and histological information is required to classify tumor grade and determine best therapies.
Adjuvant radiotherapy in WHO grade 3 oligodendroglioma is typically treated by the same conventions of grade 3 IDH-mutant astrocytoma, following maximal safe resection. Radiotherapy has shown to be effective in the management of oligodendroglioma, prolonging the duration of progression free survival among pediatric patients as compared to treatment with chemotherapy alone, but has had no significant difference in overall survival[35,112]. Recommended radiotherapy fractioning differs from anaplastic astrocytoma, however, as a result of differences in tumor prognosis. As suggested in multiple clinical trials studying radiotherapy efficacy, the initial 50.4 Gy is given in 1.8 GY fractions as compared to 2.0 Gy fractions in grade 3 astrocytoma, covering the tumor volume with a 2-cm margin[34,113].
While surgical resection followed by radiotherapy is the mainstay of management, the role of adjuvant chemotherapy has come into focus due to a growing understanding of the tumor's molecular landscape and the push towards minimizing treatment-related long-term morbidity. Recommended systemic therapy includes neoadjuvant or adjuvant PCV, concurrent TMZ, or adjuvant TMZ[96]. Recommendations are based on studies that have shown that chemotherapy with radiotherapy, either concurrently or sequentially, significantly improved 5-year overall survival compared to radiotherapy alone[114]. For primary oligodendroglioma, recommendations of PCV are administration after radiotherapy in six, one-week cycles, started within four weeks after the end of radiotherapy. This protocol has demonstrated significant improvement in overall and progression free survival in anaplastic oligodendroglioma[33]. Additionally, PCV has proven to be more effective in prolonging overall survival and 5-year progression free survival specifically in patients with 1p/19q co-deleted tumors[34,114,115]. Neoadjuvant PCV as a preliminary treatment has significantly increased survival rates in patients with 1p/19q mutated oligodendroglioma, indicating that PCV may be beneficial when administered just before radiotherapy[34].
IDH-wildtype glioblastoma (IDH-wildtype astrocytoma); WHO grade 4: Pediatric glioblastoma accounts for only approximately 3% of all pediatric CNS tumors with an incidence of around 0.85 per 100000 children in the United States[116,117]. As a result, much of the recommended treatment protocols are adopted from adult studies and clinical trials. Studies specific to glioblastoma are limited as well with most of the literature assessing HGG together. Maximal safe resection is recommended, when possible, followed by chemotherapeutic regimes and radiotherapy if indicated for the pediatric patient[117]. These tumors typically present in the second decade of life as headaches and with mass effect symptoms. Unfortunately, prognosis is poor with a 5-year survival of less than 20%[116].
For glioblastoma treatment, recommendations for radiotherapy follow similar guidelines as for grade 3 HGG. Tumor volumes are primarily defined using MRI scans, with 1-2 cm margins recommended to address undetected tumor infiltration. Radiotherapy dosing is typically 60 Gy in 2.0 Gy fractions or 59.4 Gy in 1.8 Gy fractions[118]. In certain scenarios, like extensive tumor volume or brainstem involvement, the dose may be reduced to 54-57 Gy. For initial treatment phases, 45-50.4 Gy in 1.8 Gy fractions is given, followed by a "boost" phase with 9-14.4 Gy in 1.8 Gy fractions[96,119]. In cases involving elderly patients and sometimes children, a hypo-fractionated course ranging from 25 Gy in 5 fractions to 40.05 Gy in 15 fractions may be considered, although no significant differences in survival have been identified in the pediatric population[48,120,121]. Radiotherapy is not indicated in patients younger than three years due to adverse neurocognitive, psychosocial, and behavioral effects and an increased risk of recurrence secondary malignancy[96,116].
The integration of chemotherapy in the treatment of glioblastoma has emerged as a potential means of increasing overall survival. Current recommendations include concurrent or adjuvant TMZ, with some molecular mutations potentiating the effect of this treatment. Preliminary research contrasting the outcomes of standalone radiation therapy with combined TMZ chemoradiotherapy administered at 75 mg/m2 daily and subsequently at 150-200 mg/m2 post-radiation demonstrated a prolonged median survival period in adults[36]. However, TMZ has not been found to enhance outcomes for children with high-grade astrocytoma when compared to results from the Children's Cancer Group study CCG-945[122,123]. In pediatric glioblastoma, adding 90 mg/m2 lomustine to radiotherapy and concurrent 160 mg/m2 TMZ per day for 5 d every 6 wk has been found to enhance progression-free survival rates as compared to TMZ alone, particularly among those overexpressing MGMT[124].
The aggressive nature of recurrent glioblastoma necessitates a multi-modal approach, with pharmacological intervention and research at the forefront. Bevacizumab, a monoclonal antibody targeting VEGF, has shown effectiveness in recurrent glioblastoma in adults and may be administered 10mg/kg every two weeks as a potential therapeutic agent in children. Though well tolerated, the response rate and prolongation of survival are not as effective as in the adult population[125,126]. Among adult subjects with recurrent glioblastoma, a six-week cycle of 100-130 mg/m2 lomustine has demonstrated efficacy in increasing overall survival[127]. Furthermore, the combination of bevacizumab with lomustine demonstrates increased progression-free survival as compared to lomustine monotherapy[128,129].
Several therapies have been trialed for their effectiveness in improving overall survival and progression-free survival among children, including an assessment of their tolerance and toxicity profiles. Such therapies have included irinotecan, cilengitide, and sunitinib, all presenting with uncertain efficacy warranting further research[130].
Gliomas represent a category of primary brain tumors originating from glial cells of the central nervous system that account for most brain tumors in adults. Their classification and grading, determined by histological attributes and cellular lineage, provide a foundational roadmap for therapeutic decision-making for clinicians. In adults, the treatment often comprises of surgical resection, chemotherapy, and radiotherapy, with the latter also providing benefit in the reduction of seizures and progression-free survival. Pediatric gliomas, however, present a more intricate therapeutic challenge given their variability. While total tumor resection can be curative contingent upon its size and position, some inoperable cases necessitate a structured chemotherapy regimen. This often involves vincristine, coupled with carboplatin. Should chemotherapy not be effective, Vinblastine becomes a viable alternative. Radiotherapy is another option, however, concerns of neurological and endocrinological side effects in pediatric patients must be weighed. The journey for refining treatment strategies remains ongoing, especially in exploring the optimal therapeutic approach for IDH-mutant astrocytomas and honing the precision of radiotherapy dosing for young patients. Future investigations are imperative to further our understanding and enhance outcomes.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li JC, China S-Editor: Zhang H L-Editor: A P-Editor: Yu HG
| 1. | Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1689] [Cited by in RCA: 1864] [Article Influence: 116.5] [Reference Citation Analysis (0)] |
| 2. | Gusyatiner O, Hegi ME. Glioma epigenetics: From subclassification to novel treatment options. Semin Cancer Biol. 2018;51:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 376] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 3. | Villani V, Casini B, Tanzilli A, Lecce M, Rasile F, Telera S, Pace A, Piludu F, Terrenato I, Rollo F, De Nicola F, Fanciulli M, Pallocca M, Ciliberto G, Carosi M. The Glioma-IRE project - Molecular profiling in patients with glioma: steps toward an individualized diagnostic and therapeutic approach. J Transl Med. 2023;21:215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 4. | Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011-7021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1875] [Cited by in RCA: 1932] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 5. | Cancer Genome Atlas Research Network, Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA, Rheinbay E, Miller CR, Vitucci M, Morozova O, Robertson AG, Noushmehr H, Laird PW, Cherniack AD, Akbani R, Huse JT, Ciriello G, Poisson LM, Barnholtz-Sloan JS, Berger MS, Brennan C, Colen RR, Colman H, Flanders AE, Giannini C, Grifford M, Iavarone A, Jain R, Joseph I, Kim J, Kasaian K, Mikkelsen T, Murray BA, O'Neill BP, Pachter L, Parsons DW, Sougnez C, Sulman EP, Vandenberg SR, Van Meir EG, von Deimling A, Zhang H, Crain D, Lau K, Mallery D, Morris S, Paulauskis J, Penny R, Shelton T, Sherman M, Yena P, Black A, Bowen J, Dicostanzo K, Gastier-Foster J, Leraas KM, Lichtenberg TM, Pierson CR, Ramirez NC, Taylor C, Weaver S, Wise L, Zmuda E, Davidsen T, Demchok JA, Eley G, Ferguson ML, Hutter CM, Mills Shaw KR, Ozenberger BA, Sheth M, Sofia HJ, Tarnuzzer R, Wang Z, Yang L, Zenklusen JC, Ayala B, Baboud J, Chudamani S, Jensen MA, Liu J, Pihl T, Raman R, Wan Y, Wu Y, Ally A, Auman JT, Balasundaram M, Balu S, Baylin SB, Beroukhim R, Bootwalla MS, Bowlby R, Bristow CA, Brooks D, Butterfield Y, Carlsen R, Carter S, Chin L, Chu A, Chuah E, Cibulskis K, Clarke A, Coetzee SG, Dhalla N, Fennell T, Fisher S, Gabriel S, Getz G, Gibbs R, Guin R, Hadjipanayis A, Hayes DN, Hinoue T, Hoadley K, Holt RA, Hoyle AP, Jefferys SR, Jones S, Jones CD, Kucherlapati R, Lai PH, Lander E, Lee S, Lichtenstein L, Ma Y, Maglinte DT, Mahadeshwar HS, Marra MA, Mayo M, Meng S, Meyerson ML, Mieczkowski PA, Moore RA, Mose LE, Mungall AJ, Pantazi A, Parfenov M, Park PJ, Parker JS, Perou CM, Protopopov A, Ren X, Roach J, Sabedot TS, Schein J, Schumacher SE, Seidman JG, Seth S, Shen H, Simons JV, Sipahimalani P, Soloway MG, Song X, Sun H, Tabak B, Tam A, Tan D, Tang J, Thiessen N, Triche T Jr, Van Den Berg DJ, Veluvolu U, Waring S, Weisenberger DJ, Wilkerson MD, Wong T, Wu J, Xi L, Xu AW, Zack TI, Zhang J, Aksoy BA, Arachchi H, Benz C, Bernard B, Carlin D, Cho J, DiCara D, Frazer S, Fuller GN, Gao J, Gehlenborg N, Haussler D, Heiman DI, Iype L, Jacobsen A, Ju Z, Katzman S, Kim H, Knijnenburg T, Kreisberg RB, Lawrence MS, Lee W, Leinonen K, Lin P, Ling S, Liu W, Liu Y, Lu Y, Mills G, Ng S, Noble MS, Paull E, Rao A, Reynolds S, Saksena G, Sanborn Z, Sander C, Schultz N, Senbabaoglu Y, Shen R, Shmulevich I, Sinha R, Stuart J, Sumer SO, Sun Y, Tasman N, Taylor BS, Voet D, Weinhold N, Weinstein JN, Yang D, Yoshihara K, Zheng S, Zhang W, Zou L, Abel T, Sadeghi S, Cohen ML, Eschbacher J, Hattab EM, Raghunathan A, Schniederjan MJ, Aziz D, Barnett G, Barrett W, Bigner DD, Boice L, Brewer C, Calatozzolo C, Campos B, Carlotti CG Jr, Chan TA, Cuppini L, Curley E, Cuzzubbo S, Devine K, DiMeco F, Duell R, Elder JB, Fehrenbach A, Finocchiaro G, Friedman W, Fulop J, Gardner J, Hermes B, Herold-Mende C, Jungk C, Kendler A, Lehman NL, Lipp E, Liu O, Mandt R, McGraw M, Mclendon R, McPherson C, Neder L, Nguyen P, Noss A, Nunziata R, Ostrom QT, Palmer C, Perin A, Pollo B, Potapov A, Potapova O, Rathmell WK, Rotin D, Scarpace L, Schilero C, Senecal K, Shimmel K, Shurkhay V, Sifri S, Singh R, Sloan AE, Smolenski K, Staugaitis SM, Steele R, Thorne L, Tirapelli DP, Unterberg A, Vallurupalli M, Wang Y, Warnick R, Williams F, Wolinsky Y, Bell S, Rosenberg M, Stewart C, Huang F, Grimsby JL, Radenbaugh AJ. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N Engl J Med. 2015;372:2481-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2054] [Cited by in RCA: 2343] [Article Influence: 234.3] [Reference Citation Analysis (0)] |
| 6. | Jenkins RB, Blair H, Ballman KV, Giannini C, Arusell RM, Law M, Flynn H, Passe S, Felten S, Brown PD, Shaw EG, Buckner JC. A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 2006;66:9852-9861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 510] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 7. | Lu VM, O'Connor KP, Shah AH, Eichberg DG, Luther EM, Komotar RJ, Ivan ME. The prognostic significance of CDKN2A homozygous deletion in IDH-mutant lower-grade glioma and glioblastoma: a systematic review of the contemporary literature. J Neurooncol. 2020;148:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 8. | Gerstner ER, Yip S, Wang DL, Louis DN, Iafrate AJ, Batchelor TT. Mgmt methylation is a prognostic biomarker in elderly patients with newly diagnosed glioblastoma. Neurology. 2009;73:1509-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Karschnia P, Young JS, Dono A, Häni L, Sciortino T, Bruno F, Juenger ST, Teske N, Morshed RA, Haddad AF, Zhang Y, Stoecklein S, Weller M, Vogelbaum MA, Beck J, Tandon N, Hervey-Jumper S, Molinaro AM, Rudà R, Bello L, Schnell O, Esquenazi Y, Ruge MI, Grau SJ, Berger MS, Chang SM, van den Bent M, Tonn JC. Prognostic validation of a new classification system for extent of resection in glioblastoma: A report of the RANO resect group. Neuro Oncol. 2023;25:940-954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 189] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 10. | Li YM, Suki D, Hess K, Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J Neurosurg. 2016;124:977-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 439] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 11. | Moiraghi A, Prada F, Delaidelli A, Guatta R, May A, Bartoli A, Saini M, Perin A, Wälchli T, Momjian S, Bijlenga P, Schaller K, DiMeco F. Navigated Intraoperative 2-Dimensional Ultrasound in High-Grade Glioma Surgery: Impact on Extent of Resection and Patient Outcome. Oper Neurosurg (Hagerstown). 2020;18:363-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 12. | Ekhator C, Nwankwo I, Rak E, Homayoonfar A, Fonkem E, Rak R. GammaTile: Comprehensive Review of a Novel Radioactive Intraoperative Seed-Loading Device for the Treatment of Brain Tumors. Cureus. 2022;14:e29970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 13. | Odia Y, Gutierrez AN, Kotecha R. Erratum to: Stereotactic targeted radiation therapy (STaRT) trials for brain neoplasms: A comprehensive review. Neuro Oncol. 2023;25:423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Gerritsen JKW, Zwarthoed RH, Kilgallon JL, Nawabi NL, Jessurun CAC, Versyck G, Pruijn KP, Fisher FL, Larivière E, Solie L, Mekary RA, Satoer DD, Schouten JW, Bos EM, Kloet A, Nandoe Tewarie R, Smith TR, Dirven CMF, De Vleeschouwer S, Broekman MLD, Vincent AJPE. Effect of awake craniotomy in glioblastoma in eloquent areas (GLIOMAP): a propensity score-matched analysis of an international, multicentre, cohort study. Lancet Oncol. 2022;23:802-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 15. | Han SJ, Morshed RA, Troncon I, Jordan KM, Henry RG, Hervey-Jumper SL, Berger MS. Subcortical stimulation mapping of descending motor pathways for perirolandic gliomas: assessment of morbidity and functional outcome in 702 cases. J Neurosurg. 2018;131:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Puglisi G, Sciortino T, Rossi M, Leonetti A, Fornia L, Conti Nibali M, Casarotti A, Pessina F, Riva M, Cerri G, Bello L. Preserving executive functions in nondominant frontal lobe glioma surgery: an intraoperative tool. J Neurosurg. 2018;131:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Li L, Wang Y, Li Y, Fang S, Jiang T. Role of molecular biomarkers in glioma resection: a systematic review. Chin Neurosurg J. 2020;6:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Kawaguchi T, Sonoda Y, Shibahara I, Saito R, Kanamori M, Kumabe T, Tominaga T. Impact of gross total resection in patients with WHO grade III glioma harboring the IDH 1/2 mutation without the 1p/19q co-deletion. J Neurooncol. 2016;129:505-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 19. | Sales AHA, Beck J, Schnell O, Fung C, Meyer B, Gempt J. Surgical Treatment of Glioblastoma: State-of-the-Art and Future Trends. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 20. | Esiri M. Russell and Rubinstein's pathology of tumors of the nervous system. Sixth edition. J Neurol Neurosurg Psychiatry. 2000;68:538D. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Kleihues PCWK. Pathology and genetics of tumors of nervous system. International Agency for Research on Cancer: Lyon, 2000: 314. |
| 22. | Shafqat S, Hedley-Whyte ET, Henson JW. Age-dependent rate of anaplastic transformation in low-grade astrocytoma. Neurology. 1999;52:867-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | van den Bent MJ, Afra D, de Witte O, Ben Hassel M, Schraub S, Hoang-Xuan K, Malmström PO, Collette L, Piérart M, Mirimanoff R, Karim AB; EORTC Radiotherapy and Brain Tumor Groups and the UK Medical Research Council. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366:985-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 680] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 24. | Shaw E, Arusell R, Scheithauer B, O'Fallon J, O'Neill B, Dinapoli R, Nelson D, Earle J, Jones C, Cascino T, Nichols D, Ivnik R, Hellman R, Curran W, Abrams R. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002;20:2267-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 468] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 25. | Karim AB, Maat B, Hatlevoll R, Menten J, Rutten EH, Thomas DG, Mascarenhas F, Horiot JC, Parvinen LM, van Reijn M, Jager JJ, Fabrini MG, van Alphen AM, Hamers HP, Gaspar L, Noordman E, Pierart M, van Glabbeke M. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys. 1996;36:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 439] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 26. | Baumert BG, Hegi ME, van den Bent MJ, von Deimling A, Gorlia T, Hoang-Xuan K, Brandes AA, Kantor G, Taphoorn MJB, Hassel MB, Hartmann C, Ryan G, Capper D, Kros JM, Kurscheid S, Wick W, Enting R, Reni M, Thiessen B, Dhermain F, Bromberg JE, Feuvret L, Reijneveld JC, Chinot O, Gijtenbeek JMM, Rossiter JP, Dif N, Balana C, Bravo-Marques J, Clement PM, Marosi C, Tzuk-Shina T, Nordal RA, Rees J, Lacombe D, Mason WP, Stupp R. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17:1521-1532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 359] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 27. | Bell EH, Zhang P, Shaw EG, Buckner JC, Barger GR, Bullard DE, Mehta MP, Gilbert MR, Brown PD, Stelzer KJ, McElroy JP, Fleming JL, Timmers CD, Becker AP, Salavaggione AL, Liu Z, Aldape K, Brachman DG, Gertler SZ, Murtha AD, Schultz CJ, Johnson D, Laack NN, Hunter GK, Crocker IR, Won M, Chakravarti A. Comprehensive Genomic Analysis in NRG Oncology/RTOG 9802: A Phase III Trial of Radiation Versus Radiation Plus Procarbazine, Lomustine (CCNU), and Vincristine in High-Risk Low-Grade Glioma. J Clin Oncol. 2020;38:3407-3417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 28. | Feraco P, Franciosi R, Picori L, Scalorbi F, Gagliardo C. Conventional MRI-Derived Biomarkers of Adult-Type Diffuse Glioma Molecular Subtypes: A Comprehensive Review. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Mair MJ, Geurts M, van den Bent MJ, Berghoff AS. A basic review on systemic treatment options in WHO grade II-III gliomas. Cancer Treat Rev. 2021;92:102124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 30. | van den Bent MJ, Tesileanu CMS, Wick W, Sanson M, Brandes AA, Clement PM, Erridge S, Vogelbaum MA, Nowak AK, Baurain JF, Mason WP, Wheeler H, Chinot OL, Gill S, Griffin M, Rogers L, Taal W, Rudà R, Weller M, McBain C, Reijneveld J, Enting RH, Caparrotti F, Lesimple T, Clenton S, Gijtenbeek A, Lim E, Herrlinger U, Hau P, Dhermain F, de Heer I, Aldape K, Jenkins RB, Dubbink HJ, Kros JM, Wesseling P, Nuyens S, Golfinopoulos V, Gorlia T, French P, Baumert BG. Adjuvant and concurrent temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053-22054): second interim analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2021;22:813-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 211] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 31. | Yang X, Xing Z, She D, Lin Y, Zhang H, Su Y, Cao D. Grading of IDH-mutant astrocytoma using diffusion, susceptibility and perfusion-weighted imaging. BMC Med Imaging. 2022;22:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Wick W, Roth P, Hartmann C, Hau P, Nakamura M, Stockhammer F, Sabel MC, Wick A, Koeppen S, Ketter R, Vajkoczy P, Eyupoglu I, Kalff R, Pietsch T, Happold C, Galldiks N, Schmidt-Graf F, Bamberg M, Reifenberger G, Platten M, von Deimling A, Meisner C, Wiestler B, Weller M; Neurooncology Working Group (NOA) of the German Cancer Society. Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. Neuro Oncol. 2016;18:1529-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 33. | van den Bent MJ, Brandes AA, Taphoorn MJ, Kros JM, Kouwenhoven MC, Delattre JY, Bernsen HJ, Frenay M, Tijssen CC, Grisold W, Sipos L, Enting RH, French PJ, Dinjens WN, Vecht CJ, Allgeier A, Lacombe D, Gorlia T, Hoang-Xuan K. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31:344-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 835] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 34. | Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J, Fink K, Souhami L, Laperriere N, Curran W, Mehta M. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 799] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 35. | Jaeckle KA, Ballman KV, van den Bent M, Giannini C, Galanis E, Brown PD, Jenkins RB, Cairncross JG, Wick W, Weller M, Aldape KD, Dixon JG, Anderson SK, Cerhan JH, Wefel JS, Klein M, Grossman SA, Schiff D, Raizer JJ, Dhermain F, Nordstrom DG, Flynn PJ, Vogelbaum MA. CODEL: phase III study of RT, RT + TMZ, or TMZ for newly diagnosed 1p/19q codeleted oligodendroglioma. Analysis from the initial study design. Neuro Oncol. 2021;23:457-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 36. | Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14033] [Cited by in RCA: 15829] [Article Influence: 791.5] [Reference Citation Analysis (0)] |
| 37. | Lin Z, Yang R, Li K, Yi G, Li Z, Guo J, Zhang Z, Junxiang P, Liu Y, Qi S, Huang G. Establishment of age group classification for risk stratification in glioma patients. BMC Neurol. 2020;20:310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 38. | Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23:1231-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4523] [Cited by in RCA: 6547] [Article Influence: 1636.8] [Reference Citation Analysis (1)] |
| 39. | Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10993] [Cited by in RCA: 10867] [Article Influence: 1207.4] [Reference Citation Analysis (0)] |
| 40. | Lee JH, Wee CW. Treatment of Adult Gliomas: A Current Update. Brain Neurorehabil. 2022;15:e24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 41. | Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016. Neuro Oncol. 2019;21:v1-v100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1781] [Article Influence: 356.2] [Reference Citation Analysis (0)] |
| 42. | Lee JH, Lee JH. The origin-of-cell harboring cancer-driving mutations in human glioblastoma. BMB Rep. 2018;51:481-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Melhem JM, Detsky J, Lim-Fat MJ, Perry JR. Updates in IDH-Wildtype Glioblastoma. Neurotherapeutics. 2022;19:1705-1723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 44. | Malouff TD, Peterson JL, Mahajan A, Trifiletti DM. Carbon ion radiotherapy in the treatment of gliomas: a review. J Neurooncol. 2019;145:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 45. | Souhami L, Seiferheld W, Brachman D, Podgorsak EB, Werner-Wasik M, Lustig R, Schultz CJ, Sause W, Okunieff P, Buckner J, Zamorano L, Mehta MP, Curran WJ Jr. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 351] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 46. | Malmström A, Grønberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, Abacioglu U, Tavelin B, Lhermitte B, Hegi ME, Rosell J, Henriksson R; Nordic Clinical Brain Tumour Study Group (NCBTSG). Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 897] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 47. | Roa W, Brasher PM, Bauman G, Anthes M, Bruera E, Chan A, Fisher B, Fulton D, Gulavita S, Hao C, Husain S, Murtha A, Petruk K, Stewart D, Tai P, Urtasun R, Cairncross JG, Forsyth P. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22:1583-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 597] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 48. | Roa W, Kepka L, Kumar N, Sinaika V, Matiello J, Lomidze D, Hentati D, Guedes de Castro D, Dyttus-Cebulok K, Drodge S, Ghosh S, Jeremić B, Rosenblatt E, Fidarova E. International Atomic Energy Agency Randomized Phase III Study of Radiation Therapy in Elderly and/or Frail Patients With Newly Diagnosed Glioblastoma Multiforme. J Clin Oncol. 2015;33:4145-4150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 206] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 49. | Wick A, Kessler T, Platten M, Meisner C, Bamberg M, Herrlinger U, Felsberg J, Weyerbrock A, Papsdorf K, Steinbach JP, Sabel M, Vesper J, Debus J, Meixensberger J, Ketter R, Hertler C, Mayer-Steinacker R, Weisang S, Bölting H, Reuss D, Reifenberger G, Sahm F, von Deimling A, Weller M, Wick W. Superiority of temozolomide over radiotherapy for elderly patients with RTK II methylation class, MGMT promoter methylated malignant astrocytoma. Neuro Oncol. 2020;22:1162-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 50. | Wick W, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M, Nikkhah G, Papsdorf K, Steinbach JP, Sabel M, Combs SE, Vesper J, Braun C, Meixensberger J, Ketter R, Mayer-Steinacker R, Reifenberger G, Weller M; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13:707-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 808] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 51. | Wee CW, Kim IH, Park CK, Kim N, Suh CO, Chang JH, Lim DH, Nam DH, Kim IA, Kim CY, Oh YT, Chung WK, Kim SH. Chemoradiation in elderly patients with glioblastoma from the multi-institutional GBM-molRPA cohort: is short-course radiotherapy enough or is it a matter of selection? J Neurooncol. 2020;148:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Hessissen L, Parkes J, Amayiri N, Mushtaq N, Sirachainan N, Anacak Y, Mitra D, Figaji A, Schouten-van Meeteren A, Sullivan M, Burger H, Davidson A, Bouffet E, Bailey S. SIOP PODC Adapted treatment guidelines for low grade gliomas in low and middle income settings. Pediatr Blood Cancer. 2017;64 Suppl 5 [DOI:10.1002/pbc.26737. PMID: 29297618. |
| 53. | Wisoff JH, Sanford RA, Heier LA, Sposto R, Burger PC, Yates AJ, Holmes EJ, Kun LE. Primary neurosurgery for pediatric low-grade gliomas: a prospective multi-institutional study from the Children's Oncology Group. Neurosurgery. 2011;68:1548-54; discussion 1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 54. | Dhawan S, Patil CG, Chen C, Venteicher AS. Early versus delayed postoperative radiotherapy for treatment of low-grade gliomas. Cochrane Database Syst Rev. 2020;1:CD009229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Children’s Cancer and Leukaemia Group. Guidelines for the diagnosis and management of paediatric and adolescent Low-Grade Glioma. Available from: https://www.cclg.org.uk/write/MediaUploads/Member%20area/Treatment%20guidelines/LGG_Guidelines_July_2020.pdf. |
| 56. | Brown J, Perilongo G, Shafford E, Keeling J, Pritchard J, Brock P, Dicks-Mireaux C, Phillips A, Vos A, Plaschkes J. Pretreatment prognostic factors for children with hepatoblastoma-- results from the International Society of Paediatric Oncology (SIOP) study SIOPEL 1. Eur J Cancer. 2000;36:1418-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 204] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 57. | Prados MD, Edwards MS, Rabbitt J, Lamborn K, Davis RL, Levin VA. Treatment of pediatric low-grade gliomas with a nitrosourea-based multiagent chemotherapy regimen. J Neurooncol. 1997;32:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 126] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 58. | Bag AK, Chiang J, Patay Z. Radiohistogenomics of pediatric low-grade neuroepithelial tumors. Neuroradiology. 2021;63:1185-1213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Gnekow AK, Walker DA, Kandels D, Picton S, Giorgio Perilongo, Grill J, Stokland T, Sandstrom PE, Warmuth-Metz M, Pietsch T, Giangaspero F, Schmidt R, Faldum A, Kilmartin D, De Paoli A, De Salvo GL; of the Low Grade Glioma Consortium and the participating centers. A European randomised controlled trial of the addition of etoposide to standard vincristine and carboplatin induction as part of an 18-month treatment programme for childhood (≤16 years) low grade glioma - A final report. Eur J Cancer. 2017;81:206-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 60. | Ater JL, Xia C, Mazewski CM, Booth TN, Freyer DR, Packer RJ, Sposto R, Vezina G, Pollack IF. Nonrandomized comparison of neurofibromatosis type 1 and non-neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low-grade glioma: A report from the Children's Oncology Group. Cancer. 2016;122:1928-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 61. | Packer RJ, Sutton LN, Bilaniuk LT, Radcliffe J, Rosenstock JG, Siegel KR, Bunin GR, Savino PJ, Bruce DA, Schut L. Treatment of chiasmatic/hypothalamic gliomas of childhood with chemotherapy: an update. Ann Neurol. 1988;23:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 164] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 62. | Packer RJ, Ater J, Allen J, Phillips P, Geyer R, Nicholson HS, Jakacki R, Kurczynski E, Needle M, Finlay J, Reaman G, Boyett JM. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 403] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 63. | Brack E, Kozhaeva O, Ocokoljić M, Otth M, Schoot R, Vassal G, Kjaersgaard M, Scheinemann K, Bourdeau F, Michalski A, Sehested A, Achini F, Beishuizen A, Bergamaschi L, Biondi A, Brok J, Burke A, Calaminus G, Choucair M-L, Cleirec M, Corbaciouglu S, de Rojas T, Domínguez Pinilla N, Elmaraghi C, Ferrari A, Gaspar N, Genoveva Correa Llano M, Herold N, Jazmati D, Karapiperi K. Application for the Inclusion of Low Grade Glioma and related Essential Medicines on the WHO Essential Medicines List for Children 2021 Applicants: The European Society for Paediatric Oncology (SIOP Europe). 2021 April 19. Available from: www.siope.eu. |
| 64. | Bouffet E, Jakacki R, Goldman S, Hargrave D, Hawkins C, Shroff M, Hukin J, Bartels U, Foreman N, Kellie S, Hilden J, Etzl M, Wilson B, Stephens D, Tabori U, Baruchel S. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol. 2012;30:1358-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 65. | Lassaletta A, Scheinemann K, Zelcer SM, Hukin J, Wilson BA, Jabado N, Carret AS, Lafay-Cousin L, Larouche V, Hawkins CE, Pond GR, Poskitt K, Keene D, Johnston DL, Eisenstat DD, Krishnatry R, Mistry M, Arnoldo A, Ramaswamy V, Huang A, Bartels U, Tabori U, Bouffet E. Phase II Weekly Vinblastine for Chemotherapy-Naïve Children With Progressive Low-Grade Glioma: A Canadian Pediatric Brain Tumor Consortium Study. J Clin Oncol. 2016;34:3537-3543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 173] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 66. | Lafay-Cousin L, Holm S, Qaddoumi I, Nicolin G, Bartels U, Tabori U, Huang A, Bouffet E. Weekly vinblastine in pediatric low-grade glioma patients with carboplatin allergic reaction. Cancer. 2005;103:2636-2642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 67. | Stagno V, Mallucci C, Avula S, Pizer B. The use of neo adjuvant single-agent vinblastine for tumour shrinkage in a highly vascular paediatric low-grade glioma. Br J Neurosurg. 2020;34:207-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 68. | Packer RJ, Jakacki R, Horn M, Rood B, Vezina G, MacDonald T, Fisher MJ, Cohen B. Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr Blood Cancer. 2009;52:791-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 69. | Gururangan S, Fangusaro J, Poussaint TY, McLendon RE, Onar-Thomas A, Wu S, Packer RJ, Banerjee A, Gilbertson RJ, Fahey F, Vajapeyam S, Jakacki R, Gajjar A, Goldman S, Pollack IF, Friedman HS, Boyett JM, Fouladi M, Kun LE. Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas--a Pediatric Brain Tumor Consortium study. Neuro Oncol. 2014;16:310-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 70. | Ater JL, Zhou T, Holmes E, Mazewski CM, Booth TN, Freyer DR, Lazarus KH, Packer RJ, Prados M, Sposto R, Vezina G, Wisoff JH, Pollack IF. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children's Oncology Group. J Clin Oncol. 2012;30:2641-2647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 307] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 71. | de Blank P, Bandopadhayay P, Haas-Kogan D, Fouladi M, Fangusaro J. Management of pediatric low-grade glioma. Curr Opin Pediatr. 2019;31:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 72. | Gnekow AK, Falkenstein F, von Hornstein S, Zwiener I, Berkefeld S, Bison B, Warmuth-Metz M, Driever PH, Soerensen N, Kortmann RD, Pietsch T, Faldum A. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol. 2012;14:1265-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 73. | Oh KS, Hung J, Robertson PL, Garton HJ, Muraszko KM, Sandler HM, Hamstra DA. Outcomes of multidisciplinary management in pediatric low-grade gliomas. Int J Radiat Oncol Biol Phys. 2011;81:e481-e488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 74. | Marcus KJ, Goumnerova L, Billett AL, Lavally B, Scott RM, Bishop K, Xu R, Young Poussaint T, Kieran M, Kooy H, Pomeroy SL, Tarbell NJ. Stereotactic radiotherapy for localized low-grade gliomas in children: final results of a prospective trial. Int J Radiat Oncol Biol Phys. 2005;61:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 75. | Combs SE, Thilmann C, Edler L, Debus J, Schulz-Ertner D. Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: long-term results in 172 patients treated in a single institution. J Clin Oncol. 2005;23:8863-8869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 233] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 76. | Indelicato DJ, Bradley JA, Sandler ES, Aldana PR, Sapp A, Gains JE, Crellin A, Rotondo RL. Clinical outcomes following proton therapy for children with central nervous system tumors referred overseas. Pediatr Blood Cancer. 2017;64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 77. | Acharya S, Coca K, Bowers EE, Gargone M, Merchant TE. A Pilot Study of MR-Guided Mid-Treatment Adaptive Proton Therapy for Pediatric Low-Grade Gliomas. Int J Radiat Oncol, Biol, Phys. 2018;102:S238-S238. |
| 78. | Planchard D, Smit EF, Groen HJM, Mazieres J, Besse B, Helland Å, Giannone V, D'Amelio AM Jr, Zhang P, Mookerjee B, Johnson BE. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017;18:1307-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 685] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 79. | Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Grob JJ, Chiarion Sileni V, Lebbe C, Mandalà M, Millward M, Arance A, Bondarenko I, Haanen JB, Hansson J, Utikal J, Ferraresi V, Kovalenko N, Mohr P, Probachai V, Schadendorf D, Nathan P, Robert C, Ribas A, DeMarini DJ, Irani JG, Casey M, Ouellet D, Martin AM, Le N, Patel K, Flaherty K. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1405] [Article Influence: 127.7] [Reference Citation Analysis (0)] |
| 80. | Bouffet E, Hansford J, Garré ML, Hara J, Plant-Fox A, Aerts I, Locatelli F, Lugt J Van der, Papusha L, Sahm F, Tabori U, Cohen KJ, Packer RJ, Witt O, Sandalic L, Silva ABP da, Russo MW, Hargrave DR. Primary analysis of a phase II trial of dabrafenib plus trametinib (dab + tram) in BRAF V600–mutant pediatric low-grade glioma (pLGG). J Clin Oncol. 40: LBA2002-LBA2002. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 81. | Kieran MW, Bouffet E, Tabori U, Broniscer A, Cohen K, Hansford J, Geoerger B, Hingorani P, Dunkel I, Russo M, Tseng L, Liu Q, Nebot N, Whitlock J, Hargrave D. CNS tumours The first study of dabrafenib in pediatric patients with BRAF V600–mutant relapsed or refractory low-grade gliomas. Ann Oncol. 2016;27:vi557. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 82. | Lassaletta A, Zapotocky M, Mistry M, Ramaswamy V, Honnorat M, Krishnatry R, Guerreiro Stucklin A, Zhukova N, Arnoldo A, Ryall S, Ling C, McKeown T, Loukides J, Cruz O, de Torres C, Ho CY, Packer RJ, Tatevossian R, Qaddoumi I, Harreld JH, Dalton JD, Mulcahy-Levy J, Foreman N, Karajannis MA, Wang S, Snuderl M, Nageswara Rao A, Giannini C, Kieran M, Ligon KL, Garre ML, Nozza P, Mascelli S, Raso A, Mueller S, Nicolaides T, Silva K, Perbet R, Vasiljevic A, Faure Conter C, Frappaz D, Leary S, Crane C, Chan A, Ng HK, Shi ZF, Mao Y, Finch E, Eisenstat D, Wilson B, Carret AS, Hauser P, Sumerauer D, Krskova L, Larouche V, Fleming A, Zelcer S, Jabado N, Rutka JT, Dirks P, Taylor MD, Chen S, Bartels U, Huang A, Ellison DW, Bouffet E, Hawkins C, Tabori U. Therapeutic and Prognostic Implications of BRAF V600E in Pediatric Low-Grade Gliomas. J Clin Oncol. 2017;35:2934-2941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 247] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 83. | Schreck KC, Grossman SA, Pratilas CA. BRAF Mutations and the Utility of RAF and MEK Inhibitors in Primary Brain Tumors. Cancers (Basel). 2019;11:1262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 84. | Manoharan N, Liu KX, Mueller S, Haas-Kogan DA, Bandopadhayay P. Pediatric low-grade glioma: Targeted therapeutics and clinical trials in the molecular era. Neoplasia. 2023;36:100857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 85. | Skrypek M, Foreman N, Guillaume D, Moertel C. Pilomyxoid astrocytoma treated successfully with vemurafenib. Pediatr Blood Cancer. 2014;61:2099-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 86. | Lassaletta A, Guerreiro Stucklin A, Ramaswamy V, Zapotocky M, McKeown T, Hawkins C, Bouffet E, Tabori U. Profound clinical and radiological response to BRAF inhibition in a 2-month-old diencephalic child with hypothalamic/chiasmatic glioma. Pediatr Blood Cancer. 2016;63:2038-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 87. | van Tilburg CM, Selt F, Sahm F, Bächli H, Pfister SM, Witt O, Milde T. Response in a child with a BRAF V600E mutated desmoplastic infantile astrocytoma upon retreatment with vemurafenib. Pediatr Blood Cancer. 2018;65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 88. | Grenier-Chartrand F, Barrit S, Racu ML, Luce S, Spitaels J, Sadeghi-Meibodi N, Lebrun L, Salmon I, Lefranc F, De Witte O. Dabrafenib monotherapy for a recurrent BRAFV600E-mutated TTF-1-positive posterior pituitary tumor. Acta Neurochir (Wien). 2022;164:737-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 89. | Mustansir F, Mushtaq N, Darbar A. Dabrafenib in BRAFV600E mutant pilocytic astrocytoma in a pediatric patient. Childs Nerv Syst. 2020;36:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 90. | del Bufalo F, Carai A, Figà-Talamanca L, Pettorini B, Mallucci C, Giangaspero F, Antonelli M, Badiali M, Moi L, Bianco G, Cacchione A, Locatelli F, Ferretti E, Mastronuzzi A. Response of recurrent BRAFV600E mutated ganglioglioma to Vemurafenib as single agent. J Transl Med. 2014;12:356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 91. | Hargrave DR, Bouffet E, Tabori U, Broniscer A, Cohen KJ, Hansford JR, Geoerger B, Hingorani P, Dunkel IJ, Russo MW, Tseng L, Dasgupta K, Gasal E, Whitlock JA, Kieran MW. Efficacy and Safety of Dabrafenib in Pediatric Patients with BRAF V600 Mutation-Positive Relapsed or Refractory Low-Grade Glioma: Results from a Phase I/IIa Study. Clin Cancer Res. 2019;25:7303-7311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 92. | Kieran MW, Geoerger B, Dunkel IJ, Broniscer A, Hargrave D, Hingorani P, Aerts I, Bertozzi AI, Cohen KJ, Hummel TR, Shen V, Bouffet E, Pratilas CA, Pearson ADJ, Tseng L, Nebot N, Green S, Russo MW, Whitlock JA. A Phase I and Pharmacokinetic Study of Oral Dabrafenib in Children and Adolescent Patients with Recurrent or Refractory BRAF V600 Mutation-Positive Solid Tumors. Clin Cancer Res. 2019;25:7294-7302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 93. | Coleman C, Stoller S, Grotzer M, Stucklin AG, Nazarian J, Mueller S. Pediatric hemispheric high-grade glioma: targeting the future. Cancer Metastasis Rev. 2020;39:245-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 94. | Braunstein S, Raleigh D, Bindra R, Mueller S, Haas-Kogan D. Pediatric high-grade glioma: current molecular landscape and therapeutic approaches. J Neurooncol. 2017;134:541-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 95. | Jones C, Karajannis MA, Jones DTW, Kieran MW, Monje M, Baker SJ, Becher OJ, Cho YJ, Gupta N, Hawkins C, Hargrave D, Haas-Kogan DA, Jabado N, Li XN, Mueller S, Nicolaides T, Packer RJ, Persson AI, Phillips JJ, Simonds EF, Stafford JM, Tang Y, Pfister SM, Weiss WA. Pediatric high-grade glioma: biologically and clinically in need of new thinking. Neuro Oncol. 2017;19:153-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 178] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 96. | Gajjar A, Mahajan A, Abdelbaki M, Anderson C, Antony R, Bale T, Bindra R, Bowers DC, Cohen K, Cole B, Dorris K, Ermoian R, Franson A, Helgager J, Landi D, Lin C, Metrock L, Nanda R, Palmer J, Partap S, Plant A, Pruthi S, Reynolds R, Ruggieri P, Stearns D, Storm P, Wang A, Warren K, Whipple N, Zaky W, McMillian NR, Pluchino LA. Pediatric Central Nervous System Cancers, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:1339-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 97. | Vuorinen V, Hinkka S, Färkkilä M, Jääskeläinen J. Debulking or biopsy of malignant glioma in elderly people - a randomised study. Acta Neurochir (Wien). 2003;145:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 259] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 98. | Metcalfe SE. Biopsy versus resection for malignant glioma. Cochrane Database Syst Rev. 2000;CD002034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 99. | Pirotte B, Goldman S, Dewitte O, Massager N, Wikler D, Lefranc F, Ben Taib NO, Rorive S, David P, Brotchi J, Levivier M. Integrated positron emission tomography and magnetic resonance imaging-guided resection of brain tumors: a report of 103 consecutive procedures. J Neurosurg. 2006;104:238-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 100. | Tauziède-Espariat A, Debily MA, Castel D, Grill J, Puget S, Roux A, Saffroy R, Pagès M, Gareton A, Chrétien F, Lechapt E, Dangouloff-Ros V, Boddaert N, Varlet P. The pediatric supratentorial MYCN-amplified high-grade gliomas methylation class presents the same radiological, histopathological and molecular features as their pontine counterparts. Acta Neuropathol Commun. 2020;8:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 101. | Smoll NR, Hamilton B. Incidence and relative survival of anaplastic astrocytomas. Neuro Oncol. 2014;16:1400-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 102. | Gutin PH, Iwamoto FM, Beal K, Mohile NA, Karimi S, Hou BL, Lymberis S, Yamada Y, Chang J, Abrey LE. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;75:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 103. | van den Bent MJ, Baumert B, Erridge SC, Vogelbaum MA, Nowak AK, Sanson M, Brandes AA, Clement PM, Baurain JF, Mason WP, Wheeler H, Chinot OL, Gill S, Griffin M, Brachman DG, Taal W, Rudà R, Weller M, McBain C, Reijneveld J, Enting RH, Weber DC, Lesimple T, Clenton S, Gijtenbeek A, Pascoe S, Herrlinger U, Hau P, Dhermain F, van Heuvel I, Stupp R, Aldape K, Jenkins RB, Dubbink HJ, Dinjens WNM, Wesseling P, Nuyens S, Golfinopoulos V, Gorlia T, Wick W, Kros JM. Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet. 2017;390:1645-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 273] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 104. | Brandes AA, Nicolardi L, Tosoni A, Gardiman M, Iuzzolino P, Ghimenton C, Reni M, Rotilio A, Sotti G, Ermani M. Survival following adjuvant PCV or temozolomide for anaplastic astrocytoma. Neuro Oncol. 2006;8:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 105. | PDQ Pediatric Treatment Editorial Board. Childhood Astrocytomas, Other Gliomas, and Glioneuronal/Neuronal Tumors Treatment (PDQ®): Health Professional Version. 2023 Dec 19. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002. [PubMed] |
| 106. | Nicholson HS, Kretschmar CS, Krailo M, Bernstein M, Kadota R, Fort D, Friedman H, Harris MB, Tedeschi-Blok N, Mazewski C, Sato J, Reaman GH. Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors: a report from the Children's Oncology Group. Cancer. 2007;110:1542-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 107. | Prados M, Rodriguez L, Chamberlain M, Silver P, Levin V. Treatment of recurrent gliomas with 1,3-bis(2-chloroethyl)-1-nitrosourea and alpha-difluoromethylornithine. Neurosurgery. 1989;24:806-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 108. | Norden AD, Young GS, Setayesh K, Muzikansky A, Klufas R, Ross GL, Ciampa AS, Ebbeling LG, Levy B, Drappatz J, Kesari S, Wen PY. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 618] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 109. | Chamberlain MC, Johnston S. Salvage chemotherapy with bevacizumab for recurrent alkylator-refractory anaplastic astrocytoma. J Neurooncol. 2009;91:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 110. | Byer L, Cassie Kline-Nunnally, Tihan T, Mueller S. Pediatric oligodendroglioma. In: Paleologos NA, Newton HB. Oligodendroglioma: Clinical Presentation, Pathology, Molecular Biology, Imaging, and Treatment. Amsterdam: Elsevier, 2019: 379-386. [DOI] [Full Text] |
| 111. | Straehla JP, Marcus KJ. The role of radiation in pediatric oligodendrogliomas. In: Oligodendroglioma: Clinical Presentation, Pathology, Molecular Biology, Imaging, and Treatment. Amsterdam: Elsevier, 2019: 299-306. [DOI] [Full Text] |
| 112. | Intergroup Radiation Therapy Oncology Group Trial 9402; Cairncross G, Berkey B, Shaw E, Jenkins R, Scheithauer B, Brachman D, Buckner J, Fink K, Souhami L, Laperierre N, Mehta M, Curran W. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24:2707-2714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 504] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 113. | Chang S, Zhang P, Cairncross JG, Gilbert MR, Bahary JP, Dolinskas CA, Chakravarti A, Aldape KD, Bell EH, Schiff D, Jaeckle K, Brown PD, Barger GR, Werner-Wasik M, Shih H, Brachman D, Penas-Prado M, Robins HI, Belanger K, Schultz C, Hunter G, Mehta M. Phase III randomized study of radiation and temozolomide versus radiation and nitrosourea therapy for anaplastic astrocytoma: results of NRG Oncology RTOG 9813. Neuro Oncol. 2017;19:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 114. | Shin JY, Diaz AZ. Utilization and impact of adjuvant therapy in anaplastic oligodendroglioma: an analysis on 1692 patients. J Neurooncol. 2016;129:567-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 115. | Ruff MW, Buckner JC, Johnson DR, van den Bent MJ, Geurts M. Neuro-Oncology Clinical Debate: PCV or temozolomide in combination with radiation for newly diagnosed high-grade oligodendroglioma. Neurooncol Pract. 2019;6:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 116. | Das KK, Kumar R. Pediatric Glioblastoma. In: De Vleeschouwer S. Glioblastoma [Internet]. Brisbane (AU): Codon Publications; 2017: Chapter 15. [PubMed] [DOI] [Full Text] |
| 117. | Singla AK, Madan R, Gupta K, Goyal S, Kumar N, Sahoo SK, Uppal DK, Ahuja CK. Clinical behaviour and outcome in pediatric glioblastoma: current scenario. Radiat Oncol J. 2021;39:72-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 118. | Mallick S, Gandhi AK, Joshi NP, Kumar A, Puri T, Sharma DN, Haresh KP, Gupta S, Julka PK, Rath GK, Sarkar C. Outcomes of pediatric glioblastoma treated with adjuvant chemoradiation with temozolomide and correlation with prognostic factors. Indian J Med Paediatr Oncol. 2015;36:99-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 119. | Cabrera AR, Kirkpatrick JP, Fiveash JB, Shih HA, Koay EJ, Lutz S, Petit J, Chao ST, Brown PD, Vogelbaum M, Reardon DA, Chakravarti A, Wen PY, Chang E. Radiation therapy for glioblastoma: Executive summary of an American Society for Radiation Oncology Evidence-Based Clinical Practice Guideline. Pract Radiat Oncol. 2016;6:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 166] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 120. | Jablonska PA, Diez-Valle R, Pérez-Larraya JG, Moreno-Jiménez M, Idoate MÁ, Arbea L, Tejada S, Garcia de Eulate MR, Ramos L, Arbizu J, Domínguez P, Aristu JJ. Hypofractionated radiation therapy and temozolomide in patients with glioblastoma and poor prognostic factors. A prospective, single-institution experience. PLoS One. 2019;14:e0217881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 121. | Kramer ED, Donaldson M. Survival of infants with malignant astrocytomas: a report from the Childrens Cancer Group. Cancer. 1995;76:1685-1686. [PubMed] [DOI] [Full Text] |
| 122. | Cohen KJ, Pollack IF, Zhou T, Buxton A, Holmes EJ, Burger PC, Brat DJ, Rosenblum MK, Hamilton RL, Lavey RS, Heideman RL. Temozolomide in the treatment of high-grade gliomas in children: a report from the Children's Oncology Group. Neuro Oncol. 2011;13:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 283] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 123. | Fouladi M, Hunt DL, Pollack IF, Dueckers G, Burger PC, Becker LE, Yates AJ, Gilles FH, Davis RL, Boyett JM, Finlay JL. Outcome of children with centrally reviewed low-grade gliomas treated with chemotherapy with or without radiotherapy on Children's Cancer Group high-grade glioma study CCG-945. Cancer. 2003;98:1243-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 124. | Jakacki RI, Cohen KJ, Buxton A, Krailo MD, Burger PC, Rosenblum MK, Brat DJ, Hamilton RL, Eckel SP, Zhou T, Lavey RS, Pollack IF. Phase 2 study of concurrent radiotherapy and temozolomide followed by temozolomide and lomustine in the treatment of children with high-grade glioma: a report of the Children's Oncology Group ACNS0423 study. Neuro Oncol. 2016;18:1442-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 125. | Narayana A, Kunnakkat S, Chacko-Mathew J, Gardner S, Karajannis M, Raza S, Wisoff J, Weiner H, Harter D, Allen J. Bevacizumab in recurrent high-grade pediatric gliomas. Neuro Oncol. 2010;12:985-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 126. | Cloughesy TF, Prados MD, Wen PY, Mikkelsen T, Abrey LE, Schiff D, Yung WK, Maoxia Z, Dimery I, Friedman HS. A phase II, randomized, non-comparative clinical trial of the effect of bevacizumab (BV) alone or in combination with irinotecan (CPT) on 6-month progression free survival (PFS6) in recurrent, treatment-refractory glioblastoma (GBM). J Clin Oncol. 2008;26:2010b-2010b. [RCA] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 127. | Wick W, Puduvalli VK, Chamberlain MC, van den Bent MJ, Carpentier AF, Cher LM, Mason W, Weller M, Hong S, Musib L, Liepa AM, Thornton DE, Fine HA. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28:1168-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 381] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 128. | Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC, Buter J, Honkoop AH, Boerman D, de Vos FY, Dinjens WN, Enting RH, Taphoorn MJ, van den Berkmortel FW, Jansen RL, Brandsma D, Bromberg JE, van Heuvel I, Vernhout RM, van der Holt B, van den Bent MJ. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15:943-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 563] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 129. | Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, Brandes AA, Taal W, Domont J, Idbaih A, Campone M, Clement PM, Stupp R, Fabbro M, Le Rhun E, Dubois F, Weller M, von Deimling A, Golfinopoulos V, Bromberg JC, Platten M, Klein M, van den Bent MJ. Lomustine and Bevacizumab in Progressive Glioblastoma. N Engl J Med. 2017;377:1954-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 671] [Article Influence: 83.9] [Reference Citation Analysis (0)] |
| 130. | Wyss J, Frank NA, Soleman J, Scheinemann K. Novel Pharmacological Treatment Options in Pediatric Glioblastoma-A Systematic Review. Cancers (Basel). 2022;14:2814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |