Published online Dec 24, 2024. doi: 10.5306/wjco.v15.i12.1501
Revised: September 19, 2024
Accepted: September 30, 2024
Published online: December 24, 2024
Processing time: 114 Days and 1.7 Hours
Primary squamous cell carcinoma (SCC) of the middle ear is rare, with non-keratinizing basaloid types being exceptionally uncommon. Distinguishing these cancers, often caused by viral factors (e.g., human papillomavirus or Epstein-Barr virus), or specific genetic alterations (e.g., bromodomain-containing protein 4-nu
A 55-year-old female patient first exhibited signs of illness over 10 years ago with persistent discomfort in the left external auditory canal, accompanied by skin irritation and bleeding. One month prior to seeking professional help, she expe
DEK::AFF2 gene fusion-associated NKSCC of the middle ear carries a grim prog
Core Tip: DEK::AFF2 non-keratinizing squamous cell carcinoma remains an understudied entity because of its rarity. This rare variant warrants increased attention and further exploration due to its potential severity and metastatic ability.
- Citation: Sun YW, Zhou Y, Liu XY, Shen DH. DEK::AFF2 fusion-associated middle ear non-keratinizing squamous cell carcinoma: A case report. World J Clin Oncol 2024; 15(12): 1501-1506
- URL: https://www.wjgnet.com/2218-4333/full/v15/i12/1501.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i12.1501
DEK::AFF2 fusion carcinoma is a subtype of non-keratinizing squamous cell carcinoma (NKSCC). This tumor was first described in 2019 by Yang et al[1]. It is an exceedingly rare and aggressive tumor. DEK::AFF2 NKSCC typically invades neighboring tissues, tends to recur, and may lead to nodal involvement or distal spread[2]. Most recorded instances have involved the head and neck regions, particularly affecting the nasopharyngeal cavities, ears, temporo-occipital areas, and eyes[2]. Histologically, it often presents with basaloid or non-keratinizing squamous features[3]. The emergence of this rare subtype highlights the need for increased awareness and further research due to its potential virulence and pro
Herein, we report a case of DEK::AFF2 fusion NKSCC and discuss its clinical, pathological, and molecular features to elucidate the differential diagnosis of this rare tumor subtype.
A 55-year-old female patient presented with left external auditory canal pain and bloody otorrhea, symptoms she had been experiencing for over 10 years.
The patient reported persistent discomfort in the left external auditory canal, along with bothersome itching and bleeding from the affected area. Approximately one month before seeking professional attention, she began experiencing a decline in hearing on the affected side, accompanied by a sensation of congestion or blockage in that ear. She also reported episodic ringing sounds but no episodes of vertigo. Upon examination at our outpatient clinic, an unusual growth was observed in the left external auditory canal. A subsequent pathological examination of a sample obtained via needle as
The patient had no significant past medical history.
No evident abnormalities were identified in the patient’s personal or family history.
Inspection revealed a dark red mass on the anterior wall of the left external auditory canal. A minimal watery exudate covered the mass, completely occluding the canal and obstructing visual access to the deeper ear structures, including the tympanic membrane.
No abnormalities were detected in the serum tumor markers.
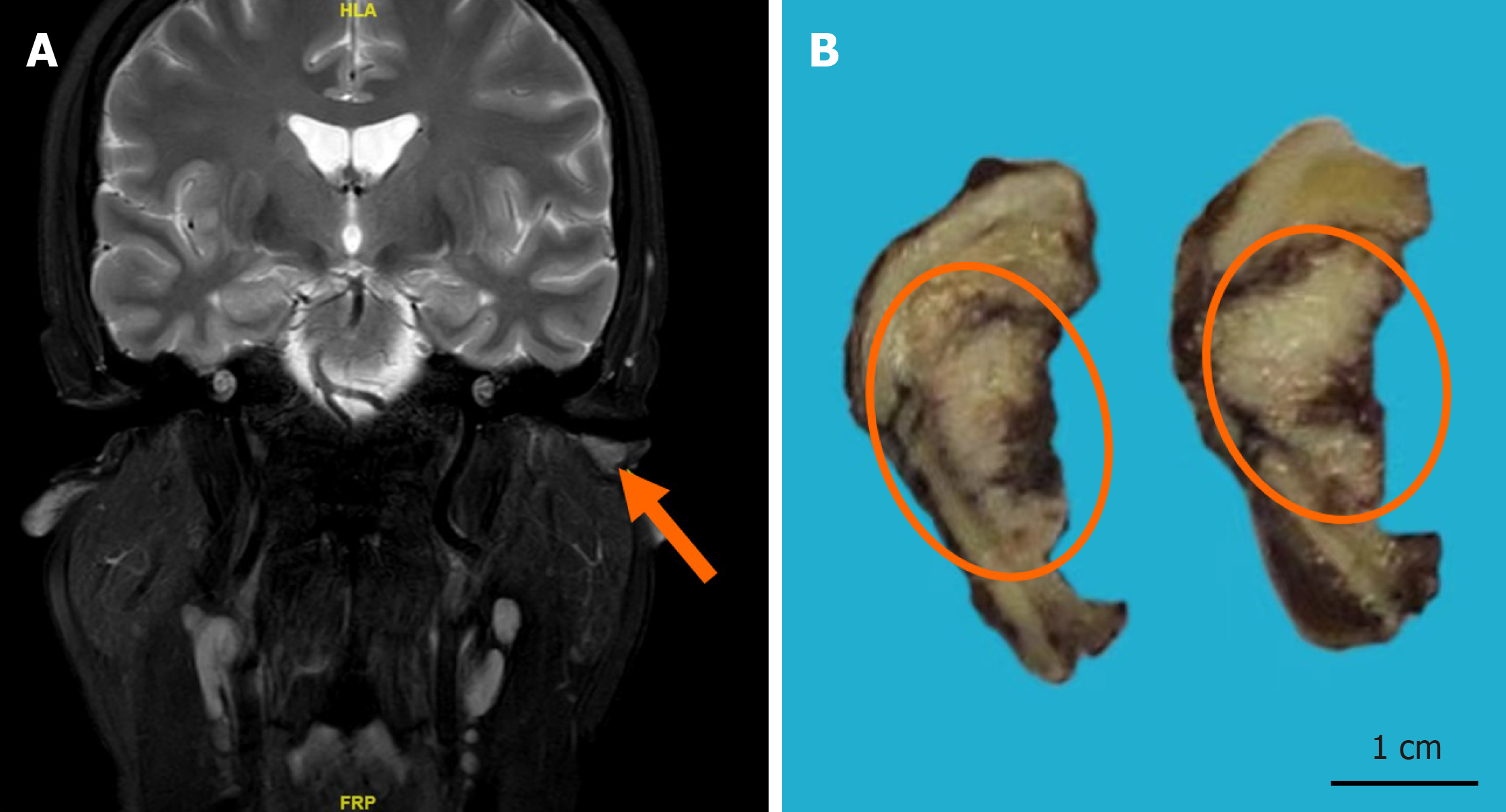
Magnetic resonance enhanced scan (November 22, 2020; our hospital): The scan revealed a neoplasm with equal T1 and long T2 signals, clear boundaries, and dimensions of approximately 1.1 cm × 0.6 cm × 0.5 cm. The enhanced scan showed contrast enhancement suspicious for osseous destruction, with closely adjacent vascular shadows observed in the anterior lower part of the left external auditory canal (Figure 1A).
DEK::AFF2 non-keratinizing squamous cell carcinoma (SCC).
We subsequently performed surgical excision of the neoplasm via mastoidectomy and sent the specimens for pathological analysis (Figure 1B). Following the surgery, the patient underwent three cycles of adjuvant local radiotherapy. The postoperative tumor bed received a total of 60 Gy, delivered in 30 fractions. Additionally, the preauricular and postauricular parotid lymphatic drainage region was irradiated with 56 Gy in 30 fractions.
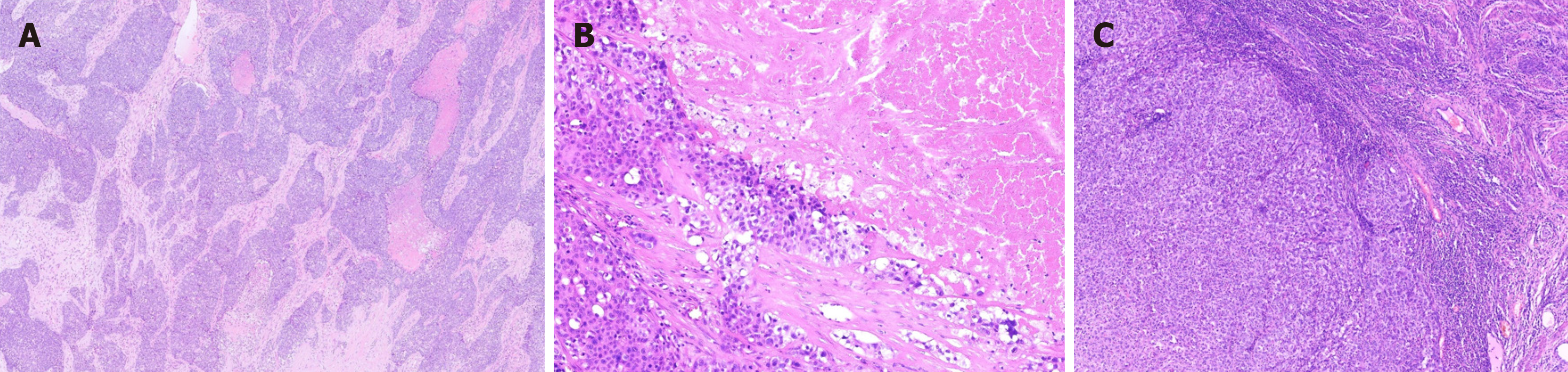
The excised tumor was grayish-red, measuring approximately 2.0 cm × 1.6 cm × 0.5 cm. The cross-section was white-gray and solid, displaying hemorrhagic areas and visible surrounding cartilage under microscopic examination (Figure 1B). Histological analysis showed the tumor manifested as an endophytic papillary carcinoma resembling transitional epithe
Non-keratinizing SCC.
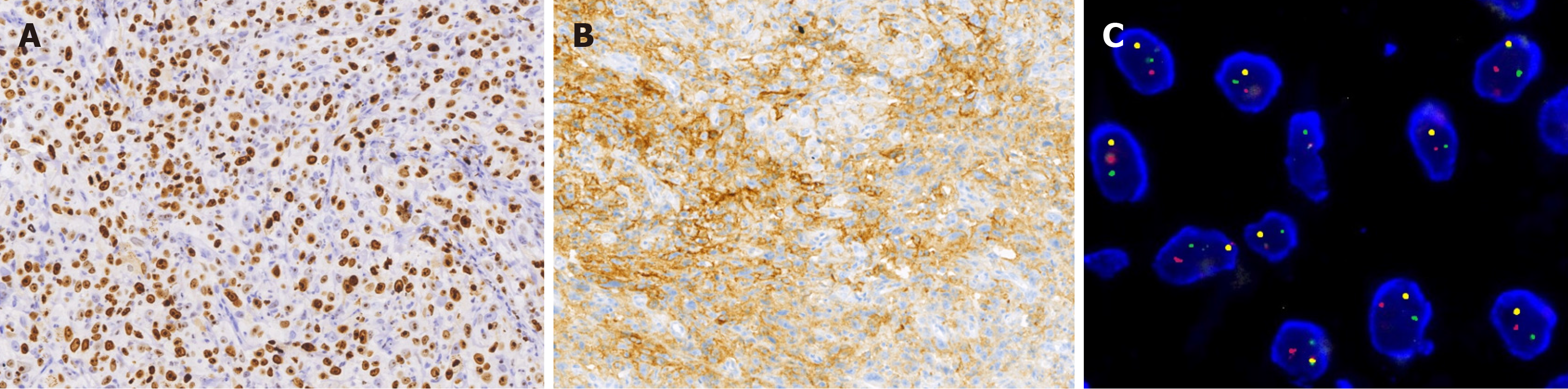
A retrospective analysis using reverse transcription polymerase chain reaction (RT-PCR) was negative for human papillomavirus (HPV). In situ hybridization for Epstein-Barr virus (EBV) encoded RNA also yielded negative results. Fluore
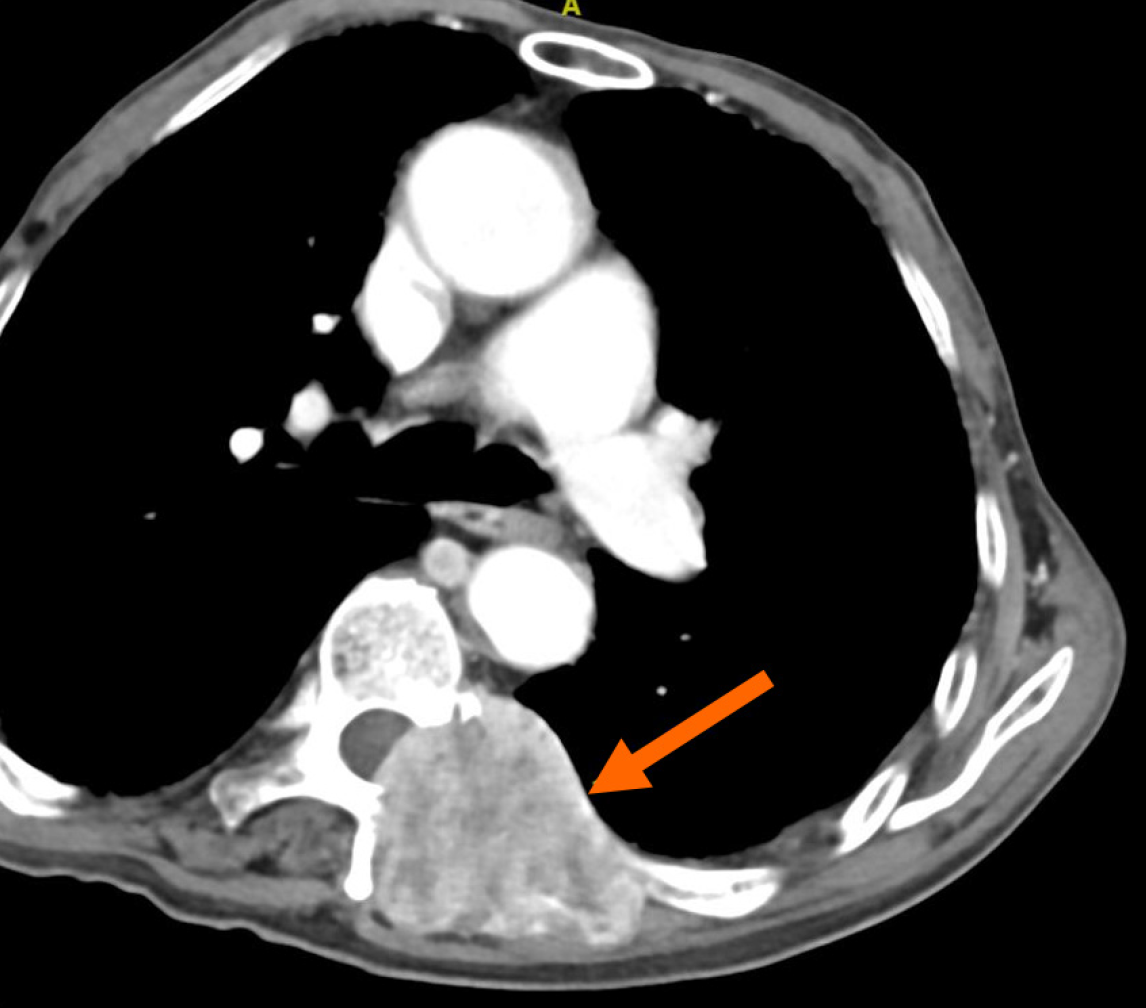
The follow-up period lasted 31 months, from the date of the initial operation until the patient's death. Metastasis to the lumbar vertebrae was detected 19 months after treatment (Figure 4). Three months after the removal of the metastatic lesion, an enlargement of the neck lymph nodes was observed during a physical examination. A biopsy confirmed cancer metastasis, and the patient passed away nine months later.
The newly identified DEK::AFF2 NKSCC represents a distinct type of cancer with specific genomic alterations, as recog
This cancer exhibits invasive behavior, risk of recurrence, and potential for progression to regional nodes or distant sites. It predominantly affects females aged between 18 years and 79 years and is mainly found in the head and neck, nasopharynx, ears, temporo-occipital area, eyes, and lungs. Notably, only four cases have involved the inner ear[6].
Histologically, nasopharyngeal keratoacanthomas share similar features, including: (1) Complex exophytic papillomatous structures that resemble inverted folds; (2) Basaloid/transitional epithelial cells with eosinophilic or biphasic cytoplasm; (3) Non-keratinization or focal keratinization; (4) Uniform round to oval nuclei; and (5) Abundant inflammatory cellular infiltrates, especially neutrophils within the epithelium and stroma, and thin fibrovascular cores forming exophytic projections where interstitial connective tissue may gather. Epithelial cells often display spongiosis and loss of cohesion, leading to pseudopapillary formation[3]. Nuclear chromatin varies from fine to vacuolated in the nucleolus, with low mitotic activity and rare necrosis. Some cases also exhibit special morphological features, such as clear cell transformation, glandular cavities secreting mucoid substances, and intracellular alterations. Kuo et al[7] reported seven instances of deceptively benign DEK::AFF2 nasal sinus SCCs that lacked distinct stromal invasion or a marked des
Immunophenotypically, the tumor cells diffusely expressed CK 5/6, p63, and p40. NUT was negative, whereas ex
The prognosis for individuals diagnosed with DEK::AFF2 fusion NKSCC is unfavorable, as approximately 20% de
DEK::AFF2 NKSCC represents a significant diagnostic challenge. In our routine diagnostic work, if we encounter complex papillary non-keratinized SCCs in the head and neck area (including the nasal cavity, paranasal sinuses, ears, and cranial base), even after ruling out HPV, EBV, SMRCB1/SMARCA4, or NUT-related malignancies, the possibility of DEK::AFF2 fusion NKSCC should be considered.
| 1. | Yang W, Lee KW, Srivastava RM, Kuo F, Krishna C, Chowell D, Makarov V, Hoen D, Dalin MG, Wexler L, Ghossein R, Katabi N, Nadeem Z, Cohen MA, Tian SK, Robine N, Arora K, Geiger H, Agius P, Bouvier N, Huberman K, Vanness K, Havel JJ, Sims JS, Samstein RM, Mandal R, Tepe J, Ganly I, Ho AL, Riaz N, Wong RJ, Shukla N, Chan TA, Morris LGT. Immunogenic neoantigens derived from gene fusions stimulate T cell responses. Nat Med. 2019;25:767-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 280] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 2. | Ruangritchankul K, Sandison A. DEK::AFF2 Fusion Carcinomas of Head and Neck. Adv Anat Pathol. 2023;30:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Skálová A, Agaimy A, Bradova M, Poorten VV, Hanna E, Guntinas-Lichius O, Franchi A, Hellquist H, Simpson RHW, Lopéz F, Nuyts S, Chiesa-Estomba C, Ng SP, Homma A, Teng Y, Leivo I, Ferlito A. Molecularly defined sinonasal malignancies: an overview with focus on the current WHO classification and recently described provisional entities. Virchows Arch. 2024;484:885-900. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Agarwal A, Bhatt AA, Bathla G, Kanekar S, Soni N, Murray J, Vijay K, Vibhute P, Rhyner PH. Update from the 5th Edition of the WHO Classification of Nasal, Paranasal, and Skull Base Tumors: Imaging Overview with Histopathologic and Genetic Correlation. AJNR Am J Neuroradiol. 2023;44:1116-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 5. | Thompson LDR, Bishop JA. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Nasal Cavity, Paranasal Sinuses and Skull Base. Head Neck Pathol. 2022;16:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 6. | Yu ST, Ye XZ, Xu BQ, Ouyang XJ, Zhang JQ, Qu LJ. [Primary DEK::AFF2 fusion positive nonkeratinizing squamous cell carcinoma of the middle ear: report of a case]. Zhonghua Binglixue Zazhi. 2023;52:1058-1060. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Kuo YJ, Lewis JS Jr, Truong T, Yeh YC, Chernock RD, Zhai C, Chen YA, Hongo T, Lee CK, Shi Q, Velez Torres JM, Geromes AB, Chu YH, Hsieh MS, Yamamoto H, Weinreb I, Hang JF. Nuclear expression of AFF2 C-terminus is a sensitive and specific ancillary marker for DEK::AFF2 carcinoma of the sinonasal tract. Mod Pathol. 2022;35:1587-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Savari O, Chang JC, Bishop JA, Sakthivel MK, Askin FB, Rekhtman N. First Report of Thoracic Carcinoma With DEK::AFF2 Rearrangement: A Case Report. J Thorac Oncol. 2022;17:1050-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Ng JKM, Chow C, Tang CY, Chan AZ, Li JJX, Chan ABW. Prevalence and interpretation of AFF immunostain of DEK::AFF2 fusion-associated papillary squamous cell carcinoma in a retrospective cohort of recurrent sinonasal papillomas. Virchows Arch. 2024;484:119-125. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Laforga JB, Abdullah B. Sinonasal DEK-rearranged Papillary Non-keratinizing Squamous Cell Carcinoma: Expanding the Emerging Entity. Indian J Otolaryngol Head Neck Surg. 2023;75:3866-3870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Lépine C, Trinquet A, Laé M, Costes-Martineau V. [Translocated sinonasal tumors]. Ann Pathol. 2024;S0242-6498(24)00007. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Larkin R, Hermsen MA, London NR Jr. Translocations and Gene Fusions in Sinonasal Malignancies. Curr Oncol Rep. 2023;25:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |