Published online Dec 24, 2024. doi: 10.5306/wjco.v15.i12.1468
Revised: August 2, 2024
Accepted: August 26, 2024
Published online: December 24, 2024
Processing time: 155 Days and 12.3 Hours
Squamous cell carcinoma of the head and neck (SCCHN) accounts for 3% of all malignant tumors in Italy. Immune checkpoint inhibitors combined with che
To perform a multicenter retrospective study on the efficacy and safety of weekly paclitaxel for SCCHN.
All patients were previously treated with at least one systemic therapy regimen, which included platinum-based therapy in the vast majority. No patient received prior immunotherapy.
Median progression-free survival (mPFS) was 3.4 months and median overall survival (mOS) was 6.5 months. Subgroup analysis was performed according to three principal prognostic factors: Smoking, alcohol consumption, and body mass index. Analysis demonstrated reduced survival, both mOS and mPFS, in the unfavorable prog
Weekly paclitaxel provided favorable survival and disease control rates, with low severe adverse events. Paclitaxel is a safe and valid therapeutic option for patients with SCCHN who received prior therapy.
Core Tip: The aim of this retrospective observational study was to evaluate the efficacy of paclitaxel as second-line treatment for patients with metastatic squamous cell carcinoma of the head and neck (SCCHN), providing unique real-world clinical experience. The observations reflect the experience of clinicians in an era before the advent of cancer immunotherapy. The results showed good efficacy of paclitaxel, and importantly, a favorable toxicity profile. These findings demonstrate that paclitaxel is a valid therapeutic option for patients with SCCHN who received prior therapy.
- Citation: Fasano M, Pirozzi M, Vitale P, Damiano V, Ronzino G, Farese S, Carfora V, Ciccarelli G, Di Giovanni I, Facchini S, Cennamo G, Caraglia M, Ciardiello F, Addeo R. Paclitaxel for second-line treatment of squamous cell carcinoma of the head and neck: A multicenter retrospective Italian study. World J Clin Oncol 2024; 15(12): 1468-1480
- URL: https://www.wjgnet.com/2218-4333/full/v15/i12/1468.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i12.1468
Squamous cell carcinoma of the head and neck (SCCHN) is the 7th most common cancer worldwide, with an estimated 350000 deaths per year. In Italy, it represents about 3% of all malignant tumors[1]. Seventy-five percent of SCCHN cases are related to smoking and alcohol[2]. Approximately 54% of patients present with advanced SCCHN at diagnosis, with a 5-year survival of about 34% in the case of regional node disease and 8% for metastatic disease. Few therapeutic options are available for recurrent/metastatic (R/M) SCCHN. Historically, the standard of care (SoC) first-line treatment was platinum-based chemotherapy. Since 2008, the EXTREME regimen (platinum + 5 fluorouracil + cetuximab) has been the standard treatment for patients with SCCHN. However, in the last several years, based on the results of the Keynote-048 trial, the treatment regimen has changed for patients with SCCHN with programmed death-ligand 1 (PD-L1) expression as determined by a combined positive score > 1. In those cases, instead of the EXTREME regimen, the physician can choose between single-agent immunotherapy with pembrolizumab or a combination of chemotherapy and immunotherapy (platinum + 5 fluorouracil + pembrolizumab) for 4-6 cycles. Thereafter, pembrolizumab is continued for main
Since 2016, immunotherapy has been standard therapy after disease progression, following the results of the CheckMate 141 and Keynote-040 trials. In the CheckMate 141 trial, nivolumab resulted in increased survival with a median overall survival (mOS) of 7.5 months vs 5.1 months and response rate (RR) of 13.3% vs 5.8% compared to ph
Considering the widespread use of immune checkpoint inhibitors (ICIs) as first-line treatment, it is necessary to identify appropriate second-line options[7]. In most cases, the physician’s choice is SoC, where paclitaxel is a still valid alternative[8,9]. Other alternatives remain docetaxel, cetuximab, or methotrexate monotherapy. Until recently, metho
Because of its increasing new role in the SCCHN continuum of care, we analyzed the efficacy and safety of weekly paclitaxel, which is most routinely used in clinical practice. To this end, we conducted a multicenter, retrospective, observational study of 107 patients with R/M SCCHN who received prior therapy at four high-volume centers in South Italy.
This multicenter, retrospective, observational study investigated weekly paclitaxel in patients with R/M SCCHN treated at the following high-volume centers in Southern Italy: Azienda Ospedaliera Universitaria Luigi Vanvitelli (Naples), Azienda Ospedaliera Universitaria Federico II (Naples), Ospedale Civile San Giovanni di Dio (Frattamaggiore, Naples), and Ospedale Vito Fazzi (Lecce). We retrospectively reviewed data from all patients diagnosed with SCCHN, who were treated with paclitaxel after at least one line of systemic therapy in these institutions between February 2015 and July 2018.
Paclitaxel was administered intravenously at 80 mg/m2 every 7 days. Dose reductions were allowed in case of toxicities as per clinical practice, and treatment was continued until disease progression according to Response Evaluation Criteria in Solid Tumours (RECIST) criteria, unacceptable toxicity, death, or consent withdrawal.
We investigated PFS, OS, and DCR. PFS was defined as the time from first paclitaxel administration to disease pro
Patient characteristics at baseline were compared using χ2 and Fisher’s exact test for categorical variables and t-test for continuous variables. In case of violation of the normality assumption, the non-parametric Mann-Whitney-Wilcoxon test was used. The median follow-up time was estimated using the reverse Kaplan-Meier method.
To study the effect of risk factors on survival, unweighted and weighted Cox proportional hazard regression models were estimated. Hazard ratios (HR) along with their 95%CI were reported. P ≤ 0.05 was considered statistically significant. P values < 0.10 were reported to the third decimal place, whereas P values ≥ 0.10 were reported to the second decimal place. Statistical analyses were performed using R version 4.0.
As per local guidelines, at the start of data collection, informed consent was not necessary for patients treated at S. Giovanni Di Dio Hospital. Accordingly, the ASL Napoli 2 Nord Ethical Committee sanctioned an acknowledgement declaration in May 2015.
A total of 107 SCCHN patients treated in our institutions were selected for the final analysis. The main population characteristics are shown in Table 1.
| Characteristics | |
| Median age in years (minimum-maximum) | 57.8 (37.8-82.7) |
| Sex | |
| Female | 15 (14) |
| Male | 92 (86) |
| Eastern cooperative oncology group performance status | |
| 0 | 4 (3) |
| 1 | 89 (84) |
| 2 | 14 (13) |
| Location of primary tumor | |
| Larynx | 27 (25) |
| Oropharynx | 33 (31) |
| Hypopharynx | 29 (27) |
| Oral cavity | 18 (17) |
| Body mass index | |
| Overweight, ≥ 25.0 | 26 (24) |
| Normal, 18.5-25.0 | 38 (36) |
| Underweight, ≤ 18.5 | 43 (40) |
| Caregiver | |
| Yes | 66 (62) |
| No | 41 (38) |
| Alcohol | |
| Yes | 41 (38) |
| No | 66 (62) |
| Smoking | |
| Yes | 45 (42) |
| No | 62 (58) |
| Human papillomavirus | |
| Yes | 11 (11) |
| No | 46 (49) |
| Unknown | 50 (40) |
Most patients were male (86%), with an Eastern cooperative oncology group performance status (PS) between 0 and 1 (87%). More than 50% of patients denied smoking or alcohol abuse (58% and 62%, respectively), which is unexpected for this group of patients. It is possible that patients lied during history collection due to perceived social stigma and because they incorrectly assumed different treatment from medical personnel. As expected, few patients were overweight, while 40% were underweight and 36% presented with normal BMI. Human papillomavirus status was only known for 60% of patients with oropharyngeal cancer. Primary tumor sites were oropharynx (31%), hypopharynx (27%), larynx (25%), and oral cavity (17%). No other head and neck site was included in this study.
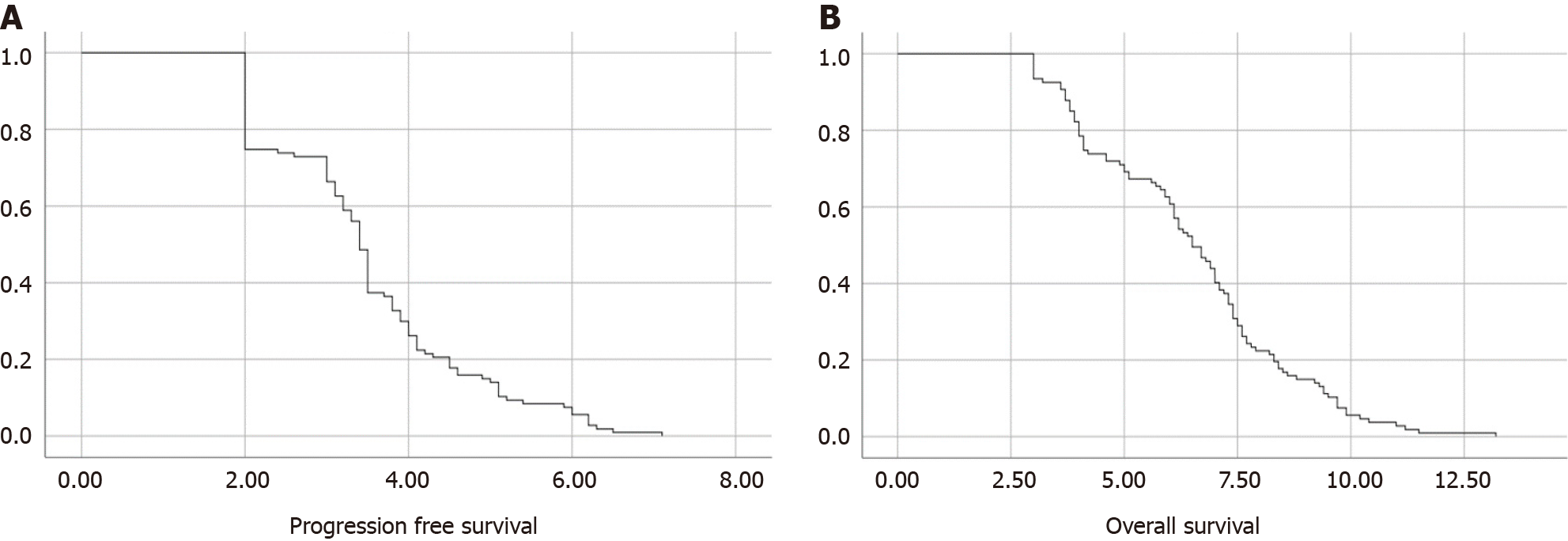
All patients were previously treated with at least one systemic therapy regimen. The main characteristics of previous treatments (cisplatin, carboplatin, cetuximab, other chemotherapy) are shown in Table 2. No patient received immunotherapy before treatment with paclitaxel. Only a small percentage of patients received further treatment after progression on paclitaxel, with few receiving immunotherapy. Thus, no further analyses were performed on subsequent treatments. Platinum-based therapy was previously administered to almost all patients, with only 5 having received other treatment regimens. A total of 48% of patients received cisplatin-based therapy, whereas 47% received carboplatin. Sixty-two percent of the patients receiving chemotherapy with a platinum backbone were also administered cetuximab, and forty-five percent of them continued with cetuximab maintenance. The median number of chemotherapy cycles was 20. The DCR from first-line maintenance was 44%. Almost all patients previously underwent at least one radiotherapy course (94%). The median PFS (mPFS) was estimated at 3.4 months (95%CI: 3.299-3.501) and the mOS was 6.5 months (95%CI: 5.921-7.079) (Figure 1).
| Chemotherapy type | |
| Cisplatin-based | 52 (48) |
| Carboplatin-based | 50 (47) |
| Cetuximab also | 66 (62) |
| Other chemotherapy | 5 (5) |
| Maintenance with cetuximab | |
| Yes | 48 (45) |
| No | 59 (55) |
| Previous radiotherapy | |
| Yes | 100 (94) |
| No | 7 (6) |
| Best response of maintenance | |
| Complete response | 1 (1) |
| Partial response | 28 (27) |
| Stabilization of disease | 18 (16) |
| Disease progression | 60 (56) |
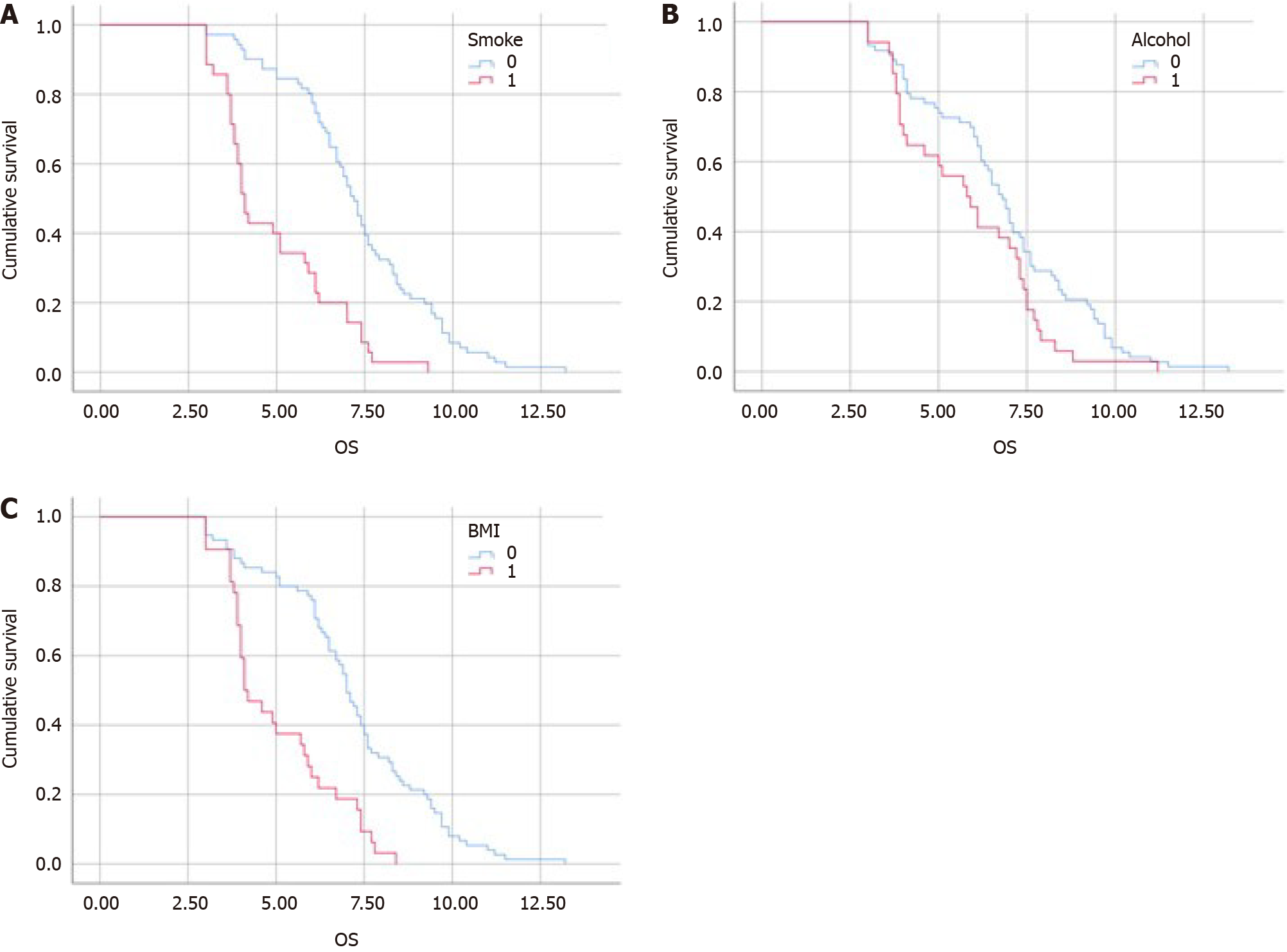
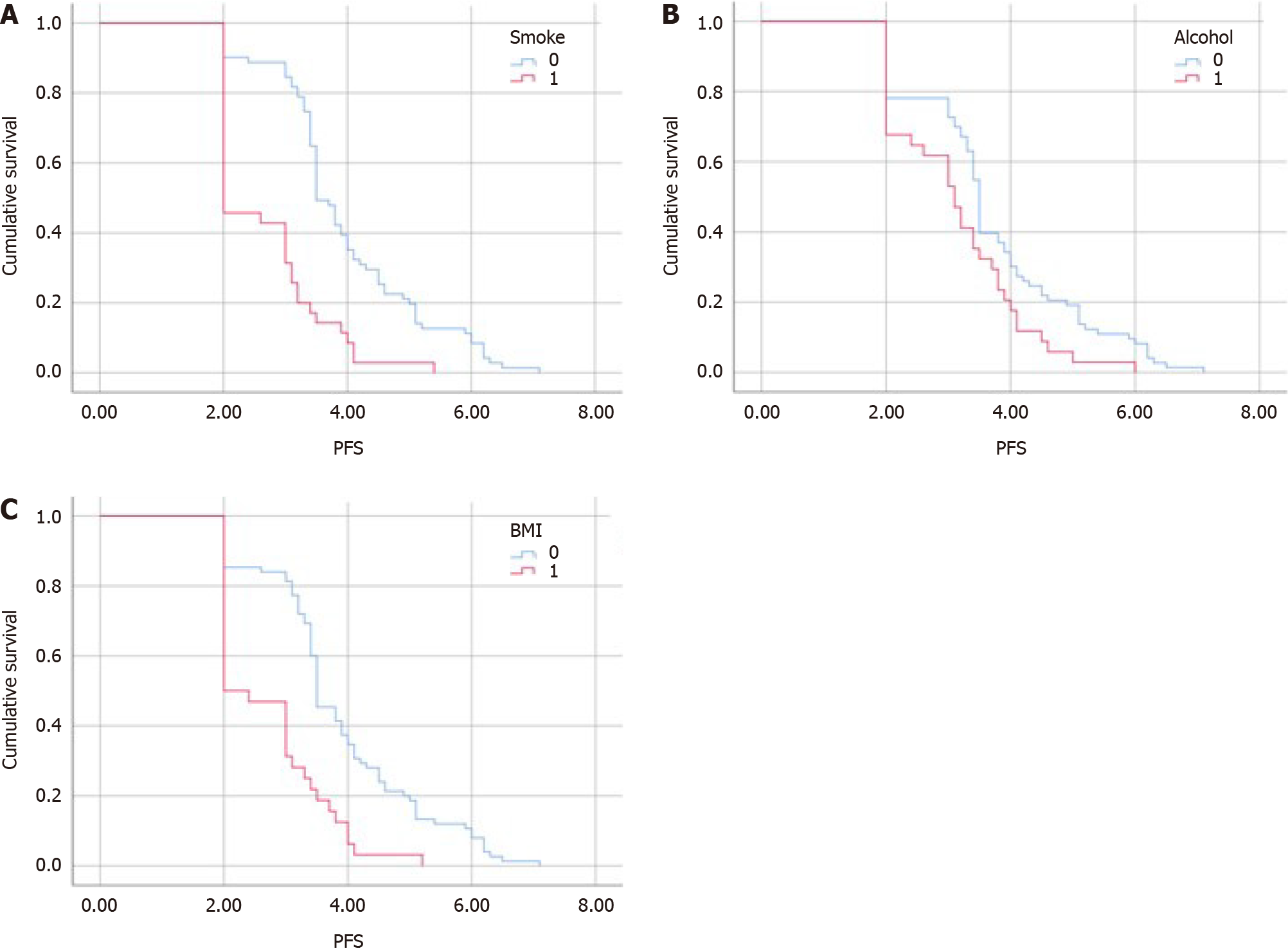
Subgroup analysis was performed according to three principal prognostic factors (Table 3): Smoking, alcohol consump
| Survival rate | Low BMI | Normal BMI | Smoking | Non-smoking | Alcohol | No alcohol |
| Median progression-free survival | 2 | 3.5 | 2.96 | 4.23 | 3.1 | 3.5 |
| Median overall survival | 4.1 | 7 | 5.47 | 7.79 | 5.8 | 6.8 |
Treatment was well tolerated overall, with 60% of patients reporting any-grade AEs. AE data are shown in Table 4. It is worth noting that only 14% of patients developed a grade 3-4 toxicity, mainly asthenia (14%), anemia (12%), and mucositis (12%). Other reported toxicities were acne-like rash, peripheral neuropathy, and neutropenia. DCR was reached in 52% of patients (55 of 107); in particular, 1 patient had CR [median duration of response (mDoR) 7 months], 25% PR (mDoR 5 months), and 26% SD (mDoR 3 months). The ORR, defined as either PR or CR, was 26% (Table 5).
| Adverse event | All grades | Grade 3-4 |
| Acne-like rash | 4 (7) | 0 |
| Anemia | 49 (46) | 14 (12) |
| Mucositis | 38 (36) | 10 (12) |
| Peripheral neuropathy | 17 (15) | 3 (2) |
| Asthenia | 42 (39) | 12 (14) |
| Neutropenia | 30 (28) | 7 (6) |
| Clinical endpoint | |
| Complete response | 1 (1) |
| Partial response | 26 (25) |
| Overall response | 27 (26) |
| Stable disease | 28 (26) |
| Disease control rate | 55 (52) |
| Progressive disease | 52 (48) |
Our data were borne out of our daily clinical practice, obtained from some of the highest-volume oncology divisions in South Italy, with consolidated experience in the treatment of SCCHN. As such, the patients’ characteristics are very heterogenous, reflecting our daily practice, and are not a strictly selected population as might be the case in a prospective clinical trial. Nevertheless, our results are in line with previous works investigating the role of paclitaxel, both alone and in combination with other drugs, in patients with SCCHN who received prior therapy. No patient was treated with checkpoint inhibitors before paclitaxel, thus permitting comparison with previous research on the subject. With the exception of a study by Vermorken et al[16] on cetuximab, our study has one of the largest sample sizes. We also analyzed our population according to the most important risk factors (i.e. smoking, alcohol consumption, BMI). In 2009, Grau et al[15] published a retrospective study on paclitaxel, resulting in an mPFS of 6.5 months, mOS of 8.5 months, and RR of 43.3%. In 2011, a Phase 2 trial by Tahara et al[17] analyzed weekly paclitaxel in a multicenter trial with an independent review committee showing a 33.3% RR, a 3.4 month mTTP, and an mOS of 14.3 months. Moreover, in 2010, Fayette et al[14] published a retrospective analysis of 66 patients treated with paclitaxel alone or in combination with carboplatin and cetuximab, finding an ORR of 30%.
Our study demonstrated a good safety profile for single-agent paclitaxel, with only 14% of patients reporting a grade 3-4 toxicity, and a DCR of 52%. Although limited by study design, the RR and mPFS from our retrospective study were significantly better than those of the nivolumab arm in the CheckMate 141 trial, a Phase 3 trial in which taxanes were part of the control arm (26% in our study, 13.3% in CheckMate 141). However, it must be noted that the higher OS data in the nivolumab arm (mOS was 7.7 months and 24-month OS rate was 16.9%) were almost triple those of the comparator arm (6.0%)[18]. Furthermore, Haddad et al[19] evaluated the use of nivolumab beyond RECIST-defined progression with clinical benefit, demonstrating an mOS benefit, reaching 12.7 months. Nivolumab also presents a better tolerability profile, with improvement in several quality-of-life domains compared to the control group[20].
Patients with advanced SCCHN who progress on platinum-based therapy often have a very poor prognosis[21], with most experiencing a high symptom burden. The widespread use of immunotherapy in the first line has led to an increasing number of patients who need a tolerable and valid option for second-line therapy. Physicians usually prefer single-agent chemotherapy to multidrug regimens due to their better tolerability.
Preclinical studies have demonstrated a synergistic effect of the association of paclitaxel and cetuximab, and several clinical trials have demonstrated a significant role for the combination, both in first and subsequent lines. In 2011, Hitt et al[22] treated 46 patients with a combination of paclitaxel and cetuximab as first-line therapy in a Phase 2 trial; the ORR was 54%, mPFS was 4.2 months, and mOS was 8.1 months. Subsequently, Jiménez et al[23] studied the combination in platinum-sensitive and platinum-refractory patients with a high mRR (66% sensitive and 44% refractory). Another retrospective analysis in 2012 also showed a good RR (38%) and OS (7.6 months) in patients who received prior therapy[24]. Nevertheless, the results of the studies have been highly variable. For example, a study of paclitaxel plus cetuximab in patients who progressed on first-line treatment of platinum-based chemotherapy and cetuximab showed an ORR of only 16.4% vs 6.2% in the paclitaxel arm, with an mOS delta of 1.3 months[25].
Thus, while the literature provides multiple lines of evidence demonstrating the efficacy of such a combination, there is a crucial need for prospective trials with a better selected population that will allow the findings to be applied to routine clinical practice. Furthermore, paclitaxel and cetuximab may have a special role as salvage therapy after ICIs (Table 6)[14-17,26-31].
| Ref. | n | Baseline characteristics | ChT regimen | Objective response rate | Overall survival | Progression-free survival |
| Vermorken, 2007 | 1031 | Median age 57 years; male 82; median KPS 80; locoregional only 52 | Cetuximab | 13 | 5.9 months | 2.3 months |
| Grau, 2009 | 60 | Median age 59.5 years; male 91.7; KPS 0 1.7; locoregional only 51.7 | Paclitaxel | 43.3 | 5.2 months | 6.2 months |
| Tahara, 2011 | 72 | Median age 61 years; male 77.8; KPS 0 66.7; locoregional only n/a | Paclitaxel | 33.3 | 14.3 months | / |
| Fayette, 2010 | 66 | Median age 60.7 years; male 89; KPS 0 6; locoregional only 58 | Paclitaxel, paclitaxel combination | 30 | 7.8 months | 3.9 months |
| Catimel, 1994 | 40 | Median age 55 years; male 32; KPS 0 11; locoregional only 26 | Docetaxel | 32 | / | / |
| Saleh, 2019 | 82 | Median age 58 years; Male 84; KPS 0 45-55; locoregional only 41 | Taxane, taxane cetuximab +/- platinum, taxane platinum, EXTREME, docetaxel-platinum-cetuximab, carboplatin cetuximab, carboplatin paclitaxel | 30 | 7.8 months | 3.6 months |
| Kurosaki, 2021 | 22 | Median age 65 years; male 59.1; KPS 0 22.7; locoregional only 31.8 | Cetuximab-paclitaxel, carboplatin fluorouracil cetuximab | 40.9 | 14.5 months | 5.2 months |
| Pestana, 2020 | 43 | Median age n/a; male 90.7; KPS 0; locoregional only 83.7 | Cetuximab, single agent ChT, ChT + cetuximab, ChT + other agents | 42 | 8.41 months | 4.24 months |
| Cabezas-Camarero, 2021 | 23 | Median age 65 years; male 73.9; KPS 0 4.3; locoregional only 17.4 | ERBITAX, EXTREME, CARBITAX, cisplatin-cetuximab | 56.5 | 12 months | 6 months |
| Harrington, 2023 | 311 | Median age; male; KPS 0; locoregional only | Taxane-based, non-taxane-based (antimetabolite, platinum-based) | N/A | N/A | Pembrolizumab alone; taxane-based: 9.8 months; non-taxane-based: 9.5 months; pembrolizumab-ChT; taxane-based: 12.7 months; non-taxane-based: 12.5 months |
In recent years, several studies have investigated cetuximab-based and paclitaxel-based chemotherapy as salvage treatment after immunotherapy progression. Cabezas-Camarero et al[29] found an ORR of 56.5% and mOS of 12 months after salvage chemotherapy with four different chemotherapy regimens, all containing cetuximab. A United States study of 43 pretreated patients, 60% of whom were platinum-refractory, demonstrated ORR for salvage chemotherapy of about 42%, 37.5% with cetuximab single-agent only[30]. A French study evaluating 82 patients demonstrated an ORR of 30% and mOS of 7.8 months, showing an improved RR and survival if immunotherapy was used in a first-line setting[31]. Furthermore, ORR increased to 53% vs 25% if cetuximab + taxane + platinum was used instead of other chemotherapy regimens. Although these results might seem underwhelming with limited survival and RR, they are in accordance with results from previous trials. For example, first-line therapy in the EXTREME trial only showed an RR of 36%, whereas in a study by Vermorken et al[16], cetuximab monotherapy in the first line and beyond resulted in an unsatisfying RR of 13%. A 2021 study by Sato et al[32] evaluated paclitaxel-based chemotherapy before and after nivolumab. In the 10 patients receiving paclitaxel after immunotherapy, there was an increased ORR and mTTP compared to the group receiving paclitaxel before immunotherapy. In fact, the ORR was 53.4% in the first group vs 34.9%. Indeed, the 4-year updated results of the Keynote-048 trial also showed improved second PFS following pembrolizumab or pembrolizumab + chemotherapy compared to cetuximab + chemotherapy in the next-line taxane subgroup (HR 0.96 and 0.67, respectively)[32]. It has been presumed that paclitaxel may have an immunomodulatory effect on synergy with ICIs activity[19] or that there is chemosensitivity restoration due to immunotherapy-induced microenvironment modification[18].
Advanced cancer is burdened by a poor PS, and indeed, patients with SCCHN frequently present with several co
Our study had some limitations. The retrospective study design is subject to a higher risk of incomplete data, information, and recall bias. For example, as aforementioned, a smaller percentage of patients confirmed smoking or alcohol consumption than what is expected from this population. Inclusion criteria were less stringent than those of a clinical trial, thus allowing for a more heterogeneous population, albeit more similar to routine clinical practice. Fur
New strategies for patients with SCCHN who received prior therapy are already under investigation[42]. Tipifarnib inhibits farnesyltransferase, blocking RAS binding to the membrane, rendering it inactive. One study investigated the efficacy of tipifarnib in patients with HRAS-mutated SCCHN, and found an ORR of 55% in the 20 patients [43]. On the other hand, while initially promising, a study of the association between cetuximab and a novel ICIs targeting the natural killer receptor NKG2A, was prematurely terminated for futility. Furthermore, tisotumab vetodin, an antibody-drug conjugate comprising a monoclonal antibody against tissue factor covalently coupled to the microtubule-disrupting monomethyl auristatin E payload, is being investigated in an open-label, Phase 2 trial (SGNTV-001, No. NCT03485209), with initial good results, as reported by Hong et al[44].
Immunotherapy combinations of anti-PD-L1 and anti-cytotoxic T lymphocyte antigen-4 have not shown any benefit over anti-PD-L1 alone. The Phase 2 CONDOR[45] and Phase 3 EAGLE trial[46] found no difference between durvalumab and durvalumab plus tremelimumab. Nivolumab plus ipilimumab also failed to reach its primary endpoint of OS in the CheckMate 651 study[47] compared to the EXTREME regimen. Immunotherapy combinations with other drugs are also being studied. A Phase 1b/2 trial of lenvatinib and pembrolizumab in 22 patients showed a PFS of 7.6 months and 24-week ORR of 36.4%[48]. These data were confirmed by a Taiwanese study with 14 patients, which showed an ORR of 28.6%, OS of 6.2 months, and PFS of 4.6 months[49]. In 2021, a study designed to evaluate the addition of inducible T cell co-stimulatory receptor agonist to pembrolizumab was prematurely terminated, with results still pending[50]. Following good preliminary results in a mouse model treated with the combination of anti-T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains and anti-PD-1/PD-L1 antibodies, two ongoing basket trials, involving a Phase 1/2 (No. NCT05060432) and a Phase 2 (No. NCT05483400), are investigating EOS-448 and tiragolumab, respectively, in combination with anti-PD-1/PD-L1.
Nevertheless, paclitaxel is still in the spotlight, and new investigations are including taxanes as part of their com
In conclusion, this study demonstrated that paclitaxel is a safe and valid therapeutic choice for patients with SCCHN who received prior therapy, as the results showed favorable survival and DCRs, and only a limited subgroup of patients reported severe AEs. These results were better than most historical cohorts in a heavily pretreated setting, suggesting paclitaxel as a significant player in these patients. We also confirmed that alcohol consumption, smoking, and malnourishment are correlated with lower survival rates. While our results need to be confirmed by future research, the literature are also promising and favor the use of taxanes and taxane-based therapies as salvage chemotherapy after prior treatment with checkpoint inhibitors. Thus, paclitaxel may emerge as a key element in the SCCHN continuum of care, with a new role as a good option for pretreated patients as salvage treatment after immunotherapy. For patient-centered care, the choice of therapeutic strategy should take this information into account.
| 1. | Machiels JP, René Leemans C, Golusinski W, Grau C, Licitra L, Gregoire V; EHNS Executive Board; ESMO Guidelines Committee; ESTRO Executive Board. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1462-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 488] [Article Influence: 97.6] [Reference Citation Analysis (0)] |
| 2. | Toporcov TN, Znaor A, Zhang ZF, Yu GP, Winn DM, Wei Q, Vilensky M, Vaughan T, Thomson P, Talamini R, Szeszenia-Dabrowska N, Sturgis EM, Smith E, Shangina O, Schwartz SM, Schantz S, Rudnai P, Richiardi L, Ramroth H, Purdue MP, Olshan AF, Eluf-Neto J, Muscat J, Moyses RA, Morgenstern H, Menezes A, McClean M, Matsuo K, Mates D, Macfarlane TV, Lissowska J, Levi F, Lazarus P, La Vecchia C, Lagiou P, Koifman S, Kjaerheim K, Kelsey K, Holcatova I, Herrero R, Healy C, Hayes RB, Franceschi S, Fernandez L, Fabianova E, Daudt AW, Curioni OA, Maso LD, Curado MP, Conway DI, Chen C, Castellsague X, Canova C, Cadoni G, Brennan P, Boccia S, Antunes JL, Ahrens W, Agudo A, Boffetta P, Hashibe M, Lee YC, Filho VW. Risk factors for head and neck cancer in young adults: a pooled analysis in the INHANCE consortium. Int J Epidemiol. 2015;44:169-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 3. | Burtness B, Rischin D, Greil R, Soulières D, Tahara M, de Castro G Jr, Psyrri A, Brana I, Basté N, Neupane P, Bratland Å, Fuereder T, Hughes BGM, Mesia R, Ngamphaiboon N, Rordorf T, Wan Ishak WZ, Ge J, Swaby RF, Gumuscu B, Harrington K. Pembrolizumab Alone or With Chemotherapy for Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma in KEYNOTE-048: Subgroup Analysis by Programmed Death Ligand-1 Combined Positive Score. J Clin Oncol. 2022;40:2321-2332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 136] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 4. | Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane P, Bratland Å, Fuereder T, Hughes BGM, Mesía R, Ngamphaiboon N, Rordorf T, Wan Ishak WZ, Hong RL, González Mendoza R, Roy A, Zhang Y, Gumuscu B, Cheng JD, Jin F, Rischin D; KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2237] [Cited by in RCA: 2078] [Article Influence: 346.3] [Reference Citation Analysis (0)] |
| 5. | Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375:1856-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3044] [Cited by in RCA: 3696] [Article Influence: 410.7] [Reference Citation Analysis (0)] |
| 6. | Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R, Burtness B, Zhang P, Cheng J, Swaby RF, Harrington KJ; KEYNOTE-040 investigators. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393:156-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 1180] [Article Influence: 196.7] [Reference Citation Analysis (0)] |
| 7. | Fasano M, Corte CMD, Liello RD, Viscardi G, Sparano F, Iacovino ML, Paragliola F, Piccolo A, Napolitano S, Martini G, Morgillo F, Cappabianca S, Ciardiello F. Immunotherapy for head and neck cancer: Present and future. Crit Rev Oncol Hematol. 2022;174:103679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 8. | A phase III randomised trial of cistplatinum, methotrextate, cisplatinum + methotrexate and cisplatinum + 5-FU in end stage squamous carcinoma of the head and neck. Liverpool Head and Neck Oncology Group. Br J Cancer. 1990;61:311-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 91] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Forastiere AA, Metch B, Schuller DE, Ensley JF, Hutchins LF, Triozzi P, Kish JA, McClure S, VonFeldt E, Williamson SK. Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: a Southwest Oncology Group study. J Clin Oncol. 1992;10:1245-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 512] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 10. | Vogl SE, Schoenfeld DA, Kaplan BH, Lerner HJ, Engstrom PF, Horton J. A Randomized Prospective Comparison of Methotrexate With a Combination of Methotrexate, Bleomycin, and Cisplatin in Head and Neck Cancer. Cancer. 1985;56:432-442. [PubMed] [DOI] [Full Text] |
| 11. | Guardiola E, Peyrade F, Chaigneau L, Cupissol D, Tchiknavorian X, Bompas E, Madroszyk A, Ronchin P, Schneider M, Bleuze JP, Blay JY, Pivot X. Results of a randomised phase II study comparing docetaxel with methotrexate in patients with recurrent head and neck cancer. Eur J Cancer. 2004;40:2071-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Fasano M, Della Corte CM, Viscardi G, Di Liello R, Paragliola F, Sparano F, Iacovino ML, Castrichino A, Doria F, Sica A, Morgillo F, Colella G, Tartaro G, Cappabianca S, Testa D, Motta G, Ciardiello F. Head and neck cancer: the role of anti-EGFR agents in the era of immunotherapy. Ther Adv Med Oncol. 2021;13:1758835920949418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 13. | Caballero M, Grau JJ, Blanch JL, Domingo-Domenech J, Auge JM, Jimenez W, Bernal-Sprekelsen M. Serum vascular endothelial growth factor as a predictive factor in metronomic (weekly) Paclitaxel treatment for advanced head and neck cancer. Arch Otolaryngol Head Neck Surg. 2007;133:1143-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Fayette J, Montella A, Chabaud S, Bachelot T, Pommier P, Girodet D, Racadot S, Montbarbon X, Favier B, Zrounba P. Paclitaxel is effective in relapsed head and neck squamous cell carcinoma: a retrospective study of 66 patients at a single institution. Anticancer Drugs. 2010;21:553-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Grau JJ, Caballero M, Verger E, Monzó M, Blanch JL. Weekly paclitaxel for platin-resistant stage IV head and neck cancer patients. Acta Otolaryngol. 2009;129:1294-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, Knecht R, Amellal N, Schueler A, Baselga J. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25:2171-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 739] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 17. | Tahara M, Minami H, Hasegawa Y, Tomita K, Watanabe A, Nibu K, Fujii M, Onozawa Y, Kurono Y, Sagae D, Seriu T, Tsukuda M. Weekly paclitaxel in patients with recurrent or metastatic head and neck cancer. Cancer Chemother Pharmacol. 2011;68:769-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington KJ, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Docampo LCI, Haddad R, Rordorf T, Kiyota N, Tahara M, Lynch M, Jayaprakash V, Li L, Gillison ML. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 574] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 19. | Haddad R, Concha-Benavente F, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Kasper S, Vokes EE, Worden F, Saba NF, Tahara M, Jayaprakash V, Lynch M, Li L, Gillison ML, Harrington KJ, Ferris RL. Nivolumab treatment beyond RECIST-defined progression in recurrent or metastatic squamous cell carcinoma of the head and neck in CheckMate 141: A subgroup analysis of a randomized phase 3 clinical trial. Cancer. 2019;125:3208-3218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 20. | Harrington KJ, Ferris RL, Blumenschein G Jr, Colevas AD, Fayette J, Licitra L, Kasper S, Even C, Vokes EE, Worden F, Saba NF, Kiyota N, Haddad R, Tahara M, Grünwald V, Shaw JW, Monga M, Lynch M, Taylor F, DeRosa M, Morrissey L, Cocks K, Gillison ML, Guigay J. Nivolumab versus standard, single-agent therapy of investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 2017;18:1104-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 315] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 21. | León X, Hitt R, Constenla M, Rocca A, Stupp R, Kovács AF, Amellal N, Bessa EH, Bourhis J. A retrospective analysis of the outcome of patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck refractory to a platinum-based chemotherapy. Clin Oncol (R Coll Radiol). 2005;17:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Hitt R, Irigoyen A, Cortes-Funes H, Grau JJ, García-Sáenz JA, Cruz-Hernandez JJ; Spanish Head and Neck Cancer Cooperative Group (TTCC). Phase II study of the combination of cetuximab and weekly paclitaxel in the first-line treatment of patients with recurrent and/or metastatic squamous cell carcinoma of head and neck. Ann Oncol. 2012;23:1016-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 23. | Jiménez B, Trigo JM, Pajares BI, Sáez MI, Quero C, Navarro V, Llácer C, Medina L, Rueda A, Alba E. Efficacy and safety of weekly paclitaxel combined with cetuximab in the treatment of pretreated recurrent/metastatic head and neck cancer patients. Oral Oncol. 2013;49:182-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Péron J, Ceruse P, Lavergne E, Buiret G, Pham BN, Chabaud S, Favier B, Girodet D, Zrounba P, Ramade A, Fayette J. Paclitaxel and cetuximab combination efficiency after the failure of a platinum-based chemotherapy in recurrent/metastatic head and neck squamous cell carcinoma. Anticancer Drugs. 2012;23:996-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Chevalier T, Daste A, Saada-Bouzid E, Loundou A, Peyraud F, Lambert T, Le Tourneau C, Peyrade F, Dupuis C, Alfonsi M, Fayette J, Reure J, Huguet F, Fakhry N, Toullec C, Salas S. Cetuximab combined with paclitaxel or paclitaxel alone for patients with recurrent or metastatic head and neck squamous cell carcinoma progressing after EXTREME. Cancer Med. 2021;10:3952-3963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Catimel G, Verweij J, Mattijssen V, Hanauske A, Piccart M, Wanders J, Franklin H, Le Bail N, Clavel M, Kaye SB. Docetaxel (Taxotere): an active drug for the treatment of patients with advanced squamous cell carcinoma of the head and neck. EORTC Early Clinical Trials Group. Ann Oncol. 1994;5:533-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 153] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Kurosaki T, Mitani S, Tanaka K, Suzuki S, Kanemura H, Haratani K, Fumita S, Iwasa T, Hayashi H, Yoshida T, Ishikawa K, Kitano M, Otsuki N, Nishimura Y, Doi K, Nakagawa K. Safety and efficacy of cetuximab-containing chemotherapy after immune checkpoint inhibitors for patients with squamous cell carcinoma of the head and neck: a single-center retrospective study. Anticancer Drugs. 2021;32:95-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Harrington KJ, Burtness B, Greil R, Soulières D, Tahara M, de Castro G Jr, Psyrri A, Brana I, Basté N, Neupane P, Bratland Å, Fuereder T, Hughes BGM, Mesia R, Ngamphaiboon N, Rordorf T, Wan Ishak WZ, Lin J, Gumuscu B, Swaby RF, Rischin D. Pembrolizumab With or Without Chemotherapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Updated Results of the Phase III KEYNOTE-048 Study. J Clin Oncol. 2023;41:790-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 252] [Article Influence: 126.0] [Reference Citation Analysis (0)] |
| 29. | Cabezas-Camarero S, Cabrera-Martín MN, Merino-Menéndez S, Paz-Cabezas M, García-Barberán V, Sáiz-Pardo Sanz M, Iglesias-Moreno M, Alonso-Ovies A, Pérez-Segura P. Safety and Efficacy of Cetuximab-Based Salvage Chemotherapy After Checkpoint Inhibitors in Head and Neck Cancer. Oncologist. 2021;26:e1018-e1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Pestana RC, Becnel M, Rubin ML, Torman DK, Crespo J, Phan J, Hanna E, Bell D, Glisson BS, Johnson JM, Lee JJ, Ferrarotto R. Response rates and survival to systemic therapy after immune checkpoint inhibitor failure in recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol. 2020;101:104523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 31. | Saleh K, Daste A, Martin N, Pons-Tostivint E, Auperin A, Herrera-Gomez RG, Baste-Rotllan N, Bidault F, Guigay J, Le Tourneau C, Saada-Bouzid E, Even C. Response to salvage chemotherapy after progression on immune checkpoint inhibitors in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Eur J Cancer. 2019;121:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 32. | Sato Y, Fukuda N, Fujiwara YU, Wang X, Urasaki T, Ohmoto A, Nakano K, Ono M, Tomomatsu J, Mitani H, Takahashi S. Efficacy of Paclitaxel-based Chemotherapy After Progression on Nivolumab for Head and Neck Cancer. In Vivo. 2021;35:1211-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Du E, Mazul AL, Farquhar D, Brennan P, Anantharaman D, Abedi-Ardekani B, Weissler MC, Hayes DN, Olshan AF, Zevallos JP. Long-term Survival in Head and Neck Cancer: Impact of Site, Stage, Smoking, and Human Papillomavirus Status. Laryngoscope. 2019;129:2506-2513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 34. | van Imhoff LC, Kranenburg GG, Macco S, Nijman NL, van Overbeeke EJ, Wegner I, Grolman W, Pothen AJ. Prognostic value of continued smoking on survival and recurrence rates in patients with head and neck cancer: A systematic review. Head Neck. 2016;38 Suppl 1:E2214-E2220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 35. | Kawakita D, Matsuo K. Alcohol and head and neck cancer. Cancer Metastasis Rev. 2017;36:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 36. | Kubrak C, Olson K, Jha N, Jensen L, McCargar L, Seikaly H, Harris J, Scrimger R, Parliament M, Baracos VE. Nutrition impact symptoms: key determinants of reduced dietary intake, weight loss, and reduced functional capacity of patients with head and neck cancer before treatment. Head Neck. 2010;32:290-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Silander E, Nyman J, Hammerlid E. An exploration of factors predicting malnutrition in patients with advanced head and neck cancer. Laryngoscope. 2013;123:2428-2434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Couch M, Lai V, Cannon T, Guttridge D, Zanation A, George J, Hayes DN, Zeisel S, Shores C. Cancer cachexia syndrome in head and neck cancer patients: part I. Diagnosis, impact on quality of life and survival, and treatment. Head Neck. 2007;29:401-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | Nakayama M, Tabuchi K, Hara A. Clinical utility of the modified Glasgow prognostic score in patients with advanced head and neck cancer. Head Neck. 2015;37:1745-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 40. | Kono T, Sakamoto K, Shinden S, Ogawa K. Pre-therapeutic nutritional assessment for predicting severe adverse events in patients with head and neck cancer treated by radiotherapy. Clin Nutr. 2017;36:1681-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 41. | de Carvalho TM, Miguel Marin D, da Silva CA, de Souza AL, Talamoni M, Lima CS, Monte Alegre S. Evaluation of patients with head and neck cancer performing standard treatment in relation to body composition, resting metabolic rate, and inflammatory cytokines. Head Neck. 2015;37:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Fasano M, Perri F, Della Corte CM, Di Liello R, Della Vittoria Scarpati G, Cascella M, Ottaiano A, Ciardiello F, Solla R. Translational Insights and New Therapeutic Perspectives in Head and Neck Tumors. Biomedicines. 2021;9:1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Ho AL, Brana I, Haddad R, Bauman J, Bible K, Oosting S, Wong DJ, Ahn MJ, Boni V, Even C, Fayette J, Flor MJ, Harrington K, Kim SB, Licitra L, Nixon I, Saba NF, Hackenberg S, Specenier P, Worden F, Balsara B, Leoni M, Martell B, Scholz C, Gualberto A. Tipifarnib in Head and Neck Squamous Cell Carcinoma With HRAS Mutations. J Clin Oncol. 2021;39:1856-1864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 136] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 44. | Hong DS, Birnbaum A, Steuer C, Taylor M, George TJ, Lacy J, Wang B, Beca F, Nicacio L, Soumaoro I, Cho M. Efficacy and Safety of Tisotumab Vedotin in Patients with Head and Neck Squamous Cell Carcinoma: Results From a Phase II Cohort. IJROBP. 2022;112:e10-e11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Siu LL, Even C, Mesía R, Remenar E, Daste A, Delord JP, Krauss J, Saba NF, Nabell L, Ready NE, Braña I, Kotecki N, Zandberg DP, Gilbert J, Mehanna H, Bonomi M, Jarkowski A, Melillo G, Armstrong JM, Wildsmith S, Fayette J. Safety and Efficacy of Durvalumab With or Without Tremelimumab in Patients With PD-L1-Low/Negative Recurrent or Metastatic HNSCC: The Phase 2 CONDOR Randomized Clinical Trial. JAMA Oncol. 2019;5:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 236] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 46. | Ferris RL, Haddad R, Even C, Tahara M, Dvorkin M, Ciuleanu TE, Clement PM, Mesia R, Kutukova S, Zholudeva L, Daste A, Caballero-Daroqui J, Keam B, Vynnychenko I, Lafond C, Shetty J, Mann H, Fan J, Wildsmith S, Morsli N, Fayette J, Licitra L. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol. 2020;31:942-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 274] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 47. | Haddad RI, Harrington K, Tahara M, Ferris RL, Gillison M, Fayette J, Daste A, Koralewski P, Zurawski B, Taberna M, Saba NF, Mak M, Kawecki A, Girotto G, Alvarez Avitia MA, Even C, Toledo JGR, Guminski A, Müller-Richter U, Kiyota N, Roberts M, Khan TA, Miller-Moslin K, Wei L, Argiris A. Nivolumab Plus Ipilimumab Versus EXTREME Regimen as First-Line Treatment for Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck: The Final Results of CheckMate 651. J Clin Oncol. 2023;41:2166-2180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 90] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 48. | Taylor MH, Rasco DW, Brose MS, Vogelzang NJ, Richey SL, Cohn AL, Richards DA, Stepan DE, Dutcus CE, Guo M, Shumaker RC, Schmidt E V. , Wirth LJ. A phase 1b/2 trial of lenvatinib plus pembrolizumab in patients with squamous cell carcinoma of the head and neck. J Clin Oncol. 2018;36:6016-6016. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 49. | Chen TH, Chang PM, Yang MH. Combination of pembrolizumab and lenvatinib is a potential treatment option for heavily pretreated recurrent and metastatic head and neck cancer. J Chin Med Assoc. 2021;84:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 50. | Hansen AR, Stanton TS, Hong MH, Cohen EEW, Mehanna HM, Chisamore MJ, Turner D, Yadavilli S, Bell K, Baccan C, Leone R, Chen H, Zhou H, Ellis CE, Ballas MS, Hoos A, Rischin D. INDUCE-3: A randomized, double-blind study of GSK3359609 (GSK609), an inducible T-cell co-stimulatory (ICOS) agonist antibody, plus pembrolizumab (PE) versus placebo (PL) plus PE for first-line treatment of PD-L1-positive recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). J Clin Oncol. 2020;38:TPS6591-TPS6591. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Soulières D, Faivre S, Mesía R, Remenár É, Li SH, Karpenko A, Dechaphunkul A, Ochsenreither S, Kiss LA, Lin JC, Nagarkar R, Tamás L, Kim SB, Erfán J, Alyasova A, Kasper S, Barone C, Turri S, Chakravartty A, Chol M, Aimone P, Hirawat S, Licitra L. Buparlisib and paclitaxel in patients with platinum-pretreated recurrent or metastatic squamous cell carcinoma of the head and neck (BERIL-1): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol. 2017;18:323-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 158] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 52. | Soulieres D, Faivre SJ, Dreyer K, Licitra LF. The BURAN study of buparlisib (AN2025) in combination with paclitaxel compared to paclitaxel alone, in patients with recurrent or metastatic head and neck squamous cell carcinoma. J Clin Oncol. 2021;39:TPS6090-TPS6090. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |