Published online May 24, 2023. doi: 10.5306/wjco.v14.i5.198
Peer-review started: January 9, 2023
First decision: January 31, 2023
Revised: February 28, 2023
Accepted: April 21, 2023
Article in press: April 21, 2023
Published online: May 24, 2023
Processing time: 129 Days and 9 Hours
The body of evidence investigating human epidermal growth factor receptor-2 (HER2) directed therapy in patients with breast cancer (BC) has been growing within the last decade. Recently, the use of tyrosine kinase inhibitors (TKIs) has been of particular interest in the treatment of human malignancies. This literature commentary is intended to highlight the most recent findings associated with the widely-studied TKI agents and their clinical significance in improving the outcomes of HER2 positive BC.
Core Tip: Newly published randomized controlled trials within the past two years have provided compelling evidence on the use of tyrosine kinase inhibitors (TKIs) such as Lapatinib, Pyrotinib, Neratinib, Tucatinib, Ruxolitinib, and Afatinib. Several of these agents were found to offer better outcomes in terms of progression-free survival when combined with other agents. While some TKIs, namely Lapatinib, and Neratinib, are supported with a large amount of data than others, the medical literature still lacks substantial evidence to draw a clinical conclusion that could modify/add to the present recommendations in human epidermal growth factor receptor-2 positive breast cancer treatment guidelines.
- Citation: Abunada A, Sirhan Z, Thyagarajan A, Sahu RP. Tyrosine kinase inhibitors and human epidermal growth factor receptor-2 positive breast cancer. World J Clin Oncol 2023; 14(5): 198-202
- URL: https://www.wjgnet.com/2218-4333/full/v14/i5/198.htm
- DOI: https://dx.doi.org/10.5306/wjco.v14.i5.198
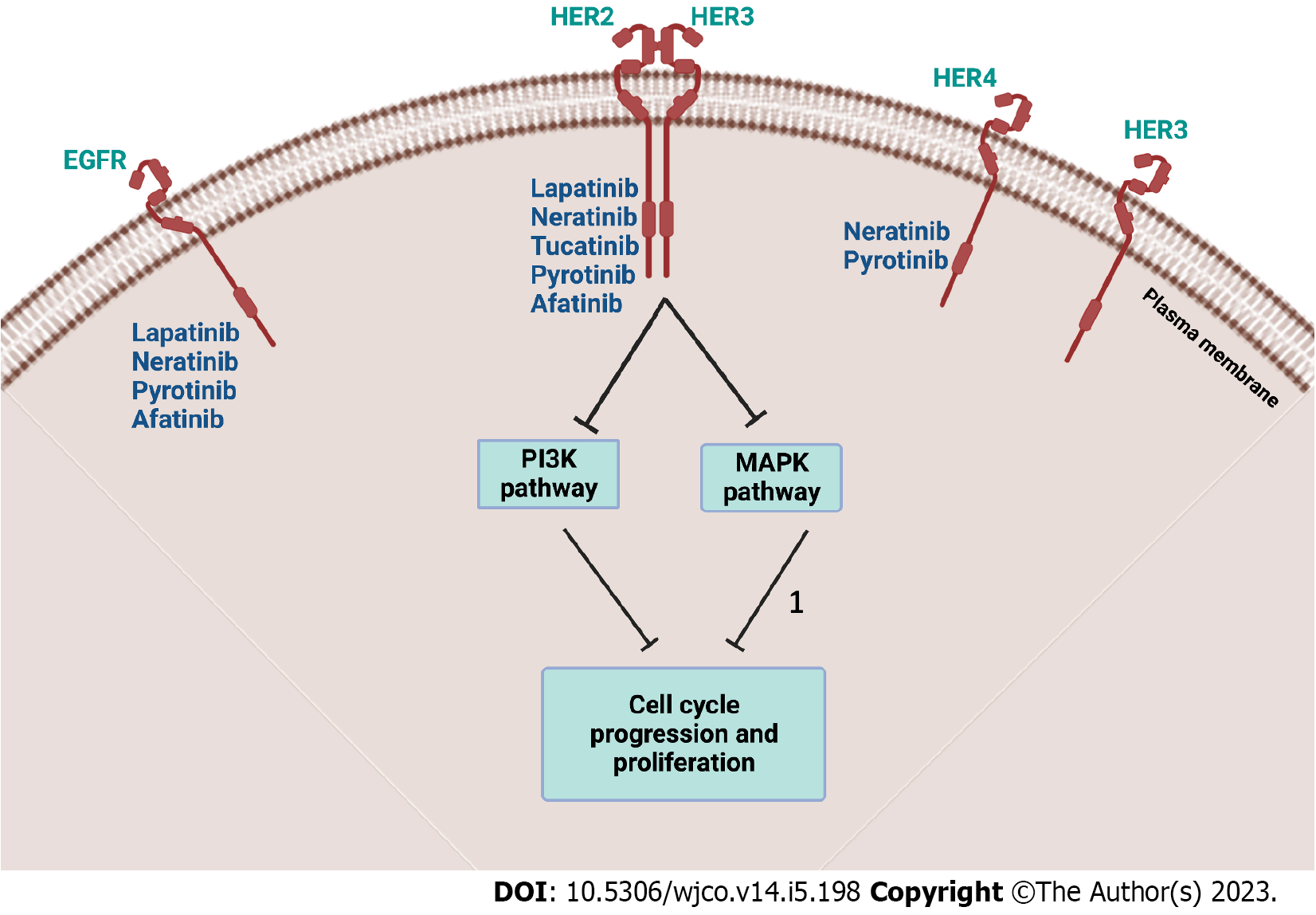
In 2022, breast cancer (BC) has been the most common cause of cancer-related mortality in women in the United States[1]. Amongst all confirmed BC cases, human epidermal growth factor receptor-2 (HER2) positive BC is estimated to comprise around 15%-20%[2]. Thus, the emergence of HER2-directed therapy, namely, humanized monoclonal antibodies (mAbs), has transformed the path of BC outcomes. The first agent, Trastuzumab, was approved by the United States Food and Drug Administration (FDA) in the past two decades and has revolutionized the treatment modalities[3]. Soon after the approval of other mAbs such as Pertuzumab, and ado-Trastuzumab emtansine, several tyrosine kinase inhibitors (TKIs) have also been approved as targeted therapies[4]. Figure 1 illustrates various TKIs and their targets. Within the last two years (2021 and 2022), significant additions to the literature were made on the use of TKIs in HER2 positive BC. This commentary aims to highlight the most recent findings published in the literature up to this date. Furthermore, since all TKIs, (e.g., Lapatinib, Neratinib, Pyrotinib, and Tucatinib) can be used to treat both early stages and metastatic BC (mBC), either in combination or as monotherapy, their addition to hospital formularies can be of benefit from a pharmacoeconomic perspective[5]. The summary highlighting the ongoing and completed/terminated clinical trials on TKIs in HER2 positive BC patients is given in Table 1.
| Study title | Conditions | Interventions | Outcome measures | NCT number |
| Pyrotinib rechallenge in HER2-positive metastatic breast cancer pretreated with Pyrotinib and Trastuzumab | HER2-positive breast cancer, metastatic breast cancer | Trastuzumab plus chemotherapy: Trastuzumab in combination with Pyrotinib plus chemotherapy | PFS, ORR, AEs | NCT05346861[14] |
| A study of Pyrotinib plus Capecitabine in patients with HER2+ metastatic breast cancer | HER2 positive metastatic breast cancer | Pyrotinib, Capecitabine | PFS, ORR, AEs, SAEs, DoR, CBR, OS | NCT02973737[15] |
| A randomized controlled trial of HER2 positive breast cancer patients treated with Lapatinib vs herceptin | HER2-positive breast cancer | Lapatinib/Herceptin | DFS, OS | NCT03085368[16] |
| Tykerb evaluation after chemotherapy (TEACH): Lapatinib versus placebo in women with early-stage breast cancer | Neoplasms, breast | Lapatinib | This clinical trial has several outcomes measures to be evaluated including DFS, OS, MDFS | NCT00374322[17] |
| Neo altto (neoadjuvant Lapatinib and/or Trastuzumab treatment optimization) study | Neoplasms, breast | Lapatinib, Trastuzumab, Paclitaxel | This clinical trial has several outcomes measures to be evaluated including OS, Par with pCR at the ToS, OR at the ToS | NCT00553358[18] |
| Lapatinib in combination with Trastuzumab versus Lapatinib monotherapy in subjects with HER2-positive metastatic breast cancer | Neoplasms, breast | Lapatinib, Trastuzumab | PFS, OS, OR, CBR, TTR, DR, change from baseline in FACT-B scores at week 4, week 12, week 16, week 24, and conclusion or withdrawal from study | NCT00320385[19] |
| Paclitaxel with/without GW572016 (Lapatinib) as first line therapy for women with advanced or metastatic breast cancer | Neoplasms, breast | Paclitaxel, GW572016 (Lapatinib) | This clinical trial has several outcomes measures to be evaluated including PFS, OS, DoR | NCT00075270[20] |
| Continued HER2 suppression with Lapatinib plus Trastuzumab vs Trastuzumab alone (terminated) | Cancer | Lapatinib, Trastuzumab | PFS, OS, Best overall response, CBR (CR, PR or SD ≥ 24 wk), AE | NCT00968968[21] |
In a recent phase III randomized controlled trials, dual HER2 blockade with Lapatinib, Trastuzumab, and an aromatase inhibitor (AI) was found to be superior compared to a single HER2 blockade with AI plus Lapatinib alone or Trastuzumab alone in terms of progression-free survival (PFS) in postmenopausal women [hazard ratio: 0.62 (95%CI: 0.45-0.88); P = 0.0063][6]. However, this trial was intended to offer an alternative regimen for patients not receiving chemotherapy, a scenario typically followed when chemotherapy is contraindicated[6]. Nevertheless, the question of whether dual blockade with Lapatinib + Trastuzumab combination can be superior to first-line chemotherapy in terms of PFS remained unanswered.
Conversely, in another phase III trial, Pyrotinib + Capecitabine combination was found to yield longer PFS [12.5 mo (95%CI: 9.7–not reached)] as compared to the arm receiving Lapatinib + Capecitabine treatment [6.8 mo (5.4–8.1); hazard ratio 0.39 (95%CI: 0.27–0.56); one-sided P < 0.0001][7]. However, unlike the above-mentioned trial, the patient population in this trial was comprised of mBC patients.
Along similar lines, when Neratinib + Capecitabine (N + C) treatment was compared to Lapatinib + Capecitabine (L + C) combination, N + C resulted in longer PFS (Median PFS = 7 mo compared to 5.4 mo; P = 0.0011)[8]. Besides, the duration of response (DoR) in N + C vs L + C was 11.1 mo vs 4.2 mo (P < 0.0001), and time to intervention for central nervous system (CNS) illness was 27.9% vs 33.8% (P = 0.039) in Asian patients with mBC who had previously received at least two HER2-directed regimens[8]. The effectiveness and safety profiles of the N + C combination in the Asian group matched those of the general population. The studies indicated that Neratinib may provide further advantages for HER2+ mBC patients treated with Trastuzumab-only regimens for their metastatic illnesses such as CNS[8].
With the scarcity of published evidence comparing the efficacy of Tucatinib to other TKIs, the question of whether it offers additional PFS benefit was investigated through one network meta-analysis[9]. The data demonstrated that the combination of Tucatinib + Trastuzumab + Capecitabine is regarded as the most effective option in improving both overall survival (OS) and PFS (P = 0.003 and P < 0.0001). With OS, the choices of Trastuzumab emtansine (P < 0.004) and Pertuzumab + Trastuzumab + Capecitabine (P = 0.011) are comparatively superior. On the other hand, Neratinib and Lapatinib resulted in greater improvement in PFS (P = 0.001) when combined with Capecitabine[9].
However, despite the promising efficacy of Tucatinib over other TKIs, it was associated with increased levels of serum creatinine, which was concerning regarding its effect on renal function. However, the increase in serum creatinine level was found to be attributed to the inhibition of tubular secretion of creatinine[10]. Importantly, one study evaluated the use of Tucatinib vs placebo when both were combined with Trastuzumab and Capecitabine. It was concluded that Tucatinib can significantly improve OS (9.1 mo longer in the Tucatinib group) and delay the progression of brain metastasis [hazard ratio, 0.55 (95%CI: 0.36-0.85)][11].
Of note, within the last two years, no additional data regarding Afatinib’s use in HER2 positive BC was published. Notably, only one study reported the benefits of Afatinib but the subjects included were not limited to BC, and those included BC patients were not HER2 positive[12]. Thus, there is no significant update regarding Afatinib’s role in HER2 positive BC treatment.
With Ruxolitinib, a class of the Janus kinase inhibitors, the first and only study performed so far with a Trastuzumab combination indicated that the tolerability data is appealing[12]. However, there was no difference in the PFS than that of Trastuzumab alone in mBC patients as compared to the historical control[13]. To draw a more robust conclusion regarding Ruxolitinib and explore its implications with TKIs, more interventional studies are warranted with larger power using randomized and prospective designs since these aspects are lacking in Ruxolitinib studies.
In conclusion, while the body of evidence currently available in the literature is still insufficient to offer recommendations in the treatment guidelines of HER2 positive BC, the existing studies concluding the benefits of TKIs promise hope for patients resistant to conventional first- and second-line treatments.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kegyes D, Romania; Merrett ND, Australia S-Editor: Li L L-Editor: A P-Editor: Zhang XD
| 1. | Miller KD, Fidler-Benaoudia M, Keegan TH, Hipp HS, Jemal A, Siegel RL. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin. 2020;70:443-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 737] [Article Influence: 147.4] [Reference Citation Analysis (0)] |
| 2. | American Cancer Society. Breast cancer HER2 status (2021) What is HER2 Status? American Cancer Society. [cited 17 April 2023]. Available from: https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-her2-status.html. |
| 3. | Collins DM, Madden SF, Gaynor N, AlSultan D, Le Gal M, Eustace AJ, Gately KA, Hughes C, Davies AM, Mahgoub T, Ballot J, Toomey S, O'Connor DP, Gallagher WM, Holmes FA, Espina V, Liotta L, Hennessy BT, O'Byrne KJ, Hasmann M, Bossenmaier B, O'Donovan N, Crown J. Effects of HER Family-targeting Tyrosine Kinase Inhibitors on Antibody-dependent Cell-mediated Cytotoxicity in HER2-expressing Breast Cancer. Clin Cancer Res. 2021;27:807-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 4. | Yang X, Wu D, Yuan S. Tyrosine Kinase Inhibitors in the Combination Therapy of HER2 Positive Breast Cancer. Technol Cancer Res Treat. 2020;19:1533033820962140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Schlam I, Swain SM. HER2-positive breast cancer and tyrosine kinase inhibitors: the time is now. NPJ Breast Cancer. 2021;7:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 156] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 6. | Johnston SRD, Hegg R, Im SA, Park IH, Burdaeva O, Kurteva G, Press MF, Tjulandin S, Iwata H, Simon SD, Kenny S, Sarp S, Izquierdo MA, Williams LS, Gradishar WJ. Phase III, Randomized Study of Dual Human Epidermal Growth Factor Receptor 2 (HER2) Blockade With Lapatinib Plus Trastuzumab in Combination With an Aromatase Inhibitor in Postmenopausal Women With HER2-Positive, Hormone Receptor-Positive Metastatic Breast Cancer: ALTERNATIVE. J Clin Oncol. 2018;36:741-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Xu B, Yan M, Ma F, Hu X, Feng J, Ouyang Q, Tong Z, Li H, Zhang Q, Sun T, Wang X, Yin Y, Cheng Y, Li W, Gu Y, Chen Q, Liu J, Cheng J, Geng C, Qin S, Wang S, Lu J, Shen K, Liu Q, Wang H, Luo T, Yang J, Wu Y, Yu Z, Zhu X, Chen C, Zou J; PHOEBE Investigators. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22:351-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 251] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 8. | Dai MS, Feng YH, Chen SW, Masuda N, Yau T, Chen ST, Lu YS, Yap YS, Ang PCS, Chu SC, Kwong A, Lee KS, Ow S, Kim SB, Lin J, Chung HC, Ngan R, Kok VC, Rau KM, Sangai T, Ng TY, Tseng LM, Bryce R, Bebchuk J, Chen MC, Hou MF. Analysis of the pan-Asian subgroup of patients in the NALA Trial: a randomized phase III NALA Trial comparing neratinib+capecitabine (N+C) vs lapatinib+capecitabine (L+C) in patients with HER2+metastatic breast cancer (mBC) previously treated with two or more HER2-directed regimens. Breast Cancer Res Treat. 2021;189:665-676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | DeBusk K, Abeysinghe S, Vickers A, Nangia A, Bell J, Ike C, Forero-Torres A, Blahna MT. Efficacy of tucatinib for HER2-positive metastatic breast cancer after HER2-targeted therapy: a network meta-analysis. Future Oncol. 2021;17:4635-4647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Topletz-Erickson AR, Lee AJ, Mayor JG, Rustia EL, Abdulrasool LI, Wise AL, Dailey B, DeChenne S, Walker LN, Alley SC, Endres CJ. Tucatinib Inhibits Renal Transporters OCT2 and MATE Without Impacting Renal Function in Healthy Subjects. J Clin Pharmacol. 2021;61:461-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Lin NU, Murthy RK, Abramson V, Anders C, Bachelot T, Bedard PL, Borges V, Cameron D, Carey LA, Chien AJ, Curigliano G, DiGiovanna MP, Gelmon K, Hortobagyi G, Hurvitz SA, Krop I, Loi S, Loibl S, Mueller V, Oliveira M, Paplomata E, Pegram M, Slamon D, Zelnak A, Ramos J, Feng W, Winer E. Tucatinib vs Placebo, Both in Combination With Trastuzumab and Capecitabine, for Previously Treated ERBB2 (HER2)-Positive Metastatic Breast Cancer in Patients With Brain Metastases: Updated Exploratory Analysis of the HER2CLIMB Randomized Clinical Trial. JAMA Oncol. 2023;9:197-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 110] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 12. | Salawu A, Hansen AR, Spreafico A, Al-Ezzi E, Webster S, Bedard PL, Doi J, Wang L, Siu LL, Abdul Razak AR. A Phase 2 Trial of Afatinib in Patients with Solid Tumors that Harbor Genomic Aberrations in the HER family: The MOBILITY3 Basket Study. Target Oncol. 2022;17:271-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Kearney M, Franks L, Lee S, Tiersten A, Makower DF, Cigler T, Mundi P, Chi DC, Goel A, Klein P, Andreopoulou E, Sparano J, Trivedi M, Accordino M, Califano A, Hershman DL, Silva J, Kalinsky K. Phase I/II trial of ruxolitinib in combination with trastuzumab in metastatic HER2 positive breast cancer. Breast Cancer Res Treat. 2021;189:177-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Zhang J. Pyrotinib Rechallenge in Her2-positive Metastatic Breast Cancer Pretreated With Pyrotinib and Trastuzumab. [accessed 2023 Apr 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT05346861 ClinicalTrials.gov Identifier: NCT05346861. |
| 15. | Jiangsu HengRui Medicine Co. A Study of Pyrotinib Plus Capecitabine in Patients With HER2+ Metastatic Breast Cancer. [accessed 2023 Apr 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02973737 ClinicalTrials.gov Identifier: NCT02973737. |
| 16. | Peking Union Medical College Hospital. A Randomized Controlled Trial of HER-2 Positive Breast Cancer Patients Treated With Lapatinib vs Herceptin. [accessed 2023 Apr 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03085368 ClinicalTrials.gov Identifier: NCT03085368. |
| 17. | GlaxoSmithKline. Tykerb Evaluation After Chemotherapy (TEACH): Lapatinib Versus Placebo In Women With Early-Stage Breast Cancer. [accessed 2023 Apr 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT00374322 ClinicalTrials.gov Identifier: NCT00374322. |
| 18. | Novartis (Novartis Pharmaceuticals). Neo ALTTO (Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimisation) Study (Neo ALTTO). [accessed 2023 Apr 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT00553358 ClinicalTrials.gov Identifier: NCT00553358. |
| 19. | GlaxoSmithKline. Lapatinib In Combination With Trastuzumab Versus Lapatinib Monotherapy In Subjects With HER2-positive Metastatic Breast Cancer. [accessed 2023 Apr 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT00320385 ClinicalTrials.gov Identifier: NCT00320385. |
| 20. | GlaxoSmithKline. Paclitaxel With / Without GW572016 (Lapatinib) As First Line Therapy For Women With Advanced Or Metastatic Breast Cancer. [accessed 2023 Apr 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT00075270 ClinicalTrials.gov Identifier: NCT00075270. |
| 21. | Novartis (Novartis Pharmaceuticals). Continued HER2 Suppression With Lapatinib Plus Trastuzumab Versus Trastuzumab Alone. [accessed 2023 Apr 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT00968968 ClinicalTrials.gov Identifier: NCT00968968. |