Published online Oct 24, 2024. doi: 10.5306/wjco.v15.i10.1366
Revised: August 19, 2024
Accepted: September 2, 2024
Published online: October 24, 2024
Processing time: 154 Days and 21.9 Hours
Robotic-assisted partial splenectomy (RAPS) is a superior approach for treating splenic cysts and splenic hemangiomas, as it preserves the immune function of the spleen and reduces the risk of overwhelming post splenectomy infection. Curren
Four patients with splenic cysts or splenic hemangiomas were treated by RAPS. Critical aspects with RAPS include carefully dissecting the splenic pedicle, accurately identifying and ligating the supplying vessels of the targeted segment, and ensuring precise hemostasis during splenic parenchymal transection. Four successful RAPS cases are presented, where the tumors were removed by pret
Four cases confirm the feasibility and superiority of RAPS for the treatment of benign splenic tumors.
Core Tip: Robotic-assisted partial splenectomy (RAPS) is a superior approach for treating splenic cysts and splenic hemangiomas, as it preserves the immune function of the spleen and reduces the risk of overwhelming post splenectomy infection. Currently, there are no standardized guidelines for performing a partial splenectomy. This paper reports four cases of benign splenic tumors managed successfully by robotic-assisted partial splenectomy. This article provides the first comprehensive account of the detailed surgical procedure. RAPS demonstrates notable advantages in the treatment of benign splenic diseases.
- Citation: Xue HM, Chen P, Zhu XJ, Jiao JY, Wang P. Robot-assisted partial splenectomy for benign splenic tumors: Four case reports. World J Clin Oncol 2024; 15(10): 1366-1375
- URL: https://www.wjgnet.com/2218-4333/full/v15/i10/1366.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i10.1366
Splenic cysts and splenic hemangiomas are rare benign tumors. The reported incidence rate is 0.07% for splenic cysts and 0.02%-0.16% for splenic hemangiomas, with a higher prevalence among females[1,2]. Typically, there are no prominent clinical symptoms. These are usually discovered incidentally during routine medical examinations. However, when the tumor diameter is larger than 5 cm, there may be a palpable mass and pain in the left upper abdomen, with a risk of rupture. Therefore, for patients with symptoms or tumors larger than 5 cm in diameter, surgical treatment is strongly recommended[3].
In the past, the standard treatment for splenic cysts and splenic hemangiomas was splenectomy. However, recent studies have revealed a correlation between splenectomy and post operative complications such as infection, intra-abdominal abscess, portal vein thrombosis, pulmonary hypertension, thrombocytosis, and venous thromboembolism[4]. Due to the increasing recognition of the immune function of the spleen, spleen preservation has become a new trend in the surgical treatment of benign splenic diseases. Among the various methods, partial splenectomy is currently the pre
Case 1: A 14-year-old female, was admitted to the hospital after an enlarged splenic cyst was incidentally detected one week prior.
Case 2: A 40-year-old female, presented to the hospital due to progressive enlargement of a splenic hemangioma for one year.
Case 3: A 63-year-old female, was admitted to the hospital due to a four-month history of recurrent abdominal pain accompanied by thrombocytosis.
Case 4: A 40-year-old female, was admitted to the hospital with a complaint of an enlarged splenic cyst incidentally detected during a routine physical examination one year prior.
Case 1: Patient 1 experienced a sensation of bloating in the upper abdomen after meals for the past week.
Case 2: Patient 2 reported no symptoms; however, regular assessments indicated an increase in the size of the tumor.
Case 3: Patient 3 reported a history of recurrent pain in the upper left abdomen that had lasted for the past four months.
Case 4: Patient 4 had a similar history to patient 2.
All four patients had no history of abdominal trauma or travel outside the local area. They were systemically well.
All four patients denied any family history of splenic tumors.
Abdominal examination of patient 1: The abdomen was soft, and a firm mass approximately 9 cm in size was palpated in the left upper abdomen, below the costal margin, with clear boundaries and no tenderness. The abdomen showed no tenderness to palpation, no rebound tenderness, and no muscle rigidity.
Abdominal examination of patient 2: Patient 2 was systemically well and had a soft abdomen without abdominal pain.
Abdominal examination of patient 3: On palpation, the abdomen was soft with mild pain in the upper left abdomen, below the rib margin. The abdomen showed no tenderness to palpation, no rebound tenderness, and no muscle rigidity.
Abdominal examination of patient 4: On palpation, a firm and well-defined mass measuring approximately 8 cm was palpable in the left upper quadrant, below the rib margin. The abdomen showed no tenderness to palpation, no rebound tenderness, and no muscle rigidity.
Patient 1’s, patient 2’s and patient 4’s blood tests revealed no abnormalities. The results of patient 3's blood test revealed a platelet count of 1084 × 109/L.
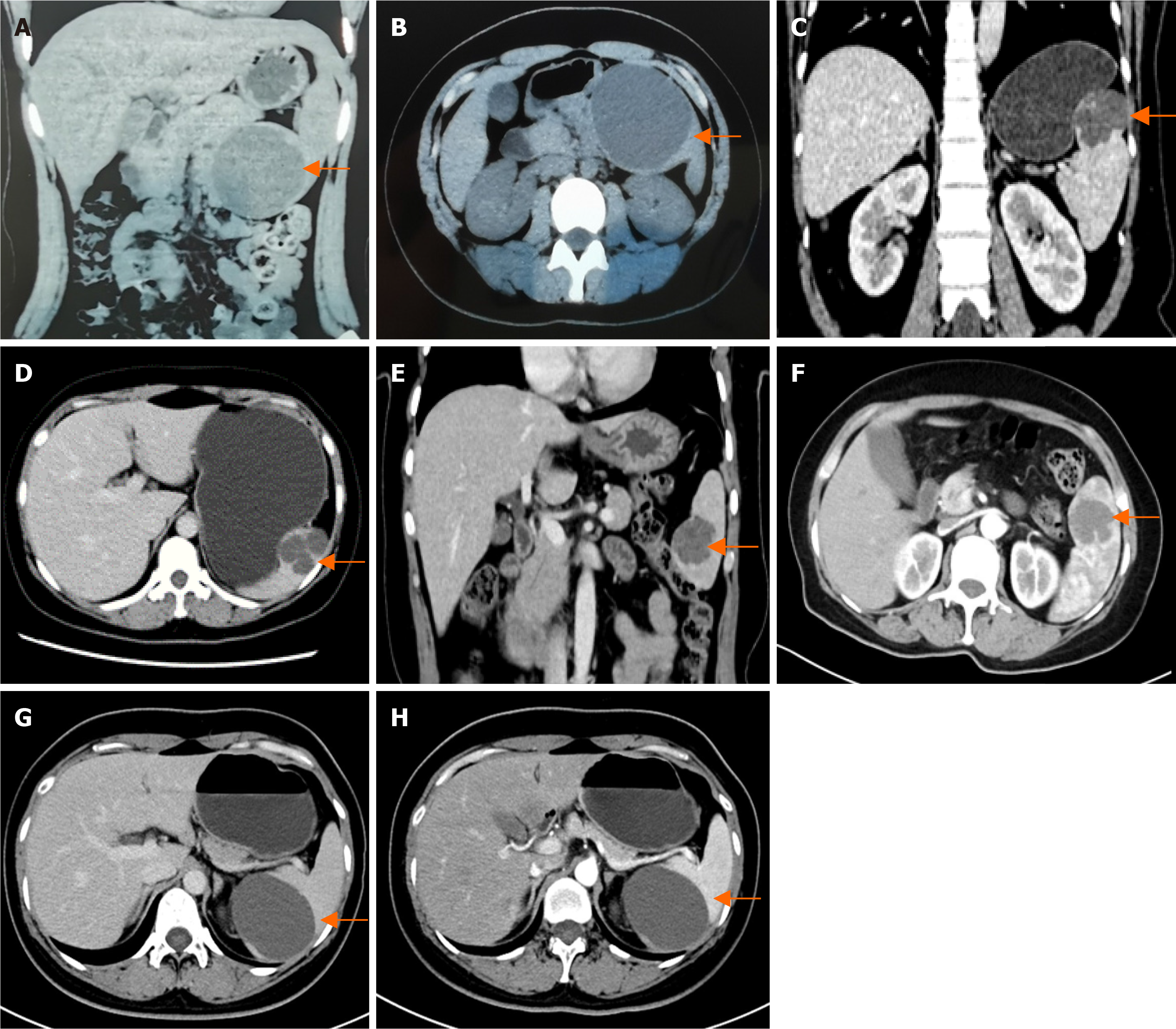
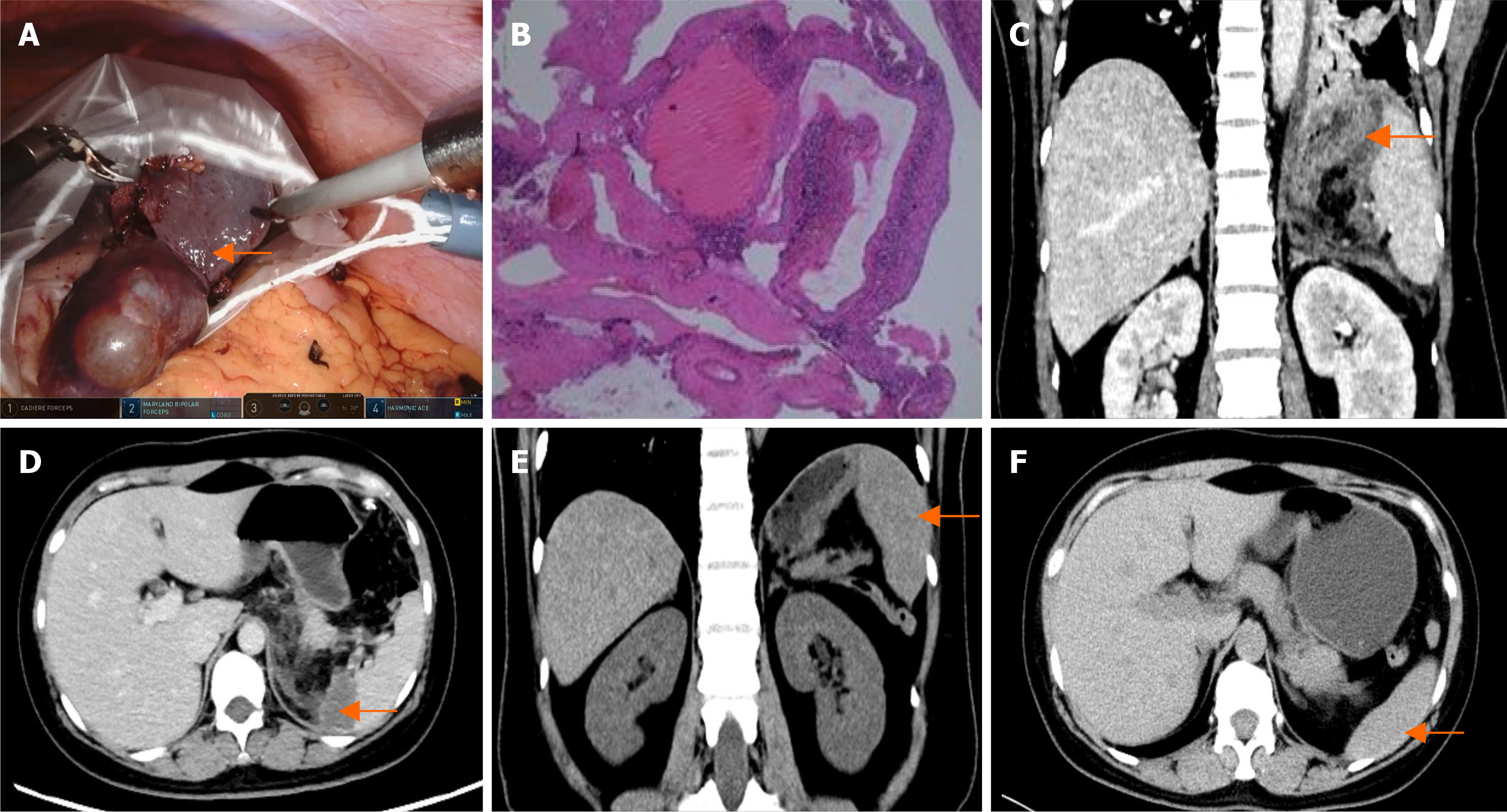
Patient 1’s computed tomography (CT) scan revealed a round, non-enhancing low-density lesion measuring approximately 90 mm in the lower pole of the spleen, which was consistent with a splenic cyst (Figure 1A and B).
Patient 2’s CT scan revealed multiple oval-shaped low-density lesions in the upper segment of the spleen, with the largest measuring approximately 52 mm. After contrast, the edges of the lesion demonstrated rings of enhancement, and, in the balance phase, they had slightly increased density, suggesting the presence of splenic hemangiomas (Figure 1C and D).
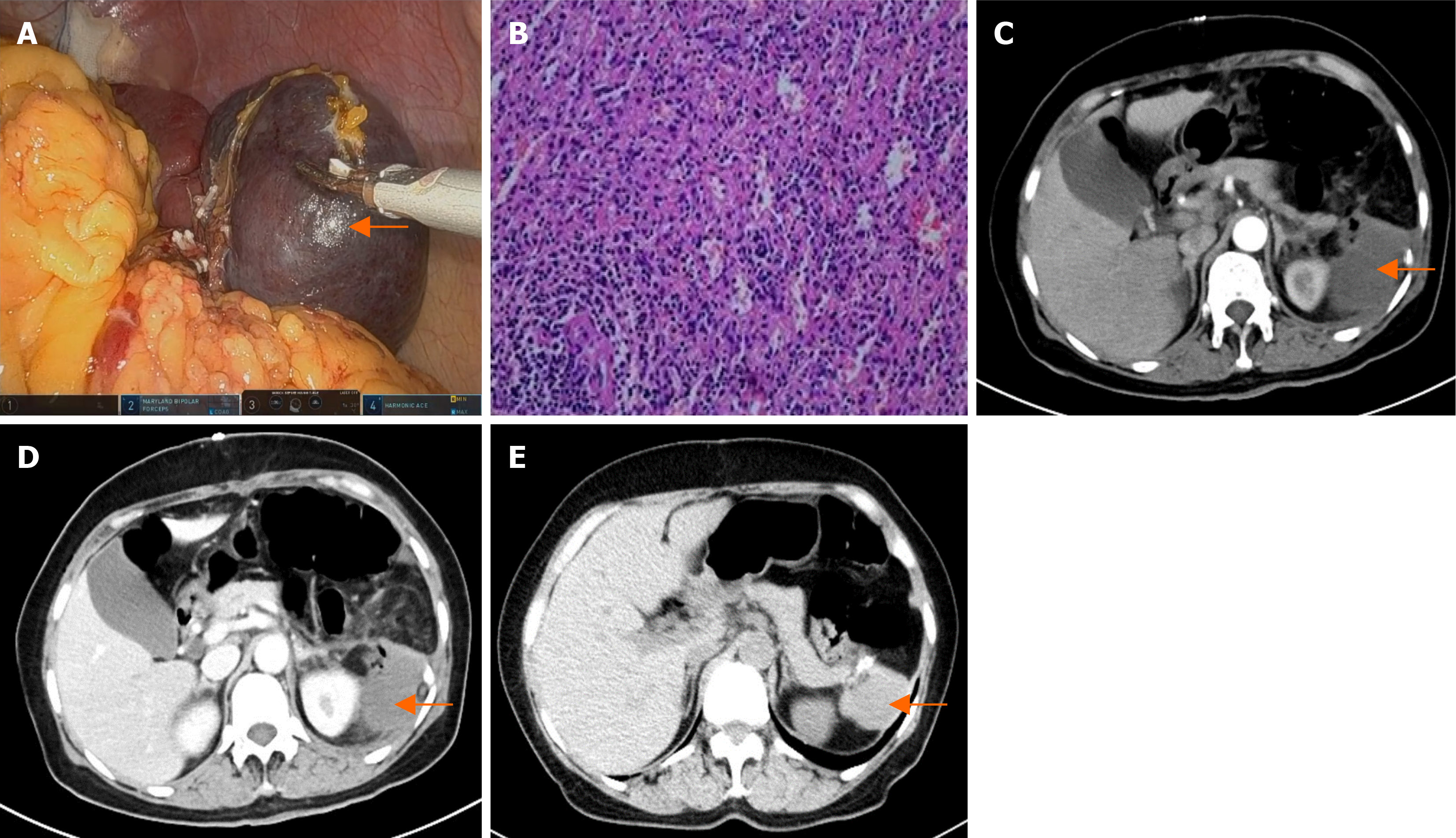
Patient 3’s magnetic resonance imaging scan revealed a 40 mm well-defined mass in the lower pole of the spleen, which appeared as a low signal on T1 weighted images and a high signal on T2 weighted images. The signal intensity increased over time, and a splenic hemangioma was considered (Figure 1E and F).
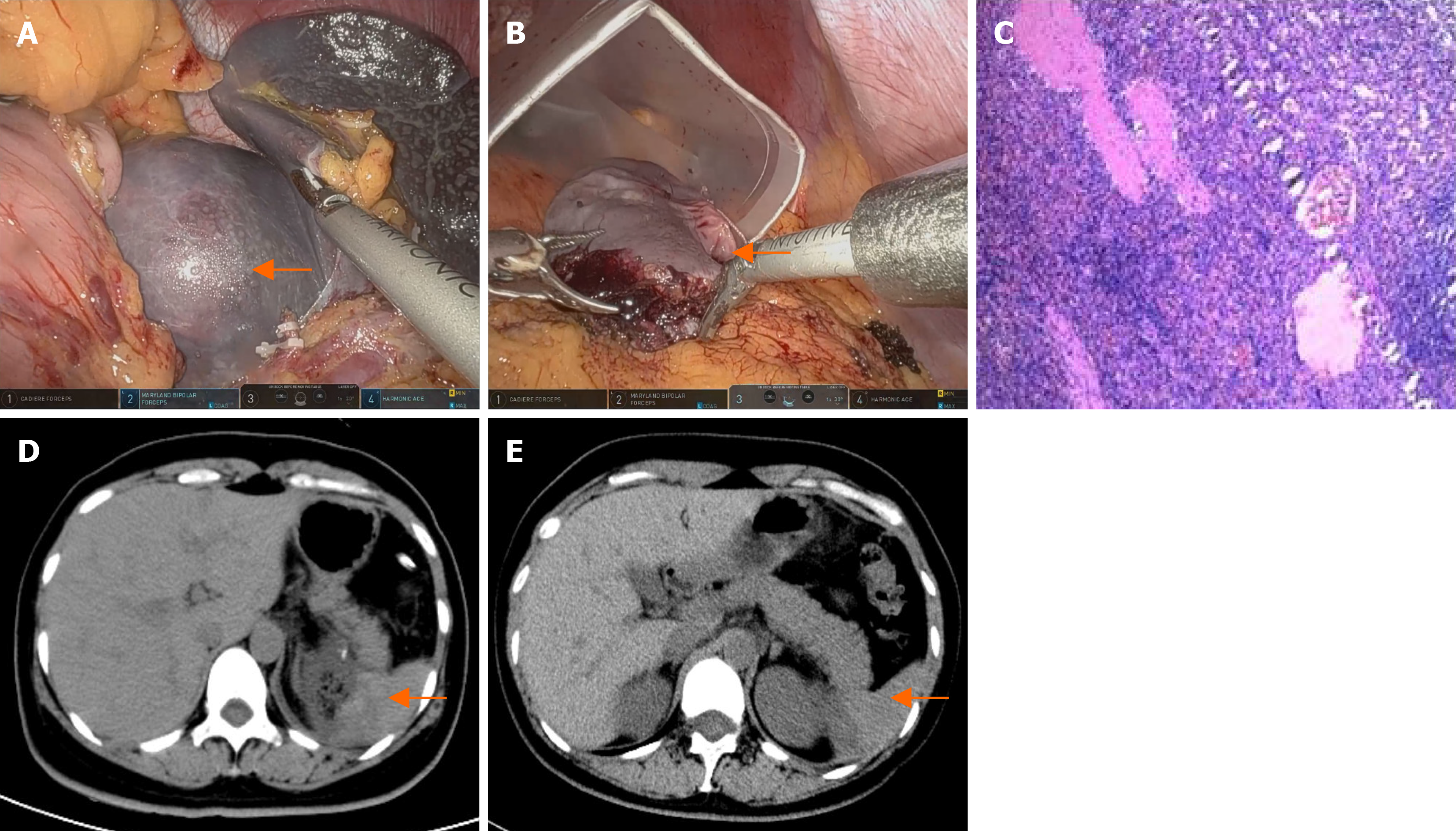
Patient 4’s CT scan revealed a round, non-enhancing low-density lesion measuring approximately 73 mm in the lower pole of the spleen, which was consistent with a splenic cyst (Figure 1G and H).
The final diagnoses of four patients were benign splenic tumors. Patient 1’s diagnosis was splenic cyst. Patient 2’s diagnosis was a splenic hemangioma. Patient 3’s diagnosis was a splenic hemangioma. Patient 4’s diagnosis was a splenic cyst.
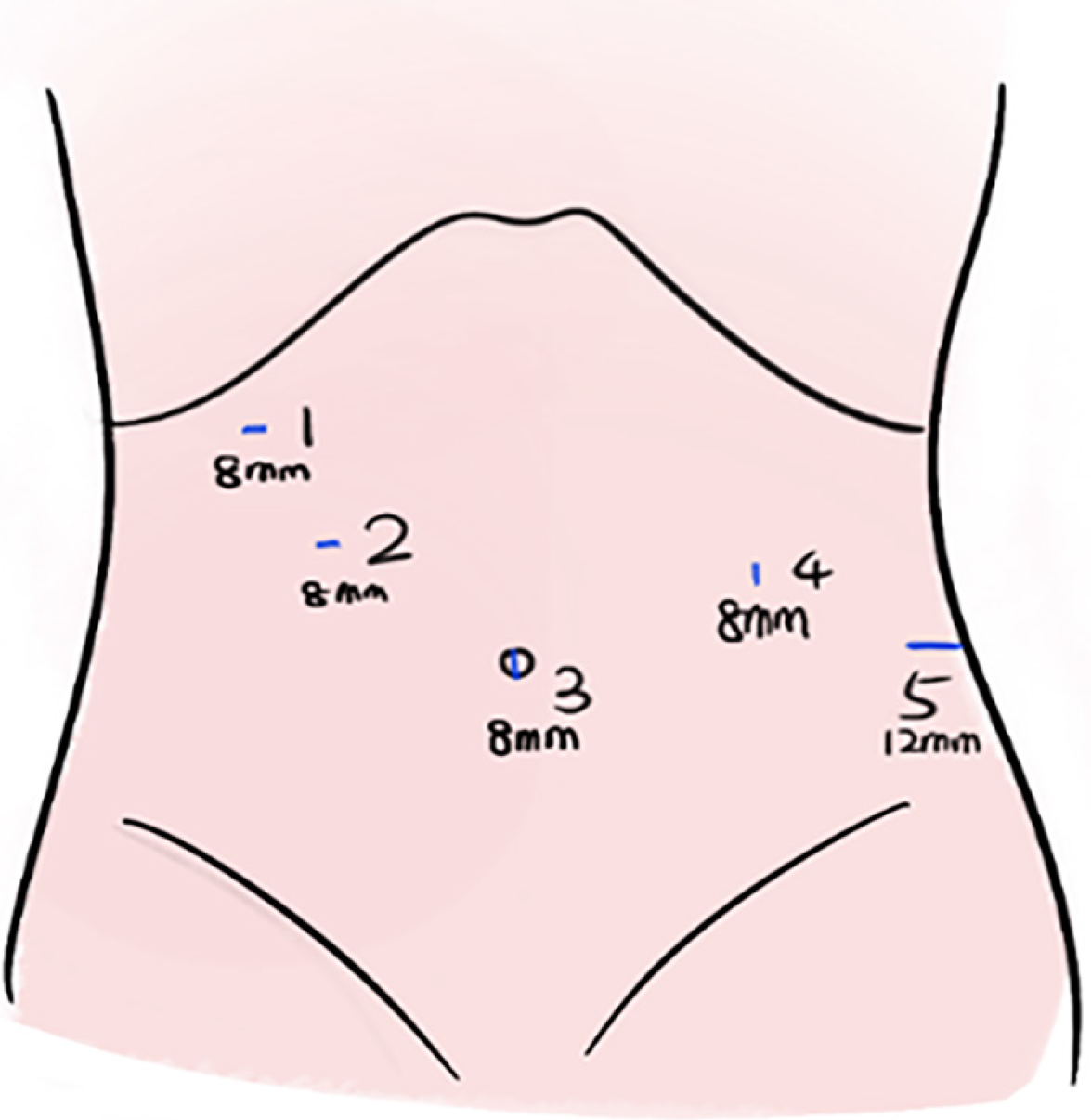
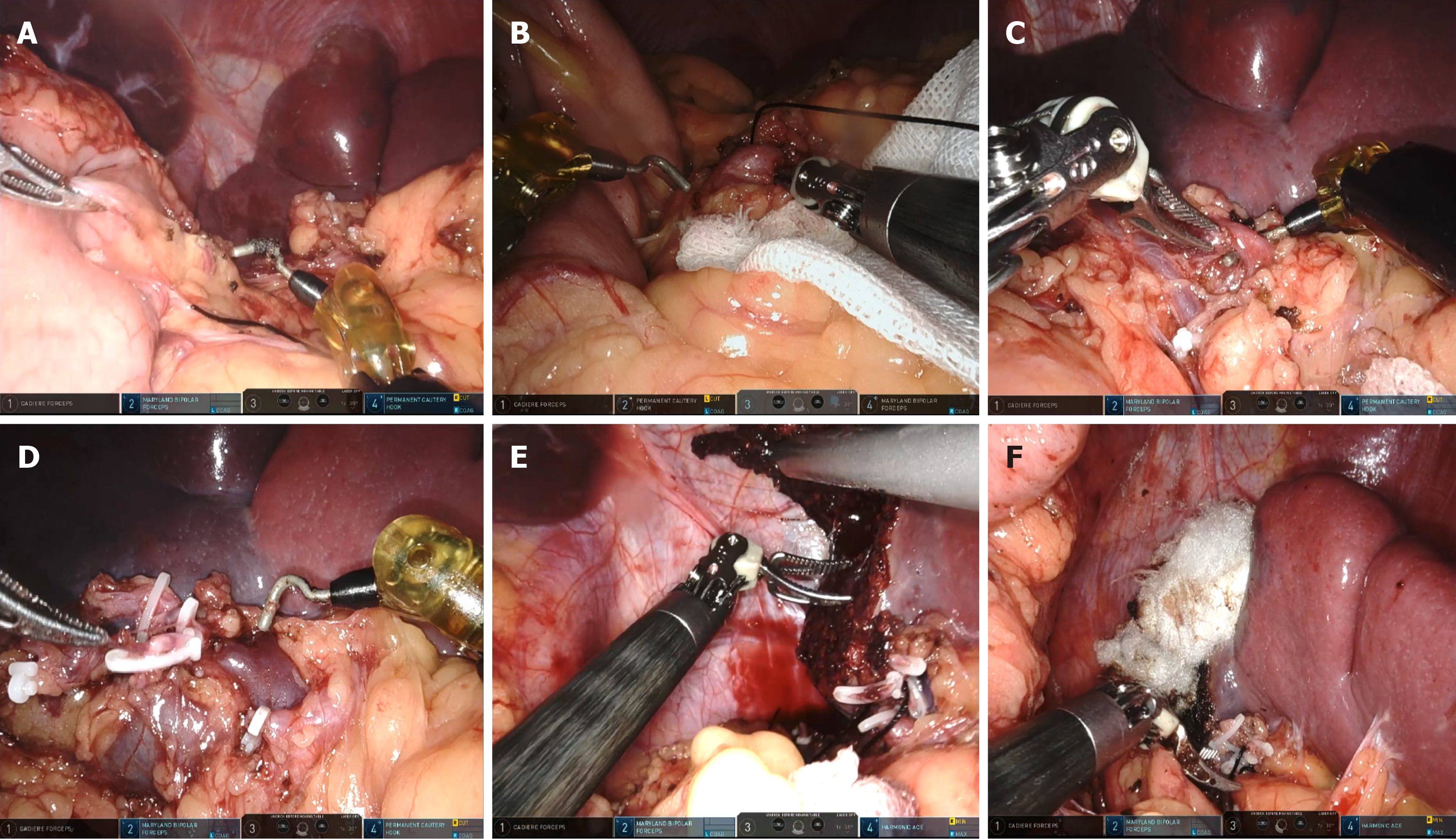
Under general anesthesia, the patient was placed in the right lateral position. RAPS surgery was performed with five ports in the upper abdomen (Figure 2). Under a 10 mmHg capnoperitoneum, the gastrocolic ligament was incised using an ultrasonic scalpel to expose the pancreas and spleen. The splenic artery was carefully dissected along the superior border of the pancreas, and then slung with nylon tape. The splenic hilum was exposed after the gastrosplenic ligament was divided. The ligaments around the segment to be pre-resected were divided, and the segment vessels of the pre-resected piece were cut. Once an ischemic line appeared on the spleen, the tumor was checked to ensure that it was localized within the ischemic line. Using an ultrasonic scalpel, the splenic parenchyma was dissected approximately 1 cm inside the inner edge of the ischemic line. Any bleeding points on the cut surface were cauterized using Maryland bipolar forceps or permanent cautery hooks. The splenic edge was treated with hemostatic gauze (Figure 3). The resected part of the spleen was placed into a specimen bag. The umbilical incision was subsequently extended to 2 cm, and the specimen bag was extracted from the body. The specimen was then fragmented by using tissue scissors for retrieval.
Four patients underwent successful surgeries; the operation duration and blood loss during the procedures are listed in Table 1.
| Operation duration (minute) | Blood loss (mL) | |
| Patient 1 | 120 | 20 |
| Patient 2 | 160 | 20 |
| Patient 3 | 145 | 100 |
| Patient 4 | 180 | 100 |
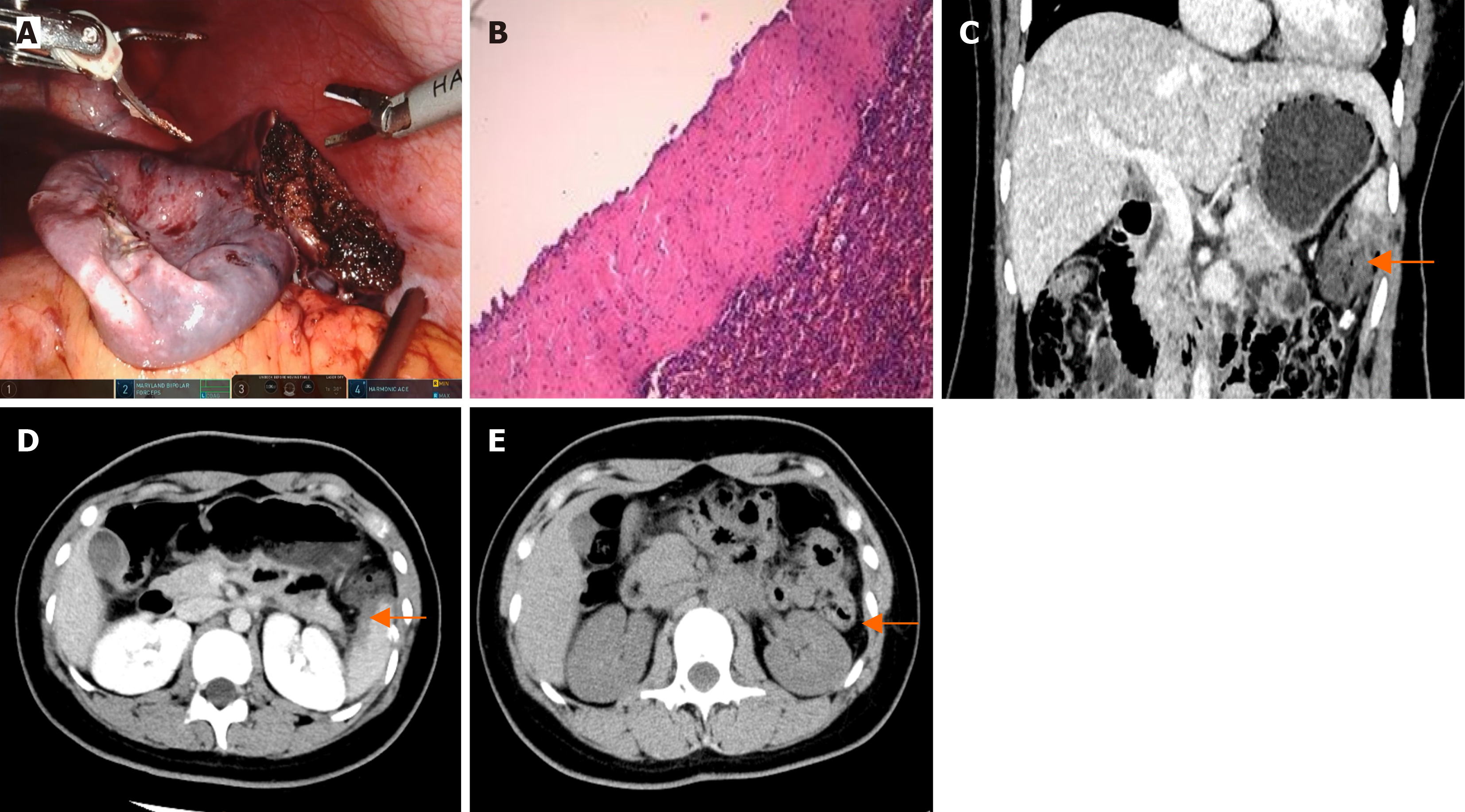
The patients recovered smoothly after the operation and were discharged on the fifth or sixth postoperative days. Histopathology confirmed that there was no evidence of malignancy. Patient 1’s pathology results indicated a primary splenic cyst (Figure 4). Patient 2’s pathology result showed splenic hemangiomas (Figure 5B). Patient 3’s pathology result indicated a splenic hemangioma (Figure 6B). Patient 4’s pathology results revealed a splenic cyst (Figure 7B).
Four patients had CT scans on the third day after surgery, and the results revealed some effusion around the spleen (Figure 4C and D, Figure 5C and D, Figure 6C and D, Figure 7D). Blood tests revealed no significant abnormalities.
A follow-up CT scan six months after surgery for the four patients revealed no signs of tumor recurrence in the spleen (Figure 4E, Figure 5E and F, Figure 6E, Figure 7E).
Splenic cysts and splenic hemangiomas are relatively rare in humans. Small spleen cysts generally show no clinical symptoms and usually do not require treatment. However, if a cyst exceeds 5 cm in diameter, surgical intervention is necessary[6]. Splenic hemangiomas are the most common benign tumors of the spleen, with an incidence of 0.02%-0.16%, and they are more common in females[4]. Hemangiomas tend to grow slowly and have a low risk of malignant trans
In 1952, King et al[8] were the first to report deaths following total splenectomy, noting severe infections that can occur afterwards. This led to the identification of overwhelming post splenectomy infection[8]. Since then, numerous studies have linked total splenectomy to complications such as postoperative infections, intra-abdominal abscesses, portal vein thrombosis, pulmonary hypertension, thrombocytosis, and venous thromboembolism.
With further research into the anatomy and function of the spleen, it has become clear that the phagocytic activity of splenic macrophages and the production of polysaccharide antibodies by B-lymphocytes are vital for infection defense[9]. Consequently, maintaining the immune function of the spleen is critical, with findings suggesting that preserving more than 25% of the splenic tissue can safeguard this function[9]. This revelation has brought partial splenectomy into the spotlight, and various reports have emerged.
Partial splenectomy has become a new trend in the surgical treatment of benign splenic diseases. This procedure is based on the segmental blood supply of the spleen. The splenic artery branches out into the splenic artery branches at the hilum of the spleen, with four types of branching patterns, including 1, 2, 3, and multiple branches, with the 2-branch and 3-branch types being the most common[10]. The arterial branches of the spleen form wedge-shaped segments that supply blood to their respective segments. There are relatively ischemic planes between these segments[11]. By ligating the corresponding vessels at the splenic hilum and cutting the spleen 0.5-1 cm inside the ischemic line, segmental ischemia can be induced, allowing for safe removal of the desired portion of the spleen[12-14].
The challenges of partial splenectomy include anatomical identification of the splenic hilum vessels, precise identification and division of the blood supply to the pre-resected segments, and precise hemostasis during splenic paren
With respect to handling the splenic section, previous methods include the use of an ultrasonic scalpel, bipolar electrocoagulation, ligating clips, stapling devices, and bipolar radiofrequency electrodes for hemostasis[12]. Bing et al[4] utili
An enlarged umbilical puncture hole was used to extract the splenic specimen. Our team has gained experience in obtaining samples via single-incision laparoscopic cholecystectomy. We extended the umbilical incision to 2 cm, opened the umbilical ring, and removed the opening of the specimen bag. The splenic tumor was carefully cut into small frag
We completed four partial splenectomies with the assistance of the Da Vinci robotic surgical system. Robotic surgery has emerged as a superior minimally invasive technique, showing great promise in treating various conditions, including splenic surgery. In 2003, Talamini et al[17] were the first to use the Da Vinci robotic system for splenectomy[17]. The first case of robot-assisted partial splenectomy was reported by Vasilescu et al[18] in 2010.
Compared to laparoscopic surgery, robotic surgery significantly reduces the operation time, intraoperative blood loss, and overall incidence of complications during the perioperative period[19,20]. Research has indicated that the average operation duration of an open partial splenectomy is 120 minutes, that of laparoscopic surgery is approximately 135 minutes, and that of robotic surgery is 150 minutes. Patients who undergo robotic surgery typically experience an average postoperative hospital stay of 6.2 days and have fewer complications[21-24]. Importantly, there is no considerable dif
The robotic surgery system offers several advantages. Firstly, it provides a higher magnification ratio, intuitive control, and a clear 3D surgical field, thereby minimizing collateral damage during the procedure[6]. Additionally, robotic arms have a better range of motion and can perform intricate maneuvers, allowing for more precise surgical techniques[21]. However, there are notable disadvantages to using the Da Vinci robot. Firstly, there is a lack of force feedback. Secondly, the cost of the robotic system is relatively high.
To summarize, robotic-assisted partial splenectomy demonstrates notable advantages in treating benign splenic diseases, significantly reducing surgical complexity. Nevertheless, the widespread adoption of robotic surgery is hindered by its high cost and limited insurance coverage. Additional clinical cases are necessary to further validate its superior benefits.
We express our deep appreciation to the participants who made this study possible. We also would like to express our sincere gratitude to editors and anonymous reviewers for their valuable comments, which have greatly improved this paper.
| 1. | Lin JL, Lin C, Wang HL, Wu SJ, Tang Y, Yang CS, Luo JW, Chi W, Fang ZT. Splenic Artery Embolization and Splenectomy for Spontaneous Rupture of Splenic Hemangioma and Its Imaging Features. Front Cardiovasc Med. 2022;9:925711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 2. | Elhardello O, Ammori BJ. Splenic pedicle control during laparoscopic de-capsulation of a giant splenic cyst. J Surg Case Rep. 2018;2018:rjx255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Chin EH, Shapiro R, Hazzan D, Katz LB, Salky B. A ten-year experience with laparoscopic treatment of splenic cysts. JSLS. 2007;11:20-23. [PubMed] |
| 4. | Bing Y, Sadula A, Xiu D, Yuan C. Laparoscopic middle segment splenectomy for central splenic hemangioma: A case report. Int J Surg Case Rep. 2020;77:925-929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | Hassoun J, Ortega G, Burkhalter LS, Josephs S, Qureshi FG. Management of nonparasitic splenic cysts in children. J Surg Res. 2018;223:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Kirih MA, Liang X, Xie Y, Cai J, Zheng J, Xu F, He S, Tao L, Abdi FA. Robot-Assisted Partial Splenectomy for Splenic Epidermoid Cyst. Case Rep Surg. 2020;2020:6245909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Giulianotti PC, Buchs NC, Addeo P, Ayloo S, Bianco FM. Robot-assisted partial and total splenectomy. Int J Med Robot. 2011;7:482-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | KING H, SHUMACKER HB Jr. Splenic studies. I. Susceptibility to infection after splenectomy performed in infancy. Ann Surg. 1952;136:239-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 612] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 9. | Van Wyck DB, Witte MH, Witte CL. Compensatory spleen growth and protective function in rats. Clin Sci (Lond). 1986;71:573-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Liu DL, Xia S, Xu W, Ye Q, Gao Y, Qian J. Anatomy of vasculature of 850 spleen specimens and its application in partial splenectomy. Surgery. 1996;119:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Esposito F, Noviello A, Moles N, Cantore N, Baiamonte M, Coppola Bottazzi E, Miro A, Crafa F. Partial splenectomy: A case series and systematic review of the literature. Ann Hepatobiliary Pancreat Surg. 2018;22:116-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Kimura K, Kurashima Y, Tanaka K, Nakanishi Y, Asano T, Ebihara Y, Noji T, Murakami S, Nakamura T, Tsuchikawa T, Okamura K, Shichinohe T, Kanno-Okada H, Hirano S. Laparoscopic partial splenectomy for splenic lymphangioma: a case report. Surg Case Rep. 2020;6:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Bada-Bosch I, Mata DP, de la Torre M, Ordóñez J, Blanco MD, de Agustin J. Laparoscopic Partial Splenectomy Assisted by Fluorescence in a 13-Year-Old Girl. European J Pediatr Surg Rep. 2020;8:e81-e85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Zhang Q, Tian Y, Duan J, Gao Z, Wang W, Yan S. 915 MHz microwave-assisted laparoscopic partial splenectomy: A case series. J Minim Access Surg. 2020;16:441-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Ouyang G, Li Y, Cai Y, Wang X, Cai H, Peng B. Laparoscopic partial splenectomy with temporary occlusion of the trunk of the splenic artery in fifty-one cases: experience at a single center. Surg Endosc. 2021;35:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Jiao J, Zhu X, Zhou C, Wang P. Single-incision laparoscopic transabdominal preperitoneal hernioplasty: 1,054 procedures and experience. Hernia. 2023;27:1187-1194. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Talamini MA, Chapman S, Horgan S, Melvin WS; Academic Robotics Group. A prospective analysis of 211 robotic-assisted surgical procedures. Surg Endosc. 2003;17:1521-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 178] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 18. | Vasilescu C, Tudor S, Popa M, Tiron A, Lupescu I. Robotic partial splenectomy for hydatid cyst of the spleen. Langenbecks Arch Surg. 2010;395:1169-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Giza DE, Tudor S, Purnichescu-Purtan RR, Vasilescu C. Robotic splenectomy: what is the real benefit? World J Surg. 2014;38:3067-3073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Liu G, Fan Y. Feasibility and Safety of Laparoscopic Partial Splenectomy: A Systematic Review. World J Surg. 2019;43:1505-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Balaphas A, Buchs NC, Meyer J, Hagen ME, Morel P. Partial splenectomy in the era of minimally invasive surgery: the current laparoscopic and robotic experiences. Surg Endosc. 2015;29:3618-3627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Vasilescu C, Stanciulea O, Tudor S. Laparoscopic versus robotic subtotal splenectomy in hereditary spherocytosis. Potential advantages and limits of an expensive approach. Surg Endosc. 2012;26:2802-2809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Costi R, Castro Ruiz C, Romboli A, Wind P, Violi V, Zarzavadjian Le Bian A. Partial splenectomy: Who, when and how. A systematic review of the 2130 published cases. J Pediatr Surg. 2019;54:1527-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Berelavichus SV, Smirnov AV, Ionkin DA, Kriger AG, Dugarova RS. [Robot-assisted and laparoscopic partial splenectomy for nonparasitic cysts]. Khirurgiia (Mosk). 2015;41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |