Published online Sep 24, 2023. doi: 10.5306/wjco.v14.i9.335
Peer-review started: June 21, 2023
First decision: August 10, 2023
Revised: August 17, 2023
Accepted: August 29, 2023
Article in press: August 29, 2023
Published online: September 24, 2023
Processing time: 90 Days and 13.2 Hours
Breast cancer is the most common malignancy in women worldwide. Triple-negative breast cancer (TNBC), refers breast cancer negative for estrogen receptor, progesterone receptor and human epidermal growth factor receptor 2, characterized by high drug resistance, high metastasis and high recurrence, treatment of which is a difficult problem in the clinical treatment of breast cancer. In order to better treat TNBC clinically, it is a very urgent task to explore the mechanism of TNBC resistance in basic breast cancer research. Pregnane X receptor (PXR) is a nuclear receptor whose main biological function is to participate in the meta
Core Tip: Treatment of triple-negative breast cancer (TNBC) is a difficult problem in the clinical treatment of breast cancer. It is a very urgent task to explore the mechanism of TNBC resistance in basic breast cancer research. Pregnane X receptor (PXR) is a nuclear receptor whose main biological function is to participate in the metabolism, transport and clearance of allobiological agents in PXR. This reviews synthesized the important role of PXR in the process of high drug resistance to TNBC chemotherapeutic drugs and related research progress.
- Citation: Rao ZZ, Tang ZW, Wen J. Advances in drug resistance of triple negative breast cancer caused by pregnane X receptor. World J Clin Oncol 2023; 14(9): 335-342
- URL: https://www.wjgnet.com/2218-4333/full/v14/i9/335.htm
- DOI: https://dx.doi.org/10.5306/wjco.v14.i9.335
Cancer and cardiovascular disease are the two leading causes of death in the world, which seriously endanger people’s physical and mental health[1]. In recent years, the incidence of cancer has been showing an upward trend worldwide, and the growth rate and mortality rate of breast cancer in women are grim[2]. According to the overall cancer data in the world in 2020[3], breast cancer has exceeded lung cancer to become the number one malignant tumor in the world, accounting for 11.7% of all different types of cancer. The incidence and mortality of breast cancer rank the first in most countries in the world. Literature reports that in 2020, the number of new breast cancer cases in the world was more than 2.26 million, and the number of deaths reached 685000, among which Chinese patients accounted for 18.4% of all cases in the world[4]. Therefore breast cancer has become the most threatening malignant tumor that endangers women’s health.
According to the different express of estrogen receptor (ER), human epidermal growth factor receptor 2 (HER-2), progesterone receptor (PR), and insufficient expression of proliferating cell nuclear antigen-67, breast cancer have been classified into several subtypes, these include: Luminal A, HER-2 overexpression, Luminal B and triple negative[5]. In all kinds of breast cancer, the type of breast cancer which is negative for PR, ER, and HER-2 is called triple-negative breast cancer (TNBC). It accounts for 10% to 20% of all types of breast cancer[6] and occurs mostly in young women[7]. TNBC mainly metastasize to the lung and brain, and its own biological characteristics make it have poor response to general local treatment and poor prognosis[8]. Although there have been great breakthroughs in the treatment of breast cancer recently, the treatment of advanced metastatic breast cancer (especially TNBC) is still a great clinical challenge. Although there are so many different subtypes in breast cancer, TNBC is the most clinically complex subtype to treat. Because the lackness of effective molecular targets, theraputic attempts for non-TNBC, such as endocrine therapy and HER2-targeted therapy, cannot benefit TNBC patients[9]. Poly (ADP-ribose) polymerase inhibitors and immune checkpoint-based immunotherapy have made important progress in preclinical and clinical research[10]. However, although these treatment strategies can benefit some patients, the overall benefit of all TNBC patients is still very limited. At present, chemotherapy is still an important treatment for TNBC[11]. However, TNBC is not all sensitive to chemotherapy, and the main reason for the failure of chemotherapy is the resistance of TNBC to chemotherapy[12]. In summary, this type of breast cancer is characterized by high degree of deterioration, high recurrence rate, high metastasis rate and low survival rate. It is particularly important to study the mechanism of chemotherapy resistance[13].
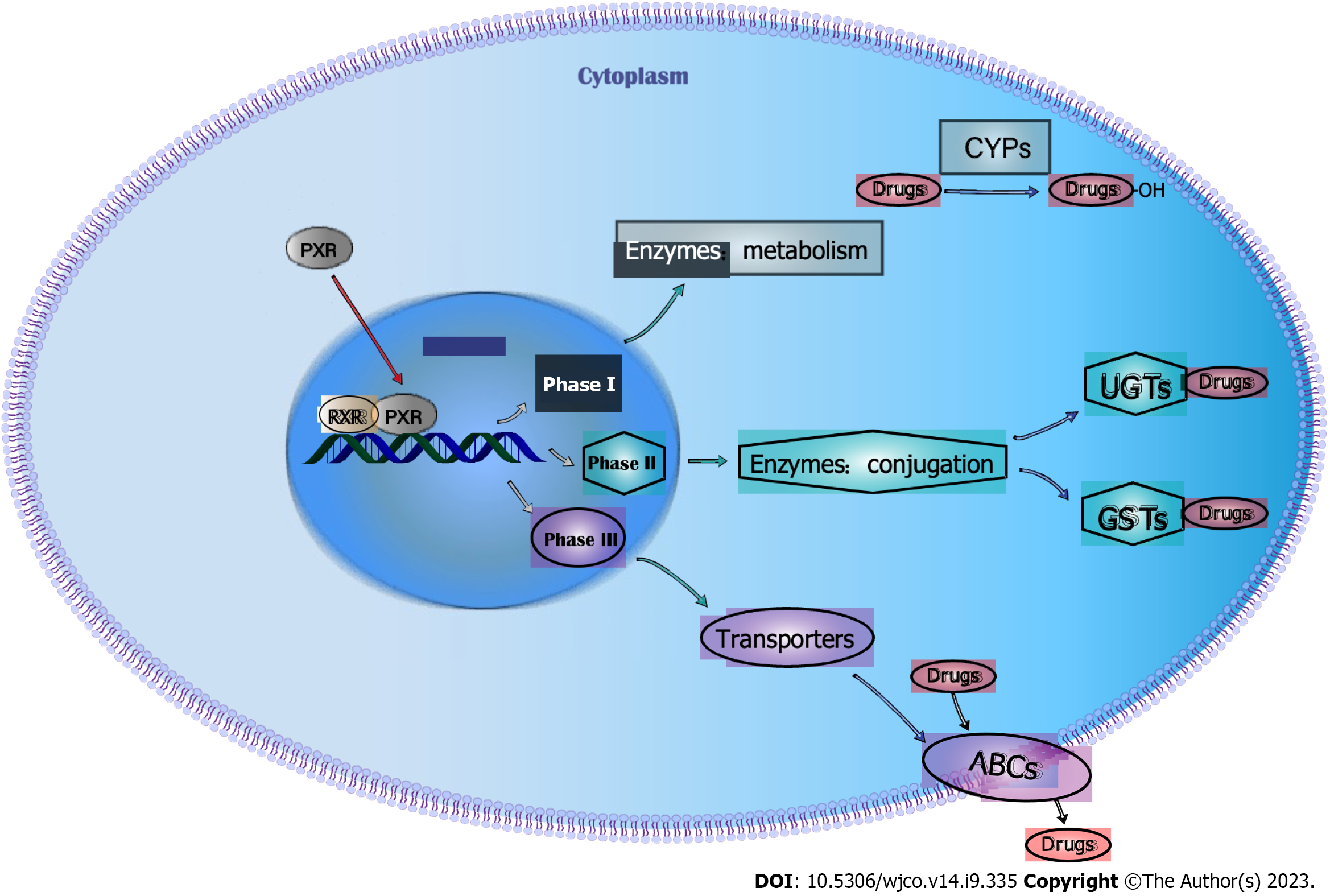
In 1998, when Kliewer et al[14] searched the mouse liver HHMI EST database, they found a sequence with high homology to the known nuclear receptor, and the protein encoded by this sequence can be activated by a series of natural or synthetic pregnane hormones, so they named it pregnane X receptor (PXR). Human PXR is expressed by the nuclear receptor subfamily 1 group I member 2 gene, located on chromosome 3q13-21, and consists of 10 exons and 9 introns, with a gene size of approximately 40 kb. In contrast to other nucleoid receptors, PXR possesses a large and somewhat flexible spherical ligand-binding domain, allowing it to bind a large number of compounds of different sizes and structures. Phosphorylation of residues at positions T248, Y249, and T422 of PXR is required for its ligand-activated function[15]. When PXR binds to its ligand, its conformation changes and activates the PXR pathway, which causes PXR to translocate from the cytoplasm to the nucleus and bind to the retinal X receptor to form a heterodimer, which in turn combine with the DNA response elements in the target gene’s specific promoter region to regulate their transcription[16]. The main biological function of PXR is to participate in the metabolism, transport and clearance of xenobiotics including chemotherapeutic drugs[17]. There are three phases involved in the metabolic process of PXR: Phase I, metabolizing enzymes; Phase II, conjugating enzymes; phase III, transporter[18] (Figure 1).
Although PXR is mainly expressed in liver, intestinal and colon tissues, it has been found that it is also expressed in normal breast tissues, and its expression level is even higher in breast cancer tissues[19]. PXR can affect the expression of drug resistance-related genes, thereby enhancing the metabolism and clearance function of chemotherapy drugs in cancer cells[20], and then plays an important role in breast cancer[21]. Studies have shown that the expression of PXR increased in docetaxel-resistant TNBC cells and tumor xenograft mice[22]. This article reviews the role of PXR in the drug resistance mechanism of TNBC.
Drug metabolizing enzymes refers a special kind of enzymes, which responsible for the metabolism function of a variety of substances such as exogenous chemicals and endogenous biological small molecules. Cytochrome P450 (CYP) is an important enzyme system involved in the metabolism of xenobiotics in cells. CYP was first discovered in rat liver microsomes in 1958[23]. CYP is named for its typical absorption peak at 450 nm wave length[24]. The rules for CYP nomenclature include: Different numbers after the family represent different families, different letters after the family represent different subfamilies, and different numbers after the subfamily represent different peptides[25]. There are 18 CYP families in human body, including 26 subfamilies and more than 50 different isoforms with catalytic functions[26]. Three families, CYP1, CYP2 and CYP3, account for nearly 70% of the human CYP family and response for most drugs’ metabolism progress. It is the dominant superfamily enzyme system not only involved in the drug metabolism phase I, but also affected drug oxidation, reduction or hydrolysis[27]. For patients with liver cancer, clarifying the expression information of CYP, strengthening the monitoring of medication, adjusting the dose and frequency of drugs, and reducing drug resistance and side effects are of great significance for the precise treatment of anticancer drugs[28].
It is demonstrated by Murray et al[29] that CYP2S1, CYP4V2, CYP3A4, and CYP26A1 were connected to the final survive rate of breast cancer patients, which also indicated the potential of CYP as a marker for the clinical results of breast cancer patients. A large number of studies have shown that CYP enzymes are related to breast cancer drug metabolism. Among them, CYP enzymes have been experimentally confirmed to be: CYP3A4, CYP3A5, CYP2C8, CYP2C9, CYP2J2, CYP1A1, CYP1B1, CYP17A1, CYP2B6, CYP2D6, CYP2C19, etc[30-33]. Alexanian et al[34] reported the lower expressions of CYP4A11 and CYP4A22 in normal breast tissues than those in TNBC tissues. Overexpression of CYP3A4 can promote the metabolism of docetaxel in triple negative breast cancer stem cells and further induce reduced accumulation of chemotherapy drugs in cancer cells, leading to cell drug resistance[22]. Two major metabolic enzymes of paclitaxel (CYP2C8, CYP3A4) and other genes involved in taxane heterogenic metabolism (e.g., CYP1B1) are associated with drug resistance in TNBC[35]. Numerous experiments have shown that CYP enzymes are significantly upregulated in TNBC patients [22,29,35]. Therefore, the association between CYP enzymes and tumor resistance in TNBC has attracted increasing attention.
It has been reported that activated PXR can transcriptically up-regulate the expression of CYP450 family members such as CYP3A4, CYP3A23, CYP2B6, CYP2B9, CYP2C55, CYP2C9 and CYP1A[36,37]. In experimental studies related to TNBC drug resistance, it has been confirmed that PXR can regulate the expression of CYP3A4, resulting in increased drug metabolism in TNBC, which is obviously related to TNBC chemotherapy resistance[22].
Conjugation enzymes in phase II of drug metabolism are mainly various transferases, such as glutathione transferase (GST) and uridine diphosphate glucuronosyltransferase (UGT)[30]. GST, as an important part of the detoxification system of the body, is responsible for catalyzing the combination of glutathione and drugs, and expelling the conjugate from the body under the action of multidrug resistant-related proteins, all of above made GST plays a detoxification role[38]. UGT is the most important enzyme involved in human phase II of drug metabolism, and about 40%-70% of drugs and traditional Chinese medicine are metabolized by UGT[39]. UGT and GST can make exogenous harmful substances into water-soluble harmless small molecular substances, and then excreted in the form of bile and urine.
In 1978, Lawrence et al[40] found that there was a glutathione peroxidase without selenium in the liver tissue of mice, named GST. The GST family plays a crucial role in cellular defense by catalyzing the coupling reaction of carcinogens to glutathione, thereby preventing cell damage. Any mutation in the gene that expresses this enzyme may alter the catalytic process, which in turn can alter drug bioavailability and may amplify or reduce drug efficacy and toxicity[41]. Multidrug resistance (MDR) mediated by the overexpression of GST is the main cause of chemotherapy failure in breast cancer[42]. Compared with non-TNBC cells, GSTP1 expression is higher in TNBC, and GSTP1 plays a crucial role in the chemoresistance of TNBC cells[43]. In GSTA1-overexpressing cancer cells, an unexpected lack of chemotherapeutic agents leads to enhanced cytotoxicity[44]. Overexpression of GSTA2 protects cancer cells from apoptosis can also induced by chemotherapeutic agents[45]. Upregulation of GSTA2 is associated with doxorubicin resistance[46]. A case-control study, which investigated children suffered acute lymphoblastic leukemia treated with different anticancer agents (vincristine, daunorubicin, cytarabine, etc.), showed that GSTM1 deficiency reduced the risk of recurrence by 18 times[47]. In addition, low survival rate was observed in patients with high GSTM1 expression who received high-dose cyclophosphamide, carmustine and cisplatin as initial chemotherapy for breast cancer[48]. Clearly, GST family is associated with drug resistance of breast cancer, and it also involved in the drug resistance of TNBC.
UGTs are a superfamily, so named because they mainly utilize uridine diphosphate glucuronic acid as a glycosyl donor. UGT catalyzes the binding of the substrate to the uridine diphosphate glucuronate group, making it more hydrophilic and conducive to elimination from the body. The human UGT superfamily is divided into two families based on nucleotide sequence similarity: UGT1A and UGT2[49]. The UGT1A gene cluster, encoded by a gene cluster located at 2q37, contains a total of 17 exons. UGT1A enzymes, especially UGT1A1, have been shown to be overexpressed in tumor tissues and play a role in anticancer drug resistance[50], as well as in TNBC[51]. Overexpression of UGT1A6 counteracts the cytotoxicity caused by the breast cancer chemotherapy drug methotrexate[52]. UGT2B7 can induce epirubicin resistance in breast cancer cells[53]. To sum up that UGT, as a conjugation enzyme in phase II of drug metabolism, plays a important role in breast cancer resistance. Although there are few reports on UGT family in TNBC, the only reports can also illustrate the role of UGT in tumor resistance.
Among the conjugated enzymes in phase II of drug metabolism, the target genes of PXR have been found to include UGT1A1, UGT1A6, UGT1A3, UGT1A4 and GSTA1, GSTA2, GSTA3, GSTM1, GSTM2, GSTM3, GSTM4[30]. The mechanism of which PXR regulates UGT and GST, further lead to drug resistance in TNBC may be one of the drug resistance mechanisms, but due to the lack of relevant reports, more experiments are needed to prove it.
The transporters in phase Ⅲ of drug metabolism are mainly adenosine triphosphate binding cassette (ABC) membrane transporters, including MDR protein, multidrug resistation-associated protein (MRP) and breast cancer resistance protein (BCRP), which are mainly involved in drug transport and clearance[54].
ABC membrane transporters affect the therapeutic effect of drugs on malignant tumors by affecting the absorption and metabolism of drugs in cells. ABC transporters use adenosine triphosphate to efflux various compounds, including chemotherapeutic drugs of different structures and properties. A variety of ABC transporters are closely related to chemotherapy resistance of solid tumors including breast cancer, and increased drug efflux mediated by ABC transporters is the most common mechanism of MDR caused by drug efflux[55]. The ABC family of membrane transporters includes seven isoforms (ABCA-ABCG), among which the MDR protein 1 (MDR1/P-gp) gene is a membrane transporter encoded by the ABCB1 gene, with a relative molecular weight of 170 KDa, composed of 1280 amino acids, and located on the cell membrane. The energy released by ATP hydrolysis can be used to transport the hydrophobic and lipophilic drugs outside the cell, when MDR1/P-gp is overexpressed, drug efflux is increased through the role of efflux pump, thereby reducing the accumulation of drugs in cells and the effect of drugs on cells, thus causing drug resistance in tumor cells[56]. Overexpression of MDR has become an important mechanism of drug resistance mediated by TNBC, which is associated with poor outcome, reduced survival rate and chemoresistance of patients[57]. The MRP gene is a membrane transporter encoded by the ABCC gene, whereas BCRP is a membrane transporter encoded by the ABCG gene. In breast cancer related studies, ABCC1, ABCC3, ABCB1 and ABCG2 are associated with drug resistance [22,30,33]. Compared with other breast cancer subtypes, tmultidrug resistance protein-1 (ABCC1/MRP1), MDR protein-8 (ABCC11/MRP8) and BCRP (ABCG2/BCRP) is significantly overexpressed in TNBC[58,59], which is closely related to chemot
PXR regulates a variety of proteins, including MDR protein (ABCB1, ABCB2), MDR associated protein (ABCC2, ABCC3, ABCC4, ABCC5) and so on. These enzymes are mainly bile acid transporters, which mediate the metabolism and excretion of bile acids, as well as the transmembrane transport and clearance of chemotherapeutic drugs[61]. Overexpression of PXR leads to increased cellular levels of resistance proteins such as ABCC1 and ABCG2[62,63]. Studies have also shown that PXR-mediated induction of ABCC2 seems to be involved in chemotherapy resistance in tamoxifen-resistant breast cancer [64,65]. PXR has been confirmed to regulate two membrane transporters ABCB1 and ABCG2 in TNBC[66]. Clearly, PXR-mediated upregulation of ABC membrane transporter family expression in TNBC cancer patients is one of the mechanisms of chemotherapy resistance in TNBC.
In conclusion, although PXR is mainly expressed in liver, intestinal and colon tissues, it is also expressed in normal breast tissues, and its expression level is even higher in breast cancer tissues[67-70]. PXR is associated with the phenotype of TNBC and is a powerful and independent poor prognostic factor[71]. PXR can accelerate the metabolism and clearance of chemotherapy drugs in TNBC through the regulation of three phases of the metabolism of chemotherapy drugs: phase I drug metabolism enzymes CYPs, phase II drug binding enzymes GSTs and UGTs, and phase III drug transporter ABCs, thus resulting in drug resistance (Table 1). Among them, experiments have confirmed that PXR can regulate the expression of CYP3A4, ABCC1, and ABCG2 in TNBC, resulting in TNBC drug resistance. In the future, researchers should focus on improving our understanding of the mechanism of PXR in TNBC drug resistance, including regulation of PXR and function of PXR independence of drug metabolism.
| Phase | Resistance-associated proteins associated with PXR | Resistance-associated proteins associated with breast cancer | Resistance associated proteins associated with TNBC | Resistance related proteins known to be regulated by PXR in TNBC | Possible regulatory targets of PXR in TNBC (unconfirmed) |
| Phase Ⅰ | CYP3A4, CYP3A23 | CYP3A4, CYP3A5 | CYP3A4 | CYP3A4 | CYP2C8 |
| Enzymes metabolism | CYP3A11, CYP2B6 | CYP2C8, CYP2C9 | CYP4A11 | ||
| CYPs | CYP2C8, CYP2C9 | CYP2J2, CYP1A1 | CYP4A22 | ||
| CYP2C19, CYP1A | CYP1B1, CYP17A1 | CYP2C8 | |||
| CYP2B9, CYP2C55 | CYP2B6, CYP2D6 | CYP1B1 | |||
| CYP2C19, CYP2S1 | |||||
| CYP4V2, CYP26A1 | |||||
| CYP4A11, CYP4A22 | |||||
| Phase Ⅱ | GSTA1, GSTA2 | GSTM1, GSTP1 | GSTP1 | ||
| Enzymes conjugation | GSTA3, GSTM1 | GSTA1, GSTA2 | |||
| GSTs | GSTM2, GSTM3 | ||||
| GSTM4 | |||||
| UGTs | UGT1A1, UGT1A6 | UGT1A, UGT2B7 | UGT1A1 | UGT1A1 | |
| UGT1A3, UGT1A4 | |||||
| Phase Ⅲ | ABCB1, ABCB2 | ABCC1, ABCC3 | ABCC1 | ABCC1 | |
| Ttansporters | ABCC1, ABCC2 | ABCB1, ABCG2 | ABCG2 | ABCG2 | |
| ABCs | ABCC3, ABCC4 | ABCC11 | ABCC11 | ||
| ABCC5, ABCG2 |
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kukongviriyapan V, Thailand; PhD CKP, India S-Editor: Qu XL L-Editor: A P-Editor: Zhang XD
| 1. | Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-3030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 924] [Cited by in RCA: 1236] [Article Influence: 309.0] [Reference Citation Analysis (0)] |
| 2. | Chong FF, Yin LY, Liu J, Guo J, Fan Y, Zhang ML, Zhang L, He MY, Zhang HM. Malnutrition increases therisk of mortality in hospitalized lung cancer patients. J NutrOncol. 2022;7: 49-57. |
| 3. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64618] [Article Influence: 16154.5] [Reference Citation Analysis (176)] |
| 4. | Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond). 2021;41:1037-1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 584] [Article Influence: 146.0] [Reference Citation Analysis (0)] |
| 5. | Burstein HJ, Curigliano G, Thürlimann B, Weber WP, Poortmans P, Regan MM, Senn HJ, Winer EP, Gnant M; Panelists of the St Gallen Consensus Conference. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol. 2021;32:1216-1235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 442] [Cited by in RCA: 499] [Article Influence: 124.8] [Reference Citation Analysis (0)] |
| 6. | Coughlin SS. Epidemiology of Breast Cancer in Women. Adv Exp Med Biol. 2019;1152:9-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 202] [Article Influence: 33.7] [Reference Citation Analysis (2)] |
| 7. | Yu KD, Ye FG, He M, Fan L, Ma D, Mo M, Wu J, Liu GY, Di GH, Zeng XH, He PQ, Wu KJ, Hou YF, Wang J, Wang C, Zhuang ZG, Song CG, Lin XY, Toss A, Ricci F, Shen ZZ, Shao ZM. Effect of Adjuvant Paclitaxel and Carboplatin on Survival in Women With Triple-Negative Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6:1390-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 146] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 8. | Zhou YD, Li J, Du L, Mahdi F, Le TP, Chen WL, Swanson SM, Watabe K, Nagle DG. Biochemical and Anti-Triple Negative Metastatic Breast Tumor Cell Properties of Psammaplins. Mar Drugs. 2018;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Yin L, Duan JJ, Bian XW, Yu SC. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020;22:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 1406] [Article Influence: 281.2] [Reference Citation Analysis (0)] |
| 10. | Lee A, Djamgoz MBA. Triple negative breast cancer: Emerging therapeutic modalities and novel combination therapies. Cancer Treat Rev. 2018;62:110-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 256] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 11. | Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13:674-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1971] [Article Influence: 219.0] [Reference Citation Analysis (0)] |
| 12. | Nedeljković M, Damjanović A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 382] [Cited by in RCA: 539] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 13. | Singh DD, Yadav DK. TNBC: Potential Targeting of Multiple Receptors for a Therapeutic Breakthrough, Nanomedicine, and Immunotherapy. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 14. | Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterström RH, Perlmann T, Lehmann JM. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1146] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 15. | Doricakova A, Novotna A, Vrzal R, Pavek P, Dvorak Z. The role of residues T248, Y249 and T422 in the function of human pregnane X receptor. Arch Toxicol. 2013;87:291-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Chen Y, Tang Y, Guo C, Wang J, Boral D, Nie D. Nuclear receptors in the multidrug resistance through the regulation of drug-metabolizing enzymes and drug transporters. Biochem Pharmacol. 2012;83:1112-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 17. | Xing Y, Yan J, Niu Y. PXR: a center of transcriptional regulation in cancer. Acta Pharm Sin B. 2020;10:197-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 18. | Baldwin WS. Phase 0 of the Xenobiotic Response: Nuclear Receptors and Other Transcription Factors as a First Step in Protection from Xenobiotics. Nucl Receptor Res. 2019;6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Verma S, Tabb MM, Blumberg B. Activation of the steroid and xenobiotic receptor, SXR, induces apoptosis in breast cancer cells. BMC Cancer. 2009;9:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Shao Z, Li Y, Dai W, Jia H, Zhang Y, Jiang Q, Chai Y, Li X, Sun H, Yang R, Cao Y, Feng F, Guo Y. ETS-1 induces Sorafenib-resistance in hepatocellular carcinoma cells via regulating transcription factor activity of PXR. Pharmacol Res. 2018;135:188-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 21. | Feng F, Jiang Q, Cao S, Cao Y, Li R, Shen L, Zhu H, Wang T, Sun L, Liang E, Sun H, Chai Y, Li X, Liu G, Yang R, Yang Z, Yang Y, Xin S, Li BA. Pregnane X receptor mediates sorafenib resistance in advanced hepatocellular carcinoma. Biochim Biophys Acta Gen Subj. 2018;1862:1017-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 22. | Qiao EQ, Yang HJ, Yu XF, Gong LJ, Zhang XP, Chen DB. Curcuma zedoaria petroleum ether extract reverses the resistance of triple-negative breast cancer to docetaxel via pregnane X receptor. Ann Transl Med. 2021;9:1389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Gajjar K, Martin-Hirsch PL, Martin FL. CYP1B1 and hormone-induced cancer. Cancer Lett. 2012;324:13-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Luthra A, Denisov IG, Sligar SG. Spectroscopic features of cytochrome P450 reaction intermediates. Arch Biochem Biophys. 2011;507:26-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Nelson DR. Cytochrome P450 nomenclature. Methods Mol Biol. 1998;107:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Pelkonen O, Turpeinen M, Hakkola J, Honkakoski P, Hukkanen J, Raunio H. Inhibition and induction of human cytochrome P450 enzymes: current status. Arch Toxicol. 2008;82:667-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 396] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 27. | Guengerich FP. Mechanisms of cytochrome P450 substrate oxidation: MiniReview. J Biochem Mol Toxicol. 2007;21:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 28. | Ul-Islam S, Ahmed MB, Shehzad A, Ul-Islam M, Lee YS. Failure of Chemotherapy in Hepatocellular Carcinoma Due to Impaired and Dysregulated Primary Liver Drug Metabolizing Enzymes and Drug Transport Proteins: What to Do? Curr Drug Metab. 2018;19:819-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Murray GI, Patimalla S, Stewart KN, Miller ID, Heys SD. Profiling the expression of cytochrome P450 in breast cancer. Histopathology. 2010;57:202-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 30. | Ihunnah CA, Jiang M, Xie W. Nuclear receptor PXR, transcriptional circuits and metabolic relevance. Biochim Biophys Acta. 2011;1812:956-963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 31. | Panigrahy D, Kaipainen A, Greene ER, Huang S. Cytochrome P450-derived eicosanoids: the neglected pathway in cancer. Cancer Metastasis Rev. 2010;29:723-735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 32. | Sneha S, Baker SC, Green A, Storr S, Aiyappa R, Martin S, Pors K. Intratumoural Cytochrome P450 Expression in Breast Cancer: Impact on Standard of Care Treatment and New Efforts to Develop Tumour-Selective Therapies. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 33. | van Eijk M, Boosman RJ, Schinkel AH, Huitema ADR, Beijnen JH. Cytochrome P450 3A4, 3A5, and 2C8 expression in breast, prostate, lung, endometrial, and ovarian tumors: relevance for resistance to taxanes. Cancer Chemother Pharmacol. 2019;84:487-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 34. | Alexanian A, Miller B, Roman RJ, Sorokin A. 20-HETE-producing enzymes are up-regulated in human cancers. Cancer Genomics Proteomics. 2012;9:163-169. [PubMed] |
| 35. | Stewart DA, Winnike JH, McRitchie SL, Clark RF, Pathmasiri WW, Sumner SJ. Metabolomics Analysis of Hormone-Responsive and Triple-Negative Breast Cancer Cell Responses to Paclitaxel Identify Key Metabolic Differences. J Proteome Res. 2016;15:3225-3240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Ding X, Staudinger JL. Induction of drug metabolism by forskolin: the role of the pregnane X receptor and the protein kinase a signal transduction pathway. J Pharmacol Exp Ther. 2005;312:849-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 37. | Miki Y, Suzuki T, Tazawa C, Blumberg B, Sasano H. Steroid and xenobiotic receptor (SXR), cytochrome P450 3A4 and multidrug resistance gene 1 in human adult and fetal tissues. Mol Cell Endocrinol. 2005;231:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | Leake CD. Annual review of pharmacology and toxicology: review of reviews. Annu Rev Pharmacol Toxicol. 1978;18:581-588. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Mano ECC, Scott AL, Honorio KM. UDP-glucuronosyltransferases: Structure, Function and Drug Design Studies. Curr Med Chem. 2018;25:3247-3255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 40. | Lawrence RA, Parkhill LK, Burk RF. Hepatic cytosolic non selenium-dependent glutathione peroxidase activity: its nature and the effect of selenium deficiency. J Nutr. 1978;108:981-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 174] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Zhang BL, Sun T, Zhang BN, Zheng S, Lü N, Xu BH, Wang X, Chen GJ, Yu DK, Lin DX. Polymorphisms of GSTP1 is associated with differences of chemotherapy response and toxicity in breast cancer. Chin Med J (Engl). 2011;124:199-204. [PubMed] [DOI] [Full Text] |
| 42. | Gangemi R, Paleari L, Orengo AM, Cesario A, Chessa L, Ferrini S, Russo P. Cancer stem cells: a new paradigm for understanding tumor growth and progression and drug resistance. Curr Med Chem. 2009;16:1688-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 43. | Yang M, Li Y, Shen X, Ruan Y, Lu Y, Jin X, Song P, Guo Y, Zhang X, Qu H, Shao Y, Quan C. CLDN6 promotes chemoresistance through GSTP1 in human breast cancer. J Exp Clin Cancer Res. 2017;36:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 44. | van Gisbergen MW, Cebula M, Zhang J, Ottosson-Wadlund A, Dubois L, Lambin P, Tew KD, Townsend DM, Haenen GR, Drittij-Reijnders MJ, Saneyoshi H, Araki M, Shishido Y, Ito Y, Arnér ES, Abe H, Morgenstern R, Johansson K. Chemical Reactivity Window Determines Prodrug Efficiency toward Glutathione Transferase Overexpressing Cancer Cells. Mol Pharm. 2016;13:2010-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Xie J, Shults K, Flye L, Jiang F, Head DR, Briggs RC. Overexpression of GSTA2 protects against cell cycle arrest and apoptosis induced by the DNA inter-strand crosslinking nitrogen mustard, mechlorethamine. J Cell Biochem. 2005;95:339-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Lee JY, Han CY, Yang JW, Smith C, Kim SK, Lee EY, Kim SG, Kang KW. Induction of glutathione transferase in insulin-like growth factor type I receptor-overexpressed hepatoma cells. Mol Pharmacol. 2007;72:1082-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 47. | Rocha JC, Cheng C, Liu W, Kishi S, Das S, Cook EH, Sandlund JT, Rubnitz J, Ribeiro R, Campana D, Pui CH, Evans WE, Relling MV. Pharmacogenetics of outcome in children with acute lymphoblastic leukemia. Blood. 2005;105:4752-4758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 48. | Salinas AE, Wong MG. Glutathione S-transferases--a review. Curr Med Chem. 1999;6:279-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 144] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 49. | Mackenzie PI, Bock KW, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, Miners JO, Owens IS, Nebert DW. Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet Genomics. 2005;15:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 630] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 50. | Pathania S, Bhatia R, Baldi A, Singh R, Rawal RK. Drug metabolizing enzymes and their inhibitors' role in cancer resistance. Biomed Pharmacother. 2018;105:53-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 51. | Li Y, Zhou Y, Mao F, Shen S, Zhao B, Xu Y, Lin Y, Zhang X, Cao X, Chen C, Zhang J, Sun Q. miR-452 Reverses Abnormal Glycosylation Modification of ERα and Estrogen Resistance in TNBC (Triple-Negative Breast Cancer) Through Targeting UGT1A1. Front Oncol. 2020;10:1509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | de Almagro MC, Selga E, Thibaut R, Porte C, Noé V, Ciudad CJ. UDP-glucuronosyltransferase 1A6 overexpression in breast cancer cells resistant to methotrexate. Biochem Pharmacol. 2011;81:60-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Parmar S, Stingl JC, Huber-Wechselberger A, Kainz A, Renner W, Langsenlehner U, Krippl P, Brockmöller J, Haschke-Becher E. Impact of UGT2B7 His268Tyr polymorphism on the outcome of adjuvant epirubicin treatment in breast cancer. Breast Cancer Res. 2011;13:R57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | van den Heuvel-Eibrink MM, Sonneveld P, Pieters R. The prognostic significance of membrane transport-associated multidrug resistance (MDR) proteins in leukemia. Int J Clin Pharmacol Ther. 2000;38:94-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2565] [Cited by in RCA: 2764] [Article Influence: 145.5] [Reference Citation Analysis (0)] |
| 56. | Abolhoda A, Wilson AE, Ross H, Danenberg PV, Burt M, Scotto KW. Rapid activation of MDR1 gene expression in human metastatic sarcoma after in vivo exposure to doxorubicin. Clin Cancer Res. 1999;5:3352-3356. [PubMed] |
| 57. | Abd El-Aziz YS, Spillane AJ, Jansson PJ, Sahni S. Role of ABCB1 in mediating chemoresistance of triple-negative breast cancers. Biosci Rep. 2021;41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 58. | Yamada A, Ishikawa T, Ota I, Kimura M, Shimizu D, Tanabe M, Chishima T, Sasaki T, Ichikawa Y, Morita S, Yoshiura K, Takabe K, Endo I. High expression of ATP-binding cassette transporter ABCC11 in breast tumors is associated with aggressive subtypes and low disease-free survival. Breast Cancer Res Treat. 2013;137:773-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 59. | Kumar H, Gupta NV, Jain R, Madhunapantula SV, Babu CS, Kesharwani SS, Dey S, Jain V. A review of biological targets and therapeutic approaches in the management of triple-negative breast cancer. J Adv Res. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 62] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 60. | Sharom FJ. ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics. 2008;9:105-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 722] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 61. | Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 699] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 62. | Revathidevi S, Sudesh R, Vaishnavi V, Kaliyanasundaram M, MaryHelen KG, Sukanya G, Munirajan AK. Screening for the 3'UTR Polymorphism of the PXR Gene in South Indian Breast Cancer Patients and its Potential Role in Pharmacogenomics. Asian Pac J Cancer Prev. 2016;17:3971-3977. [PubMed] |
| 63. | Nabekura T, Kawasaki T, Jimura M, Mizuno K, Uwai Y. Microtubule-targeting anticancer drug eribulin induces drug efflux transporter P-glycoprotein. Biochem Biophys Rep. 2020;21:100727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Bhagyaraj E, Ahuja N, Kumar S, Tiwari D, Gupta S, Nanduri R, Gupta P. TGF-β induced chemoresistance in liver cancer is modulated by xenobiotic nuclear receptor PXR. Cell Cycle. 2019;18:3589-3602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 65. | Kodama S, Negishi M. Pregnane X receptor PXR activates the GADD45beta gene, eliciting the p38 MAPK signal and cell migration. J Biol Chem. 2011;286:3570-3578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 66. | Yang H, Ren L, Wang Y, Bi X, Li X, Wen M, Zhang Q, Yang Y, Jia Y, Li Y, Zang A, Wei Y, Dai G. FBI-1 enhanced the resistance of triple-negative breast cancer cells to chemotherapeutic agents via the miR-30c/PXR axis. Cell Death Dis. 2020;11:851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 67. | Chen Y, Tang Y, Chen S, Nie D. Regulation of drug resistance by human pregnane X receptor in breast cancer. Cancer Biol Ther. 2009;8:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 68. | Dotzlaw H, Leygue E, Watson P, Murphy LC. The human orphan receptor PXR messenger RNA is expressed in both normal and neoplastic breast tissue. Clin Cancer Res. 1999;5:2103-2107. [PubMed] |
| 69. | Meyer zu Schwabedissen HE, Tirona RG, Yip CS, Ho RH, Kim RB. Interplay between the nuclear receptor pregnane X receptor and the uptake transporter organic anion transporter polypeptide 1A2 selectively enhances estrogen effects in breast cancer. Cancer Res. 2008;68:9338-9347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 70. | Miki Y, Suzuki T, Kitada K, Yabuki N, Shibuya R, Moriya T, Ishida T, Ohuchi N, Blumberg B, Sasano H. Expression of the steroid and xenobiotic receptor and its possible target gene, organic anion transporting polypeptide-A, in human breast carcinoma. Cancer Res. 2006;66:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 71. | Theocharis S, Giaginis C, Gourzi S, Alexandrou P, Tsourouflis G, Sarantis P, Danas E, Michail A, Tsoukalas N, Pergaris A, Politis PK, Nakopoulou L. High Pregnane X Receptor (PXR) Expression Is Correlated with Poor Prognosis in Invasive Breast Carcinoma. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |