Published online Oct 24, 2024. doi: 10.5306/wjco.v15.i10.1315
Revised: August 15, 2024
Accepted: August 23, 2024
Published online: October 24, 2024
Processing time: 194 Days and 4.7 Hours
Although the occurrence of multiple primary cancers (MPC) is not exceedingly common, it is not rare in clinical practice. In recent years, there has been a notable increase in its incidence. The frequent confusion between MPC and tumor metastasis or recurrence often leads to delays in diagnosis and treatment. This study aimed to enhance understanding of MPC, improve diagnostic accuracy, guide precise clinical treatment, and implement a case management nursing model (CMNM) to facilitate quick patient recovery.
A 61-year-old female patient presented with persistent upper abdominal pain lasting over 2 months. Gastroscopy revealed the presence of both gastric and duodenal cancers. Following a thorough evaluation, the patient underwent pancreaticoduodenectomy, cholecystectomy, and total gastrectomy. Post-surgery, an individualized case management nursing approach was applied, leading to a successful recovery. Three months after the surgery, follow-up examinations showed no signs of recurrence.
The CMNM effectively promoted rapid patient recovery, enhanced the quality of orthopedic nursing services, and accelerated postoperative recovery, ultimately leading to increased patient satisfaction with nursing care.
Core Tip: Accurate diagnosis of multiple primary cancers, coupled with multidisciplinary collaboration to formulate tailored diagnosis and treatment plans and the implementation of individualized patient care, significantly contributed to rapid recovery and favorable prognosis.
- Citation: Liu D, Li SC. Nursing of a patient with multiple primary cancers: A case report and review of literature. World J Clin Oncol 2024; 15(10): 1315-1323
- URL: https://www.wjgnet.com/2218-4333/full/v15/i10/1315.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i10.1315
Multiple primary cancers (MPC), also known as duplicate cancers, are characterized by the simultaneous or sequential occurrence of two or more primary malignant tumors within a single or multiple organs in the same individual. MPC is classified based on the interval between the diagnoses of the two cancers: Synchronous MPC, where the interval is 6 months or less, and asynchronous MPC, where the interval exceeds 6 months[1]. In China, MPC most frequently involves the digestive system, followed by occurrences in the head and neck, lungs, breast, and thyroid. Research evidence indicates that when MPC initially manifests in the digestive system, the subsequent primary cancer is most often also a digestive system tumor, with the respiratory and urogenital systems being the next most common sites[2].
There is no standardized treatment protocol for MPC, but it is generally agreed that treatment should be tailored based on factors such as tumor type, location, progression stage, and the patient’s overall condition[3]. Surgical intervention is typically the preferred treatment for MPC. Although performing simultaneous surgeries can be complex and traumatic, postoperative management should be carefully intensified to promote early recovery.
The case management nursing model (CMNM) is a structured approach that emphasizes collaboration, systematization, and specificity in patient care. By integrating resources, CMNM provides personalized nursing care, allowing patients to receive comprehensive and continuous services during complex treatments. This model addresses the individualized needs of patients, making nursing care more personalized and effective, thereby enhancing the quality of patient care and promoting postoperative recovery[4].
A 61-year-old female patient was admitted due to persistent upper abdominal pain lasting over 2 months.
In April 2023, she initially presented with dull upper abdominal pain of unknown origin. The pain was intermittent and accompanied by three episodes of vomiting blood, each time containing approximately 10 mL of fresh blood. Seeking medical attention at Hospital A, a gastroscopy examination revealed gastric cancer, multiple stomach polyps, and duodenitis.
Following treatments such as hemostasis, acid suppression, and fluid replacement, the symptoms improved, and there was no further occurrence of blood vomiting. However, the patient continued to experience discomfort in the upper abdomen. Given her age and perceived physical intolerance, the family, after discussion, opted against surgery or chemotherapy. Subsequently, the patient sought traditional Chinese medicine treatment at our hospital for 2 months.
On July 3, 2023, the patient revisited our hospital for an outpatient checkup, revealing persistent gastric cancer and newly identified duodenal carcinoma in the gastroscopy examination. Throughout this period, the patient reported no additional symptoms, such as fever or chills. Returning for further treatment on July 10, 2023, the patient was admitted to the hospital with a diagnosis of “duodenal cancer”.
The patient had a history of hypertension for over a decade, which was managed with oral nifedipine sustained-release tablets. There was no medical history of diabetes, coronary artery disease, tuberculosis, or other infectious diseases. Additionally, the patient had no history of trauma, surgery, blood transfusions, or drug allergies.
The patient had no history of smoking, alcohol consumption, or exposure to radiation or toxins. The patient’s parents were deceased, with the causes of death unknown. Within the family, the eldest brother had kidney cancer, the second brother had stomach cancer, and the second sister had lung cancer.
Throughout the course of the illness, the patient consistently exhibited clear consciousness, a stable mental state, an average appetite, regular sleep patterns, reduced physical strength, and normal bowel movements.
A specialized examination revealed no apparent abnormalities in the cardiopulmonary system. The patient exhibited a soft abdomen, experienced mild tenderness in the upper abdomen, and did not report any rebound pain.
Tumor markers: Carcinoembryonic antigen: 3.4 ng/mL; Carbohydrate antigen 19-9: 25.65 U/mL; Carbohydrate antigen 125: 12.79 ng/mL; Ferritin: 13.76 ng/mL; Carbohydrate antigen 50: 9.53 IU/mL.
Blood routine: Platelet count: 37.2 × 109/L.
Electrolyte liver and kidney function: Albumin: 38.9 g/L.
Antibodies: Hepatitis A, B, and C antibodies: HBcAb (+) (positive). All others were negative.
Urinary routine: Positive for occult blood.
Coagulation function: Within normal range.
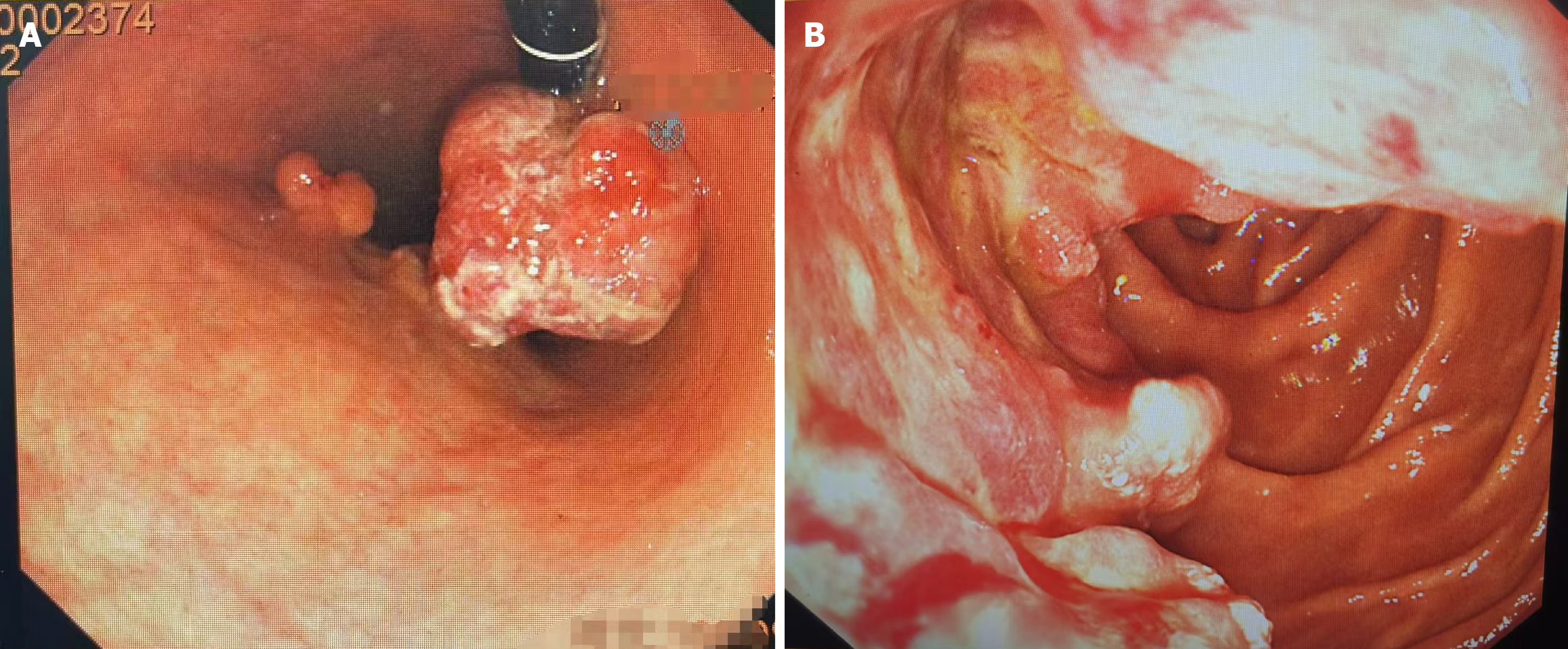
Gastroscopy: Multiple gastric masses and duodenal carcinoma were detected (Figure 1).
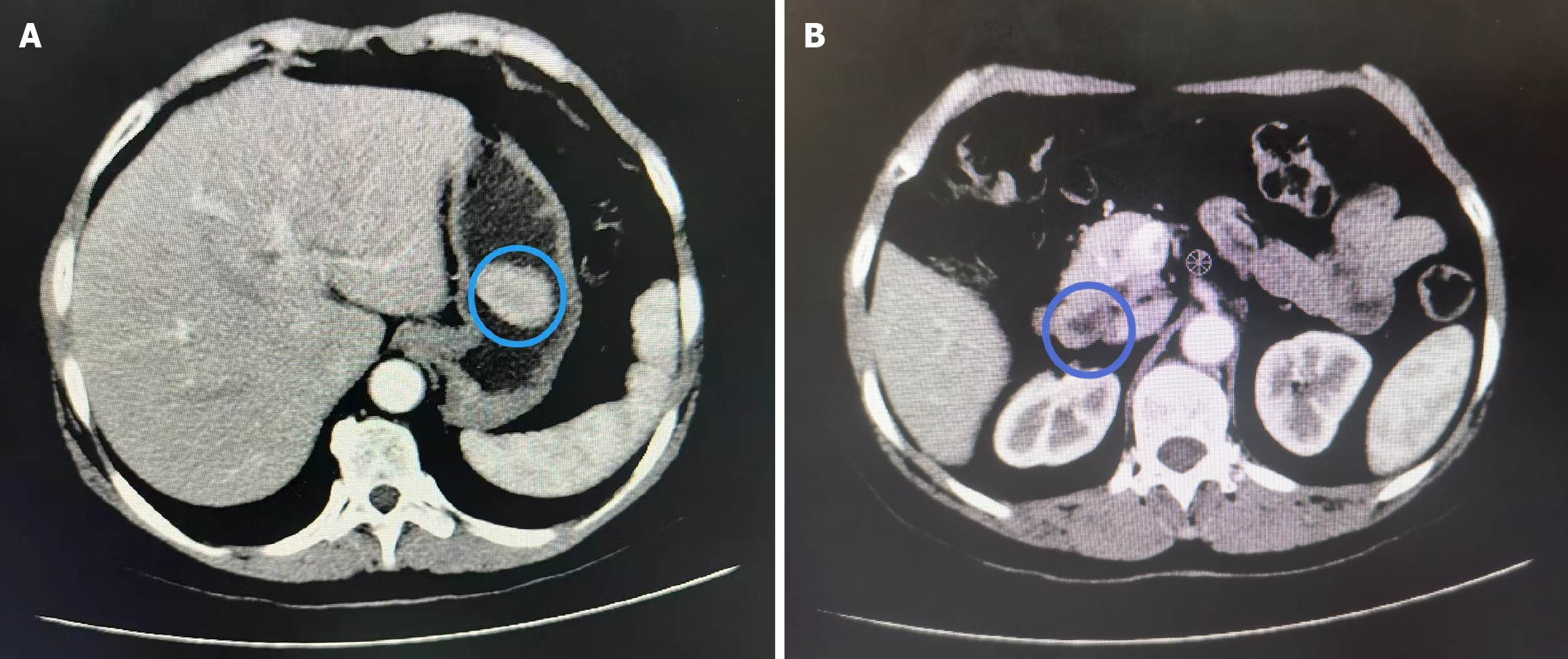
Computed tomography scan: A complete abdominal computed tomography scan (Figure 2) indicated the presence of a soft tissue mass in the gastric cavity. This finding should be correlated with the results of gastroscopy. Additionally, the thickening of the duodenal wall in the horizontal segment raised concerns about a high likelihood of malignancy.
Given the patient’s medical history, laboratory tests, and imaging findings, the primary diagnoses under consideration were duodenal carcinoma and gastric cancer. In terms of a differential diagnosis, it’s worth noting that based on the patient’s pathological diagnosis, there appeared to be no necessity for further differentiation.
After the examination on July 18, 2023, pancreaticoduodenectomy, cholecystectomy, and total gastrectomy were performed.
The postoperative pathological report, following the resection of the entire stomach, duodenum, and partial pancreas, revealed six distinct masses. The pathological analysis identified different tumor types and grades, including intramucosal adenoma, adenocarcinoma grade II with mucinous adenocarcinoma invading the submucosal layer, and high-grade intraepithelial neoplasia of the glandular epithelium. Additionally, adenocarcinoma grade II-III was observed in the ampulla and pancreatic tissue, involving the duodenal muscle layer, with visible nerve invasion and no evident vascular tumor thrombus.
Formation of the CMNM team: The CMNM team was composed of a head nurse for the department, two primary nurses, two to three senior nurses, and one to two physicians.
Formulation of the CMNM plan: A comprehensive and personalized nursing plan was meticulously developed, drawing on clinical experience and evidence-based medicine. This plan was tailored to the patient’s specific condition, physical health, and psychological well-being. From the initial consultation to discharge, the plan covered the entire care process, including the prevention and management of complications, subsequent treatment phases, and follow-up care. The patient was supported throughout with personalized, one-on-one nursing care, ensuring continuity and consistency in their treatment and recovery journey.
Preoperative evaluation: The patient’s vital signs, past history, medication history, psychological status, diet, sleep, bowel movements, family economic and social relationships, etc., were comprehensively evaluated. Special attention was paid to whether there was a history of vomiting blood, and meticulous care was taken to attend to the oral cavity.
Psychological nursing: Vomiting blood and persistent upper abdominal discomfort, unalleviated by active medication treatment, induced fear and anxiety in the patient. It became crucial to offer psychological counseling, listen patiently, facilitate physical and mental relaxation, and provide explanations about the disease and its treatment plans to both the patient and their family members. This approach aimed to all deviate nervousness, instill patient confidence, foster active cooperation with treatment, and encourage participation in enjoyable activities such as listening to music or engaging in play. These interventions have been proven to effectively enhance the patient’s cognitive function, diminish mental stress, and alleviate worry[5].
Pain care: Pain assessment is initiated upon the patient’s recent admission and has been consistently and comprehensively conducted as part of daily systematic assessments. Patients are educated on the use of digital assessment scales, language assessment scales, Wong-Baker facial expression pain scales, and similar tools for effective pain evaluation. In instances of mild pain, there is a focus on reinforcing communication and interaction with patients. Studies[6,7] have indicated that listening to calming and gentle music can effectively alleviate both anxiety and pain.
For patients experiencing moderate to severe pain, it becomes imperative to inform a doctorand adhere to their recommendations for the reasonable use of painkillers. A physical examination revealed mild abdominal tenderness, which did not impede the patient’s ability to rest or engage in daily activities. The patient was provided with psychological counseling, and music tailored to their personal preferences and cultural background was played, contributing to the alleviation of preoperative anxiety and pain.
Respiratory preparation: Research findings indicate that respiratory complications account for 38.3% of postoperative issues following open surgery[8]. Preoperative patient education, focusing on smoking cessation and lung expansion procedures, has been established as crucial in reducing the risk of postoperative pulmonary complications[9]. Patman[10] have demonstrated that incorporating preoperative lung pre-rehabilitation along with postoperative lung enhance mentre habilitation training for surgical patients significantly enhances respiratory function, lowers the incidence of postoperative pulmonary complications, promotes early recovery, and shortens hospital stays.
Further studies[11] support that perioperative respiratory training can effectively decrease the occurrence of pulmonary complications after laparoscopic colorectal cancer surgery. All patients scheduled for elective surgery, including those undergoing laparoscopic colorectal cancer surgery, are advised to undergo perioperative respiratory training. This training regimen encompasses deep breathing, cough exercises, balloon-blowing exercises, and pursed lip breathing exercises.
Upon admission, the patient was introduced to lip-tightening breathing training involving quick inhalation through the nose and slow exhalation, maintaining a 1:2 ratio of inhalation to exhalation time. This training occurred twice a day for 15 minutes per session. Additionally, abdominal breathing exercises, conducted while sitting upright or lying down, entailed slow and deep nasal inhalation, inward abdominal contraction during exhalation, and a breath ingrate of 7-8 times per minute, with training sessions lasting 10 minutes twice a day. Activity endurance training, such as flat walking and stair climbing, was performed twice daily for 20-30 minutes per session, with attention to breathing during exercise. In cases of noticeable breathing difficulty, the patient was advised to take short rests before resuming exercise. The training regimen also included resistance breathing activities like blowing a balloon, along with effective coughing techniques such as explosive coughing, segmented coughing, and episodic coughing.
Preoperative intestinal preparation: The patient was advised to diligently adhere to the enhanced recovery after surgery (ERAS) requirements for preoperative intestinal preparation. This involved fasting fora period of 6 hours, and a further 2 hours of fasting immediately preceding anesthesia induction. Exceptions to this standard fasting protocol are made for patients with delayed gastric emptying or gastrointestinal motility disorders, as well as those undergoing emergency surgery[12].
Disease observation: Postoperative electrocardiographic monitoring was conducted to closely track changes in the patient’s heart rate, blood pressure, pulse, respiration, oxygen saturation, consciousness, facial expression, urine output, and drainage volume.
Care of pipelines: Care of pipelines is crucial during the postoperative period. It involves maintaining the unobstructed status of each pipeline, carefully observing the color, nature, and quantity of drainage fluid, ensuring proper fixation, and utilizing the suture fixation method. This method involves using surgical sutures to secure the drainage tube on both sides of the tube’s exit on the skin to prevent folding and detachment. Additionally, effective secondary fixation is implemented, incorporating catheter fixation patches to prevent any unintended detachment of the pipelines.
Respiratory management: Routinely welling of a nasogastric tube is not recommended for postoperative gastric cancer patients. If indwelling becomes necessary, it should be done cautiously to eliminate the risk of postoperative bleeding and anastomotic leakage. Early removal of the gastric tube within 24 hours after surgery is encouraged as it facilitates sputum excretion, promotes lung function recovery, and reduces the likelihood of postoperative pulmonary infections. Ensuring effective postoperative analgesia is crucial for patient recovery and plays a significant role in decreasing the occurrence of pulmonary infections[13]. Postoperative guidance involves encouraging patients to continue respiratory exercises, providing assistance in turning over, and patting the back to facilitate phlegm expulsion. Bilateral abdominal compression is identified as a simple and effective method to alleviate postoperative cough pain, promote sputum excretion, and contribute to preventing postoperative pulmonary complications[14]. Nebulization inhalation is ad
Patients are encouraged to start getting out of bed and moving around on the first day after surgery. Daily activity goals are set, and the amount of activity is gradually increased following the doctor’s recommendations. This proactive approach aims to enhance overall patient recovery and minimize postoperative complications.
Pain management: Effective postoperative pain management not only aims to minimize patient discomfort but also promotes early activity and functional recovery, ultimately resulting in a shorter hospital stay, reduced medical expenses, and improved hospital satisfaction[15].
Multimodal analgesia involves the integration of various analgesic methods, such as patient-controlled intravenous analgesia, oral medication, intravenous injection, intramuscular injection, systemic analgesic drugs, as well as local infiltration anesthesia, peripheral nerve block, thoracic paravertebral nerve block, and epidural block anesthesia. This comprehensive approach also includes the combination of different analgesic drugs like opioids, nonsteroidal anti-inflammatory drugs, acetaminophen, and anti-neuropathic pain medications[16].
As part of this multimodal analgesia strategy, the patient underwent ultrasound-guided transverse abdominis plane nerve block during surgery. Subsequently, postoperative pain management involved the use of a patient-controlled analgesia pump in combination with other analgesic measures. This integrated approach ensured a more comprehensive and effective pain relief regimen for the patient.
Deep vein thrombosis prophylaxis: Venous thromboembolism (VTE) is a common complication following surgical procedures. Studies[17] indicate that the incidence of postoperative VTE in patients undergoing abdominal surgery for cancer is approximately 19.95%. Perioperative risk assessment for VTE using the Caprini score in general surgery inpatients is recommended[18]. For general surgical patients with either low or higher risk, VTE prevention measures are suggested, including the selection of mechanical and/or medication-based preventive approaches. Adjustments to preventive measures should be made in a timely manner[19]. In the case described, the postoperative patient scored 10 points on the Caprini score. Preventive measures implemented included ensuring adequate fluid intake to prevent blood viscosity, avoiding venous punctures in the lower limbs, guiding the patient in performing ankle pump exercises, adhering to medical advice for 30 minutes of intermittent inflation pressure therapy daily, and continuously wearing gradient pressure elastic socks (pressure level I: 16-22 mmHg) around the clock. After ruling out the risk of bleeding on the second day post-surgery, subcutaneous injections of enoxaparin sodium (4000 IU) were administered.
Nutrition support: The nutrition risk screening (NRS) 2002 has been endorsed by European countries as the preferred tool for nutritional risk assessment in hospitalized patients, and its significance has been acknowledged by various societies, including those in the United States and China. Research studies[20] have demonstrated the safety and feasibility of early oral feeding after total gastrectomy. This practice not only promotes the recovery of gastrointestinal function but also enhances post operative nutritional status. The ERAS guidelines emphasize the importance of early postoperative recovery through oral feeding, considering it a safe and crucial aspect of the overall recovery process. In cases where oral intake falls short of the target nutrient level, sequential consideration of enteral nutrition and parenteral nutrition is recommended[21].
The patient in question initiated the use of NRS 2002 for nutritional assessment upon admission, resulting in a score of 3, indicating nutritional risk. Prior to surgery, efforts were made to improve the patient’s nutritional status by enhancing their dietary structure. Six hours after the surgery, glucose infusion was administered via a nasojejunal nutrition tube, progressively transitioning from a liquid diet to oral feeding.
Bleeding: Duodenal resection (PD) is a complex surgical procedure associated with a heightened risk of postoperative complications, notably post-PD hemorrhage (PPH), which stands as a significant risk factor for perioperative mortality. Research findings indicate that the incidence of PPH is approximately 10.11%, with a related mortality rate of 9.72%[22].
Close monitoring of vital signs, careful observation of drainage fluid color and quantity, meticulous care of drainage tubes to ensure unobstructed drainage, vigilant attention to patient test indicators, and prompt notification of doctors in the event of any abnormalities are crucial measures. Early gastrointestinal bleeding is more commonly observed in areas such as the pancreas, gallbladder, gastrointestinal anastomosis, or gastrointestinal stump. In cases of coagulation dysfunction or jaundice, intravenous infusion of vitamin K1, fibrinogen, and other hemostatic drugs may be employed. Additionally, somatostatin and its analogs can be used to reduce gastrointestinal blood supply, lower portal venous pressure, and prevent or treat gastrointestinal and portal venous bleeding. When hemodynamics are stable, an endoscopic examination can be performed to identify the bleeding site, and initial hemostasis treatment may be administered. In cases where endoscopic treatment proves ineffective, or hemodynamics are unstable, timely surgical intervention is recommended. Delayed abdominal bleeding is often secondary to complications like pancreatic fistula and abdominal infection, with severe bleeding being more prevalent. Intervention is the preferred treatment for delayed abdominal bleeding. In situations where abdominal bleeding coexists with pancreatic fistula and abdominal infection, adequate drainage should be ensured during intervention treatment[23].
Pancreatic fistula: A study[24] reveals that the incidence of pancreatic fistula is 15.3%. To address this, it is essential to enhance the monitoring of drainage fluid after pancreatic surgery, with a focus on the concentration of amylase in the drainage fluid, an important monitoring indicator. Starch levels in the drainage fluid should be checked, and the amount of drainage fluid should be closely monitored on postoperative days 1, 3, and 5. A high drainage flow rate indicates increased leakage from the pancreatic stump, which may stimulate the anastomotic opening and increase the risk of pancreatic fistula. Continuous observation of the color change in drainage fluid is crucial. The presence of brown, dark red, or yellow viscous liquid, along with a drainage flow rate exceeding 100 mL/day, should raise alertness to the potential occurrence of pancreatic fistula.
Abdominal pain, often the initial symptom of postoperative pancreatic fistula, requires heightened vigilance during the nursing process. The patient’s abdominal condition should be observed every 2-4 hours, and even mild abdominal pain should be taken seriously. To control infection, the initiation of empirical use of broad-spectrum antibiotics and the conducting of drainage fluid cultivation are recommended. Antibiotic usage should be adjusted based on the results of drug sensitivity tests. Additionally, strategies such as the inhibition of pancreatic exocrine secretion, fasting with water, gastrointestinal decompression, and the application of somatostatin and its analogs should be employed. Nutritional support is crucial for managing post operative pancreatic fistula[23].
Anastomotic fistula: Complete gastrectomy stands out as the primary risk factor for the occurrence of esophagojejunal anastomotic fistula (EJF). Research indicates that the incidence of EJF is 5.8%[25]. This complication is associated with a prolonged hospital stay and can have a negative impact on long-term survival. The management of EJF emphasizes early detection, which is crucial for achieving the best possible outcome[26]. Treatment measures for EJF are categorized into three main groups: Conservative, endoscopic, and surgical. Conservative treatment involves clinical support, the administration of broad-spectrum antibiotics and antifungal therapy to control infection, appropriate drainage, and early initiation of nutritional support[27].
Biliary fistula: The primary principle of treatment for bile fistulas is ensuring smooth drainage[28]. Simultaneously, it is essential to enhance nutritional support and prevent infection, as a significant proportion of bile fistulas can spontaneously heal. If drainage is not optimal or bile accumulates in the abdominal cavity, the placement of a catheter for drainage may be necessary. In cases where non-surgical treatment proves ineffective, another open surgery can be considered. This surgical intervention aims to establish routine external rain age and appropriately repair the fistula.
Abdominal infection: If, after 3 days or more post-surgery, patients exhibit persistent symptoms like chills, high fever, and abdominal distension lasting over 24 hours, along with significant increases in white blood cell count, procalcitonin, and hypersensitive C-reactive protein levels in laboratory examinations, and imaging reveals fluid accumulation in the abdominal cavity, a diagnosis of abdominal infection is plausible. The confirmation of this diagnosis can be achieved by detecting bacteria or fungi in abdominal drainage or puncture fluid. In such cases, it is imperative to initiate early anti-infective treatment promptly. This proactive approach is essential to reduce the mortality rate associated with septic shock[23].
The patient’s postoperative recovery was satisfactory, with no reported abnormalities identified in the abdominal magnetic resonance imaging conducted 3 months after the surgery. Ongoing regular follow-up appointments are being maintained to monitor the patient’s progress.
In the case of the described patient, the preoperative examination conclusively identified both gastric cancer and duodenal adenocarcinoma.
Gastric cancer is a malignant tumor originating from gastric mucosal epithelial cells, with the highest incidence rate in east Asian countries and regions. In China, gastric cancer ranks third among malignant tumors[29]. When gastric cancer patients are found to have other systemic tumors, it is essential to remain vigilant regarding the possibility of multiple in situ cancers.
When considering whether surgery should be performed simultaneously for patients with MPC, factors such as tumor staging, lesion location, surrounding conditions, reconstruction method, lymph node clearance range, and patient tolerance are taken into account. The survival period of patients with MPC is dependent on the most malignant tumor and is not directly related to the number of multiple primary tumors. As a result, some patients may have multiple tumors simultaneously but still manage to survive for an extended period[30].
Pancreatic cancer holds the 8th position in the incidence rate of malignant tumors in men, the 12th in women, and the 6th in the mortality rate of malignant tumors in China, according to the 2016 statistics released by the national cancer center of China in 2022. Pancreatic cancer is challenging to diagnose in the early stages, has a low surgical resection rate, and exhibits highly malignant biological behavior, leading to a poor prognosis. Surgical resection remains the only effective method to provide a cure opportunity and long-term survival for pancreatic cancer. The scope of radical surgery involves the primary tumor and regional lymph node dissection[31].
Primary duodenal carcinoma refers to a malignant tumor originating from the duodenal mucosa epithelium, and its incidence rate is low. For precancerous lesions of the duodenum and early duodenal cancer, endoscopic minimally invasive resection is an important treatment method, with efficacy being determined[32]. However, there is a lack of recommended norms and guidelines for the treatment plan for advanced duodenal carcinoma[33].
Considering the patient’s overall situation, auxiliary examinations, and pathological results, no distant metastasis or severe local invasion of the liver was detected. The hepatobiliary surgery department of our hospital enlisted external department professors to carry out a comprehensive and scientific analysis and evaluation of the patient’s condition. The feasibility of the surgical plan and specific methods was explored through multiple demonstrations. Ultimately, the patient underwent pancreaticoduodenectomy, cholecystectomy, and total gastrectomy successfully.
Case-based nursing management involves the establishment of a dedicated case management team that guides primary nurses in the deliberate and focused execution of their nursing responsibilities. By assessing, monitoring, and ensuring the implementation of health education, this approach guarantees that patients receive effective nursing support throughout their perioperative period. This method not only improves the overall quality of care but also facilitates a quicker recovery for patients following surgery. Upon discharge, the patient was generally cured, with a good overall condition, and no abnormalities were observed in the follow-up examination conducted 3 months post-surgery. Regular follow-up is ongoing.
| 1. | Fu B, Zhang JR, Wu H, Zhang YM. Simultaneous resection of synchronous multiple primary cancers: A case report and literature review. Asian J Surg. 2023;46:1489-1491. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Lyu JM, Xiong HC, Wu B, Zhou XQ, Hu J. [Clinical analysis of 138 multiple primary cancers diagnosed of digestive system malignant tumor initially]. Zhonghua Zhong Liu Za Zhi. 2018;40:147-150. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Wang S, Liu Z, Wang G, Jiang Z, Guan X, Wang X. [Research status of multiple primary carcinoma]. Zhongliu Yanjiu Yu Linchuang. 2018;30:645-648. [DOI] [Full Text] |
| 4. | Yu H, Zha Q, Zhang S, Hu Y, Jin Y, Shen Y, Wang S, Fang Q. [Application research in breast-conserving surgery for breast cancer in the whole process of the whole case management mode]. Chongqing Yixue. 2023;52:508-512, 517. [DOI] [Full Text] |
| 5. | Sun X, Zhong W, Lu J, Zhuang W. Influence of Psychological Nursing Intervention on Psychological State, Treatment Compliance, and Immune Function of Postoperative Patients with Rectal Cancer. J Oncol. 2021;2021:1071490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Tola YO, Chow KM, Liang W. Effects of non-pharmacological interventions on preoperative anxiety and postoperative pain in patients undergoing breast cancer surgery: A systematic review. J Clin Nurs. 2021;30:3369-3384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 7. | Lee HY, Nam ES, Chai GJ, Kim DM. Benefits of Music Intervention on Anxiety, Pain, and Physiologic Response in Adults Undergoing Surgery: A Systematic Review and Meta-analysis. Asian Nurs Res (Korean Soc Nurs Sci). 2023;17:138-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Inukai K, Usui A, Amano K, Kayata H, Mukai N, Tsunetoshi Y, Nakata Y. Perioperative Factors Associated With Respiratory Complications Following Open Abdomen Management. Respir Care. 2020;65:1663-1667. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Chandler D, Mosieri C, Kallurkar A, Pham AD, Okada LK, Kaye RJ, Cornett EM, Fox CJ, Urman RD, Kaye AD. Perioperative strategies for the reduction of postoperative pulmonary complications. Best Pract Res Clin Anaesthesiol. 2020;34:153-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Patman S. Preoperative physiotherapy education prevented postoperative pulmonary complications following open upper abdominal surgery. BMJ Evid Based Med. 2019;24:74-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Qin PP, Jin JY, Wang WJ, Min S. Perioperative breathing training to prevent postoperative pulmonary complications in patients undergoing laparoscopic colorectal surgery: A randomized controlled trial. Clin Rehabil. 2021;35:692-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Chinese Society of Surgery, Chinese Society of Anesthesiology. [Clinical Practice Guidelines for Accelerated Rehabilitation Surgery in China (2021) (IV)]. Xiehe Yixue Zazhi. 2021;12:650-657. [DOI] [Full Text] |
| 13. | Bai D, Xiang W, Chen XZ, Hu JK. [Risk factors of postoperative pulmonary infection of gastric cancer and perioperative intervention measures]. Zhonghua Wei Chang Wai Ke Za Zhi. 2021;24:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Shimoyama H, Sugiyama M, Suzuki Y, Teruya K, Ohki A, Kishiki T, Takeuchi H, Sakamoto Y, Sunami E, Abe N. Bilateral Flank Compression Maneuver for Reducing Pain on Coughing after Abdominal Surgery: A Prospective Study. J Am Coll Surg. 2021;233:459-466.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Expert Committee on Accelerated Rehabilitation Surgery of the Medical Management Center of the National Health Commission; Clinical Pharmacist Expert Committee of the Zhejiang Medical Association; Pharmaceutical Professional Committee of the Zhejiang Pharmaceutical Association Hospital. [Expert Consensus on Clinical Application of Non steroidal Anti inflammatory Drugs in Perioperative Period of Accelerated Rehabilitation Surgery in China]. Zhonghua Putong Waike Zazhi. 2019;34:283-288. [DOI] [Full Text] |
| 16. | Zhu Y, Lin L, Liao H, Zhou J, Liu H, Yu H, Liu L. [Consens us of Perioperative Pain Management Experts in Chinese Thoracic Surgery (2018 Edition)]. Zhongguo Xiongxinxueguan Waike Linchuang Zazhi. 2018;25:921-928. [DOI] [Full Text] |
| 17. | Theochari CA, Theochari NA, Mylonas KS, Papaconstantinou D, Giannakodimos I, Spartalis E, Patelis N, Schizas D. Venous Thromboembolism Following Major Abdominal Surgery for Cancer: A Guide for the Surgical Intern. Curr Pharm Des. 2022;28:787-797. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Bartlett MA, Mauck KF, Stephenson CR, Ganesh R, Daniels PR. Perioperative Venous Thromboembolism Prophylaxis. Mayo Clin Proc. 2020;95:2775-2798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 19. | Segon YS, Summey RD, Slawski B, Kaatz S. Surgical venous thromboembolism prophylaxis: clinical practice update. Hosp Pract (1995). 2020;48:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Lu YX, Wang YJ, Xie TY, Li S, Wu D, Li XG, Song QY, Wang LP, Guan D, Wang XX. Effects of early oral feeding after radical total gastrectomy in gastric cancer patients. World J Gastroenterol. 2020;26:5508-5519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, Laviano A, Ljungqvist O, Lobo DN, Martindale RG, Waitzberg D, Bischoff SC, Singer P. ESPEN practical guideline: Clinical nutrition in surgery. Clin Nutr. 2021;40:4745-4761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 354] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 22. | Gao HQ, Li BY, Ma YS, Tian XD, Zhuang Y, Yang YM. [Risk factors and treatment strategies for postoperative bleeding after pancreaticoduodenectomy]. Zhonghua Xiaohua Waike Zazhi. 2022;21:492-499. [DOI] [Full Text] |
| 23. | Study Group of Pancreatic Surgery in China Society of Surgery of Chinese Medical Association; Pancreatic Disease Committee of China Research Hospital Association; Editorial Board of Chinese Journal of Surgery. [The guideline for prevention and treatment of common complications after pancreatic surgery (2022)]. Zhonghua Wai Ke Za Zhi. 2023;61:1-18. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Paik KY, Oh JS, Kim EK. Amylase level after pancreaticoduodenectomy in predicting postoperative pancreatic fistula. Asian J Surg. 2021;44:636-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Malgras B, Dokmak S, Aussilhou B, Pocard M, Sauvanet A. Management of postoperative pancreatic fistula after pancreaticoduodenectomy. J Visc Surg. 2023;160:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (1)] |
| 26. | Barchi LC, Ramos MFKP, Pereira MA, Dias AR, Ribeiro-Júnior U, Zilberstein B, Cecconello I. Esophagojejunal anastomotic fistula: a major issue after radical total gastrectomy. Updates Surg. 2019;71:429-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Ding X, Zhang C, Li X, Liu T, Ma Y, Yin M, Li C, Zhou G, Wu G. The three-tube method via precise interventional placement for esophagojejunal anastomotic fistula after gastrectomy: a single-center experience. World J Surg Oncol. 2023;21:236. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Xu XS, Wang XA, Chen W, Liu YB. [The treatment strategies for complications after pancreaticoduodenectomy]. Zhonghua Yi Xue Za Zhi. 2022;102:3658-3662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent. 2022;2:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 852] [Cited by in RCA: 951] [Article Influence: 317.0] [Reference Citation Analysis (1)] |
| 30. | Yao HB, Shao QS. [Progress in the diagnosis and treatment of gastric cancer with multiple primary cancers]. Zhonghua YiXue Zazhi. 2020;100:2075-2077. [DOI] [Full Text] |
| 31. | Pancreatic cancer Professional Committee of China Anti Cancer Association. [Guidelines for Integrated Diagnosis and Treatment of pancreatic cancer of China Anti Cancer Association (simplified version)]. Zhongguo Zhongliu Linchuang. 2023;50:487-496. [DOI] [Full Text] |
| 32. | Wang Y, Khizar H, Zhou H, Jin H, Lou Q, Zhang X, Yang J. Endoscopic treatment for early duodenal papillary carcinoma: long-term outcomes. J Gastroenterol Hepatol. 2024;39:1367-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Wang RJ, Xia DD, Liu CY. [Clinical and pathological characteristics, chemotherapy efficacy, and survival analysis of 54 cases of advanced duodenal cancer]. Zhongliu Yaoxue. 2023;13:325-331. [DOI] [Full Text] |