Published online Jul 24, 2023. doi: 10.5306/wjco.v14.i7.247
Peer-review started: April 24, 2023
First decision: June 14, 2023
Revised: June 14, 2023
Accepted: July 3, 2023
Article in press: July 3, 2023
Published online: July 24, 2023
Processing time: 85 Days and 17.6 Hours
Thyroid cancer is the most common endocrine malignancy. While there has been no appreciable increase in the observed mortality of well-differentiated thyroid cancer, there has been an overall rise in its incidence worldwide over the last few decades. Patients with papillary thyroid carcinoma (PTC) and clinical evidence of central (cN1) and/or lateral lymph node metastases require total thyroidectomy plus central and/or lateral neck dissection as the initial surgical treatment. Nodal status in PTC patients plays a crucial role in the prognostic evaluation of the recurrence risk. The 2015 guidelines of the American Thyroid Association (ATA) have more accurately determined the indications for therapeutic central and lateral lymph node dissection. However, prophylactic central neck lymph node dissection (pCND) in negative lymph node (cN0) PTC patients is controversial, as the 2009 ATA guidelines recommended that CND “should be considered” routinely in patients who underwent total thyroidectomy for PTC. Although the current guidelines show clear indications for therapeutic CND, the role of pCND in cN0 patients with PTC is still debated. In small solitary papillary carcinoma (T1, T2), pCND is not recommended unless there are high-risk prediction factors for recurrence and diffuse nodal spread (extrathyroid extension, mutation in the BRAF gene). pCND can be considered in cN0 disease with advanced primary tumors (T3 or T4) or clinical lateral neck disease (cN1b) or for staging and treatment planning purposes. The role of the preoperative evaluation is fund-amental to minimizing the possible detrimental effect of overtreatment of the types of patients who are associated with low disease-related morbidity and mortality. On the other hand, it determines the choice of appropriate treatment and determines if close monitoring of patients at a higher risk is needed. Thus, pCND is currently recommended for T3 and T4 tumors but not for T1 and T2 tumors without high-risk prediction factors of recurrence.
Core Tip: Nodal status in papillary thyroid cancer patients plays an important role in the prognosis of risk for recurrence. Preoperative evaluation is crucial for minimizing the possible risk of injury from overtreatment. Undoubtedly, therapeutic central neck dissection in addition to total thyroidectomy should be performed if there is positive lymph node involvement. The role of prophylactic central neck lymph node dissection in patients with papillary thyroid carcinoma with negative lymph nodes has been debated. It is currently recommended for T3 and T4 tumors but not for T1 and T2 tumors without high-risk prediction factors of recurrence.
- Citation: Pavlidis ET, Pavlidis TE. Role of prophylactic central neck lymph node dissection for papillary thyroid carcinoma in the era of de-escalation. World J Clin Oncol 2023; 14(7): 247-258
- URL: https://www.wjgnet.com/2218-4333/full/v14/i7/247.htm
- DOI: https://dx.doi.org/10.5306/wjco.v14.i7.247
Well-differentiated thyroid cancer is the most common endocrine malignancy, with approximately 570000 new cases annually. Furthermore, papillary thyroid carcinoma constitutes 90% of the new cases of thyroid cancer[1]. In comparison to statistics from the previous decade, there is now an over 100% increase in its incidence worldwide. This upsurge is somewhat due not only to increasing human exposure to defined incriminated factors for the development of thyroid carcinoma but possibly also to increases in health care utilization and imaging practices (ultrasound, fine needle aspiration), which can efficiently detect small asymptomatic nodules that otherwise remain undiagnosed[2,3].
The incidence of thyroid cancer reaches its peak between the fourth and fifth decade of life, with a predominance of women with a mean ratio of 4/1[4]. The overall five-year survival rate of thyroid carcinoma reaches over 95%, which could be characterized as excellent. Thus, it is one of the most amenable malignancies to treatment. The incidence of deaths in the United States is only 0.5 per 100,000 population and has not changed significantly from 1975 to 2009[5]. Despite the increasing incidence and due to widespread high-sensitivity screening practices, there is no described increase in mortality, which supports that well-differentiated thyroid cancer is in fact being overdiagnosed[6,7].
In the last version of the American Thyroid Association (ATA) guidelines, total thyroidectomy remains the preferred management method for tumors with a diameter above 4 cm or with a diameter under 4 cm but with high-risk features. It is widely established that high-risk features, including a family history of thyroid carcinoma, prior neck irradiation, extrathyroid extension, multifocality, and central lymph node involvement, with or without lateral lymph node neck involvement, require more extended surgical resections (total thyroidectomy with or without lymph node dissection)[8].
Papillary thyroid microcarcinomas are defined as papillary thyroid carcinomas (PTCs) of 1 cm or less in size. It has been reported that they are related to extremely low local or regional recurrence rates (2%-6%) and an even lower disease-specific mortality of less than 1%[9]. Since the majority of newly diagnosed thyroid carcinomas are microcarcinomas, there is growing pressure to stage the risk and minimize possible injury from the overtreatment of low-risk thyroid disease. To this effect, the American Thyroid Association indicates lobectomy as an alternative and less invasive approach in its new guidelines, as well as to minimize the major complications of total thyroidectomy, mainly hypocalcemia and recurrent laryngeal nerve palsy[8].
Lymph node metastases are common in papillary thyroid cancer, occurring in 20%–50% of patients, and they mostly occur in the central compartment of the neck (level VI). Lymph node metastases are also known to be an independent risk factor for local recurrence[10]. Lymph node dissection of the central, i.e., levels VI, VII with lateral compartments of the neck, i.e., Levels II to V will undoubtedly be recommended if there is a confirmed presence of lymph node metastases[8]. The necessity of prophylactic central neck lymph node dissection remains contested and in an ongoing controversy in the era of de-escalation.
This narrative review evaluates the role of prophylactic central neck lymph node dissection in well-differentiated thyroid carcinoma.
The study was based on the data of an extensive literature review from PubMed until March 2023, focusing on the comparison of the efficacy and surgical safety of its prophylactic performance. Only full-text papers published in the English language were included. Since the aim of this review was to study the efficacy and oncological completeness of thyroidectomy with or without central neck lymph node dissection for well-differentiated thyroid carcinoma, studies for nonmalignant thyroid pathologies were excluded.
The central neck compartment is anatomically composed of level VI and the upper part of level VII. Level VI is bounded cranially by the hyoid bone, caudally by the upper margin of the sternum, and laterally by the left and right common carotid arteries. The anterior border of the superficial layer of the deep cervical fascia is the posterior margin of the sternothyroid muscle. The posterior border is the prevertebral fascia, the deep layer of the deep cervical fascia. Near the origin of the right brachiocephalic artery, which forms the lower edge, the caudal of level VI is extended to level VII. Four groups constitute the lymph nodes of the central neck compartment, i.e., the prelaryngeal (Delphian), pretracheal, right paratracheal, and left paratracheal lymph nodes. Accurate central neck lymph node dissection requires imperatively meticulous complete removal of both the prelaryngeal and pretracheal regions and those of at least one paratracheal region, either left or right. In the case of the involvement of both paratracheal regions, central neck dissection should be bilateral[10].
Patients with clinical positivity for lymph node metastasis need to undergo therapeutic central neck dissection. Metastatic lymph node involvement is usually revealed either preoperatively, by ultrasound imaging, or intraoperatively by frozen section biopsy. Prophylactic central neck dissection means removal of all lymph nodes in both levels VI and VII, despite a negative preoperative diagnosis for suspected findings (cN0). The so-called berry picking, i.e., the one by one excision of only the lymph nodes with the appearance of metastasis that are in the sites that are not apparently healthy, should not be considered as an option and should be avoided[11].
The resection of paratracheal lymph nodes constitutes one of the most challenging technical parts of central neck dissection because of the necessary preservation of the anatomical integrity of all crucial structures in this region, specifically the recurrent laryngeal nerve and parathyroid glands with their vascularity. The latter deals mainly with the inferior parathyroid gland, as almost 90% of cases receive blood supply from the inferior thyroid artery, which lies beneath the area, in which all contained lymph nodes are planned to be dissected. In contrast, preservation of the upper parathyroid gland is somewhat easier during paratracheal lymph node dissection, especially when its blood supply comes exclusively from the superior thyroid artery[8,10].
The last guidelines of the American Thyroid Association state that the relevant high-risk factors from the history, i.e., rapid growth of nodules, sudden swallowing dysfunction, or dysphonia, must be investigated[8]. In addition, much relevant information can be obtained at the time of the patient’s examination. There is agreement among authors that age equal to or more than 45 years, female sex, familial history of thyroid carcinoma, and previous neck irradiation are considered predisposing factors for developing thyroid carcinoma, as shown in Table 1[12-14].
| No. | Parameter |
| 1 | Tumor size > 4 cm |
| 2 | Family history of thyroid carcinoma |
| 3 | Previous neck irradiation |
| 4 | Multifocality |
| 5 | Extrathyroid extension |
| 6 | Rapid growth of nodules |
| 7 | Sudden swallowing dysfunction or dysphonia |
| 8 | Age ≥ 45 yr |
| 9 | Female gender |
Undoubtedly, a precise preoperative diagnosis is a necessary condition for successfully planning the operative strategy. It is imperative to examine all the central and lateral neck lymph nodes in patients with well-differentiated thyroid carcinoma preoperatively, as well as to examine the central compartment intraoperatively. The preoperative fundamental diagnostic tools include an ultrasound scan of high resolution and fine-needle aspiration cytology of all suspected nodes[15].
Determining the levels of thyroglobulin in the aspiration material from suspicious lymph nodes significantly increases the sensitivity of the whole diagnostic evaluation. The sensitivity and specificity to detect lateral lymph node metastasis are sometimes higher compared to the central compartment; the referred sensitivity of the lateral is 93.8%, which is in contrast to that of the central compartment, which is 30%[16]. This notable difference is attributed to the complex anatomy of the central compartment. The central lymph nodes are not only smaller in diameter than the lateral lymph nodes but are also located in a groove between the esophagus, trachea, and thyroid. The interpretation of ultrasound findings is undoubtedly operator dependent, and much expertise is needed. Moreover, the presence of lymphocytic thyroiditis (Hashimoto’s disease) changes, which may be accompanied by inflammatory lymphadenopathy in majority of cases and may further interfere with the interpretation of the examination findings[17].
If a suspicion of extrathyroid spread exists that is accompanied by infiltration of a neighboring structure (larynx, esophagus, trachea and the main blood vessels in the neck) or if there is possible infiltration of the mediastinal and retropharyngeal lymph nodes, then a computed tomography (CT) scan of the head, neck and thorax needs to be performed. Magnetic resonance imaging of the head and neck could occasionally be a reliable alternative to CT scan; nonetheless, for the central compartment, it is less enlightening when compared to CT scan[15].
In patients with papillary thyroid cancer and clinical evidence of central with or without lateral lymph node metastases (cN1), the necessary initial treatment includes, central lymph node dissection with or without lateral neck dissection, in addition to total thyroidectomy. Level V lymph node dissection is mandatory in cases of central lymph node involvement. Additionally, it is necessary when ipsilateral with or without bilateral therapeutic lateral neck dissection, including levels IIa, III, IV, and Vb, is required[8,10,18,19]. This is because of the pivotal role of the nodal status in patients with papillary thyroid cancer, which is mainly due to its prognostic contribution to the risk of recurrence[8]. The 2015 American Thyroid Association guidelines update, in contrast to those of 2009, more precisely determines the recommendations for therapeutic central and lateral lymph node dissection when they are clinically evident. Prophylactic central neck dissection can be considered in negative central lymph node (cN0) disease of large primary tumors (T3, T4), in clinical lateral lymph node disease (cN1b), or for staging purposes to define the plan of treatment strategy. This clarified aspect is important, as the 2009 guidelines recommended that routine level VI lymph node dissection “should be considered” in all patients undergoing total thyroidectomy for papillary thyroid cancer regardless of positive or negative nodal status[9]. This decisive statement has led to controversy, as many surgeons took it as an interpretation of the recommended surgery.
Well-differentiated thyroid carcinoma includes not only papillary but also follicular carcinomas. However, the latter has mainly hematogenous metastases and only occasional (less than 5% of cases) regional lymphatic metastases of the neck[20]. The most common locations for distant hematogenous metastases are the lungs and brain[21], with a metastasis rate that fluctuates between 6% and 20% of cases[22,23]. To this effect, there is no need for prophylactic central neck dissection in follicular cancers, except for those cases in which there are clinically evident central neck metastases.
Lymphatic metastases of papillary thyroid carcinoma are mainly located in the regional lymph nodes. They most often affect the lymph nodes of level VI (paratracheal) and, in distant time, those of the lateral neck compartment, specifically levels III and IV, and extremely rarely level I[24,25]. In the absence of central lymph node metastases, escaped lateral lymph node metastases have been reported with an overall incidence of 20%[26]. They are associated, in most cases, with carcinoma located in the superior thyroid third, which mainly has metastases in lymph node levels II and III[27]. Notably, approximately 83% of the cases with lateral lymph node involvement also have microscopic metastases of the ipsilateral central lymph nodes, and 4% of them cannot be revealed by any preoperative diagnostic tool. In this clinical scenario, a need exists for at least ipsilateral prophylactic central neck dissection regardless of the negative clinical status[28].
In 5%-10% of cases of papillary thyroid carcinoma, palpatory clinical evidence of regional metastatic disease (macroscopic disease) exists at the time of diagnosis. The use of more sophisticated diagnostic approaches, including high-resolution ultrasound with fine-needle aspiration biopsy, may increase the former incidence by up to 30%[29]. Hematoxylin and eosin staining, the classical histopathological tool, can reveal positive typical lymph nodes in 30% to 50% of patients with papillary thyroid carcinoma who underwent elective central with lateral lymph node dissection[30]. There are studies in which an additional immunohistochemical evaluation of the resection specimen revealed microscopic metastases in up to 90% of cases[31,32]. These reports sustain the aspect that papillary thyroid carcinoma in most cases is accompanied by microscopic dissemination of the disease at the time of diagnosis and does not usually exhibit clinical evidence.
The results from studies such as Tisell et al[33] and Barczyńsk et al[34] have suggested that prophylactic central neck dissection has a positive effect on patient survival, mainly by reducing the probability of locoregional recurrence. The effect of lymph node status in recurrence and survival in PTC is shown in Table 2. Such a recurrence is based on macroscopic metastases with infiltration beyond the thyroid caps, a larger number of either positive or negative nodes in the overall lymph nodes included in the performed dissection, as well as the existence of five or more nodes with metastasis in the initial specimen[35]. However, the recurrence ratio could be described as very low in the presence of microscopic metastases[36]. Although the incidence of nodal micrometastases in the central compartment ranges from 38% to 80%, the probability of local nodal recurrence is below 3.8%, and central neck dissection is either performed or not[37,38].
| Ref. | Patients (Nu) | Trial | Survival |
| Tisell et al[33], 1996 | 195 | Single center retrospective study | Increased in ≥ 45 yr; Unaffected in < 45 yr |
| Zaydfudim et al[20], 2008 | 30504 | United States Registry, Surveillance | Increased in ≥ 45 yr; Unaffected in < 45 yr |
| Lundgren et al[38], 2006 | 5123 | Swedish Registry Surveillance | Increased |
In addition, the studies from Lundgren et al[38], including 5123 patients, and Zaydfudim et al[20], including 33088 patients, assessed the existence of metastases in the central and lateral compartments and documented a reduced survival rate. The recognized risk factors were age > 45 years in papillary cancer patients, male sex, metastases > 3 cm in size accompanied by spread beyond the thyroid caps, and the histopathological type of diffuse invasive follicular carcinoma[20,39,40]. According to the aforementioned, the selection of initial operative management has gained great importance; it should not be required to use only the tumor size as a criterion to determine the surgical plan.
Although there has been a worldwide agreement that lateral lymph node dissection should be preserved only in clinical N1b cases, the role of prophylactic central lymph node dissection in cN0 papillary thyroid carcinoma is still debated[40-45]. However, the preoperative evaluation of lymph nodes can be characterized as challenging. According to a meta-analysis from Liang et al[46], who included 23 “high-quality” studies, the proportion of central neck lymph node metastases fluctuates between 16.7% and 82.3% in those patients who underwent prophylactic central neck dissection.
Taking into account these broad ranges in the rate of central neck metastases, obtaining a high-quality evidence-based recommendation concerning prophylactic central neck dissection could be defined as demanding. A reason that could explain this heterogeneity in the literature’s results could be the difference in the expertise of obtaining a preoperative assessment by ultrasound, the plan of surgical management, and the histopathological evaluation. The basic assertions that are in favor of prophylactic central lymph node dissection concern the better staging accuracy, a more precise allocation to radioiodine treatment and the more reduced levels of postoperative thyroglobulin, possibly contributing to a decrease in the recurrence risk[46,47].
Otherwise, the basic argument against the abovementioned is the increased potential for complications, mainly hypoparathyroidism and laryngeal nerve injury[40,48]. A more conservative approach, i.e., ipsilateral (IpsiCND) central neck dissection, provides a lower rate of complications and was proposed in patients with clinical unilateral papillary thyroid carcinoma. It includes removal of the prelaryngeal, pretracheal and paratracheal lymph nodes on the same side as the tumor[49].
Even if the preoperative evaluation of the central lymph node compartment has not confirmed nodal metastasis, it must not prevent the surgeon from sending any suspicious node for intraoperative frozen-section histopathological assessment, and the assessment should not be based only on an intraoperative clinical inspection and palpation. Depending on the outcome of the frozen-section biopsy, a decision on therapeutic central lymph node dissection can be made. Several authors have verified that the sensitivity and specificity of intraoperative frozen-section biopsy may reach 100%[50]. Nevertheless, even in experienced hands, only approximately 26% of the confirmed metastases of the lymph nodes could be revealed based only on the intraoperative clinical evaluation[51].
In consonance with the novel scientific evolution, there is no reason to perform ipsilateral prophylactic central neck dissection for small solitary (T1, T2) well-differentiated thyroid carcinomas. The incriminated factors for the development of locoregional metastases include the larger diameter thyroid carcinomas (T3, T4), multifocality, a tall cell, a diffuse sclerotic and insular tumor that represents an aggressive subtype[52-56] as well as positivity for BRAF gene mutations on genetic testing[57]. In such scenarios, ipsilateral prophylactic central neck dissection is recommended. Nonetheless, the majority of the aforementioned data concerning possible malignancy are available only postoperatively after a precise tumor histopathological evaluation. Unfortunately, that accurate information is not available in advance for determining the plan of the extent of operative resection, thus carrying out a prophylactic central neck dissection is ultimately required.
A reliable alternative could be a prophylactic ipsilateral neck dissection frozen section examination, as proposed by Raffaelli et al[58]. Taking into consideration the highly accurate rate of frozen section evaluation of the ipsilateral central lymph node compartment in assessing the nodal status of negative cases with papillary thyroid cancer (up to 90%), they hypothesized that the frozen section assessment of ipsilateral central lymph node dissection could be valuable to modulate the extension of surgical resection. Undoubtedly, if there is an occurrence of hidden ipsilateral central lymph node metastasis, then total thyroidectomy and therapeutic central compartment dissection will become mandatory. Currently, in a case control study (unpublished data), they adopted such operative tactics personalizing the extent of the attempted resection in patients with small (T1) papillary thyroid carcinoma, without both multifocality and central lymph node involvement. This evaluation included 60 patients with personalized management who were scheduled for initial lobectomy only. The results, as described, confirmed that frozen section evaluation of ipsilateral central lymph node dissection may be effective and accurate in identifying patients who could benefit from bilateral central neck dissection. Therefore, the advantages include the lower risk of recurrence and subsequently the reduced need for a second more complicated operation[58].
Another factor that supports the debate concerning prophylactic central neck dissection is the intraoperative and postoperative morbidity that accompany such a procedure. Actually, the question that arises is whether the benefits exceed the potential harm. It is well known and demonstrated that the percentage of complications, namely, recurrent laryngeal nerve injuries and hypocalcemia, is increased after total thyroidectomy in cases accompanied by central lymph node dissection[59-61].
Lee et al[62], including 103 patients, stated that ipsilateral central neck dissection is accompanied by fewer complications, especially temporary and permanent hypocalcemia, compared to bilateral dissection. On the other hand, a meta-analysis from Chisholm et al[63], including 1132 patients, supports that there is no statistically significant increase in the percentages of complications, especially when neck dissection is performed by an endocrine surgeon. Zhu et al[64] drew the same results after evaluating nine relevant studies including 2298 patients.
Over the last two decades, a notable increase in papillary thyroid cancer and multifocal lesions as well as the coexistence of Hashimoto’s chronic thyroiditis was found. In addition, there was a gradual decrease in the papillary thyroid carcinoma sizes and subsequently an increase in micropapillary carcinoma[65]. The latter has led to controversy regarding the possible increase in lymph node metastasis reflecting central lymph node dissection. However, a recent study showed that multifocal lesions were not accompanied by a relevant increase in lymph node metastasis, but bilateral multifocality was associated with more aggressive clinical behavior and tumor histopathology. Thus, in this case, prophylactic central lymph node dissection is indicated, despite preoperative or intraoperative negative lymph node involvement[66].
The incidence of lymph node metastasis posterior to the right recurrent laryngeal nerve was estimated at 6%, making it necessary to thoroughly investigate this possibility in tumors of the lower pole that are greater than 0.5 cm in size[67]. Based on the recommendation by the American Thyroid Association for routine dissection of this lymph node[8], there is an increased risk of nerve injury and palsy in these tumors. Endoscopic thyroidectomy may offer an alternative safer approach[68].
A recent meta-analysis including 15 studies showed that total thyroidectomy with prophylactic lymph node dissection for papillary thyroid carcinoma was related to a lower local recurrence rate but a higher risk of permanent hypocalcemia and transient hypoparathyroidism than total thyroidectomy alone. There were no significant differences in transient hypocalcemia, permanent hypoparathyroidism, both temporary and permanent vocal cord paralysis, and recurrent laryngeal nerve injury[69]. The results of the above mentioned studies using routine prophylactic central neck lymph node dissection in PTC are shown in Table 3.
| Ref. | Patients (Nu) | Trial | Findings |
| Barczyński et al[34], 2013 | 640 | Single center retrospective study | Bilateral pCND increases 10-yr disease-specific survival and locoregional control, No increased risk of permanent morbidity |
| Lee et al[62], 2007 | 103 | Single center retrospective study | Increased transient hypocalcemia in bilateral than ipsilateral pCND |
| Chisholm et al[63], 2009 | 1132 | Meta-analysis | No increased permanent morbidity |
| Zhu et al[64], 2013 | 2298 | Meta-analysis | No more complications |
| Wang et al[69], 2023 | 2080 | Meta-analysis | Reduced local recurrence; Higher risk of permanent hypocalcemia and transient hypopara-thyroidism; No significant differences in transient hypocalcemia, permanent hypopara-thyroidism, both temporary and permanent vocal cord paralysis, and recurrent laryngeal nerve injury |
Hashimoto’s thyroiditis may cause reactive hyperplasia of the central lymph nodes in patients with papillary thyroid cancer. Nevertheless, in this autoimmune thyroiditis, there are often false-positive findings on ultrasound, which lead to possible overtreatment and complications[70].
Several predisposing factors for potential central lymph node metastasis in T1-T2 papillary thyroid carcinoma have been recognized. Thus, predictive nomograms have been developed, and they can be useful in planning the extent of operative strategy[71-75].
They include age (less than 44 years), gender (male), race (white and other nonblack people), size of the tumor (larger than 10 mm), multiple focal lesions, and minimal extrathyroid extension[71].
The least absolute shrinkage and selection operator -based model includes age (equal to or more than 55 years), nodular goiter, mutations in the BRAF gene, and Hashimoto’s thyroiditis as the most important factors[72].
The preoperative ultrasound suspicious findings (size of lymph node more than 5 mm, microcalcification, cystic degeneration, round shape, abnormal boundary, and cortical thickening) in addition to clinical data constitute another model[73]. Some statistical data of ultrasound signs are shown in Table 4[74].
| Sign | Sensitivity, % | Specificity, % |
| Microcalcifications | 5-69 | 93-100 |
| Cystic degeneration | 10-34 | 91-100 |
| Vascularity peripheral | 40-86 | 57-93 |
| Hyperechogenicity | 30-87 | 43-95 |
| Shape round | 37 | 70 |
Papillary thyroid carcinoma that is located in the isthmus exhibits aggressiveness and is related to poor prognosis. A nomogram including incriminated factors for metastatic lymph nodes and worse outcome (gender, age, size of malignant lesion, thyroid cap invasion, and Hashimoto’s thyroiditis)[75], as for any other location of high-risk patients[76], predicts recurrence[77].
Hypervascularity in ultrasound is an independent risk factor for recurrence in papillary thyroid carcinoma[78].
The ratio of fibrinogen to neutrophile percentage has been proposed as another independent risk factor for recurrence in patients with the coexistence of papillary thyroid carcinoma and diabetes mellitus type 2[79].
Multifocality (presence of two or more foci) of papillary thyroid carcinoma was determined to be a risk factor for an increased rate of central lymph node metastasis (44.57%) and lateral lymph node metastasis (17.17%)[80].
A radiomics nomogram based on ultrasound features, sex, age, BRAF gene V600E mutation, and extrathyroid extension predicts lymph node metastasis in papillary thyroid carcinoma[81].
For stage pT1α papillary thyroid microcarcinoma, multivariate analyses have demonstrated that younger age, male sex, and subcapsular location of the lesion were predictive factors for central lymph node metastasis[82].
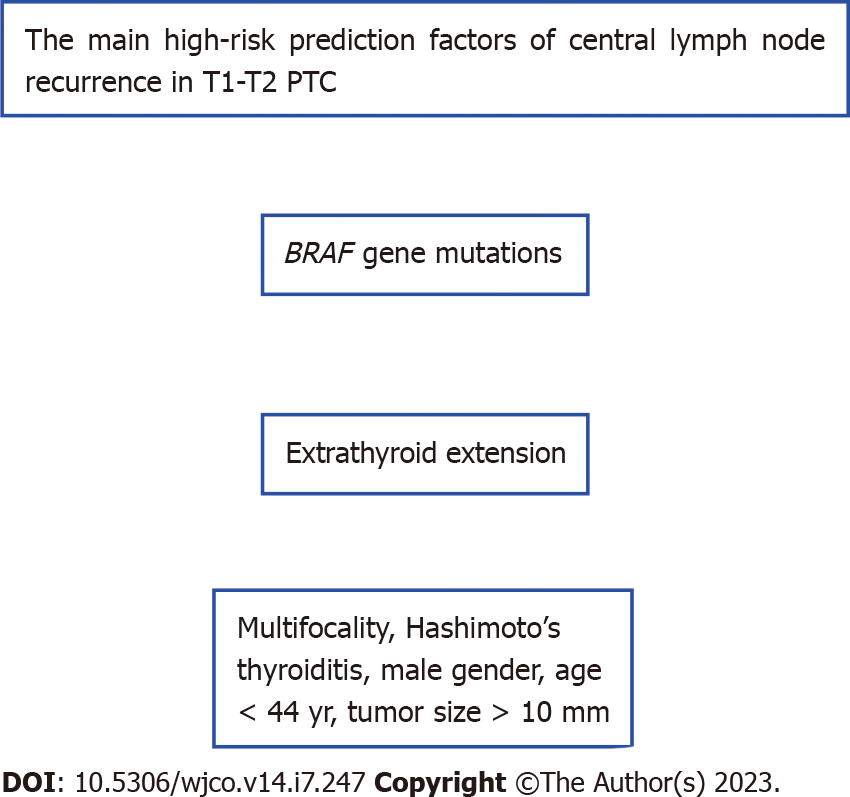
Based on the above mentioned studies, the main high-risk prediction factors of central lymph node recurrence in T1-T2 PTC are shown in Figure 1.
Small papillary thyroid carcinoma (equal to or less than 10 mm in diameter) was found to be a prediction factor for not detecting lymph node metastases, as shown in a recent study. Multivariate analyses have also showed that the values of stimulated thyroglobulin are related to shorter recurrence-free survival[83]. Thus, it must be considered a reliable prediction factor for recurrence.
Despite the presence of metastasis in the lateral neck lymph nodes, dissection of the central lymph nodes is not always necessary. A multivariate analysis showed that papillary thyroid carcinoma located in the center of the lobe and fewer than 4 positive lateral lymph nodes were protective factors against central lymph node involvement, which is subsequently a positive prognostic factor[84].
Patients with papillary thyroid carcinoma and a negative preoperative investigation for central lymph node involvement and who underwent total thyroidectomy alone without planned prophylactic lymph node dissection but with an incidentally removed lymph node positive for metastasis in the specimen biopsy had a worse course and high rate of treatment failure. In such a case, as shown in a recent large and detailed trial, the cumulative disease-free survival (DFS) was significantly lower (61.8%) vs 93.9%, and the cumulative survival was 79% vs 96% within the following 60 mo in the patients without metastasis in their incidentally removed lymph nodes[85]. Thus, a positive incidental lymph node is considered a significant risk factor for a worse outcome.
Nevertheless, the utility of intraoperative ultrasound is important for the assessment of lymph node status. Small lymph nodes (2-3 mm in size) may be evaluated adequately for metastatic spread by high-resolution neck ultrasound. The recurrence rate and subsequent need for reoperation in patients with papillary thyroid cancer and negative central lymph node involvement has been limited by the intraoperative prediction of lateral lymph nodes via ultrasound and their prophylactic dissection[86].
It seems from all the above mentioned that preoperative evaluation is crucial for minimizing the possible risk of injury from overtreatment in the majority of patients who otherwise have a low risk of disease-specific mortality and morbidity, whereas properly treating and monitoring those patients at higher risk is important since in some cases, nodal metastases are found in the surgical specimen. Apparently, molecular genomic assessment of diagnostic cytology samples could be more informative when dealing with the aggressive behavior of well-differentiated thyroid carcinoma to reliably modulate the extent of the initial surgery. Ipsilateral central neck dissection frozen section examination could be a reliable intraoperative method to assess the nodal status.
Although there is a clear indication for therapeutic central neck dissection according to the current guidelines, the role of prophylactic treatment in cN0 patients with papillary thyroid carcinoma is still debated. In follicular thyroid carcinoma, which usually has hematogenous metastases, there is no need for prophylactic central lymph node dissection. In small solitary papillary carcinoma (T1, T2), prophylactic central neck dissection is not recommended, as it does not provide benefits regarding prolonged survival, while this simultaneously provides a significant increase in the postoperative complication risk concerning either temporary or permanent complications, such as recurrent laryngeal nerve palsy and hypoparathyroidism. Prophylactic central lymph node dissection has been recommended in large papillary thyroid carcinomas (T3 and T4 tumors) or small ones (T1 and T2 tumors) related to high-risk prediction factors of recurrence and diffuse nodal spread, such as in extrathyroid extension or when there is a mutation in the BRAF gene.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Al-Ani RM, Iraq; He XH, China; Șurlin VM, Romania S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55820] [Article Influence: 7974.3] [Reference Citation Analysis (132)] |
| 2. | La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, Negri E. Thyroid cancer mortality and incidence: a global overview. Int J Cancer. 2015;136:2187-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 703] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 3. | Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide Thyroid-Cancer Epidemic? The Increasing Impact of Overdiagnosis. N Engl J Med. 2016;375:614-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 772] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 4. | Sentieri Working Group. [Sentieri: mortality, cancer incidence and hospital discharges. Summary]. Epidemiol Prev. 2014;38:5-7. [PubMed] |
| 5. | Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 1097] [Article Influence: 99.7] [Reference Citation Analysis (0)] |
| 6. | Hoang JK, Nguyen XV, Davies L. Overdiagnosis of thyroid cancer: answers to five key questions. Acad Radiol. 2015;22:1024-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 7. | Esserman LJ, Thompson IM, Reid B, Nelson P, Ransohoff DF, Welch HG, Hwang S, Berry DA, Kinzler KW, Black WC, Bissell M, Parnes H, Srivastava S. Addressing overdiagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol. 2014;15:e234-e242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 373] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 8. | Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10769] [Cited by in RCA: 9676] [Article Influence: 1075.1] [Reference Citation Analysis (1)] |
| 9. | Kovatch KJ, Hoban CW, Shuman AG. Thyroid cancer surgery guidelines in an era of de-escalation. Eur J Surg Oncol. 2018;44:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | American Thyroid Association Surgery Working Group; American Association of Endocrine Surgeons,; American Academy of Otolaryngology-Head and Neck Surgery; American Head and Neck Society, Carty SE, Cooper DS, Doherty GM, Duh QY, Kloos RT, Mandel SJ, Randolph GW, Stack BC Jr, Steward DL, Terris DJ, Thompson GB, Tufano RP, Tuttle RM, Udelsman R. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid. 2009;19:1153-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 405] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 11. | Musacchio MJ, Kim AW, Vijungco JD, Prinz RA. Greater local recurrence occurs with "berry picking" than neck dissection in thyroid cancer. Am Surg. 2003;69:191-6; discussion 196. [PubMed] |
| 12. | Matsuzu K, Sugino K, Masudo K, Nagahama M, Kitagawa W, Shibuya H, Ohkuwa K, Uruno T, Suzuki A, Magoshi S, Akaishi J, Masaki C, Kawano M, Suganuma N, Rino Y, Masuda M, Kameyama K, Takami H, Ito K. Thyroid lobectomy for papillary thyroid cancer: long-term follow-up study of 1,088 cases. World J Surg. 2014;38:68-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 228] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 13. | Hay ID, Thompson GB, Grant CS, Bergstralh EJ, Dvorak CE, Gorman CA, Maurer MS, McIver B, Mullan BP, Oberg AL, Powell CC, van Heerden JA, Goellner JR. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940-1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg. 2002;26:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 491] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 14. | Ganly I, Nixon IJ, Wang LY, Palmer FL, Migliacci JC, Aniss A, Sywak M, Eskander AE, Freeman JL, Campbell MJ, Shen WT, Vaisman F, Momesso D, Corbo R, Vaisman M, Shaha A, Tuttle RM, Shah JP, Patel SG. Survival from Differentiated Thyroid Cancer: What Has Age Got to Do with It? Thyroid. 2015;25:1106-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 15. | Yeh MW, Bauer AJ, Bernet VA, Ferris RL, Loevner LA, Mandel SJ, Orloff LA, Randolph GW, Steward DL; American Thyroid Association Surgical Affairs Committee Writing Task Force. American Thyroid Association statement on preoperative imaging for thyroid cancer surgery. Thyroid. 2015;25:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 16. | Hwang HS, Orloff LA. Efficacy of preoperative neck ultrasound in the detection of cervical lymph node metastasis from thyroid cancer. Laryngoscope. 2011;121:487-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 247] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 17. | Yoo YH, Kim JA, Son EJ, Youk JH, Kwak JY, Kim EK, Park CS. Sonographic findings predictive of central lymph node metastasis in patients with papillary thyroid carcinoma: influence of associated chronic lymphocytic thyroiditis on the diagnostic performance of sonography. J Ultrasound Med. 2013;32:2145-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Podnos YD, Smith D, Wagman LD, Ellenhorn JD. The implication of lymph node metastasis on survival in patients with well-differentiated thyroid cancer. Am Surg. 2005;71:731-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 309] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 19. | Wang LY, Ganly I. Nodal metastases in thyroid cancer: prognostic implications and management. Future Oncol. 2016;12:981-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Zaydfudim V, Feurer ID, Griffin MR, Phay JE. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery. 2008;144:1070-7; discussion 1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 329] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 21. | Parameswaran R, Shulin Hu J, Min En N, Tan WB, Yuan NK. Patterns of metastasis in follicular thyroid carcinoma and the difference between early and delayed presentation. Ann R Coll Surg Engl. 2017;99:151-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Lin JD, Huang MJ, Juang JH, Chao TC, Huang BY, Chen KW, Chen JY, Li KL, Chen JF, Ho YS. Factors related to the survival of papillary and follicular thyroid carcinoma patients with distant metastases. Thyroid. 1999;9:1227-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Schlumberger M, Challeton C, De Vathaire F, Travagli JP, Gardet P, Lumbroso JD, Francese C, Fontaine F, Ricard M, Parmentier C. Radioactive iodine treatment and external radiotherapy for lung and bone metastases from thyroid carcinoma. J Nucl Med. 1996;37:598-605. [PubMed] |
| 24. | Noguchi S, Noguchi A, Murakami N. Papillary carcinoma of the thyroid. I. Developing pattern of metastasis. Cancer. 1970;26:1053-1060. [PubMed] [DOI] [Full Text] |
| 25. | Caron NR, Tan YY, Ogilvie JB, Triponez F, Reiff ES, Kebebew E, Duh QY, Clark OH. Selective modified radical neck dissection for papillary thyroid cancer-is level I, II and V dissection always necessary? World J Surg. 2006;30:833-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 26. | Park JH, Lee YS, Kim BW, Chang HS, Park CS. Skip lateral neck node metastases in papillary thyroid carcinoma. World J Surg. 2012;36:743-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Bumber B, Marjanovic Kavanagh M, Jakovcevic A, Sincic N, Prstacic R, Prgomet D. Role of matrix metalloproteinases and their inhibitors in the development of cervical metastases in papillary thyroid cancer. Clin Otolaryngol. 2020;45:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Khafif A, Ben-Yosef R, Abergel A, Kesler A, Landsberg R, Fliss DM. Elective paratracheal neck dissection for lateral metastases from papillary carcinoma of the thyroid: is it indicated? Head Neck. 2008;30:306-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Stulak JM, Grant CS, Farley DR, Thompson GB, van Heerden JA, Hay ID, Reading CC, Charboneau JW. Value of preoperative ultrasonography in the surgical management of initial and reoperative papillary thyroid cancer. Arch Surg. 2006;141:489-94; discussion 494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 266] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 30. | Boi F, Baghino G, Atzeni F, Lai ML, Faa G, Mariotti S. The diagnostic value for differentiated thyroid carcinoma metastases of thyroglobulin (Tg) measurement in washout fluid from fine-needle aspiration biopsy of neck lymph nodes is maintained in the presence of circulating anti-Tg antibodies. J Clin Endocrinol Metab. 2006;91:1364-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 143] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 31. | Arturi F, Russo D, Giuffrida D, Ippolito A, Perrotti N, Vigneri R, Filetti S. Early diagnosis by genetic analysis of differentiated thyroid cancer metastases in small lymph nodes. J Clin Endocrinol Metab. 1997;82:1638-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Qubain SW, Nakano S, Baba M, Takao S, Aikou T. Distribution of lymph node micrometastasis in pN0 well-differentiated thyroid carcinoma. Surgery. 2002;131:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 162] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Tisell LE, Nilsson B, Mölne J, Hansson G, Fjälling M, Jansson S, Wingren U. Improved survival of patients with papillary thyroid cancer after surgical microdissection. World J Surg. 1996;20:854-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 150] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Barczyński M, Konturek A, Stopa M, Nowak W. Prophylactic central neck dissection for papillary thyroid cancer. Br J Surg. 2013;100:410-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 35. | Schneider DF, Mazeh H, Chen H, Sippel RS. Lymph node ratio predicts recurrence in papillary thyroid cancer. Oncologist. 2013;18:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 36. | Bardet S, Malville E, Rame JP, Babin E, Samama G, De Raucourt D, Michels JJ, Reznik Y, Henry-Amar M. Macroscopic lymph-node involvement and neck dissection predict lymph-node recurrence in papillary thyroid carcinoma. Eur J Endocrinol. 2008;158:551-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 37. | Gršić K, Bumber B, Curić Radivojević R, Leović D. Prophylactic Central Neck Dissection in Well-differentiated Thyroid Cancer. Acta Clin Croat 2020; 59: 87-95. |
| 38. | Lundgren CI, Hall P, Dickman PW, Zedenius J. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer. 2006;106:524-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 530] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 39. | Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A. A novel classification system for patients with PTC: addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery. 2004;135:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 294] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 40. | Bonnet S, Hartl D, Leboulleux S, Baudin E, Lumbroso JD, Al Ghuzlan A, Chami L, Schlumberger M, Travagli JP. Prophylactic lymph node dissection for papillary thyroid cancer less than 2 cm: implications for radioiodine treatment. J Clin Endocrinol Metab. 2009;94:1162-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 197] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 41. | Sancho JJ, Lennard TW, Paunovic I, Triponez F, Sitges-Serra A. Prophylactic central neck disection in papillary thyroid cancer: a consensus report of the European Society of Endocrine Surgeons (ESES). Langenbecks Arch Surg. 2014;399:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 42. | Raffaelli M, De Crea C, Sessa L, Giustacchini P, Revelli L, Bellantone C, Lombardi CP. Prospective evaluation of total thyroidectomy versus ipsilateral versus bilateral central neck dissection in patients with clinically node-negative papillary thyroid carcinoma. Surgery. 2012;152:957-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 43. | Raffaelli M, De Crea C, Sessa L, Giustacchini P, Bellantone R, Lombardi CP. Can intraoperative frozen section influence the extension of central neck dissection in cN0 papillary thyroid carcinoma? Langenbecks Arch Surg. 2013;398:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Raffaelli M, De Crea C, Sessa L, Fadda G, Bellantone C, Lombardi CP. Ipsilateral Central Neck Dissection Plus Frozen Section Examination Versus Prophylactic Bilateral Central Neck Dissection in cN0 Papillary Thyroid Carcinoma. Ann Surg Oncol. 2015;22:2302-2308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 45. | Sessa L, Lombardi CP, De Crea C, Tempera SE, Bellantone R, Raffaelli M. Risk Factors for Central Neck Lymph Node Metastases in Micro- Versus Macro- Clinically Node Negative Papillary Thyroid Carcinoma. World J Surg. 2018;42:623-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Liang J, Li Z, Fang F, Yu T, Li S. Is prophylactic central neck dissection necessary for cN0 differentiated thyroid cancer patients at initial treatment? A meta-analysis of the literature. Acta Otorhinolaryngol Ital. 2017;37:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 47. | Shaha AR. Prophylactic central compartment dissection in thyroid cancer: a new avenue of debate. Surgery. 2009;146:1224-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Hughes DT, White ML, Miller BS, Gauger PG, Burney RE, Doherty GM. Influence of prophylactic central lymph node dissection on postoperative thyroglobulin levels and radioiodine treatment in papillary thyroid cancer. Surgery. 2010;148:1100-6; discussion 1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 49. | Giordano D, Valcavi R, Thompson GB, Pedroni C, Renna L, Gradoni P, Barbieri V. Complications of central neck dissection in patients with papillary thyroid carcinoma: results of a study on 1087 patients and review of the literature. Thyroid. 2012;22:911-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 269] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 50. | Viola D, Materazzi G, Valerio L, Molinaro E, Agate L, Faviana P, Seccia V, Sensi E, Romei C, Piaggi P, Torregrossa L, Sellari-Franceschini S, Basolo F, Vitti P, Elisei R, Miccoli P. Prophylactic central compartment lymph node dissection in papillary thyroid carcinoma: clinical implications derived from the first prospective randomized controlled single institution study. J Clin Endocrinol Metab. 2015;100:1316-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 218] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 51. | Lee DH, Yoon TM, Kim HK, Lee JK, Kang HC, Lim SC. Intraoperative Frozen Biopsy of Central Lymph Node in the Management of Papillary Thyroid Microcarcinoma. Indian J Otolaryngol Head Neck Surg. 2016;68:56-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Scherl S, Mehra S, Clain J, Dos Reis LL, Persky M, Turk A, Wenig B, Husaini H, Urken ML. The effect of surgeon experience on the detection of metastatic lymph nodes in the central compartment and the pathologic features of clinically unapparent metastatic lymph nodes: what are we missing when we don't perform a prophylactic dissection of central compartment lymph nodes in papillary thyroid cancer? Thyroid. 2014;24:1282-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 53. | Zhang L, Wei WJ, Ji QH, Zhu YX, Wang ZY, Wang Y, Huang CP, Shen Q, Li DS, Wu Y. Risk factors for neck nodal metastasis in papillary thyroid microcarcinoma: a study of 1066 patients. J Clin Endocrinol Metab. 2012;97:1250-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 218] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 54. | Koo BS, Choi EC, Yoon YH, Kim DH, Kim EH, Lim YC. Predictive factors for ipsilateral or contralateral central lymph node metastasis in unilateral papillary thyroid carcinoma. Ann Surg. 2009;249:840-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 55. | Roh JL, Kim JM, Park CI. Central lymph node metastasis of unilateral papillary thyroid carcinoma: patterns and factors predictive of nodal metastasis, morbidity, and recurrence. Ann Surg Oncol. 2011;18:2245-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 56. | Horvatic Herceg G, Herceg D, Kralik M, Kulic A, Bence-Zigman Z, Tomic-Brzac H, Bracic I, Kusacic-Kuna S, Prgomet D. Urokinase plasminogen activator and its inhibitor type-1 as prognostic factors in differentiated thyroid carcinoma patients. Otolaryngol Head Neck Surg. 2013;149:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Howell GM, Nikiforova MN, Carty SE, Armstrong MJ, Hodak SP, Stang MT, McCoy KL, Nikiforov YE, Yip L. BRAF V600E mutation independently predicts central compartment lymph node metastasis in patients with papillary thyroid cancer. Ann Surg Oncol. 2013;20:47-52. [RCA] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 58. | Raffaelli M, Tempera SE, Sessa L, Lombardi CP, De Crea C, Bellantone R. Total thyroidectomy versus thyroid lobectomy in the treatment of papillary carcinoma. Gland Surg. 2020;9:S18-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 59. | Palestini N, Borasi A, Cestino L, Freddi M, Odasso C, Robecchi A. Is central neck dissection a safe procedure in the treatment of papillary thyroid cancer? Our experience. Langenbecks Arch Surg. 2008;393:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 60. | Díez JJ, Anda E, Sastre J, Pérez Corral B, Álvarez-Escolá C, Manjón L, Paja M, Sambo M, Santiago Fernández P, Blanco Carrera C, Galofré JC, Navarro E, Zafón C, Sanz E, Oleaga A, Bandrés O, Donnay S, Megía A, Picallo M, Sánchez Ragnarsson C, Baena-Nieto G, García JCF, Lecumberri B, de la Vega MS, Romero-Lluch AR, Iglesias P. Prevalence and risk factors for hypoparathyroidism following total thyroidectomy in Spain: a multicentric and nation-wide retrospective analysis. Endocrine. 2019;66:405-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 61. | Machens A, Elwerr M, Thanh PN, Lorenz K, Schneider R, Dralle H. Impact of central node dissection on postoperative morbidity in pediatric patients with suspected or proven thyroid cancer. Surgery. 2016;160:484-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Lee YS, Kim SW, Kim SK, Kang HS, Lee ES, Chung KW. Extent of routine central lymph node dissection with small papillary thyroid carcinoma. World J Surg. 2007;31:1954-1959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 63. | Chisholm EJ, Kulinskaya E, Tolley NS. Systematic review and meta-analysis of the adverse effects of thyroidectomy combined with central neck dissection as compared with thyroidectomy alone. Laryngoscope. 2009;119:1135-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 64. | Zhu W, Zhong M, Ai Z. Systematic evaluation of prophylactic neck dissection for the treatment of papillary thyroid carcinoma. Jpn J Clin Oncol. 2013;43:883-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Bakar B, Taşar P, Kırdak T, Kılıçturgay S. What has changed in the last 20 years in the postoperative specimen findings of the papillary thyroid cancer cases? A retrospective analysis. Turk J Surg. 2022;38:345-352. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 66. | Ozdemir K, Harmantepe AT, Gonullu E, Kocer B, Bayhan Z. Should multifocality be an indication for prophylactic central neck dissection in papillary thyroid cancer? Updates Surg. 2023;75:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 67. | Yang H, Tao L. Lymph Node Posterior to the Right Recurrent Laryngeal Nerve Metastasis in Right Lobe T1a Papillary Thyroid Carcinoma: A Retrospective Cohort Study. Cancer Control. 2023;30:10732748221149819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 68. | Si L, Mei H, Wang Q, Wang F, Sha S, He Z, Ke J. Surgical outcomes of different approaches to dissection of lymph nodes posterior to right recurrent laryngeal nerve: a retrospective comparative cohort study of endoscopic thyroidectomy via the areolar approach and via the axillo-breast approach. Gland Surg. 2022;11:1936-1945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (2)] |
| 69. | Wang Y, Xiao Y, Pan Y, Yang S, Li K, Zhao W, Hu X. The effectiveness and safety of prophylactic central neck dissection in clinically node-negative papillary thyroid carcinoma patients: A meta-analysis. Front Endocrinol (Lausanne). 2022;13:1094012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 70. | Tan HL, Nyarko A, Duan SL, Zhao YX, Chen P, He Q, Zhang ZJ, Chang S, Huang P. Comprehensive analysis of the effect of Hashimoto's thyroiditis on the diagnostic efficacy of preoperative ultrasonography on cervical lymph node lesions in papillary thyroid cancer. Front Endocrinol (Lausanne). 2022;13:987906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | Sun Y, Sun W, Xiang J, Zhang H. Nomogram for predicting central lymph node metastasis in T1-T2 papillary thyroid cancer with no lateral lymph node metastasis. Front Endocrinol (Lausanne). 2023;14:1112506. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 72. | Zhao F, Wang P, Yu C, Song X, Wang H, Fang J, Zhu C, Li Y. A LASSO-based model to predict central lymph node metastasis in preoperative patients with cN0 papillary thyroid cancer. Front Oncol. 2023;13:1034047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 73. | Huang J, Li Z, Zhong Q, Fang J, Chen X, Zhang Y, Huang Z. Developing and validating a multivariable machine learning model for the preoperative prediction of lateral lymph node metastasis of papillary thyroid cancer. Gland Surg. 2023;12:101-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 74. | Mizrachi A, Shaha AR. Lymph Node Dissection for Differentiated Thyroid Cancer. Mol Imaging Radionucl Ther. 2017;26:10-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 75. | Zhao Y, Shi W, Dong F, Wang X, Lu C, Liu C. Risk prediction for central lymph node metastasis in isolated isthmic papillary thyroid carcinoma by nomogram: A retrospective study from 2010 to 2021. Front Endocrinol (Lausanne). 2022;13:1098204. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 76. | Li F, Zhou FJ, Zhu TW, Qiu HL, Zhang XT, Ruan BW, Huang DY. Nomogram for predicting skip metastasis in cN0 papillary thyroid cancer patients at increased risk of lymph node metastasis. Adv Clin Exp Med. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 77. | Jang SW, Park JH, Kim HR, Kwon HJ, Lee YM, Hong SJ, Yoon JH. Recurrence Risk Evaluation in Patients with Papillary Thyroid Carcinoma: Multicenter Machine Learning Evaluation of Lymph Node Variables. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 78. | Li W, Li Y, Long M, Li J, Ma J, Luo Y. Vascularity depicted by contrast-enhanced ultrasound predicts recurrence of papillary thyroid cancer. Eur J Radiol. 2023;159:110667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 79. | Zheng D, Yang J, Qian J, Jin L, Huang G. Fibrinogen-to-Neutrophil Ratio as a New Predictor of Central Lymph Node Metastasis in Patients with Papillary Thyroid Cancer and Type 2 Diabetes Mellitus. Cancer Manag Res. 2022;14:3493-3505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 80. | Zhang T, He L, Wang Z, Dong W, Sun W, Zhang P, Zhang H. Risk factors of cervical lymph node metastasis in multifocal papillary thyroid cancer. Front Oncol. 2022;12:1003336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 81. | Wen Q, Wang Z, Traverso A, Liu Y, Xu R, Feng Y, Qian L. A radiomics nomogram for the ultrasound-based evaluation of central cervical lymph node metastasis in papillary thyroid carcinoma. Front Endocrinol (Lausanne). 2022;13:1064434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 82. | Tagliabue M, Giugliano G, Mariani MC, Rubino M, Grosso E, Chu F, Calastri A, Maffini FA, Mauri G, De Fiori E, Manzoni MF, Ansarin M. Prevalence of Central Compartment Lymph Node Metastases in Papillary Thyroid Micro-Carcinoma: A Retrospective Evaluation of Predictive Preoperative Features. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 83. | Ryu YJ, Kwon SY, Lim SY, Na YM, Park MH. Predictive Factors for Skip Lymph Node Metastasis and Their Implication on Recurrence in Papillary Thyroid Carcinoma. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 84. | Graceffa G, Orlando G, Cocorullo G, Mazzola S, Vitale I, Proclamà MP, Amato C, Saputo F, Rollo EM, Corigliano A, Melfa G, Cipolla C, Scerrino G. Predictors of Central Compartment Involvement in Patients with Positive Lateral Cervical Lymph Nodes According to Clinical and/or Ultrasound Evaluation. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Pinheiro RA, Leite AK, Cavalheiro BG, de Mello ES, Kowalski LP, Matos LL. Incidental Node Metastasis as an Independent Factor of Worse Disease-Free Survival in Patients with Papillary Thyroid Carcinoma. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 86. | Shen Y, Li X, Tao L, Chen Y, Xie R. Clinical Efficacy of Intraoperative Ultrasound for Prophylactic Lymphadenectomy of the Lateral Cervical Neck in Stage CN0 Papillary Thyroid Cancer: A Prospective Study. J Invest Surg. 2023;36:2154416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |