Published online Aug 24, 2023. doi: 10.5306/wjco.v14.i8.297
Peer-review started: May 10, 2023
First decision: June 7, 2023
Revised: June 19, 2023
Accepted: July 19, 2023
Article in press: July 19, 2023
Published online: August 24, 2023
Processing time: 103 Days and 17.7 Hours
Immune cells play an important role in regulating the behavior of tumor cells. According to emerging evidence, six-transmembrane epithelial antigen of the prostate 4 (STEAP4) performs a crucial part in tumor microenvironmental immune response and tumorigenesis, and serves as the potential target for cellular and antibody immunotherapy. However, the immunotherapeutic role of STEAP4 in gastric cancer (GC) remains unclear.
To investigate the expression of STEAP4 in GC and its relationship with immune infiltrating cells, and explore the potential value of STEAP4 as an immune prognostic indicator in GC.
The expression level of STEAP4 was characterized by immunohistochemistry in tumors and adjacent non-cancerous samples in 96 GC patients. Tumor Immune Estimation Resource was used to study the correlation between STEAP4 and tumor immune infiltration level and immune infiltration gene signature. R package was used to analyze the relationship between STEAP4 expression and immune and stromal scores in GC (GSE62254) by the ESTIMATE algorithm, and Kaplan-Meier Plotter and Gene Expression Profiling Interactive Analysis were applied to analyze the effect of STEAP4 on clinical prognosis.
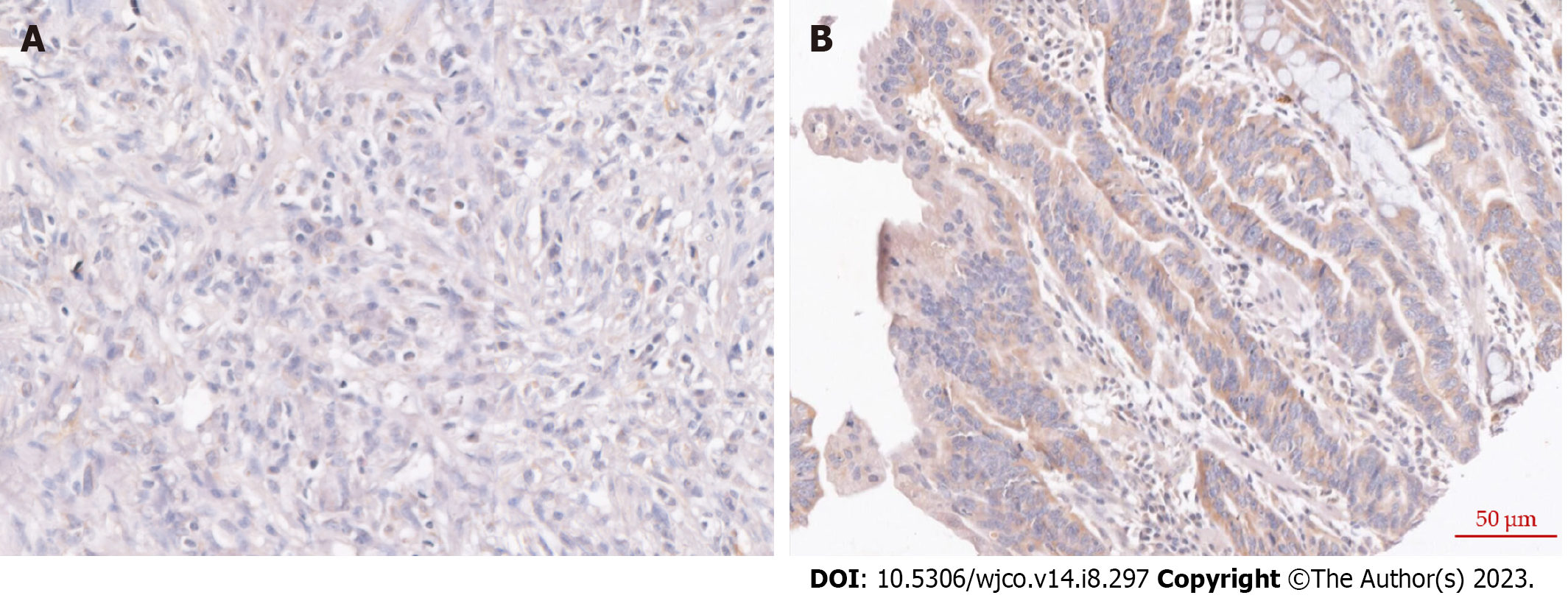
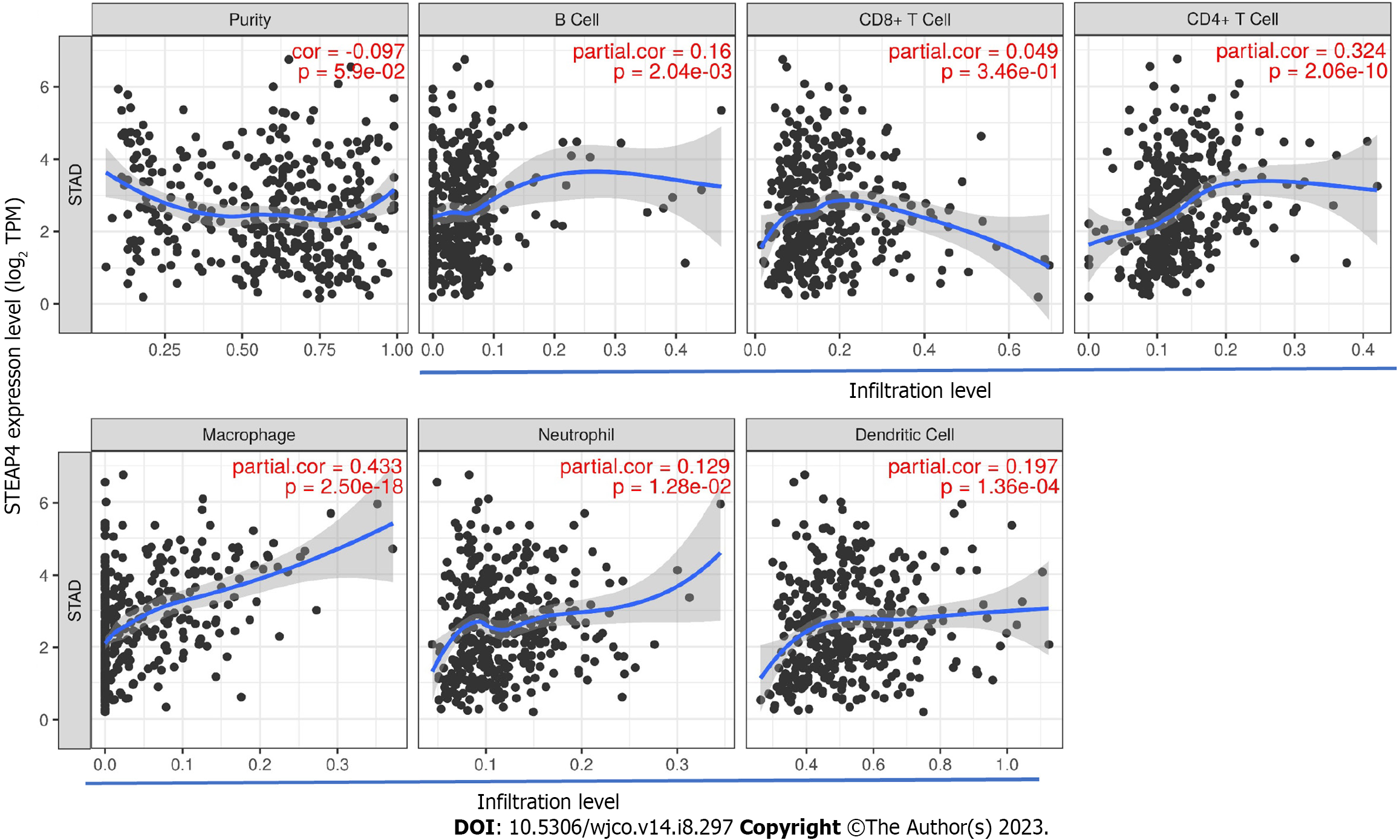
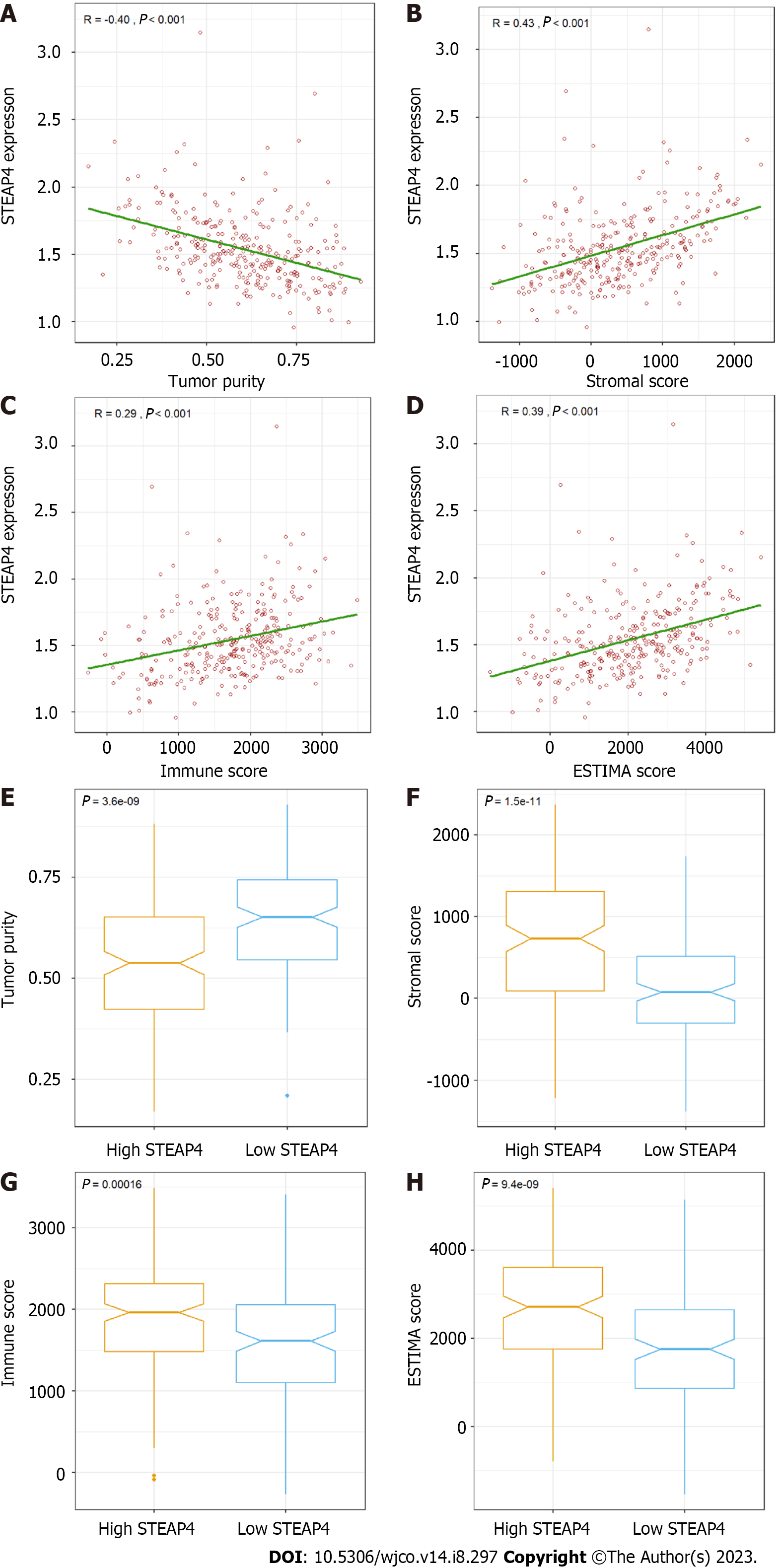
Immunohistochemistry analysis showed that STEAP4 expression was higher in GC tissues than in adjacent tissues, and STEAP4 expression was positively correlated with the clinical stage of GC. In GC, the expression of STEAP4 was positively correlated with the infiltration levels of B cells, CD4+ T cells, macrophages, neutrophils, and dendritic cells. The expression level of STEAP4 was strongly correlated with most of the immune markers. In addition, STEAP4 expression was inversely correlated with tumor purity, but correlated with stromal score (r = 0.43, P < 0.001), immune score (r = 0.29, P < 0.001) and estimate score (r = 0.39, P < 0.001). Moreover, stromal, immune, and estimate scores were higher in the STEAP4 high expression group, whereas tumor purity was higher in the STEAP4 Low expression group. The relationship between STEAP4 expression and prognosis of patients with GC was further investigated, and the results showed that high STEAP4 expression was associated with poor overall survival and disease-free survival. In addition, Kaplan-Meier Plotter showed that high expression of STEAP4 was significantly correlated with poor survival of patients with GC.
The current findings suggest an oncogenic role for STEAP4 in GC, with significantly high levels being associated with poor prognosis. Investigation of the GC tumor microenvironment suggests the potential function of STEAP4 is connected with the infiltration of diverse immune cells, which may contribute to the regulation of the tumor microenvironment. In conclusion, STEAP4 may serve as a potential therapeutic target for GC to improve the immune infiltration, as well as serve as a prognostic biomarker for judging the prognosis and immune infiltration status of GC.
Core Tip: The present study analyzed the expression level of six-transmembrane epithelial antigen of the prostate 4 (STEAP4) in gastric cancer (GC) and found that high STEAP4 expression is significantly associated with poor survival of patients. STEAP4 is positively correlated with immune infiltration of different types of immune cells, and has strong correlations with most immune markers. STEAP4 may become a potential biomarker for predicting the prognosis of GC patients.
- Citation: Fang ZX, Hou YY, Wu Z, Wu BX, Deng Y, Wu HT, Liu J. Immune responses of six-transmembrane epithelial antigen of the prostate 4 functions as a novel biomarker in gastric cancer. World J Clin Oncol 2023; 14(8): 297-310
- URL: https://www.wjgnet.com/2218-4333/full/v14/i8/297.htm
- DOI: https://dx.doi.org/10.5306/wjco.v14.i8.297
Gastric cancer (GC), the fifth most common malignant tumor, is the second leading cause of cancer-related death worldwide[1,2]. Although the overall survival (OS) of GC patients has improved with standardized extended (D2) lymphadenectomy and the implementation of chemotherapy and targeted therapy, its survival rate is still less than 30%[3,4]. However, recent studies have shown that immune-involved mechanisms play a certain critical role in gastric tumors, and immunotherapy is considered a promising strategy for the therapeutics of gastric tumors[5]. In addition, Zhang et al[6] found that tumor-infiltrating lymphocytes can affect the prognosis and efficacy of chemotherapy and immunotherapy in GC patients. Therefore, there is an urgent need to elucidate the mechanism of tumor-immune interaction in GC, and to identify novel prognostic targets for immunotherapy.
Six-transmembrane epithelial antigen of the prostate 4 (STEAP4) consists of an N-terminal oxidoreductase domain and a six-helix transmembrane domain, serving as a transmembrane protein involved in metal reductase transport of copper and iron[7,8]. It is reported that high expression of STEAP4 is correlated with the pathogenesis of cancer and metabolic diseases[9-11]. STEAP4 is not only involved in the occurrence and development of breast cancer[12,13], but is also related to the inflammatory response of colon cancer[14]. It is also found that STEAP4 is highly expressed in prostate cancer tissues, serving as a promising prognostic indicator[15]. Nevertheless, the effect of STEAP4 in GC development and the mechanisms involved remain unclear.
In this study, the expression of STEAP4 and its correlation with the prognosis of GC patients are comprehensively analyzed. Moreover, the relevance between STEAP4 and different tumor-infiltrating immune cells and immune cell markers is also examined to clarify the essential role of STEAP4 in GC and provide a potential relationship and mechanism between STEAP4 and tumor-immune interactions.
Tissue array (XT17-037, OUTDO, China) recruited total 96 cases of GC, including 84 pairs of GC tissues and corresponding adjacent tissues, and 12 extra GC samples. This investigation of STEAP4 in GC was approved by the Ethics Committee of Shantou University Medical College.
The protocol for immunohistochemical staining was conducted as described previously[16]. The primary antibody used was anti-STEAP4 antibody in 1:400 diluent (Proteintech 11944-AP). The sections were visualized and evaluated independently under a bright-field microscope (PerkinElmer Vectra, United States) by two investigators with no prior knowledge of the patient information. The evaluation of STEAP4 expression was based on the sum of the scores from the staining intensities (0-3 indicating colorless, light yellow, brown and dark) and the percentage of positive cells (0-4 for 0%, 1% to 25%, 26% to 50%, 51% to 74%, and 76% to 100%), and the patients were divided into two groups based on the sum score results[17].
Gene Expression Profiling Interactive Analysis (GEPIA) (http://gepia.cancer-pku.cn/index.html), an interactive network from TCGA and GTEx projects was used to further analyze the expression level of STEAP4, in TCGA expression data, in different clinical stages of GC[18]. The survival information of GC patients was also evaluated based on STEAP4 expression in the GEPIA datasets.
Tumor Immune Estimation Resource (TIMER) (https://cistrome.shinyapps.io/timer/) is an online dataset for systematic analysis of immune infiltration in various types of cancer[19]. The correlation between STEAP4 level and the abundance of infiltrating immune cells was analyzed using gene modules in the database. In addition, the correlation between STEAP4 level and biomarkers of tumor-infiltrating immune cells was also investigated, with scatterplots and Spearman’s value for estimated statistical signifcance. Gene markers of tumor-infiltrating immune cells included CD8+ T cells, CD4+T cells, B cells, monocytes, TAMs, M1 macrophages, M2 macrophages, neutrophils, natural killer cells (NK), dendritic cells (DCs), T-helper 1 (Th1) cells, T-helper 2 (Th2) cells, and follicle-helper T (Tfh) cells, T-helper 17 (Th17) cells, Tregs and exhausted T cells[20-22].
The “ESTIMATE” algorithm of R package was used to calculate the immune score and stromal score of the GSE62254 dataset (n = 300), which was helpful for the evaluation of immune and stromal constitute in tumors. The immune and stromal scores were also calculated by STEAP4 expression in immune and stromal cells in GC.
Kaplan-Meier Plotter (http://kmplot.com/analysis/) was applied to analyze the correlation between STEAP4 and survival rate of GC[23]. Hazard ratios (HRs) and log-rank P values for 95% confidence intervals were calculated simultaneously.
SPSS software was used for χ2 or Fisher’s exact probability tests to analyze the relationship of STEAP4 level and clinic information of GC patients. To investigate the prognosis of GC patients, the Kaplan-Meier survival curve was conducted, along with log-rank test. Differences were achieved with P < 0.05.
To investigate the expression profiling of STEAP4 in GC tissues, cancerous tissues and adjacent normal tissues were obtained from GC patients. Representative images of STEAP4 expression are shown in Figure 1. Based on the quantitation of STEAP4 expression levels in GC, a significantly high level of STEAP4 in GC tissues was found, compared with corresponding adjacent normal tissues (P = 0.0056) (Table 1).
| Case (n) | STEAP4 | χ2 | P value | ||
| Low (%) | High (%) | ||||
| Tumor | 84 | 20 (23.81) | 64 (76.19) | 7.674 | 0.0056 |
| Normal | 84 | 37 (44.05) | 47 (55.95) | ||
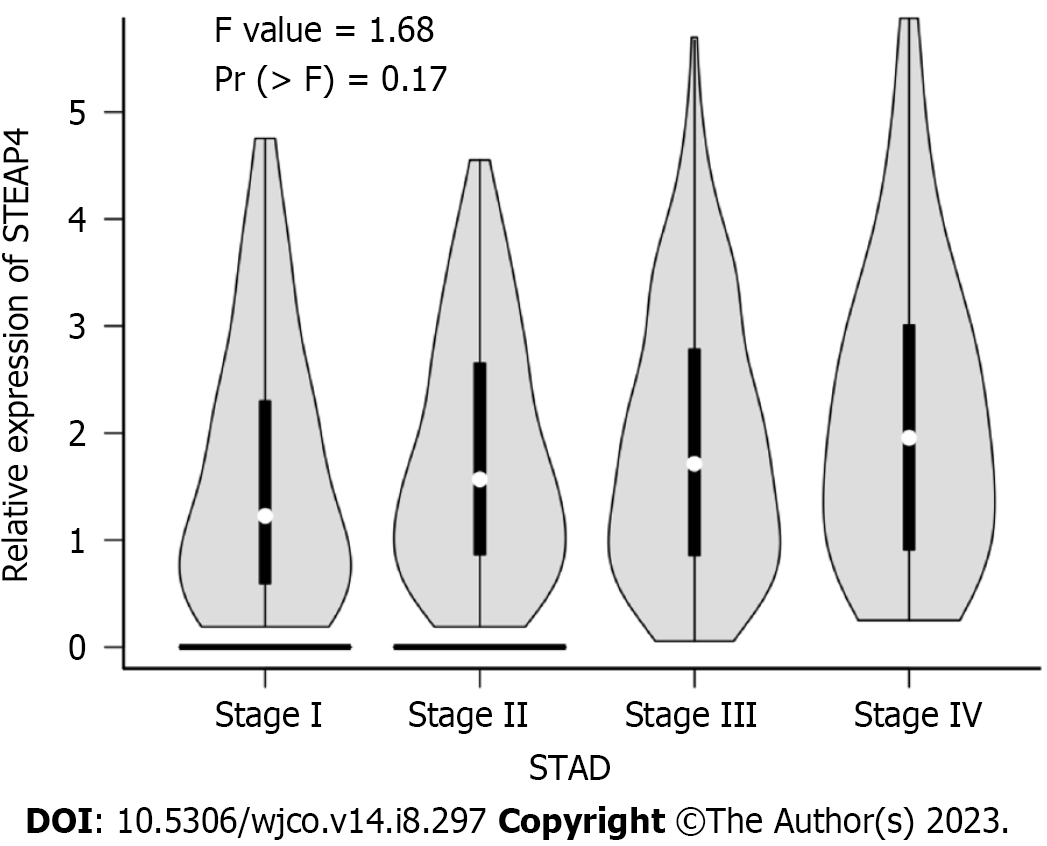
The expression level of STEAP4 in 96 GC patients was further analyzed with their clinicopathological parameters (Table 2). Although no statistical significance was found between the expression level of STEAP4 and the clinicopathologic parameters, including age of diagnosis, gender, lymph node status, vascular invasion and clinical stage (P > 0.05), the proportion of patients with high STEAP4 expression tended to increase with the progression of pathological stage, and high STEAP4 expression tended to be associated with lymph node metastasis and vascular invasion, indicating the potential contribution of STEAP4 to the progression of GC. The GEPIA database, regarding mRNA expression, was used to verify the relationship of STEAP4 with clinical stage of GC. Interestingly, there was no significant difference in the expression of STEAP4 between 4 different clinical stages. However, an increased expression of STEAP4 was found in Stage III and Stage IV, compared with Stage I and Stage II, predicting the potential promoting role of STEAP4 in GC (Figure 2).
| Clinical parameters | STEAP4 | P value | |
| Low (%) | High (%) | ||
| Age | |||
| < 60 | 9 (25.0) | 27 (75.0) | 0.5662 |
| ≥ 60 | 12 (20.0) | 48 (80.0) | |
| Gender | |||
| Female | 6 (19.4) | 25 (80.6) | 0.6800 |
| Male | 15 (23.1) | 50 (76.9) | |
| T | |||
| T1-3 | 15 (23.8) | 48 (76.2) | 0.5264 |
| T4 | 6 (18.2) | 27 (81.8) | |
| N | |||
| N0 | 7 (35.0) | 13 (65.0) | 0.1105 |
| N1-N3 | 14 (18.4) | 62 (81.8) | |
| M | |||
| M0 | 21 (22.3) | 73 (77.7) | 0.9999 |
| M1 | 0 (0) | 2 (100) | |
| Vascular invasion | |||
| No | 18 (26.1) | 51 (73.9) | 0.1105 |
| Yes | 3 (11.1) | 24 (88.9) | |
| Clinical stage | |||
| Phase 1 | 2 (25.0) | 6 (75.0) | 0.5900 |
| Phase 2 | 8 (29.6) | 19 (70.4) | |
| Phase 3 | 11 (18.6) | 48 (81.4) | |
| Phase 4 | 0 (0) | 2 (100) | |
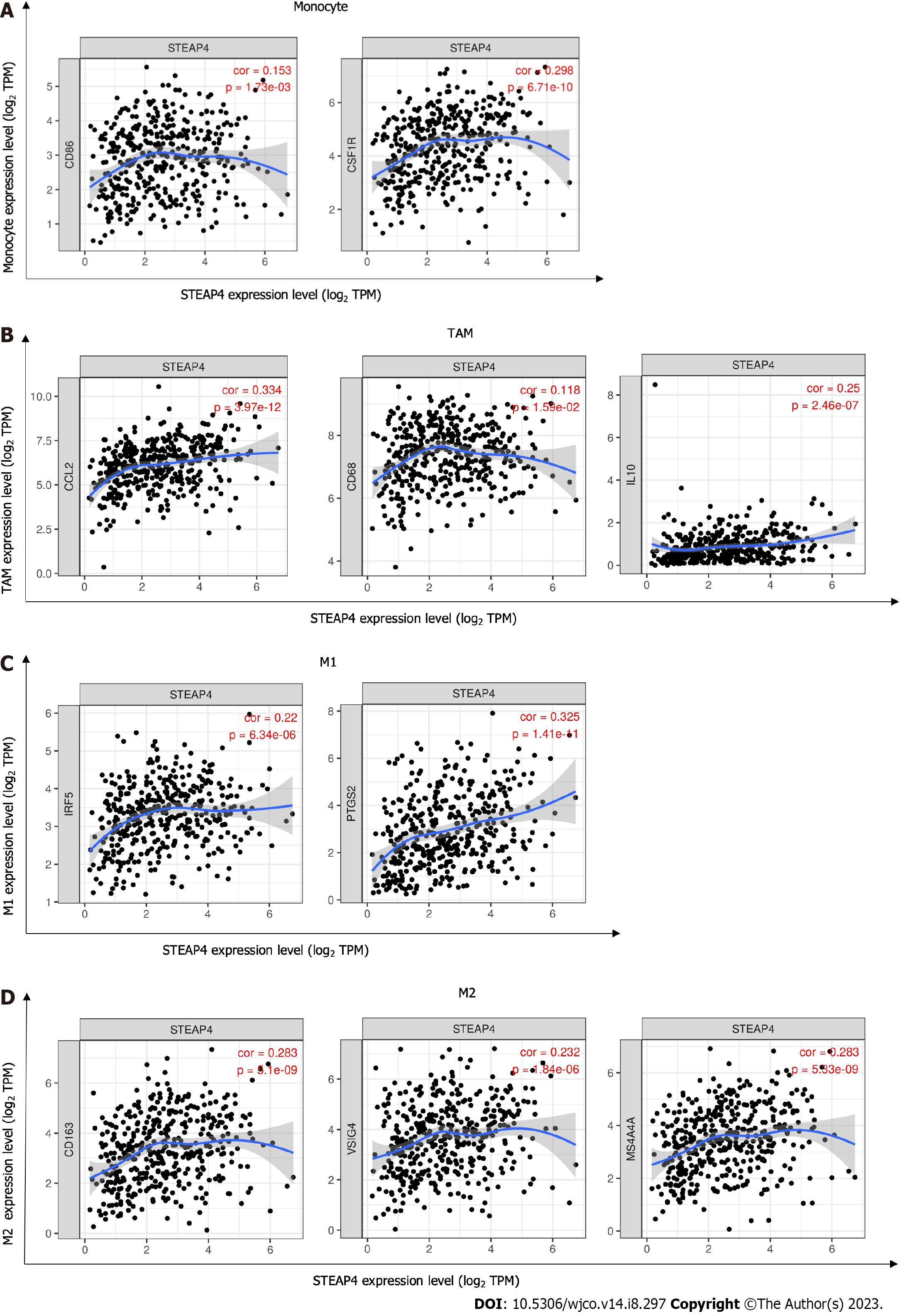
Considering that tumor purity is an important factor affecting immune infiltration of clinical tumor samples analyzed by genomic approaches[24], it is of interest to investigate the tumor microenvironment-related immune infiltration with STEAP4 levels. Interestingly, STEAP4 expression levels were found to be associated with higher immune infiltration in GC. The level of STEAP4 expression was positively associated with that of immune-infiltrating cells, including B cells, CD4+ T cells, neutrophils, macrophages and dendritic cells (Figure 3).
External verification was conducted on the GSE62254 dataset, with 300 GC samples, using the ESTIMATE algorithm in R software. Based on the features, stromal and immune scores were generated to reflect the proportion of stroma and immune cells, respectively, and single sample gene set enrichment analysis was used to combine the two to measure tumor purity. In Figure 4A-D, it is revealed that STEAP4 expression was inversely correlated with tumor purity and stromal score (r = 0.43, P < 0.001), immune score (r = 0.29, P < 0.001) and ESTIMATE score (r = 0.39, P < 0.001). In addition, stromal, immune, and estimate scores all increased with high STEAP4 expression (Figure 4F-H), whereas tumor purity was accompanied by low STEAP4 expression (Figure 4E).
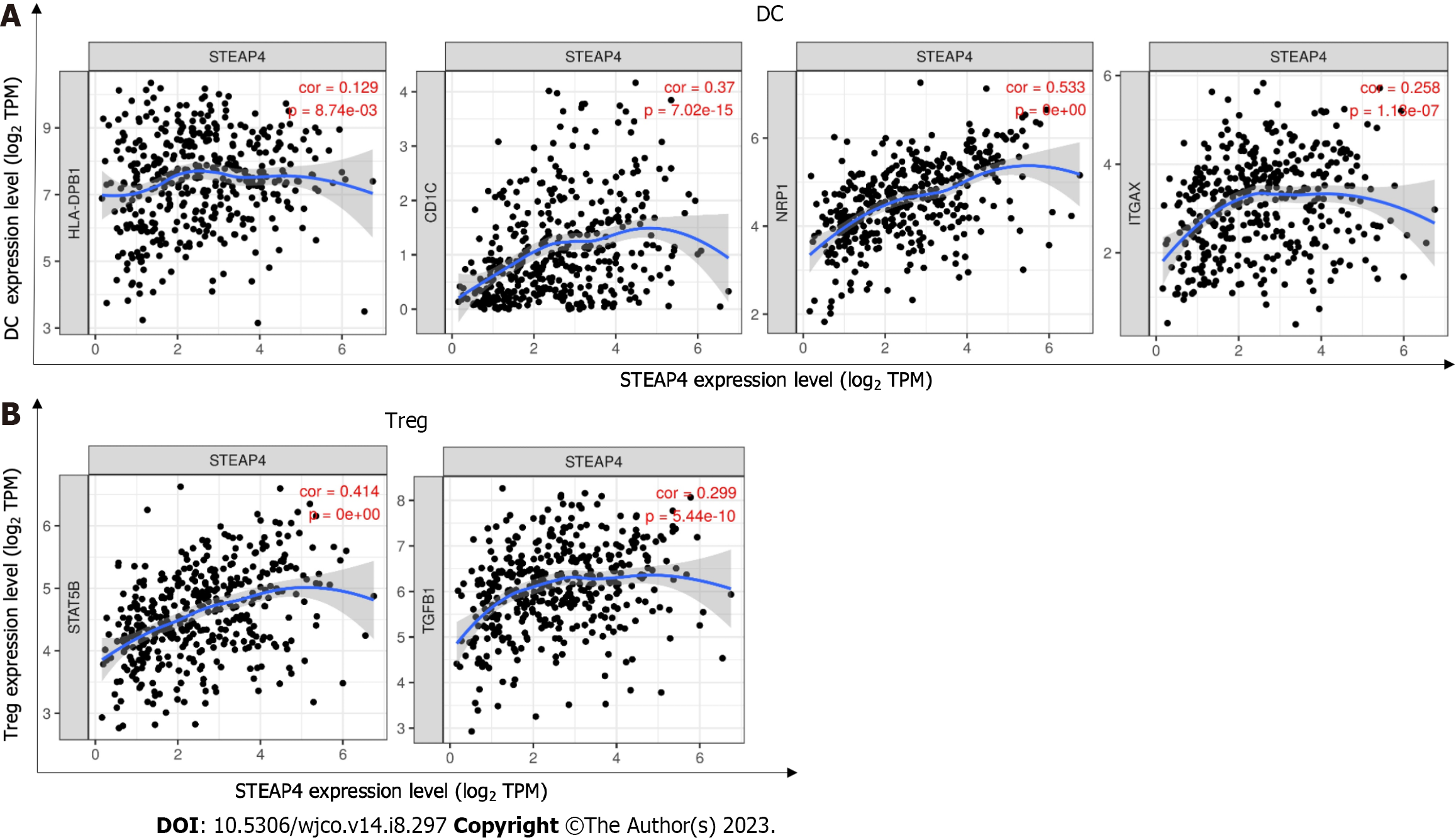
Due to the positive correlation between STEAP4 and immune infiltration was found in GC, further investigation was conducted to uncover the role of STEAP4 in the development of GC, and specify the subtype of immune cells associated with STEAP4. Diverse immunomarker sets were analyzed in TIMER database to verify the relationship of STEAP4 level with immune-infiltrating cells. After adjustment for purity, STEAP4 expression levels were significantly correlated with most of the immune marker sets of various immune cells and different T cells (Table 3).
| Immune cells | Gene markers | STAD | |||
| None | Tumor purity | ||||
| Cor | P value | Cor | P value | ||
| CD8+ T cells | CD8A | 0.083 | 0.0925 | 0.039 | 0.455 |
| CD8B | 0.039 | 0.427 | 0.012 | 0.823 | |
| CD4+ T cells | CD3D | 0.003 | 0.959 | -0.059 | 0.254 |
| CD3E | 0.043 | 0.382 | -0.022 | 0.670 | |
| CD2 | 0.048 | 0.325 | -0.006 | 0.914 | |
| B cells | CD19 | 0.152 | P < 0.01 | 0.127 | P < 0.05 |
| CD79A | 0.185 | P < 0.001 | 0.148 | P < 0.01 | |
| Monocytes | CD86 | 0.153 | P < 0.01 | 0.109 | P < 0.05 |
| CD115 (CSF1R) | 0.298 | P < 0.001 | 0.261 | P < 0.001 | |
| TAMs | CCL2 | 0.334 | P < 0.001 | 0.296 | P < 0.001 |
| CD68 | 0.118 | P < 0.05 | 0.085 | 0.0981 | |
| IL10 | 0.250 | P < 0.001 | 0.217 | P < 0.001 | |
| M1 macrophages | INOS (NOS2) | -0.113 | P < 0.05 | -0.142 | P < 0.01 |
| IRF5 | 0.220 | P < 0.001 | 0.201 | P < 0.001 | |
| COX2 (PTGS2) | 0.325 | P < 0.001 | 0.312 | P < 0.001 | |
| M2 macrophages | CD163 | 0.283 | P < 0.001 | 0.248 | P < 0.001 |
| VSIG4 | 0.232 | P < 0.001 | 0.203 | P < 0.001 | |
| MS4A4A | 0.479 | P < 0.001 | 0.194 | 0.0999 | |
| Neutrophils | CD66b (CEACAM8) | -0.109 | 0.338 | -0.089 | 0.452 |
| CD11b (ITGAM) | 0.407 | P < 0.001 | 0.118 | 0.320 | |
| CCR7 | 0.384 | P < 0.001 | 0.118 | 0.319 | |
| Natural killer cells | KIR2DL1 | 0.087 | 0.0768 | 0.078 | 0.129 |
| KIR2DL3 | 0.055 | 0.262 | 0.042 | 0.410 | |
| KIR2DL4 | -0.065 | 0.184 | -0.088 | 0.087 | |
| KIR3DL1 | 0.079 | 0.107 | 0.084 | 0.102 | |
| KIR3DL2 | 0.073 | 0.138 | 0.063 | 0.217 | |
| KIR3DL3 | -0.071 | 0.148 | -0.06 | 0.240 | |
| KIR2DS4 | 0.019 | 0.693 | 0.012 | 0.819 | |
| Dendritic cells | HLA-DPB1 | 0.129 | P < 0.01 | 0.08 | 0.120 |
| HLA-DQB1 | 0.025 | 0.609 | -0.032 | 0.532 | |
| HLA-DRA | 0.086 | 0.0789 | 0.046 | 0.376 | |
| HLA-DPA1 | 0.108 | P < 0.05 | 0.066 | 0.199 | |
| BDCA-1 (CD1C) | 0.370 | P < 0.001 | 0.351 | P < 0.001 | |
| BDCA-4 (NRP1) | 0.533 | P < 0.001 | 0.504 | P < 0.001 | |
| CD11c (ITGAX) | 0.258 | P < 0.001 | 0.217 | P < 0.001 | |
| Th1 | T-bet (TBX21) | 0.050 | 0.307 | 0.008 | 0.881 |
| STAT4 | 0.204 | P < 0.001 | 0.172 | P < 0.001 | |
| STAT1 | -0.051 | 0.304 | -0.070 | 0.174 | |
| IFN-γ (IFNG) | -0.195 | P < 0.001 | -0.229 | P < 0.001 | |
| IFN-α (TNF) | 0.005 | 0.921 | -0.045 | 0.387 | |
| Th2 | GATA3 | 0.230 | P < 0.001 | 0.205 | P < 0.001 |
| STAT6 | 0.122 | P < 0.01 | 0.119 | P < 0.05 | |
| STAT5A | 0.220 | P < 0.001 | 0.184 | P < 0.001 | |
| IL13 | 0.038 | 0.436 | 0.049 | 0.339 | |
| Tfh | BCL6 | 0.470 | P < 0.001 | 0.451 | P < 0.001 |
| IL21 | -0.031 | 0.529 | -0.055 | 0.285 | |
| Th17 | STAT3 | 0.337 | P < 0.001 | 0.310 | P < 0.001 |
| IL17A | -0.268 | P < 0.001 | -0.278 | P < 0.001 | |
| Treg | FOXP3 | 0.025 | 0.616 | -0.028 | 0.589 |
| CCR8 | 0.158 | P < 0.01 | 0.129 | P < 0.05 | |
| STAT5B | 0.414 | P < 0.001 | 0.383 | P < 0.001 | |
| TGFβ (TGFB1) | 0.299 | P < 0.001 | 0.266 | P < 0.001 | |
| T cell exhaustion | PD-1 (PDCD1) | -0.060 | 0.221 | -0.114 | P < 0.05 |
| CTLA4 | -0.072 | 0.141 | -0.125 | P < 0.05 | |
| LAG3 | -0.119 | P < 0.01 | -0.170 | P < 0.001 | |
| TIM-3 (HAVCR2) | 0.124 | P < 0.05 | 0.082 | 0.112 | |
| GZMB | -0.121 | P < 0.01 | -0.169 | P < 0.001 | |
Interestingly, the expression levels of gene markers for B cells, monocytes, TAMs, M1 and M2 macrophages and other immune cells were correlated with the expression of STEAP4. Specifically, it was found that the expression level of CD19, B cell CD79A, CD86, monocyte CD115, TAM CCL2 and IL-10, M1 macrophage IRF5 and PTGS2, and M2 macrophage CD163, VSIG4, and MS4A4A were significantly correlated with STEAP4 expression (P < 0.01) (Table 3, Figure 5), suggesting a function of STEAP4 in regulating the infiltration of macrophages during the progression of GC.
DCs promote tumor metastasis by increasing the activity of Treg cells and decreasing the activity of CD8+ T cells[25]. Here, high expression of STEAP4 was correlated with a high degree of DC infiltration, and DC markers such as HLA-DPB1, CD1C, NRP1 and ITGAX were also significantly correlated with STEAP4 expression (P < 0.01). In addition, STEAP4 was positively correlated with, that is STAT5B and TGFB1, biomarkers of Treg cells (Table 3, Figure 6), indicating a close relationship between STEAP4 and DC and Treg cell infiltration. However, whether STEAP4 can also mediate DC and tumor metastasis needs further research.
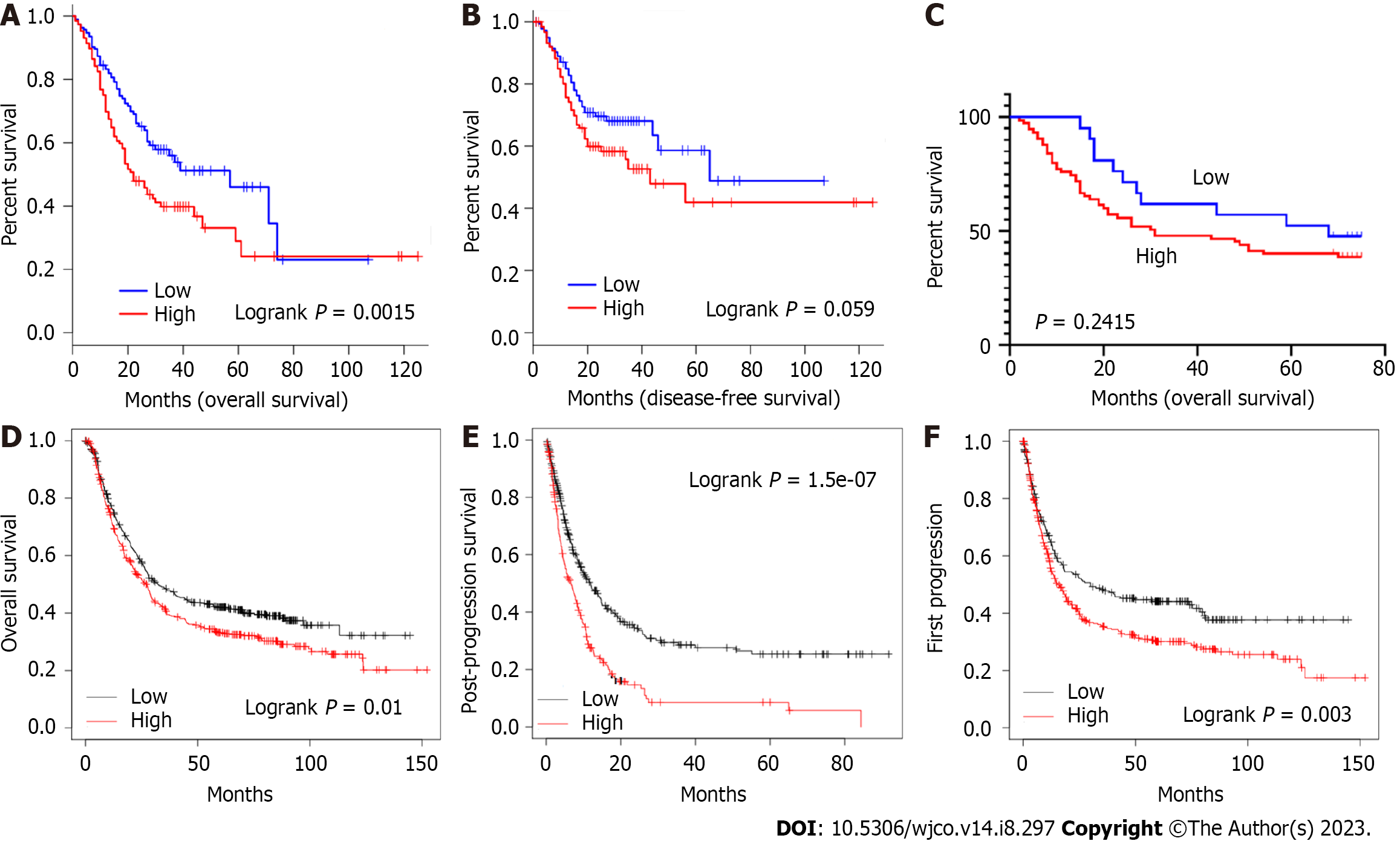
Based on the increased level of STEAP4 expression in GC, the prognostic value of STEAP4 was also evaluated on survival rate by using GEPIA database. It is worth noting that the expression of STEAP4 affects prognosis in all GC patients, and patients with high expression of STEAP4 have poor OS (P = 0.0015) and disease-free survival (DFS) (P = 0.059) (Figure 7A and B).
For immunohistochemical staining of STEAP4, it is showed that STEAP4 expression was not significantly correlated with OS. Although the difference did not meet statistical criteria (P > 0.05), high expression of STEAP4 tended to predict shorter OS in patients with GC, suggesting that STEAP4 protein levels could be used as a predictor of survival in patients with GC (Figure 7C). For further investigation, the Kaplan-Meier Plotter database was also applied to evaluate the prognostic signature of STEAP4. Interestingly, poor prognosis [OS: HR = 1.25, 95%CI: 1.05-1.48, P = 0.01; post-progression survival (PPS): HR = 1.8, 95%CI: 1.44-2.25, P = 1.5e-07; first progression (FP): HR = 1.38, 95%CI: 1.11-1.70, P = 0.003] was correlated with higher STEAP4 expression, suggesting that the level of STEAP4 influences the prognosis of GC patients (Figure 7D-F).
It is accepted that STEAP4 is an inflammatory metal reductase to catalyze the reduction of copper and iron, and the oxidation of NADPH. It has been shown that STEAP4 expression can promote the uptake of iron and copper, which can only be transported in reduced form through the cell membrane to exert their effects[9,14,26]. Liao et al[9] recently reported higher levels of cellular copper can enhance and maintain the activation of NF-κB, which leads to the production of inflammatory cytokines and chemokines, and Zhao et al[27] found STEAP4-mediated chemokine and cytokine induction enhances recruitment and activation of immune cells. As an important type of malignancy in gastrointestinal tract, GC is significantly associated with inflammatory and immune infiltration, both of which interact with the tumor microenvironment[28]. However, the regulatory factors in GC are not well characterized regarding inflammatory and immune infiltration.
Here, current research focused on STEAP4, a reductase related to oxidation, and its role in the progression of GC. We found that changes in STEAP4 expression levels are associated with the prognosis of GC, predicting poor prognosis of GC patients. Interestingly, high STEAP4 expression had a tendency to promote lymph node metastasis and vascular invasion, proposing STEAP4 as a predictor of tumor metastasis. In addition, we also show that in GC, the level of immune infiltration and multiple immune marker sets are correlated with STEAP4 expression level, and STEAP4 expression is positively correlated with stromal cells and immune cells of the tumor microenvironment. Thus, studies demonstrating the potential role of STEAP4 in tumor immunology and its use as a cancer biomarker provide insight.
In current investigation, we used a GC tissue microarray to determine the expression level of STEAP4 in GC and its adjacent tissues, and prognosis. Based on the immunohistochemical analysis, STEAP4 is highly expressed in GC compared with normal tissues, and is associated with poor prognosis. Although there was a significant correlation between STEAP4 expression and clinicopathological parameters, patients with high STEAP4 expression tended to have a higher pathological stage, lymph node metastasis and vascular invasion. Analysis of the GC cohort in TCGA showed that increased expression of STEP4 is associated with higher clinical stage. Furthermore, analysis of data from GEPIA and Kaplan-Meier Plotter revealed that high levels of STEAP4 expression are associated with high hazard ratios of OS, DFS, PPS, and FP. Together, these findings suggest that STEAP4 may be a prognostic biomarker in GC.
Another important aspect of this study is that STEAP4 expression correlates with different levels of immune infiltration in GC. Our results show a moderate to strong positive correlation between the infiltration levels of M1/M2 macrophages and DCs with STEAP4 expression levels in GC, implicating a potential regulatory function of STEAP4 in tumor-associated macrophage infiltration. Moreover, there is a significant correlation between STEAP4 expression and the regulation of several markers of helper T cells (Trf, Th17, and Treg), and it is known that the recruitment of regulatory T cells (Tregs) is another mechanism of immunosuppression[29]. Tumor cells secrete chemokines to attract Tregs and promote tumor angiogenesis[30], indicating that STEAP4 is a potential source for regulating T cell function in GC.
In addition, ESTIMATE algorithm analysis showed that high STEAP4 expression is positively correlated with stromal cells and immune cells. Interestingly, cancer develops in a complex tissue environment, and they rely on this en
The present study found that STEAP4 is a cancer-promoting factor in GC and can be used as a prognostic indicator in GC patients. GC patients with high expression of STEAP4 have a shorter survival time, and may play an important role in immune cell infiltration in GC patients, as well as serve as a prognostic biomarker.
Six-transmembrane epithelial antigen of the prostate 4 (STEAP4), a transmembrane protein involved in metal reductase transport of copper and iron, has been reported as a potential target for cellular and antibody immunotherapy.
Few studies on STEAP4 in gastric cancer (GC), which may play a role in the immune response to the occurrence and development of GC.
The expression of STEAP4 in GC tissues and its correlation with the level of tumor immune infiltration were comprehensively analyzed and to explore the potential immune effect of STEAP4 in GC.
The protein expression level, clinicopathological parameters and prognosis of STEAP4 in tumor and adjacent tissues of GC patients were detected by immunohistochemistry. An online database was used to study the correlation between STEAP4 and the level of tumor immunoinfiltration and the characteristics of immunoinfiltration genes. The relationship between STEAP4 expression and immune and stromal scores in the GC was analyzed by ESTIMATE algorithm.
Immunohistochemistry analysis showed that STEAP4 was highly expressed in GC and was positively correlated with the clinical stage of GC. The infiltration levels of immune cells such as B cells, CD4+ T cells, macrophages, neutrophils and dendritic cells were positively correlated with STEAP4. The expression level of STEAP4 was strongly correlated with most of the immune markers. In addition, the ESTIMATED algorithm analysis showed that the stromal, immune and estimated scores were higher in the group with high expression of STEAP4, while the tumor purity was higher in the STEAP4 Low expression group. The relationship between STEAP4 expression and prognosis of GC patients was further studied, and the results showed that high STEAP4 expression had shorter overall survival and disease-free survival. Moreover, Kaplan-Meier Plotter showed that high expression of STEAP4 was associated with poor survival in patients with GC.
STEAP4 is indicated as a potential immune indicator of GC, targeting STEAP4 may provide a new therapeutic method for GC patients.
The comprehensive analysis of STEAP4 function in GC still needs to explore the mechanism by which STEAP4 plays an immune role in GC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Liu Q, China; Sato T, Japan; Senchukova M, Russia S-Editor: Chen YL L-Editor: A P-Editor: Zhang XD
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55814] [Article Influence: 7973.4] [Reference Citation Analysis (132)] |
| 2. | Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71:264-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 1085] [Article Influence: 271.3] [Reference Citation Analysis (0)] |
| 3. | Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 1307] [Article Influence: 87.1] [Reference Citation Analysis (1)] |
| 4. | Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11065] [Cited by in RCA: 12186] [Article Influence: 1523.3] [Reference Citation Analysis (3)] |
| 5. | Chen Y, Jia K, Sun Y, Zhang C, Li Y, Zhang L, Chen Z, Zhang J, Hu Y, Yuan J, Zhao X, Gong J, Dong B, Zhang X, Li J, Shen L. Predicting response to immunotherapy in gastric cancer via multi-dimensional analyses of the tumour immune microenvironment. Nat Commun. 2022;13:4851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 129] [Reference Citation Analysis (0)] |
| 6. | Zhang H, Liu H, Shen Z, Lin C, Wang X, Qin J, Qin X, Xu J, Sun Y. Tumor-infiltrating Neutrophils is Prognostic and Predictive for Postoperative Adjuvant Chemotherapy Benefit in Patients With Gastric Cancer. Ann Surg. 2018;267:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 7. | Ohgami RS, Campagna DR, McDonald A, Fleming MD. The Steap proteins are metalloreductases. Blood. 2006;108:1388-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 501] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 8. | Grunewald TG, Bach H, Cossarizza A, Matsumoto I. The STEAP protein family: versatile oxidoreductases and targets for cancer immunotherapy with overlapping and distinct cellular functions. Biol Cell. 2012;104:641-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Liao Y, Zhao J, Bulek K, Tang F, Chen X, Cai G, Jia S, Fox PL, Huang E, Pizarro TT, Kalady MF, Jackson MW, Bao S, Sen GC, Stark GR, Chang CJ, Li X. Inflammation mobilizes copper metabolism to promote colon tumorigenesis via an IL-17-STEAP4-XIAP axis. Nat Commun. 2020;11:900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 10. | Cherner JA, Cloud ML, Offen WW, Latz JE. Comparison of nizatidine and cimetidine as once-nightly treatment of acute duodenal ulcer. Nizatidine Multicenter Duodenal Ulcer Study Group. Am J Gastroenterol. 1989;84:769-774. [PubMed] |
| 11. | Wellen KE, Fucho R, Gregor MF, Furuhashi M, Morgan C, Lindstad T, Vaillancourt E, Gorgun CZ, Saatcioglu F, Hotamisligil GS. Coordinated regulation of nutrient and inflammatory responses by STAMP2 is essential for metabolic homeostasis. Cell. 2007;129:537-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Wu HT, Chen WJ, Xu Y, Shen JX, Chen WT, Liu J. The Tumor Suppressive Roles and Prognostic Values of STEAP Family Members in Breast Cancer. Biomed Res Int. 2020;2020:9578484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Chen WJ, Wu HT, Li CL, Lin YK, Fang ZX, Lin WT, Liu J. Regulatory Roles of Six-Transmembrane Epithelial Antigen of the Prostate Family Members in the Occurrence and Development of Malignant Tumors. Front Cell Dev Biol. 2021;9:752426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Xue X, Bredell BX, Anderson ER, Martin A, Mays C, Nagao-Kitamoto H, Huang S, Győrffy B, Greenson JK, Hardiman K, Spence JR, Kamada N, Shah YM. Quantitative proteomics identifies STEAP4 as a critical regulator of mitochondrial dysfunction linking inflammation and colon cancer. Proc Natl Acad Sci U S A. 2017;114:E9608-E9617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Burnell SEA, Spencer-Harty S, Howarth S, Bodger O, Kynaston H, Morgan C, Doak SH. Utilisation of the STEAP protein family in a diagnostic setting may provide a more comprehensive prognosis of prostate cancer. PLoS One. 2019;14:e0220456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Liu J, Wei XL, Huang WH, Chen CF, Bai JW, Zhang GJ. Cytoplasmic Skp2 expression is associated with p-Akt1 and predicts poor prognosis in human breast carcinomas. PLoS One. 2012;7:e52675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Fang ZX, Li CL, Chen WJ, Wu HT, Liu J. Potential of six-transmembrane epithelial antigen of the prostate 4 as a prognostic marker for colorectal cancer. World J Gastrointest Oncol. 2022;14:1675-1688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Tang Z, Li C, Kang B, Gao G, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98-W102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5550] [Cited by in RCA: 7081] [Article Influence: 885.1] [Reference Citation Analysis (0)] |
| 19. | Chen D, Sun Q, Zhang L, Zhou X, Cheng X, Zhou D, Ye F, Lin J, Wang W. The lncRNA HOXA11-AS functions as a competing endogenous RNA to regulate PADI2 expression by sponging miR-125a-5p in liver metastasis of colorectal cancer. Oncotarget. 2017;8:70642-70652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Siemers NO, Holloway JL, Chang H, Chasalow SD, Ross-MacDonald PB, Voliva CF, Szustakowski JD. Genome-wide association analysis identifies genetic correlates of immune infiltrates in solid tumors. PLoS One. 2017;12:e0179726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 21. | Danaher P, Warren S, Dennis L, D'Amico L, White A, Disis ML, Geller MA, Odunsi K, Beechem J, Fling SP. Gene expression markers of Tumor Infiltrating Leukocytes. J Immunother Cancer. 2017;5:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 555] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 22. | Sousa S, Määttä J. The role of tumour-associated macrophages in bone metastasis. J Bone Oncol. 2016;5:135-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 23. | Lánczky A, Nagy Á, Bottai G, Munkácsy G, Szabó A, Santarpia L, Győrffy B. miRpower: a web-tool to validate survival-associated miRNAs utilizing expression data from 2178 breast cancer patients. Breast Cancer Res Treat. 2016;160:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 590] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 24. | Yoshihara K, Shahmoradgoli M, Martínez E, Vegesna R, Kim H, Torres-Garcia W, Treviño V, Shen H, Laird PW, Levine DA, Carter SL, Getz G, Stemke-Hale K, Mills GB, Verhaak RG. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4:2612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3056] [Cited by in RCA: 6443] [Article Influence: 585.7] [Reference Citation Analysis (0)] |
| 25. | Sawant A, Hensel JA, Chanda D, Harris BA, Siegal GP, Maheshwari A, Ponnazhagan S. Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. J Immunol. 2012;189:4258-4265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 26. | Jiang C, Wu B, Xue M, Lin J, Hu Z, Nie X, Cai G. Inflammation accelerates copper-mediated cytotoxicity through induction of six-transmembrane epithelial antigens of prostate 4 expression. Immunol Cell Biol. 2021;99:392-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Zhao J, Liao Y, Miller-Little W, Xiao J, Liu C, Li X, Kang Z. STEAP4 expression in CNS resident cells promotes Th17 cell-induced autoimmune encephalomyelitis. J Neuroinflammation. 2021;18:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Li J, Xia Y, Sun B, Zheng N, Li Y, Pang X, Yang F, Zhao X, Ji Z, Yu H, Chen F, Zhang X, Zhao B, Jin J, Yang S, Cheng Z. Neutrophil extracellular traps induced by the hypoxic microenvironment in gastric cancer augment tumour growth. Cell Commun Signal. 2023;21:86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 29. | Maharaj K, Uriepero A, Sahakian E, Pinilla-Ibarz J. Regulatory T cells (Tregs) in lymphoid malignancies and the impact of novel therapies. Front Immunol. 2022;13:943354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 30. | Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 790] [Cited by in RCA: 1068] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 31. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47109] [Article Influence: 3364.9] [Reference Citation Analysis (5)] |
| 32. | Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2921] [Cited by in RCA: 3361] [Article Influence: 258.5] [Reference Citation Analysis (0)] |
| 33. | Melero I, Rouzaut A, Motz GT, Coukos G. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov. 2014;4:522-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 355] [Article Influence: 32.3] [Reference Citation Analysis (0)] |