Published online Nov 24, 2024. doi: 10.5306/wjco.v15.i11.1435
Revised: September 5, 2024
Accepted: September 27, 2024
Published online: November 24, 2024
Processing time: 130 Days and 14.9 Hours
Renal angiomyolipoma and renal cell carcinoma are the most common benign and malignant tumors of the kidney respectively, and the preoperative differential diagnosis is crucial due to the wide difference in treatment methods. Fat-poor renal angiomyolipoma is a relatively rare type of in renal angiomyolipoma. Its fat imaging features are not obvious, and it is easily misdiagnosed as renal cell carcinoma.
We report the case of a 41-year-old man who complained of osphyalgia. Subse
The use of fluorescent laparoscopy can effectively help intraoperative manage
Core Tip: We used the PINPOINT fluorescent laparoscopic system intraoperatively. The fluorescence pattern provided by intravenous indocyanine green helped to suggest the final diagnosis and effectively guided surgical decision making, avoiding nephrectomy for benign renal tumors. In the fluorescence mode, we only blocked the ectopic blood vessels supplying the tumor, thus minimizing the loss of renal function.
- Citation: Tang JE, Wang RJ, Fang ZH, Zhu PY, Yao JX, Yang H. Treatment of fat-poor renal angiomyolipoma with ectopic blood supply by fluorescent laparoscopy: A case report and review of literature. World J Clin Oncol 2024; 15(11): 1435-1443
- URL: https://www.wjgnet.com/2218-4333/full/v15/i11/1435.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i11.1435
Kidney tumors are mostly found incidentally by B-ultrasound or computed tomography (CT). Renal angiomyolipoma (RAML) is the most common benign tumor of the kidney, RAML is usually composed of smooth muscle, blood vessels, and mature adipose tissue. Most cases, the typical RAML can be achieved by color Doppler ultrasound (Dopplerul-trasound), CT or magnetic resonance imaging (MRI) to diagnose. However, approximately 4%–5% of fat-poor RAML could not be diagnosed by the above examinations[1]. Fat-poor RAML is a relatively rare type of renal angiomyolipoma[2], and the proportion of fat components in its tumor is < 20%, which is easily misdiagnosed as renal cell carcinoma[3]. At present, only a few cases have been reported about fat-poor RAML, however, fat-poor RAML with independent branches of the abdominal aorta has not been reported. We performed a partial nephrectomy with the aid of fluorescent laparoscopy.
A 41-year-old man patient, who complained of osphyalgia in the right waist for more than 1 month, was admitted to the hospital in June 2023.
The patient complained of right low back pain discomfort for more than 1 month with no apparent cause. He was unconcerned by it at the time. The patient reported no nausea, vomiting, numbness in the extremities or trunk, muscle weakness, or urinary disruption. However, the symptoms were repeated, and then he came to the hospital for further examination.
There is no obvious history of past illness related to this disease.
There is no obvious personal or family history related to this disease.
On admission to the hospital, he was conscious; his vital signs were stable, and no enlarged lymph nodes were discovered in the neck or behind the ears. Both muscle strength and muscle tension of the extremities were normal, and voluntary activities were normal. There was no significant palpable mass in the abdomen.
Routine blood tests, blood biochemistry, tumor markers, immune markers, infection markers, routine urine tests and routine stool tests showed no significant abnormalities.
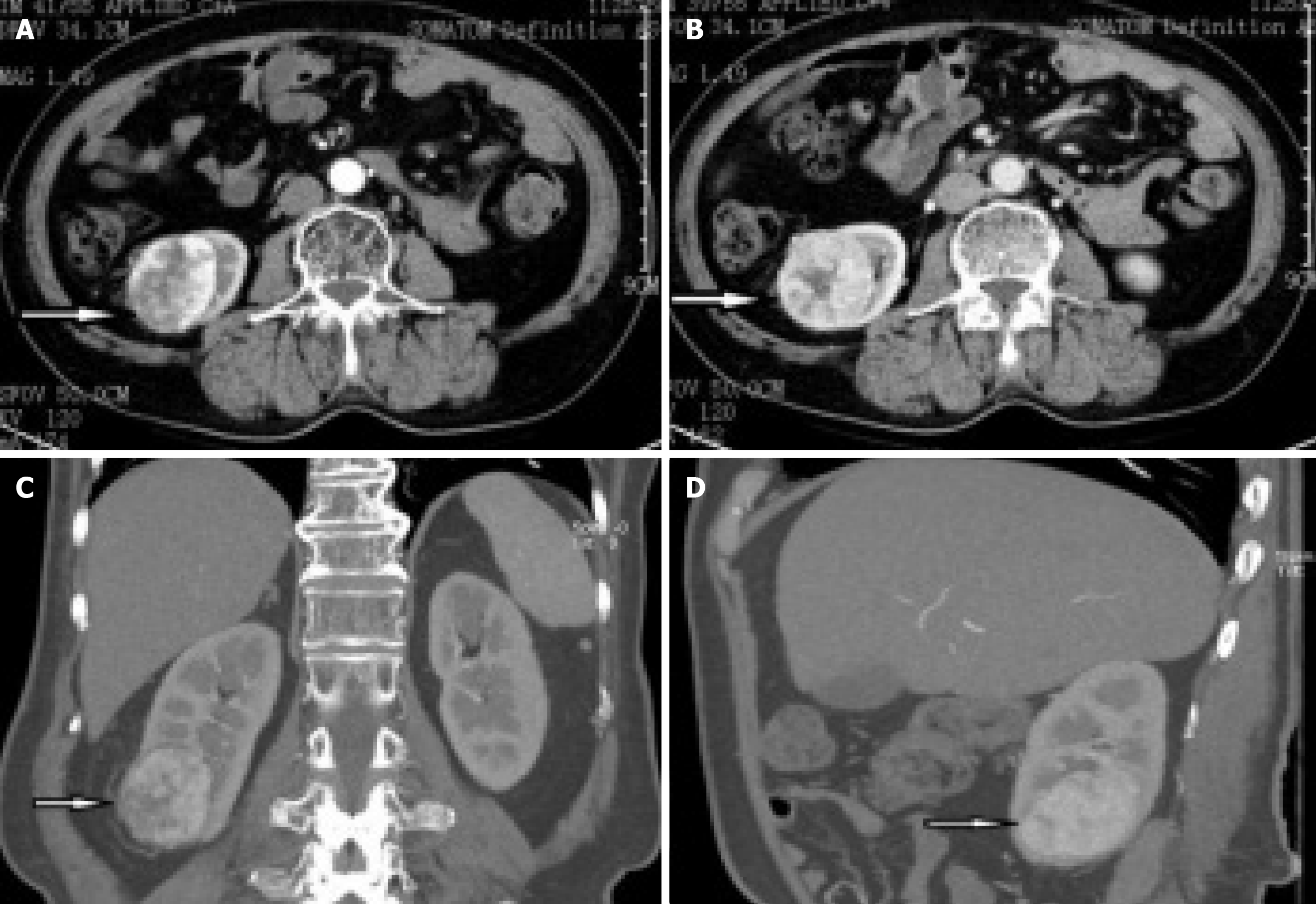
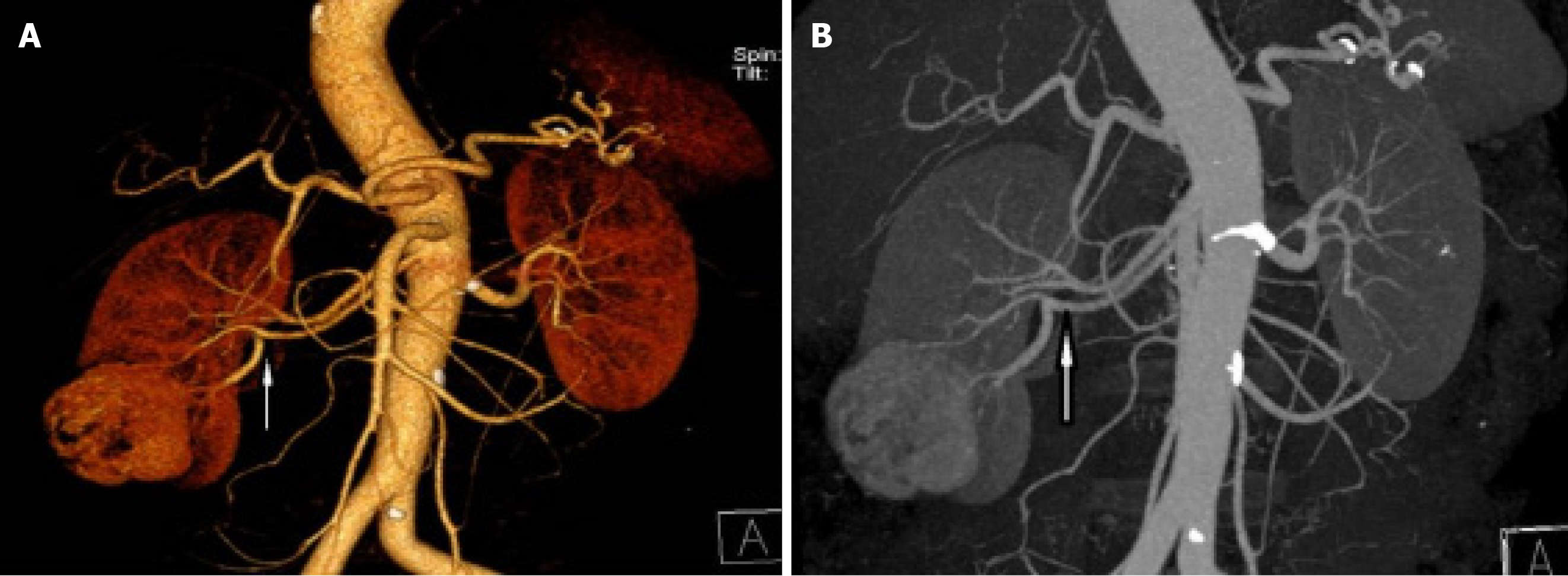
B-ultrasound showed a right renal mass with a few high echoic areas, considered renal cell carcinoma, subsequent abdominal contrast-enhanced CT revealed that a heterogeneous mass was seen in the lower pole of the right kidney, with the size of about 53 mm × 47 mm, which had moderately uneven enhancement (Figure 1). There was significant enhancement in the arterial phase and decreased density in the venous and excretory post-contrast phases. Of special interest was the mass with ectopic kidney arterial blood supply that was enhanced on CT (Figure 2A and B). The ectopic artery was emitted from the abdominal aorta, below the renal arteries, no significant stenosis and extruded of the renal artery.
Right kidney tumor.
Based on all the examination results, we initially considered the mass as renal cell carcinoma, so the patient requested a radical nephrectomy.
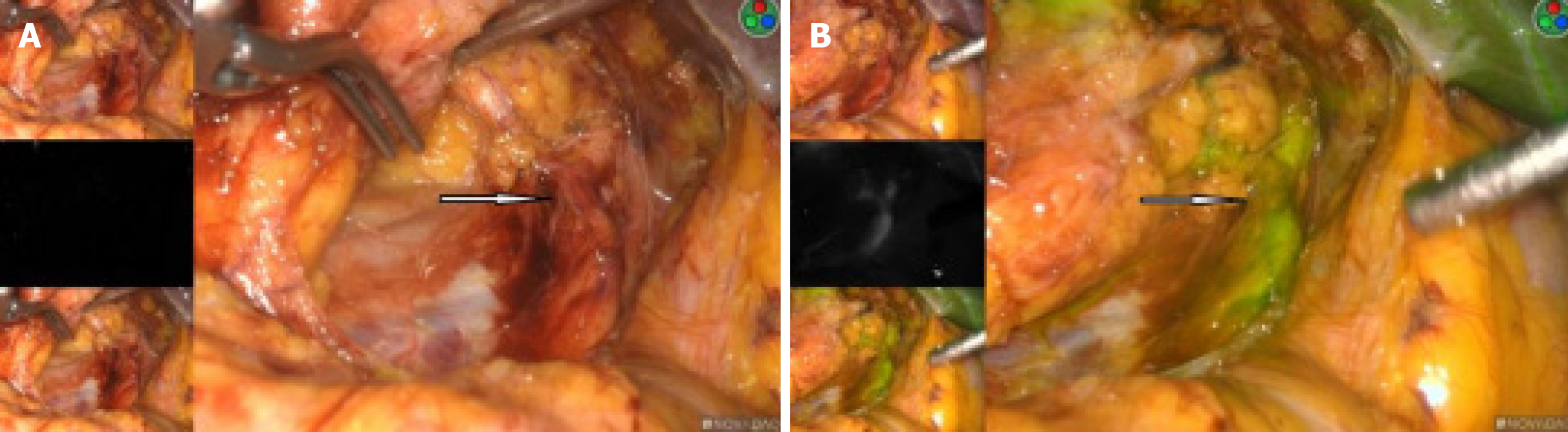
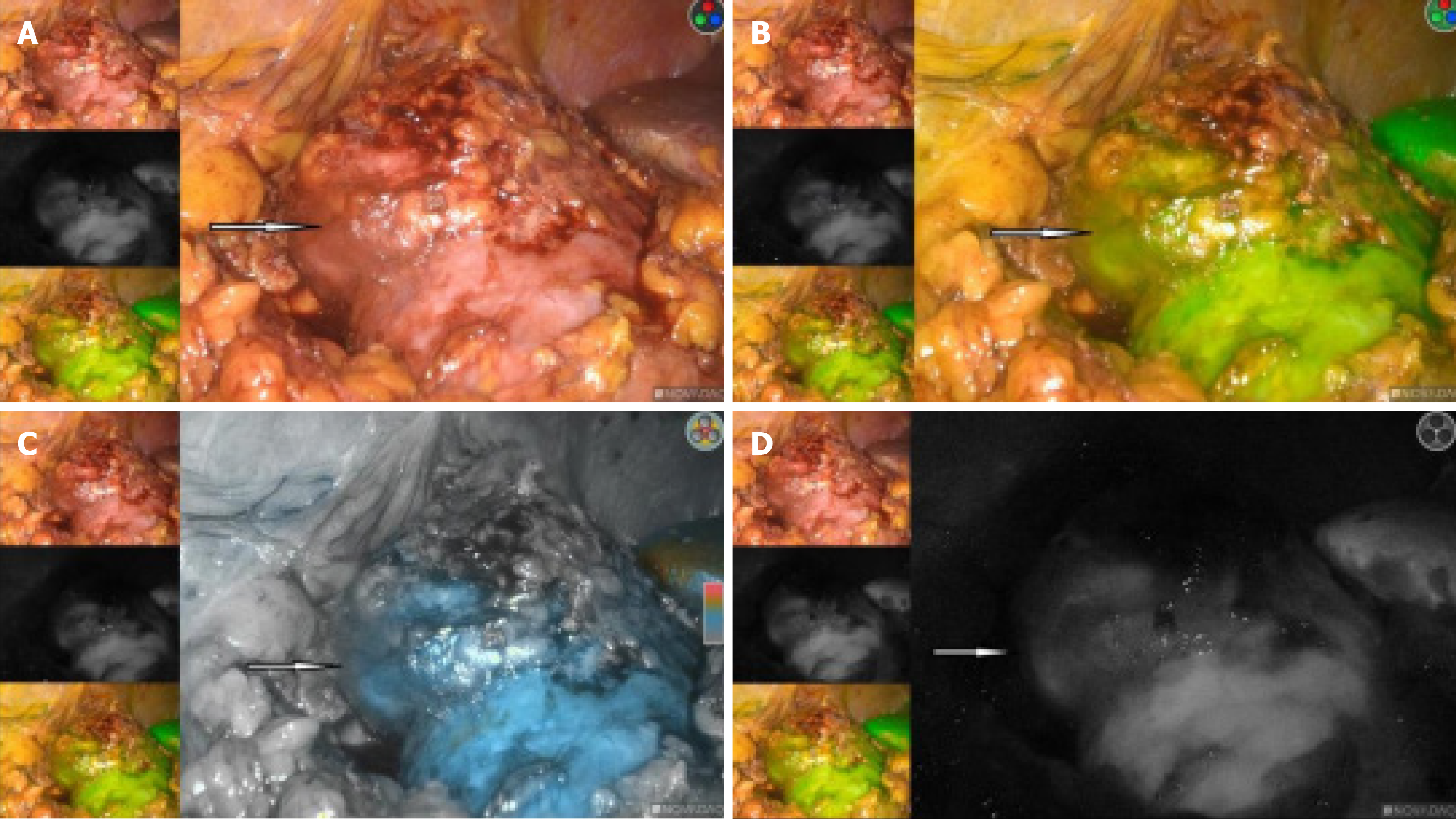
The right kidney tumor was explored under laparoscopy, and radical nephrectomy would be performed if necessary. We used the PINPOINT fluorescent laparoscopic system (Novadaq Technologies, Mississauga, ON Canada) intraoperatively. An 2 mL of 2.5 mg/mL indocyanine green (ICG) (IC-Green, Akorn Pharmaceuticals, Lake Forest, IL, United States) solution was given intravenously for renal angiography. This allowed us to successfully identify the ectopic arterial vessels from the abdominal aorta, and clearly show that it supplied the middle and lower parts of the blood to the right kidney (Figure 3A and B). Also in the fluorescence mode, the renal tumor had almost the same color as the surrounding normal tissue, and the border is hard to recognize in the light of green or black and white (Figure 4A-D).
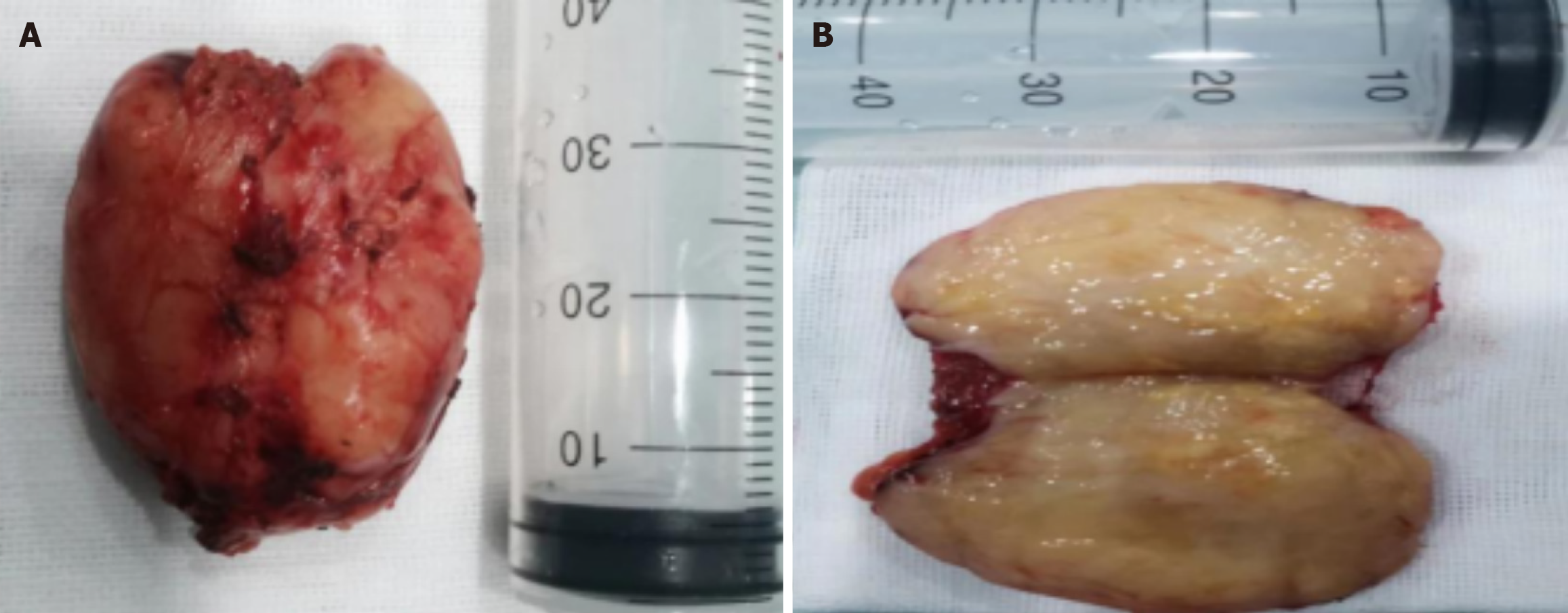
Our previous study[4] showed that renal cell carcinoma looked dark in the fluorescence mode. While other kidney tumors, such as renal angiomyolipoma, looked as bright as the normal renal tissues. Then, with the consent of the patient's family members, we performed a partial nephrectomy blocking the right renal heterotopic artery using fluorescent laparoscopy, the tumor was removed successfully and intact (Figure 5A and B). The time of renal artery ischemia during right partial nephrectomy is 35 minutes.
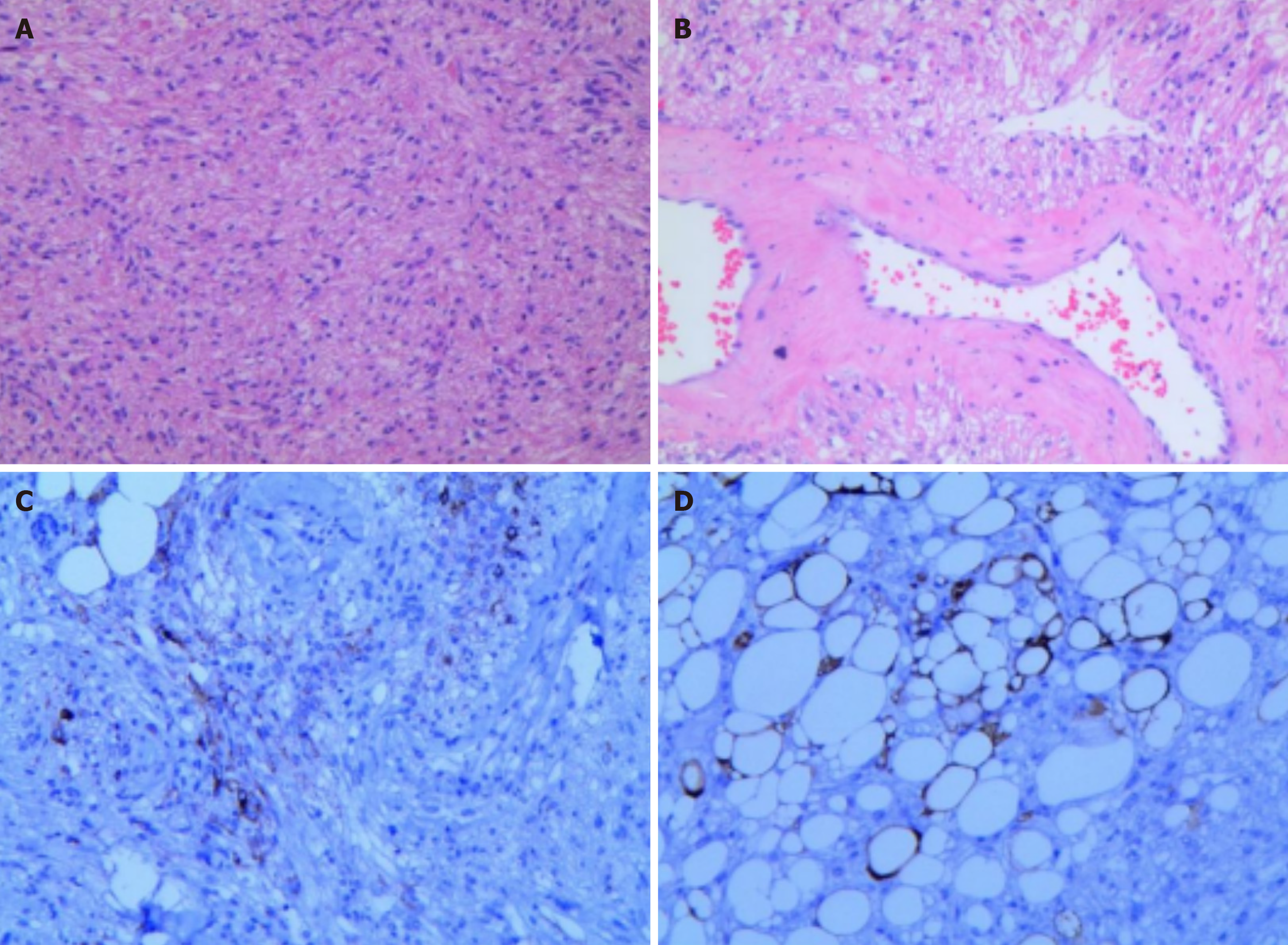
Histopathological images showed that the tumors was mainly composed of spindle cells and fat spindle cells with moderate cell density and a strip-like arrangement. The internal morphology of the tumor is diverse, with scattered thick-walled blood vessels and a small amount of mature adipose tissue, and a diffuse distribution of tumor cells between the blood vessels, closely related to the blood vessels and growing around the blood vessels. Immunohistochemical images showed: HMB 45 (+), SMA (+), C34 (vascular endothelial cells +), vim (+), S100 (adipocyte +), Ki-67 (+ 2%). The histological and immunohistochemical findings confirmed that it was a fat-poor RAML (Figure 6A-D). The fluorescence pattern provided by intravenous ICG can help suggest the final diagnosis, effectively guide the surgical decision-making, and avoid preoperative imaging diagnosis leading to nephrectomy for benign renal tumors.
The patient was followed up for 12 months postoperatively, and no recurrence and metastasis were found. The renal function was not significantly increased than before surgery and was within the reference range.
Due to advancements in modern imaging during the last years, nowadays more than 70% of kidney tumours are detected incidentally[5]. Renal cell carcinoma (RCC) is a common sporadic renal tumor that generally requires surgical resection. About 20% of solid renal tumors are benign, and RAML is more common[6]. RAML is mainly composed of mature spindle smooth muscle cells, deformed blood vessels and fat in different proportions. The proportion of various components varies greatly in different cases. Typical RAML often contains visible fat, while RCC has rare fat components[7], which can identify typical RAML and RCC. However, approximately 4%-5% of fat-poor RAML cannot diagnose[1] by the detection techniques described above. It is difficult to distinguish between atypical or fat-poor RAML and RCC on imaging, which easily leads to benign RAML being misdiagnosed as malignant RCC.
RAML is a relatively rare tumor with an incidence of less than 0.2%[8] and is the most common benign renal tumor. The disease was first reported by Fischer in 1911 and named[9] by Morgan in 1951. It was previously considered a hamartoma, but recent evidence suggests that RAML is a monoclonal rather than a tumor of polyclonal origin. Now, RAML is thought to originate from perivascular epithelioid cells of the neural crest and belongs to a member of the family of "perivascular epithelioid cell tumors". Currently, RAML is further classified into different tumor subtypes, each with its own unique pathological features, imaging features, and clinical manifestations[2].
Incidental RAML occurs in middle-aged women aged 40-60 years old, the ratio between women and men is about 4: 1, usually in one side of the kidney, Most asymptomatic during physical examination[1]. RAML may be associated by tuberous sclerosis (TSC), which tends to be multifocal and larger. Clinically, TSC occurs in approximately 20%-30% of patients with RAML, and approximately 50% of TSC patients develop RAML[1].
Fat-poor RAML is a relatively rare type of RAML[2]. The proportion of fat components in its tumors is < 20%, accounting for about 4.5% of all RAML. Its fat imaging features are not obvious, and it is easily misdiagnosed as renal cell carcinoma[10]. Most of the patients do not have lumbago. It is found in physical examination that its main components are smooth muscle and blood vessels, with very little fat composition, which is difficult to distinguish from renal cancer (especially clear cell carcinoma) in ordinary CT scan. Therefore, preoperative diagnosis of RAML is extremely important.
This patient was a 41-year-old male with B-ultrasound and enhanced CT both suggestive of right kidney tumor, considering malignancy, CT scans revealed that a heterogeneous mass was seen in the lower pole of the right kidney, with the size of about 53 mm × 47 mm. There was significant enhancement in the arterial phase and decreased density in the venous and excretory post-contrast phases. While showing no significant fat imaging. Of special interest was the mass with ectopic kidney arterial blood supply that was enhanced on CT. The ectopic artery was emitted from the abdominal aorta, below the renal arteries. Some scholars believe that whether the renal epithelioid angiomyolipoma with independent blood supply is more aggressive behavior and malignant tendency to change, so we initially considered the mass as renal cell carcinoma, and tended to undergo radical nephrectomy.
B-ultrasound is the preferred screening and diagnostic method for renal tumors, but it is still difficult to identify some atypical cases due to its limited display of tiny blood vessels and blood flow in the lesion[11]. Intraoperative ultrasonography (US) is a commonly used technique for tumor differentiation during surgical procedures. In renal tumors, intraoperative US plays a crucial role in identifying and distinguishing various types of kidney masses, aiding surgeons in making real-time decisions and ensuring optimal treatment outcomes. During partial nephrectomy for renal tumors, intraoperative US helps in differentiating between malignant and benign lesions based on their characteristics such as size, shape, echogenicity, vascularity, and margins. But there are also some limitations to consider. One limitation is the potential challenge in accurately distinguishing between benign and malignant renal masses based solely on intraoperative US imaging characteristics. While certain features such as vascularity, shape, and margins can provide clues to the nature of the tumor, these findings may not always be definitive.
CT with contrast enhancement is the most commonly used radiologic method to diagnose RAML[12]. However, there are bleeding, cystic changes, small lesions or fat-poor in the tumor, and CT scan often cannot accurately measure the internal adipose tissue, which is easy to cause misdiagnosis[13]. Biphasic helical CT may be useful in differentiating RAML with minimal fat from RCC, with homogeneous tumor enhancement and prolonged enhancement pattern being the most valuable CT findings[14]. Some studies shown that the fat-poor RAML had a significantly higher mean attenuation value compared with that of renal cell carcinoma on unenhanced CT scans. In addition, significant differences were found between fat-poor RAML and RCC with regard to wash-in and enhancement ratios on contrast-enhanced CT scans[15]. Kim et al[16] established a diagnostic scoring system by comparing the ratio of long-to-short diameter, enhancement characteristics, tumor attenuation on unenhanced scan, tumor margin, calcification, age, and sex, to distinguish fat-poor RAML and RCC with high accuracy, sensitivity and specificity. Someone also identified RAML and RCC by intratumoral blood volume[17]. Lassel et al[18] combined demographic, CT enhanced characteristics on fat-poor RAML and showed specificity > 95%, In conclusion, there is no method to confirm fat-poor RAML through a single CT image, and multiple imaging methods are needed to improve its diagnostic rate.
MRI is the examination method for distinguishing fat-poor RAML from RCC after ultrasound and CT. Currently, various MRI techniques can be used to diagnose fat-poor RAML. RCC often showed significantly hypersignal on T2 weighted imaging (T2WI), fat-poor RAML often shows low and slightly lower signal, and the signal is more uniform. Choi et al[19] suggested that the low signal of fat-poor RAML on T2WI correlated with the smooth muscle content in the lesion. Schieda et al[20] confirmed that the signal intensity (SI) of the lesion on T2-weighted MR images is important for the identification of fat-poor RAML[21]. However, Hindman et al[22] reported that there were no differences between minimal fat RAML and clear cell RCC for the SI index. Kang et al[23] noted that evaluation of apparent diffusion coefficient values can help to determine between benign and malignant lesions of renal tumors[23-25]. However, there are no characteristics MRI images of fat-poor RAML.
Studies have reported experience with percutaneous renal needle biopsy and obtained meaningful results[26], but it has not yet become a routine examination method and has concerns about the risk of causing tumor metastasis and spread. However, current technologies rely on pre- and intraoperative 3D reconstruction, which ensures safer selective arterial clamping[27]. By utilizing three-dimensional reconstruction technology, surgeons can better plan their approach, visualize the optimal clamping location, and navigate the surgical field with enhanced accuracy. This ultimately leads to a more targeted and meticulous procedure, minimizing potential complications such as excessive bleeding and post
In recent years, infrared fluorescence technology with indocyanine green as contrast agent has been applied in urology[28]. Some studies have reported that infrared fluorescence imaging can clearly show the size and location of renal tumors during operation, and improve the ability to deal with complex renal tumors[29]. Previous studies showed that the loss of ICG-based fluorescence only appeared in renal clear cell carcinoma[30]. Our clinical practice also presented that only renal clear cell carcinoma looked dark in the fluorescence mode. While other renal tumors looked as bright as the normal renal tissues. In this case, we used the PINPOINT fluorescent laparoscopic system intraoperatively, the tumor looked as bright as the normal renal tissues, so we performed a partial nephrectomy. The final pathology suggested a fat-poor RAML, thus avoiding nephrectomy. At the same time, we finely identified the tumor supply vessels through fluorescent navigation, thus only blocking the arteries supplying the tumor during surgery, preventing intraoperative bleeding due to malocclusion and minimizing the loss of renal function.
Preoperative differentiation of fat-poor RAML from RCC remains a difficult imaging problem, and a percutaneous renal biopsy can be selected for the diagnosis of highly suspected fat-poor RAML. For patients who do not accept or cannot undergo percutaneous renal tumor biopsy, The use of fluorescent laparoscopy can effectively help intraoperative management, and the fluorescence pattern provided by intravenous ICG can help suggest the final diagnosis, effectively guide the surgical decision-making, and avoid preoperative imaging diagnosis leading to nephrectomy for benign renal tumors, through fluorescent navigation of tumor supply vessel precise block, minimize the loss of renal function.
| 1. | Katabathina VS, Vikram R, Nagar AM, Tamboli P, Menias CO, Prasad SR. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics. 2010;30:1525-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Jinzaki M, Silverman SG, Akita H, Nagashima Y, Mikami S, Oya M. Renal angiomyolipoma: a radiological classification and update on recent developments in diagnosis and management. Abdom Imaging. 2014;39:588-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 226] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 3. | Chong J, Zhang J, Ning C, Zhang L, Zhao W, Sun Y. Fat-poor renal angiomyolipoma combined with pseudoaneurysm: a case report. Ann Palliat Med. 2021;10:2343-2348. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Wang R, Tang J, Chen Y, Fang Z, Shen J. The clinical value of indocyanine green fluorescence navigation system for laparoscopic partial nephrectomy in the case of complex renal clear cell carcinoma (R.E.N.A.L score ≥7). J Cancer. 2021;12:1764-1769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Chow WH, Devesa SS, Warren JL, Fraumeni JF Jr. Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1218] [Cited by in RCA: 1157] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 6. | Patel MD, Ascher SM, Horrow MM, Pickhardt PJ, Poder L, Goldman M, Berland LL, Pandharipande PV, Maturen KE. Management of Incidental Adnexal Findings on CT and MRI: A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol. 2020;17:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Ramamurthy NK, Moosavi B, McInnes MD, Flood TA, Schieda N. Multiparametric MRI of solid renal masses: pearls and pitfalls. Clin Radiol. 2015;70:304-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 8. | Nelson CP, Sanda MG. Contemporary diagnosis and management of renal angiomyolipoma. J Urol. 2002;168:1315-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 261] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 9. | Sun R, Zhao S, Jiang H, Jiang H, Dai Y, Zhang C, Wang S. Imaging Tool for Predicting Renal Clear Cell Carcinoma Fuhrman Grade: Comparing R.E.N.A.L. Nephrometry Score and CT Texture Analysis. Biomed Res Int. 2021;2021:1821876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 10. | Garg PK, Jain BK, Kumar A, Bhatt S, Vibhav V. Fat poor angiomyolipoma with lymphadenopathy: Diagnostic dilemma. Urol Ann. 2012;4:126-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Kabakci N, Igci E, Secil M, Yorukoglu K, Mungan U, Celebi I, Kirkali Z. Echo contrast-enhanced power Doppler ultrasonography for assessment of angiogenesis in renal cell carcinoma. J Ultrasound Med. 2005;24:747-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Wang C, Li X, Peng L, Gou X, Fan J. An update on recent developments in rupture of renal angiomyolipoma. Medicine (Baltimore). 2018;97:e0497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Bauman TM, Potretzke AM, Wright AJ, Vetter JM, Potretzke TA, Figenshau RS. Patient and nonradiographic tumor characteristics predicting lipid-poor angiomyolipoma in small renal masses: Introducing the BEARS index. Investig Clin Urol. 2017;58:235-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Kim JK, Park SY, Shon JH, Cho KS. Angiomyolipoma with minimal fat: differentiation from renal cell carcinoma at biphasic helical CT. Radiology. 2004;230:677-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 234] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 15. | Xie P, Yang Z, Yuan Z. Lipid-poor renal angiomyolipoma: Differentiation from clear cell renal cell carcinoma using wash-in and washout characteristics on contrast-enhanced computed tomography. Oncol Lett. 2016;11:2327-2331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Kim MH, Lee J, Cho G, Cho KS, Kim J, Kim JK. MDCT-based scoring system for differentiating angiomyolipoma with minimal fat from renal cell carcinoma. Acta Radiol. 2013;54:1201-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Chen C, Liu Q, Hao Q, Xu B, Ma C, Zhang H, Shen Q, Lu J. Study of 320-slice dynamic volume CT perfusion in different pathologic types of kidney tumor: preliminary results. PLoS One. 2014;9:e85522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Lassel EA, Rao R, Schwenke C, Schoenberg SO, Michaely HJ. Diffusion-weighted imaging of focal renal lesions: a meta-analysis. Eur Radiol. 2014;24:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Choi HJ, Kim JK, Ahn H, Kim CS, Kim MH, Cho KS. Value of T2-weighted MR imaging in differentiating low-fat renal angiomyolipomas from other renal tumors. Acta Radiol. 2011;52:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Schieda N, Dilauro M, Moosavi B, Hodgdon T, Cron GO, McInnes MD, Flood TA. MRI evaluation of small (<4cm) solid renal masses: multivariate modeling improves diagnostic accuracy for angiomyolipoma without visible fat compared to univariate analysis. Eur Radiol. 2016;26:2242-2251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Kay FU, Canvasser NE, Xi Y, Pinho DF, Costa DN, Diaz de Leon A, Khatri G, Leyendecker JR, Yokoo T, Lay AH, Kavoussi N, Koseoglu E, Cadeddu JA, Pedrosa I. Diagnostic Performance and Interreader Agreement of a Standardized MR Imaging Approach in the Prediction of Small Renal Mass Histology. Radiology. 2018;287:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 22. | Hindman N, Ngo L, Genega EM, Melamed J, Wei J, Braza JM, Rofsky NM, Pedrosa I. Angiomyolipoma with minimal fat: can it be differentiated from clear cell renal cell carcinoma by using standard MR techniques? Radiology. 2012;265:468-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 23. | Kang SK, Zhang A, Pandharipande PV, Chandarana H, Braithwaite RS, Littenberg B. DWI for Renal Mass Characterization: Systematic Review and Meta-Analysis of Diagnostic Test Performance. AJR Am J Roentgenol. 2015;205:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Li H, Liang L, Li A, Hu Y, Hu D, Li Z, Kamel IR. Monoexponential, biexponential, and stretched exponential diffusion-weighted imaging models: Quantitative biomarkers for differentiating renal clear cell carcinoma and minimal fat angiomyolipoma. J Magn Reson Imaging. 2017;46:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Li H, Li A, Zhu H, Hu Y, Li J, Xia L, Hu D, Kamel IR, Li Z. Whole-Tumor Quantitative Apparent Diffusion Coefficient Histogram and Texture Analysis to Differentiation of Minimal Fat Angiomyolipoma from Clear Cell Renal Cell Carcinoma. Acad Radiol. 2019;26:632-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Halverson SJ, Kunju LP, Bhalla R, Gadzinski AJ, Alderman M, Miller DC, Montgomery JS, Weizer AZ, Wu A, Hafez KS, Wolf JS Jr. Accuracy of determining small renal mass management with risk stratified biopsies: confirmation by final pathology. J Urol. 2013;189:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 27. | Della Corte M, Cerchia E, Allasia M, Marquis A, Linari A, Mandaletti M, Ruggiero E, Sterrantino A, Quarello P, Catti M, Fagioli F, Gontero P, Gerocarni Nappo S. A Bosniak III Cyst Unmasking Tubulocystic Renal Cell Carcinoma in an Adolescent: Management with Selective Arterial Clamping and Robotic Enucleation. Surg. 2024;5:415-422. [DOI] [Full Text] |
| 28. | Hekman MCH, Rijpkema M, Langenhuijsen JF, Boerman OC, Oosterwijk E, Mulders PFA. Intraoperative Imaging Techniques to Support Complete Tumor Resection in Partial Nephrectomy. Eur Urol Focus. 2018;4:960-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Bjurlin MA, McClintock TR, Stifelman MD. Near-infrared fluorescence imaging with intraoperative administration of indocyanine green for robotic partial nephrectomy. Curr Urol Rep. 2015;16:20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Soga N, Inoko A, Furusawa J, Ogura Y. Evaluation to Differentiate between Tumor Lesions and the Parenchyma in Partial Nephrectomies for Renal Tumors Based on Quantitative Fluorescence Imaging Using Indocyanine Green Dye. Curr Urol. 2019;13:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |