INTRODUCTION
Gastric cancer is the fifth most common cancer and the third deadliest cancer worldwide[1]. Traditionally, the therapeutic approach has consisted of using three major treatment methods: surgery, chemotherapy, and radiation therapy. Over the years, this perspective has changed due to the introduction of immune-checkpoint inhibitors (ICIs), which have impacted the conventional paradigm by providing significantly prolonged overall survival (OS) and progression-free survival (PFS) in patients with untreatable illnesses[2].
Like other gastrointestinal (GI) tract cancers, this type of neoplasia commonly metastasizes to the liver as a result of the GI tract’s venous drainage via the portal vein. This pathway for cancer spread contributes to the poor prognosis, as indicated by a 3-year survival rate of less than 10%[3]. While metastatic tumor cells circulate throughout the circulatory system, they are more prone to become entrapped in the liver sinusoidal circulation due to its fenestrated regulation sinusoidal cells and lack of organized basement membrane. Once extravasated, the circulating tumor cells (CTCs) encounter the liver’s relatively immunotolerant microenvironment, which creates a basis for a pre-metastatic niche to promote proliferation and colonization of CTCs.
The immunotolerant state of the liver is linked to multiple mechanisms including T cell inactivation and apoptosis induced by Kupffer Cells, which have a direct and indirect apoptotic effect through NKT cell stimulation; additionally, once extravasated in Disse space, CTCs encounter a low perfusion, low pressure, highly nutrient space excellent for proliferation and progression. Not limited to these reasons, the metastatic foci are more likely to develop in the liver than in other organs[4,5].
Immune mediated tumor treatment has become a hot topic in the last decade, starting from a well-studied and proven ability of cancer cells to induce immunotolerance and avoid destruction. While under normal immunocompetent conditions, T cells effectors can recognize cancer cell antigens and control progression; however, T cell recruitment, extravasation, and intercellular migration is dependent on mechanisms that can be faulted in the tumor microenvironment. Selected clones of cancer cells may be able to express peptides such as CXCL12 and CXCR4, which deliver chemorepellent signals to T cells, avoiding T cell adherence. More so, reduced expression of intercellular adhesion molecules (i.e. ICAM-1) restricts T cell migration.
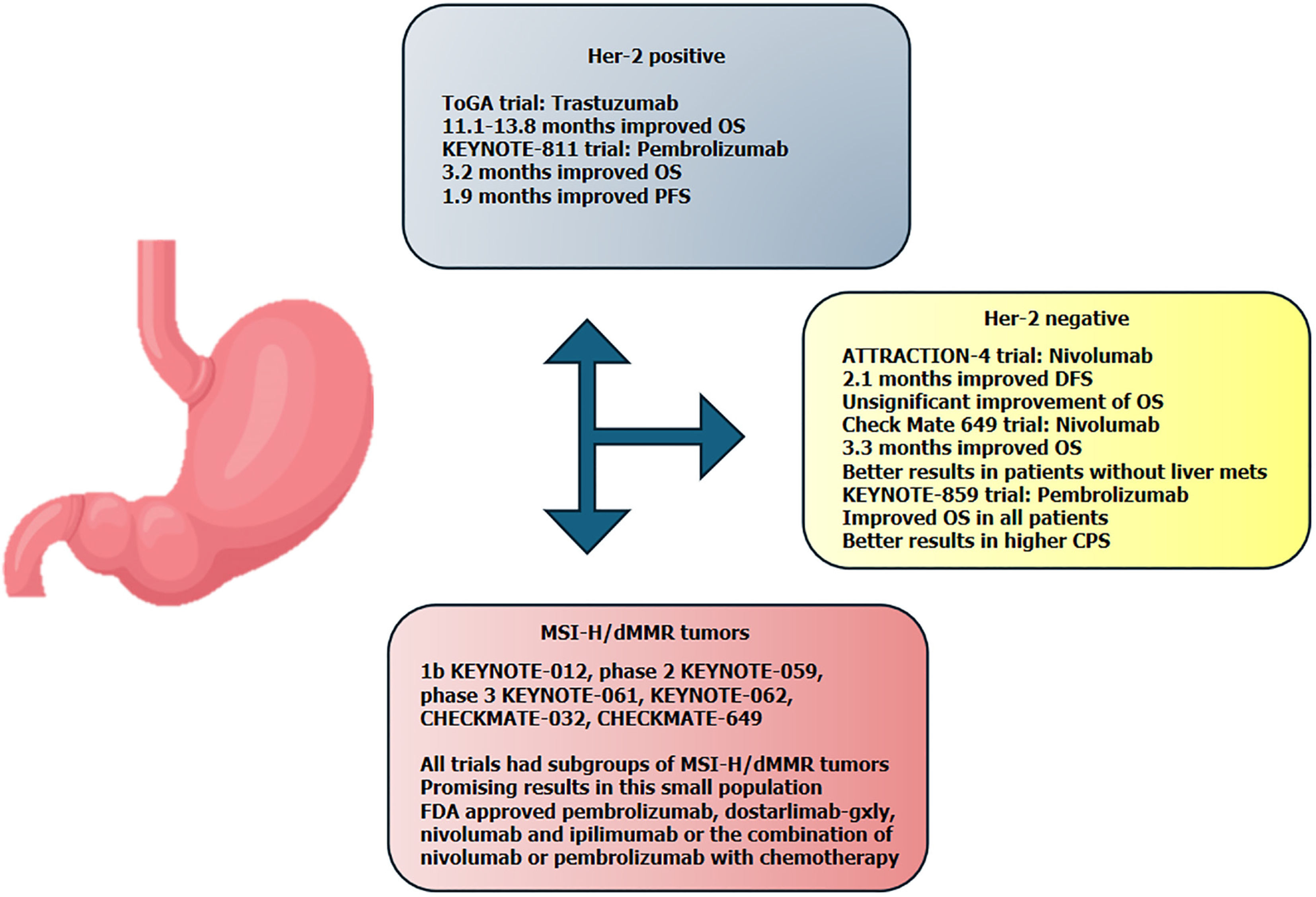
Downregulation of CD8+ and CD4+ T cells is a key factor in cancer progression. This is why many immune therapies are targeted in promoting T cells recognition of cancer cells and enabling effector T cell activation either by using tumor targeting antibodies, which act as receptors for T cell recruitment, or by engineering T cells with tumor antigen receptors[6]. Immunotherapy has now emerged as a promising treatment option for this patient group, offering new hope where standard therapies have limited effectiveness. Clinical trials have shown that agents can improve survival rates and quality of life for gastric cancer patients with liver metastases, marking a significant advancement in the treatment landscape (Figure 1). As research progresses, immunotherapy continues to be refined and combined with other treatments, aiming to offer even better outcomes for patients facing this challenging diagnosis.
Figure 1 Overview of immunotherapy results in the main clinical trials.
CPS: Combined positive score; DFS: Disease-free survival; FDA: Food and Drug Administration; OS: Overall survival; PFS: Progression-free survival.
Combined chemo-immunotherapy has been widely proven to be more effective than single treatment. The cytotoxic effects of traditional systemic therapy work in a synergistic fashion with the improved immune response, ignited by targeted immune therapies, slowing cancer progression, reducing drug resistance, and improving patient survival. Standard chemotherapy has a direct cytotoxic effect causing disruption of DNA and cell death. The immunogenic dying cells trigger cytotoxic lymphocyte responses against the antigens expressed on the surface of dying cells. This antitumor response also leads to acquisition of immunological memory enabling fulminant future reactions when facing similar antigens expressed by cancer cells. Overall, the process of cancer cell apoptosis caused by chemotherapy opens a plethora of chemo-mechanisms that upregulate the immune system and improve immune surveillance[7]. Similarly, radiotherapy may be used effectively and synergistically with immune therapies, as radiotherapy has a better effect in immunocompetent organisms, and through its destructive, cytotoxic effect, radiotherapy remodels the tumor microenvironment enabling a better antitumor immune response[8].
When transitioning to palliative treatments, clinicians weigh a multitude of factors, including patient preferences and performance status, to tailor the most appropriate treatment approach. In this context, the choice of preferred regimens is informed by the molecular characteristics of the tumor, such as Her-2 status, MMR/MSI status, and PD-L1 CPS.
HER-2 POSITIVE TUMORS
Based on the ToGA trial, the preferred treatment for metastatic gastric cancer Her-2 positive is the combination of chemotherapy (fluoropyrimidine plus cisplatin) plus trastuzumab. The addition of trastuzumab to chemotherapy prolongs the OS from 11.1 months to 13.8 months with a hazard ratio of 0.74. Since the combination treatment does not add significant toxicity, the combination of trastuzumab and chemotherapy has become the standard treatment[9] (Figure 1).
After 11 years of waiting, the Food and Drug Administration (FDA) approved the use of pembrolizumab based on the interim analyses of KEYNOTE-811. In this trial, naïve patients diagnosed with Her-2 positive metastatic gastric or gastro-esophageal junction adenocarcinoma were randomized 1:1 to receive pembrolizumab or a placebo in combination with the standard treatment, trastuzumab plus chemotherapy (fluoropyrimidine and platinum based). The treatment was administered every 3 weeks, up to 35 cycles, or until disease progression, unacceptable toxicity, or consent withdrawal. The main stratification factors were region, PD-L1 expression, and type of chemotherapy. The primary endpoints were PFS and OS. At the third interim analysis, the median PFS was 10.0 months in the pembrolizumab group and 8.1 months in the placebo group, while the median OS was 20.0 months and 16.8 months, respectively. This did not meet the prespecified criteria for significance. Although the patients had more diarrhea in the pembrolizumab group (58% vs 51%), the incidence of nausea was similar in both arms (44%) and more anemia was reported in the placebo arm (31% vs 33%)[10].
HER-2 NEGATIVE TUMORS
In Her-2 negative metastatic gastric cancers, the preferred options are the doublet of chemotherapy (platinum plus fluoropyrimidine) or the combinations of chemotherapy (oxaliplatin based) plus nivolumab for tumors with combined positive score (CPS) ≥ 5 or chemotherapy plus pembrolizumab for tumors with CPS ≥ 1. In the ATTRACTION-4 trial, patients with advanced Her-2 negative gastric or gastro-esophageal junction cancer the therapeutic scheme included capecitabine, oxaliplatin (CAPOX), and nivolumab or placebo, regardless of PD-L1 expression. Patients were stratified based on PD-L1 expression, Eastern Cooperative Oncology Group performance status, disease status, and geographical region. The primary endpoints were PFS and OS. In both arms, 36% of the patients had liver metastases. After a median follow-up of 11.6 months, the median PFS was 10.45 months in the nivolumab group and 8.34 months in the placebo group. After a median follow-up of 26.6 months, the median OS was 17.45 months in the nivolumab arm and 17.15 months in the placebo arm. In the subgroup analysis, the hazard ratio for PFS was 0.59 for the patients with liver metastases and 0.79 for the patients without liver metastases, favoring the combination of nivolumab plus chemotherapy. Regarding OS, the hazard ratio was 0.76 for patients with liver metastases and 1.00 for patients without liver metastases. Treatment-related serious adverse events were reported in 25% of the nivolumab arm patients and 14% in the placebo arm. Based on these results, although the OS was not statistically significantly prolonged, the combination of chemotherapy and nivolumab is an option for treatment of patients with advanced gastric or gastro-esophageal cancers with negative expression for Her-2[11].
In the phase 3 trial, Check Mate 649, patients with previously untreated Her2 negative gastric, gastro-esophageal junction, or esophageal adenocarcinoma were randomized regardless of PD-L1 expression to receive nivolumab plus chemotherapy (CAPOX or FOLFOX) or nivolumab plus ipilimumab or chemotherapy alone. Of all patients with CPS ≥ 5 (n = 955), 407 had liver metastases and in all randomized patients, 614 had liver metastases. The primary endpoint for nivolumab plus chemotherapy vs chemotherapy alone were OS or PFS by blinded independent central review, in patients with a CPS of 5 or more. The OS was significantly improved in the nivolumab plus chemotherapy group (hazard ratio of 0.71). Also, the PFS (hazard ratio of 0.68) was improved in patients with CPS ≥ 5, and the analysis showed benefits in terms of OS and PFS for the population with CPS ≥ 1. The median OS for patients with liver metastases and CPS ≥ 5 was 13.1 months in the nivolumab group and 9.8 months in the chemotherapy alone group. When compared with patients that did not have liver metastases, we can see a difference in median OS in patients treated with nivolumab plus chemotherapy: 13.1 months for patients with liver metastases and 15.5 for patients without liver metastases. In the randomized patient group (n = 1581), the median OS for the population with liver metastases in the nivolumab group was 12.5 months and 14.2 months for the patients without liver metastases. The adverse events were not substantially different in the two groups. The time to symptom deterioration was in the favor of nivolumab arm[2] (Figure 1).
In November 2023, the FDA approved the use of pembrolizumab in combination with chemotherapy (fluoropyrimidine and platinum based) as a first-line treatment in patients with unresectable or metastatic Her2-negative gastric or gastro-esophageal junction cancers, based on the results of the phase 3 trial KEYNOTE-859. In this study, patients were randomized to receive either pembrolizumab or a placebo, in combination with chemotherapy (cisplatin plus 5-flourouracil or CAPOX). The primary endpoint was OS in the intent-to-treat (ITT) population and in the population with CPS ≥ 1 and CPS ≥ 10. Liver metastases were present in 39.7% of the pembrolizumab arm and 39.4% of the placebo arm. The median OS was longer in the ITT population for the pembrolizumab group (12.9 months vs 11.5 months, with a hazard ratio of 0.78). The same trend was observed in the CPS ≥ 1 population (13.0 months vs 11.4 months) and in the CPS ≥ 10 population (15.7 months vs 11.8 months). The results for the general population were in favor of the pembrolizumab arm, with a hazard ratio of 0.83 for the population with liver metastases, and 0.73 for the population without liver metastases, and OS and a hazard ratio of 0.75 for both populations (with or without liver metastases) for PFS. The benefit appears to increase when a higher CPS is noticed; for example, in the CPS ≥ 10 population, the hazard ratio for OS was 0.69 for patients with liver metastases[12].
MSI-H/DMMR TUMORS (MICROSATELLITE INSTABILITY-HIGH/DEFICIENT MISMATCH REPAIR) AND OTHER PREDICTIVE MARKERS
Gastric cancer is very heterogenous from a biologic point of view. In a small population (4%) in a metastatic setting, tumors may be MSI-H or dMMR. Increasing evidence suggests that patients with MSI-H/dMMR tumors may derive benefit from immunotherapy. These data rely on the trials that show the response to ICI independent of the site of the primary tumor or from trials that enrolled patients with gastric cancer. For example, trials like the phase 1b KEYNOTE-012, phase 2 KEYNOTE-059, phase 3 KEYNOTE-061, KEYNOTE-062, CHECKMATE-032, CHECKMATE-649, and others showed promising results in this small population. Based on these findings, the FDA approved the use of pembrolizumab, dostarlimab-gxly, nivolumab, and ipilimumab, or the combination of nivolumab or pembrolizumab with chemotherapy[13-21]. Due to the small number of patients, data for patients with liver metastases are lacking (Figure 1).
PD-L1 expression was evaluated in all trials, while other markers, like tumor mutation burden, Epstein–Barr virus status, liquid markers (e.g., circulating tumor DNA, CTCs), gut microbiota, and tumor microenvironment markers are still being researched. Since a significant proportion of patients do not benefit from immunotherapy, further trials are needed in the hope of finding new predictive biomarkers[22].
OLIGOMETASTATIC DISEASE
The role of surgery in metastatic gastric cancer is limited to the gastrectomy performed in symptomatic cases. The purpose of this intervention in asymptomatic patients is controversial[23]. Recently, a group of experts tried to define oligometastatic disease, mostly defined as less than 3 metastases or 1 extra-regional lymph node station. For this situation, an agreement among the experts was that immunotherapy should be considered after systemic therapy and local treatment. This serves as a starting point for future trials and, due to a lack of evidence, these recommendations should not be used outside of a clinical trial[24,25].
IMPACT OF LIVER METASTASIS ON IMMUNOTHERAPY
The Check Mate 649, ATTRACTION-2, ATTRACTION-4, or REGONIVO trials included heterogenous groups of patients with varying disease burden and significant failures, suggesting that treatment should be tailored. In subgroup analysis, in the REGONIVO trial[26], patients with liver metastases had a response rate of 41.7%, whereas patients with lung metastases without liver involvement had a response rate of 80%. Liver metastasis disrupts liver-triggered immunity. Cytotoxic CD8+ T cells are the main contributors to a robust antitumor immune response, and have become the pivot point around which immune therapies have been developed[27,28]. ICIs, which are the norm in gastric cancer immunotherapy, act by blocking the inhibitory effect on CD8+ regulation, promoting a more sustained cytotoxic, anti-cancer immune response. Their effect, however, may be weakened in cases of liver metastasis[29,30].
The liver is a highly immunogenic and immunotolerant organ, being a main recruiter of T cells. Within its sinusoidal circulation, T cells tolerize themselves to various nutrient, bacterial, and viral antigens. However, if this is disrupted by tumor growth, the recruitment of T cells capable of reacting through a sustained immune response will be reduced[26]. This is why some have suggested and proven through experimental preclinical studies that immunotherapy is less effective in cases with liver metastases.
Based on this theory, Liu et al[31] tested the long-term outcomes of immunotherapy in a case control fashion between gastric cancer patients with liver metastases vs without. Their results reiterate preclinical studies by showing a worse PFS and OS in the first group, although we must be aware that the groups were not matched, which may increase the risk of selection bias. This is either by associated comorbidities, which will influence OS/PFS, or by burden of disease through multivisceral metastases or bilobar/multisegmental liver metastases. Regardless, this is one of the very few clinical studies to comparatively assess the efficacy of ICIs and ignite further debate on selective indications of immunotherapy.
Another promising treatment method, although in its early stages of clinical research, is chimeric antigen receptor (CAR) T therapy, which is based on the ability of cancer cells to deviate the elimination phase of the immune response. Patient T cells are collected and genetically engineered via a viral vector to present a specific antigen receptor, making them capable of better recognizing and clearing tumor-associated antigens. CAR-T therapy has shown promising results in the treatment of hematologic malignancies and a wide array of solid tumors, including gastric cancer[32,33]. Studies[34] have designed CAR-T cells directed toward the Her-2 receptor, known to be overexpressed in gastric cancer. The activation of anti-Her-2 CAR-T cells was adequate in in vitro studies, but did not reach clinical studies. Not only T Cells, but macrophage or NK cells, may be used as antitumor therapy effectors.
CONCLUSION
Although the benefits of immunotherapy are indisputable in the treatment of advanced gastric cancer and the toxicity is generally easy to control, the most controversial aspect remains the financial toxicity. Even when only direct costs are estimated, the price of the medication means that, in most financial analyses, immunotherapy cannot be considered cost-effective compared to chemotherapy alone, at least as a first-line treatment[20]. The building body of evidence suggests that patients with liver metastases have a worse prognosis, likely due to the lack of recruitment and apoptosis of CD8+ T cells; however, there may still be a clinical benefit.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: Romania
Peer-review report’s classification
Scientific Quality: Grade B, Grade C
Novelty: Grade B, Grade B
Creativity or Innovation: Grade B, Grade B
Scientific Significance: Grade B, Grade B
P-Reviewer: Liu Z S-Editor: Liu H L-Editor: Filipodia P-Editor: Zhao YQ