Published online Oct 24, 2024. doi: 10.5306/wjco.v15.i10.1256
Revised: August 10, 2024
Accepted: August 22, 2024
Published online: October 24, 2024
Processing time: 188 Days and 10.4 Hours
In their recent study published in the World Journal of Clinical Cases, the article found that minimally invasive laparoscopic surgery under general anesthesia demonstrates superior efficacy and safety compared to traditional open surgery for early ovarian cancer patients. This editorial discusses the integration of machine learning in laparoscopic surgery, emphasizing its transformative po
Core Tip: Integration of machine learning in laparoscopic surgery revolutionizes patient care, enhancing surgical precision and personalized treatment. Advanced imaging techniques, robotic systems, and virtual reality simulations powered by machine learning algorithms optimize procedural techniques and training methods. However, challenges such as data privacy and algorithm bias must be addressed for responsible deployment. Collaborations between clinicians, engineers, and data scientists drive innovation, shaping a future where minimally invasive surgery is safer, more effective, and accessible to all.
- Citation: Ardila CM, González-Arroyave D. Precision at scale: Machine learning revolutionizing laparoscopic surgery. World J Clin Oncol 2024; 15(10): 1256-1263
- URL: https://www.wjgnet.com/2218-4333/full/v15/i10/1256.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i10.1256
Minimally invasive laparoscopic surgery represents a significant advancement in surgical techniques, offering numerous advantages over traditional open surgery. In this innovative approach, surgeons utilize small incisions and specialized instruments equipped with cameras to perform complex procedures with enhanced precision and efficiency. This transformative method has revolutionized surgical practice across various medical specialties, ranging from general surgery to gynecology and urology[1-3].
One of the most notable benefits of laparoscopic surgery is the reduction in postoperative pain experienced by patients. Unlike open surgery, which involves larger incisions and greater tissue trauma, laparoscopic procedures cause minimal disruption to surrounding tissues, resulting in less pain during the recovery period. Additionally, laparoscopic surgery is associated with shorter hospital stays and faster recovery times compared to traditional open surgery[4-6]. By minimizing tissue trauma and reducing the risk of complications such as infection and blood loss, patients undergoing laparoscopic procedures can typically return to their normal activities sooner, leading to improved quality of life and reduced healthcare costs. Furthermore, unlike open surgery, which requires large incisions to access the surgical site, laparoscopic procedures are performed through small keyhole incisions. These smaller incisions result in less scarring, reduced risk of wound complications, and improved cosmetic outcomes for patients[3-5]. Moreover, laparoscopic surgery is associated with decreased blood loss compared to traditional open surgery. The minimally invasive approach allows for better visualization of blood vessels and precise control of bleeding, leading to improved patient safety and reduced need for blood transfusions. Due to its minimally invasive nature, laparoscopic surgery carries a lower risk of complications such as wound infections, hernias, and adhesions compared to open surgery[1-4]. The smaller incisions and reduced tissue trauma minimize the likelihood of postoperative complications, contributing to better patient outcomes and satisfaction. This innovative approach has transformed surgical practice, enabling surgeons to perform complex procedures with greater precision and efficiency while enhancing patient outcomes and quality of life. As technology continues to advance and techniques evolve, the future of laparoscopic surgery holds promise for further improvements in patient care and surgical innovation[3-6].
Throughout this exploration, the critical role of machine learning in laparoscopic surgery is delved into. It discusses how machine learning algorithms are integrated into the field to improve surgical outcomes, enhance decision-making processes, and optimize procedural techniques. Additionally, it explores how machine learning algorithms enhance imaging techniques like augmented reality and real-time tissue classification, providing clearer visualization of internal structures. Furthermore, it highlights the significance of machine learning in developing personalized treatment plans based on individual patient characteristics, such as anatomy and medical history. It also addresses how machine learning-driven virtual reality simulations and robotic surgical systems offer realistic training opportunities for surgeons. Moving forward, it acknowledges the challenges and ethical considerations associated with integrating machine learning into laparoscopic surgery, including data privacy concerns and algorithm bias. Finally, it discusses future directions and opportunities in the field, emphasizing emerging technologies and potential collaborations to further advance machine learning-driven laparoscopic surgery. Through these discussions, it aims to provide a comprehensive understanding of the transformative impact of machine learning on laparoscopic surgery and its implications for patient care.
Machine learning is rapidly being integrated into the field of laparoscopic surgery, revolutionizing the way surgeons approach procedures, analyze data, and make decisions. By harnessing the power of advanced algorithms and vast datasets, machine learning offers several key benefits for improving surgical outcomes, enhancing decision-making, and optimizing procedural techniques[7-9].
Machine learning algorithms excel at analyzing vast amounts of data to identify patterns, trends, and correlations that may not be apparent to human observers. In laparoscopic surgery, these algorithms can analyze diverse data sources such as patient demographics, preoperative imaging studies, intraoperative video feeds, and postoperative outcomes. By identifying subtle cues and patterns in this data, machine learning algorithms can help surgeons make more informed decisions and anticipate potential complications during procedures[4-7].
Machine learning algorithms can provide real-time decision support to surgeons during laparoscopic procedures. By continuously analyzing intraoperative data such as tissue characteristics, anatomical structures, and instrument movements, these algorithms can offer suggestions and recommendations to guide surgical interventions[6-9]. For example, machine learning algorithms can help identify optimal instrument trajectories, highlight critical structures to avoid, and provide feedback on tissue viability, thereby enhancing surgical precision and reducing the risk of errors.
Machine learning algorithms can leverage patient data to predict surgical outcomes and stratify patients based on their risk profiles. By analyzing factors such as comorbidities, surgical history, and preoperative imaging findings, these algorithms can estimate the likelihood of postoperative complications, such as bleeding, infections, or organ injury. This predictive capability enables surgeons to tailor treatment plans, allocate resources more efficiently, and proactively manage high-risk patients to optimize outcomes[10-13].
Machine learning algorithms can facilitate personalized surgical planning by synthesizing patient-specific data and generating optimized procedural strategies. By considering factors such as anatomical variability, disease pathology, and surgeon preferences, these algorithms can propose customized surgical approaches tailored to individual patient characteristics. This personalized approach enhances surgical efficiency, minimizes intraoperative variability, and maximizes patient safety and satisfaction[9-12].
Machine learning enables continuous learning and improvement in laparoscopic surgery by analyzing outcomes data and iteratively refining surgical techniques. By capturing real-world data from surgical procedures, including intraoperative video recordings, surgical instrument usage, and patient outcomes, machine learning algorithms can identify areas for optimization and adaptation. This iterative learning process fosters innovation, accelerates the dissemination of best practices, and drives continuous improvement in surgical care[8-11].
Overall, the integration of machine learning into laparoscopic surgery holds tremendous promise for improving surgical outcomes, enhancing decision-making, and optimizing procedural techniques. By leveraging advanced algorithms and vast datasets, machine learning empowers surgeons to perform procedures with greater precision, efficiency, and safety, ultimately benefiting patients and advancing the field of minimally invasive surgery[6-9].
Machine learning algorithms are playing a transformative role in enhancing imaging techniques in laparoscopic surgery, enabling surgeons to visualize internal structures with greater clarity, precision, and efficiency. Through the development of advanced imaging modalities such as augmented reality, computer-assisted navigation, and real-time tissue classification, machine learning is revolutionizing the way surgeons perceive and interact with intraoperative imaging data[2,4,8,10].
Machine learning algorithms are facilitating the integration of augmented reality into laparoscopic surgery, allowing surgeons to overlay virtual information onto real-world images of the surgical field. Augmented reality (AR) systems equipped with machine learning capabilities can accurately register preoperative imaging data (such as computed tomography scans or magnetic resonance imaging) with intraoperative video feeds, enabling surgeons to visualize anatomical structures and pathology in real-time.
By superimposing virtual guidance cues, three-dimensional reconstructions, or instrument tracking information onto the laparoscopic view, AR enhances spatial awareness, facilitates navigation, and assists in the precise localization of target structures during complex procedures[4,10,14].
Machine learning algorithms are powering computer-assisted navigation systems that provide real-time guidance and feedback to surgeons during laparoscopic procedures.
These systems leverage preoperative imaging data and intraoperative tracking sensors to create a dynamic surgical navigation environment. By integrating machine learning-based algorithms for image registration, motion tracking, and anatomical segmentation, computer-assisted navigation systems can accurately localize surgical instruments relative to target structures and provide real-time feedback on instrument positioning and trajectory. This enhances surgical precision, minimizes the risk of errors, and enables surgeons to perform complex procedures with greater confidence and efficiency[4,10,15].
Machine learning algorithms are being utilized for real-time tissue classification during laparoscopic surgery, enabling surgeons to differentiate between various tissue types based on their visual characteristics. By analyzing intraoperative video feeds and incorporating features such as color, texture, and morphology, machine learning algorithms can classify tissues (e.g., blood vessels, organs, and tumors) with high accuracy. Real-time tissue classification algorithms provide valuable feedback to surgeons, allowing them to identify critical structures, assess tissue viability, and tailor their surgical approach accordingly. This enhances surgical precision, reduces the risk of inadvertent tissue damage, and facilitates more effective tissue dissection and manipulation[4,10,16].
Overall, the integration of machine learning algorithms into imaging techniques in laparoscopic surgery is revolutionizing the way surgeons visualize and interact with intraoperative data. By enabling augmented reality visualization, computer-assisted navigation, and real-time tissue classification, machine learning is enhancing surgical precision, improving procedural efficiency, and ultimately advancing the field of minimally invasive surgery. As these technologies continue to evolve, they hold promise for further enhancing patient outcomes and shaping the future of laparoscopic surgical practice[12-15].
Machine learning algorithms are revolutionizing the development of personalized treatment plans in laparoscopic surgery by leveraging patient data to predict outcomes, identify potential complications, and tailor surgical approaches based on individual characteristics such as anatomy, pathology, and medical history[14-17]. Through the analysis of diverse datasets encompassing patient demographics, preoperative imaging studies, genetic profiles, and clinical outcomes, machine learning algorithms can extract actionable insights and inform personalized decision-making in the following ways.
Machine learning algorithms can analyze patient data to predict surgical outcomes with a high degree of accuracy. By training on historical data from similar cases, these algorithms can identify patterns and correlations between patient characteristics and postoperative outcomes. For example, machine learning models can predict the likelihood of complications such as bleeding, infections, or organ injury based on factors such as patient age, comorbidities, and preoperative imaging findings. This predictive capability enables surgeons to anticipate potential challenges, optimize perioperative management strategies, and counsel patients effectively about their expected outcomes[11-14].
Machine learning algorithms can identify patients at higher risk of postoperative complications by analyzing their individual risk factors and medical history. By integrating data from electronic health records, imaging studies, and laboratory tests, machine learning models can detect subtle indicators of complications such as impaired tissue perfusion, inflammatory markers, or anatomical anomalies. Early identification of high-risk patients allows for proactive intervention, targeted monitoring, and implementation of preventive measures to mitigate the risk of complications and improve patient safety[12-15].
Machine learning algorithms can tailor surgical approaches based on individual patient characteristics to optimize outcomes and minimize risks.
By analyzing patient anatomy, pathology, and imaging data, machine learning models can recommend customized surgical techniques, instrument selection, and procedural parameters. For example, machine learning algorithms can assist in surgical planning by identifying optimal incision sites, defining anatomical landmarks, and simulating virtual procedures to anticipate potential challenges. This personalized approach enhances surgical precision, reduces intraoperative variability, and maximizes the likelihood of achieving desired outcomes while minimizing the risk of adverse events[12-15].
Machine learning algorithms can provide real-time, adaptive decision support to surgeons during laparoscopic procedures. By continuously analyzing intraoperative data such as tissue characteristics, instrument movements, and patient responses, these algorithms can offer context-sensitive recommendations and feedback to guide surgical interventions. For instance, machine learning models can alert surgeons to deviations from the planned surgical trajectory, highlight critical structures to avoid, and provide guidance on optimal tissue manipulation techniques based on real-time feedback. This adaptive decision support enhances surgical safety, facilitates optimal tissue preservation, and fosters continuous learning and improvement in surgical practice[12-15].
By leveraging advanced analytics and predictive modeling, these algorithms empower surgeons to optimize patient care, enhance surgical precision, and improve outcomes in minimally invasive surgery. As technology continues to advance and datasets expand, machine learning holds promise for further enhancing personalized medicine and driving innovation in surgical practice.
Machine learning-driven virtual reality simulations and robotic surgical systems are revolutionizing surgical skill enhancement and training by providing surgeons with realistic training experiences, personalized learning opportunities, and remote collaboration capabilities. These technologies empower surgeons to refine their skills, master complex procedures, and improve patient outcomes in a safe and controlled environment, ultimately advancing the field of laparoscopic surgery and improving the quality of surgical care worldwide[13-16]. Table 1 depicts the integration of machine learning into surgical skill enhancement and training, emphasizing the principal advantages and capabilities of each technology.
| Technology | Description | Advantages |
| Virtual reality simulations | Machine learning-driven virtual reality simulations replicate real-life surgical scenarios in a highly immersive and interactive environment | Practice procedural techniques, refine skills, and familiarize with complex anatomy without patient involvement. Enhance realism with dynamic tissue behavior, haptic feedback, and adaptive learning algorithms. Improve proficiency, coordination, and muscle memory in a risk-free environment |
| Robotic surgical systems | Robotic surgical systems integrate machine learning algorithms to augment capabilities and enhance surgeon performance | Offer intuitive interfaces, precise control, and enhanced visualization. Analyze intraoperative data to optimize performance and outcomes. Facilitate hands-on training, skill assessment, and proficiency evaluation. Suitable for surgeons at all experience levels, from novice to expert |
| Personalized training curriculum | Machine learning algorithms tailor training curricula to individual learning needs and skill levels | Analyze trainee performance data to provide targeted feedback and recommendations. Optimize learning outcomes and accelerate skill acquisition. Focus on areas of weakness and provide opportunities for deliberate practice |
| Remote training and collaboration | Machine learning-enabled virtual reality and robotic systems support remote training and collaboration | Participate in training sessions, workshops, and simulations from anywhere in the world. Foster knowledge exchange and skill transfer among surgeons globally. Enhance accessibility to high-quality surgical education and facilitate continuous professional development. Promote interdisciplinary collaboration and remote learning opportunities |
Acknowledging the challenges and ethical considerations associated with integrating machine learning into laparoscopic surgery is crucial for ensuring the responsible and ethical deployment of these technologies.
Laparoscopic surgery generates vast amounts of sensitive patient data, including medical records, imaging studies, and intraoperative video feeds. Ensuring the privacy and security of this data is paramount to protect patient confidentiality and comply with regulatory requirements such as Health Insurance Portability and Accountability Act. Machine learning algorithms require access to large and diverse datasets for training and validation. However, the use of patient data for algorithm development raises concerns about data privacy, consent, and potential risks of data breaches or unauthorized access[4,10].
Machine learning algorithms are susceptible to biases inherent in the data used for training. Biases in the data can lead to algorithmic biases that perpetuate disparities or inequalities in patient care. In the context of laparoscopic surgery, algorithmic biases could manifest in differential treatment recommendations, diagnostic errors, or disparities in access to surgical interventions based on factors such as race, ethnicity, or socioeconomic status[4,10].
The integration of machine learning algorithms into medical devices and surgical workflows is subject to regulatory oversight by agencies such as the Food and Drug Administration or European Medicines Agency. Obtaining regulatory approval for machine learning-based medical devices or software applications requires rigorous validation of safety, efficacy, and performance. Navigating the regulatory pathway can be time-consuming, resource-intensive, and challenging due to the evolving nature of machine learning technologies[4,10,18,19].
Machine learning models in laparoscopic surgery must undergo continuous validation and refinement to ensure their reliability, generalizability, and clinical utility. Validating machine learning models involves testing their performance on diverse patient populations, assessing robustness to variations in data acquisition, and evaluating real-world effectiveness in clinical practice. Ongoing refinement of machine learning models is necessary to address issues such as overfitting, drift in performance over time, and adaptation to evolving clinical practices or technological advancements[13-16].
Machine learning algorithms are often regarded as "black boxes" due to their complex and opaque decision-making processes. Lack of transparency and interpretability in algorithmic outputs can undermine trust, hinder clinical adoption, and raise ethical concerns. In laparoscopic surgery, transparent and interpretable machine learning models are essential for enabling surgeons to understand the rationale behind algorithmic recommendations, verify their accuracy, and make informed clinical decisions[14-17].
Addressing these challenges and ethical considerations requires a multidisciplinary approach involving clinicians, data scientists, ethicists, policymakers, and regulatory authorities. Collaborative efforts are needed to develop ethical guidelines, regulatory frameworks, and best practices for the responsible integration of machine learning into laparoscopic surgery, ensuring that these technologies enhance patient care while upholding ethical principles and safe
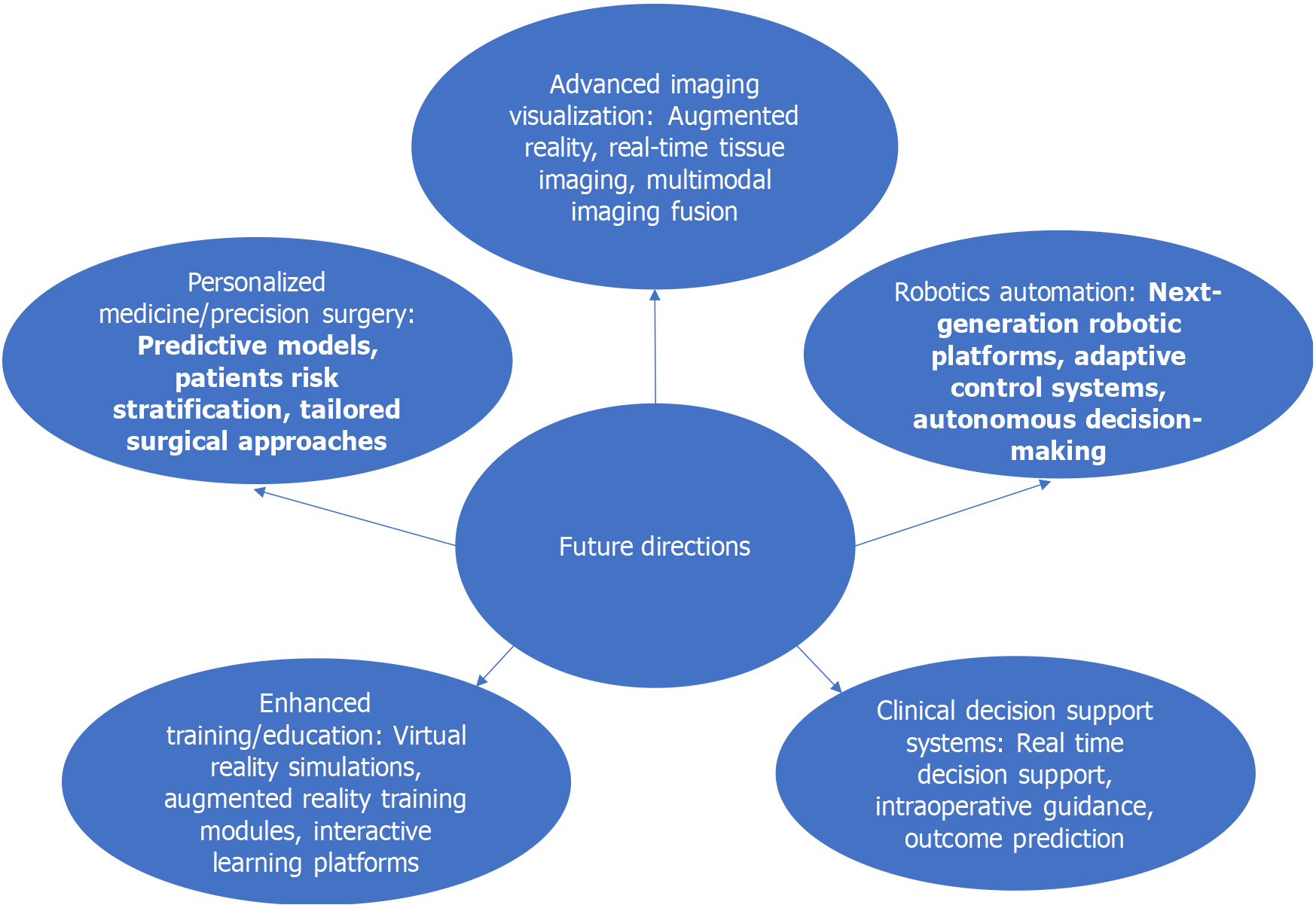
The future of machine learning-driven laparoscopic surgery holds tremendous promise for advancing surgical practice and improving patient outcomes. As technology continues to evolve and interdisciplinary collaborations flourish, several key directions and opportunities emerge for further innovation and progress in the field.
Emerging technologies such as augmented reality, real-time tissue imaging, and multimodal imaging fusion hold the potential to revolutionize visualization in laparoscopic surgery. Collaborations between engineers, imaging specialists, and surgeons can drive the development of novel imaging modalities that provide enhanced visualization of anatomical structures, facilitate real-time tissue characterization, and improve surgical navigation.
Robotics and automation are poised to play an increasingly prominent role in laparoscopic surgery, enabling precise instrument control, dexterity, and ergonomic advantages for surgeons. Collaborations between robotic engineers, software developers, and surgical teams can lead to the development of next-generation robotic platforms with advanced machine learning algorithms for adaptive control, predictive analytics, and autonomous decision-making.
Machine learning algorithms offer opportunities for personalized treatment planning, patient risk stratification, and tailored surgical approaches based on individual patient characteristics. Collaborations between clinicians, data scientists, and geneticists can leverage patient data to develop predictive models, identify biomarkers, and optimize treatment strategies for personalized medicine in laparoscopic surgery.
Virtual reality simulations, augmented reality training modules, and interactive learning platforms powered by machine learning algorithms can revolutionize surgical education and training. Collaborations between educators, simulation specialists, and technology developers can create immersive training environments, personalized learning pathways, and remote training opportunities to enhance surgical proficiency and optimize patient safety.
Machine learning-driven clinical decision support systems can assist surgeons in real-time decision-making, intraoperative guidance, and outcome prediction. Collaborations between clinicians, data scientists, and informaticians can develop intelligent decision-support tools that integrate seamlessly into surgical workflows, provide actionable insights, and improve clinical outcomes.
As depicted in Figure 1, the future of laparoscopic surgery is poised for remarkable advancements across various domains. From advanced imaging and visualization techniques to personalized medicine and precision surgery, innovative technologies driven by machine learning hold the potential to revolutionize surgical practice. Let's explore the opportunities and directions shaping the future of minimally invasive surgery.
The future of machine learning-driven laparoscopic surgery is characterized by interdisciplinary collaborations, technological advancements, and a commitment to advancing patient care. By harnessing the collective expertise of clinicians, engineers, and data scientists, we can unlock new frontiers in surgical innovation, optimize treatment outcomes, and pave the way for a future where minimally invasive surgery is safer, more effective, and accessible to all.
| 1. | Kolbinger FR, Rinner FM, Jenke AC, Carstens M, Krell S, Leger S, Distler M, Weitz J, Speidel S, Bodenstedt S. Anatomy segmentation in laparoscopic surgery: comparison of machine learning and human expertise - an experimental study. Int J Surg. 2023;109:2962-2974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Zygomalas A, Kalles D, Katsiakis N, Anastasopoulos A, Skroubis G. Artificial Intelligence Assisted Recognition of Anatomical Landmarks and Laparoscopic Instruments in Transabdominal Preperitoneal Inguinal Hernia Repair. Surg Innov. 2024;31:178-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 3. | Zhao Z, Chen Z, Voros S, Cheng X. Real-time tracking of surgical instruments based on spatio-temporal context and deep learning. Comput Assist Surg (Abingdon). 2019;24:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Sone K, Tanimoto S, Toyohara Y, Taguchi A, Miyamoto Y, Mori M, Iriyama T, Wada-Hiraike O, Osuga Y. Evolution of a surgical system using deep learning in minimally invasive surgery (Review). Biomed Rep. 2023;19:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 5. | Cai ZH, Zhang Q, Fu ZW, Fingerhut A, Tan JW, Zang L, Dong F, Li SC, Wang SL, Ma JJ. Magnetic resonance imaging-based deep learning model to predict multiple firings in double-stapled colorectal anastomosis. World J Gastroenterol. 2023;29:536-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Qin X, Chen C, Liu Y, Hua XH, Li JY, Liang MJ, Wu F. Efficacy and safety of minimally invasive laparoscopic surgery under general anesthesia for ovarian cancer. World J Clin Cases. 2024;12:1569-1577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Wang X, Li Y, Kwok KW. A Survey for Machine Learning-Based Control of Continuum Robots. Front Robot AI. 2021;8:730330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Wagner M, Bihlmaier A, Kenngott HG, Mietkowski P, Scheikl PM, Bodenstedt S, Schiepe-Tiska A, Vetter J, Nickel F, Speidel S, Wörn H, Mathis-Ullrich F, Müller-Stich BP. A learning robot for cognitive camera control in minimally invasive surgery. Surg Endosc. 2021;35:5365-5374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Jung WJ, Kwak KS, Lim SC. Vision-Based Suture Tensile Force Estimation in Robotic Surgery. Sensors (Basel). 2020;21:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Yang C, Zhao Z, Hu S. Image-based laparoscopic tool detection and tracking using convolutional neural networks: a review of the literature. Comput Assist Surg (Abingdon). 2020;25:15-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Xu K, Chen Z, Jia F. Unsupervised binocular depth prediction network for laparoscopic surgery. Comput Assist Surg (Abingdon). 2019;24:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | López-Casado C, Bauzano E, Rivas-Blanco I, Pérez-Del-Pulgar CJ, Muñoz VF. A Gesture Recognition Algorithm for Hand-Assisted Laparoscopic Surgery. Sensors (Basel). 2019;19:5182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Bareum Choi, Kyungmin Jo, Songe Choi, Jaesoon Choi. Surgical-tools detection based on Convolutional Neural Network in laparoscopic robot-assisted surgery. Annu Int Conf IEEE Eng Med Biol Soc. 2017;2017:1756-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Padovan E, Marullo G, Tanzi L, Piazzolla P, Moos S, Porpiglia F, Vezzetti E. A deep learning framework for real-time 3D model registration in robot-assisted laparoscopic surgery. Int J Med Robot. 2022;18:e2387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Luo H, Yin D, Zhang S, Xiao D, He B, Meng F, Zhang Y, Cai W, He S, Zhang W, Hu Q, Guo H, Liang S, Zhou S, Liu S, Sun L, Guo X, Fang C, Liu L, Jia F. Augmented reality navigation for liver resection with a stereoscopic laparoscope. Comput Methods Programs Biomed. 2020;187:105099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | Baltussen EJM, Kok END, Brouwer de Koning SG, Sanders J, Aalbers AGJ, Kok NFM, Beets GL, Flohil CC, Bruin SC, Kuhlmann KFD, Sterenborg HJCM, Ruers TJM. Hyperspectral imaging for tissue classification, a way toward smart laparoscopic colorectal surgery. J Biomed Opt. 2019;24:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 17. | Johnston SS, Morton JM, Kalsekar I, Ammann EM, Hsiao CW, Reps J. Using Machine Learning Applied to Real-World Healthcare Data for Predictive Analytics: An Applied Example in Bariatric Surgery. Value Health. 2019;22:580-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | O'Sullivan OE, O'Reilly BA. Robot-assisted surgery:--impact on gynaecological and pelvic floor reconstructive surgery. Int Urogynecol J. 2012;23:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Becker CM, Bokor A, Heikinheimo O, Horne A, Jansen F, Kiesel L, King K, Kvaskoff M, Nap A, Petersen K, Saridogan E, Tomassetti C, van Hanegem N, Vulliemoz N, Vermeulen N; ESHRE Endometriosis Guideline Group. ESHRE guideline: endometriosis. Hum Reprod Open. 2022;2022:hoac009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 687] [Article Influence: 229.0] [Reference Citation Analysis (0)] |