Published online Mar 24, 2023. doi: 10.5306/wjco.v14.i3.99
Peer-review started: November 20, 2022
First decision: January 3, 2023
Revised: February 10, 2023
Accepted: March 1, 2023
Article in press: March 1, 2023
Published online: March 24, 2023
Processing time: 119 Days and 6.5 Hours
Myeloproliferative neoplasms (MPNs) are defined as clonal disorders of the hematopoietic stem cell in which an exaggerated production of terminally differentiated myeloid cells occurs. Classical, Philadelphia-negative MPNs, i.e., polycythemia vera, essential thrombocythemia and primary myelofibrosis, exhibit a propensity towards the development of thrombotic complications that can occur in unusual sites, e.g., portal, splanchnic or hepatic veins, the placenta or cerebral sinuses. The pathogenesis of thrombotic events in MPNs is complex and requires an intricate mechanism involving endothelial injury, stasis, elevated leukocyte adhesion, integrins, neutrophil extracellular traps, somatic mutations (e.g., the V617F point mutation in the JAK2 gene), microparticles, circulating endothelial cells, and other factors, to name a few. Herein, we review the available data on Budd-Chiari syndrome in Philadelphia-negative MPNs, with a particular focus on its epidemiology, pathogenesis, histopathology, risk factors, classification, clinical presentation, diagnosis, and management.
Core Tip: Myeloproliferative neoplasms (MPNs) are defined as clonal disorders of the hematopoietic stem cell in which an exaggerated production of terminally differentiated myeloid cells occurs. MPNs are characterized by a propensity towards the development of thrombotic complications, including Budd-Chiari syndrome (BCS). Herein, we review the available data on BCS in MPNs, with a particular focus on its epidemiology, pathogenesis, histopathology, risk factors, classification, clinical presentation, diagnosis, and management.
- Citation: Găman MA, Cozma MA, Manan MR, Srichawla BS, Dhali A, Ali S, Nahian A, Elton AC, Simhachalam Kutikuppala LV, Suteja RC, Diebel S, Găman AM, Diaconu CC. Budd-Chiari syndrome in myeloproliferative neoplasms: A review of literature. World J Clin Oncol 2023; 14(3): 99-116
- URL: https://www.wjgnet.com/2218-4333/full/v14/i3/99.htm
- DOI: https://dx.doi.org/10.5306/wjco.v14.i3.99
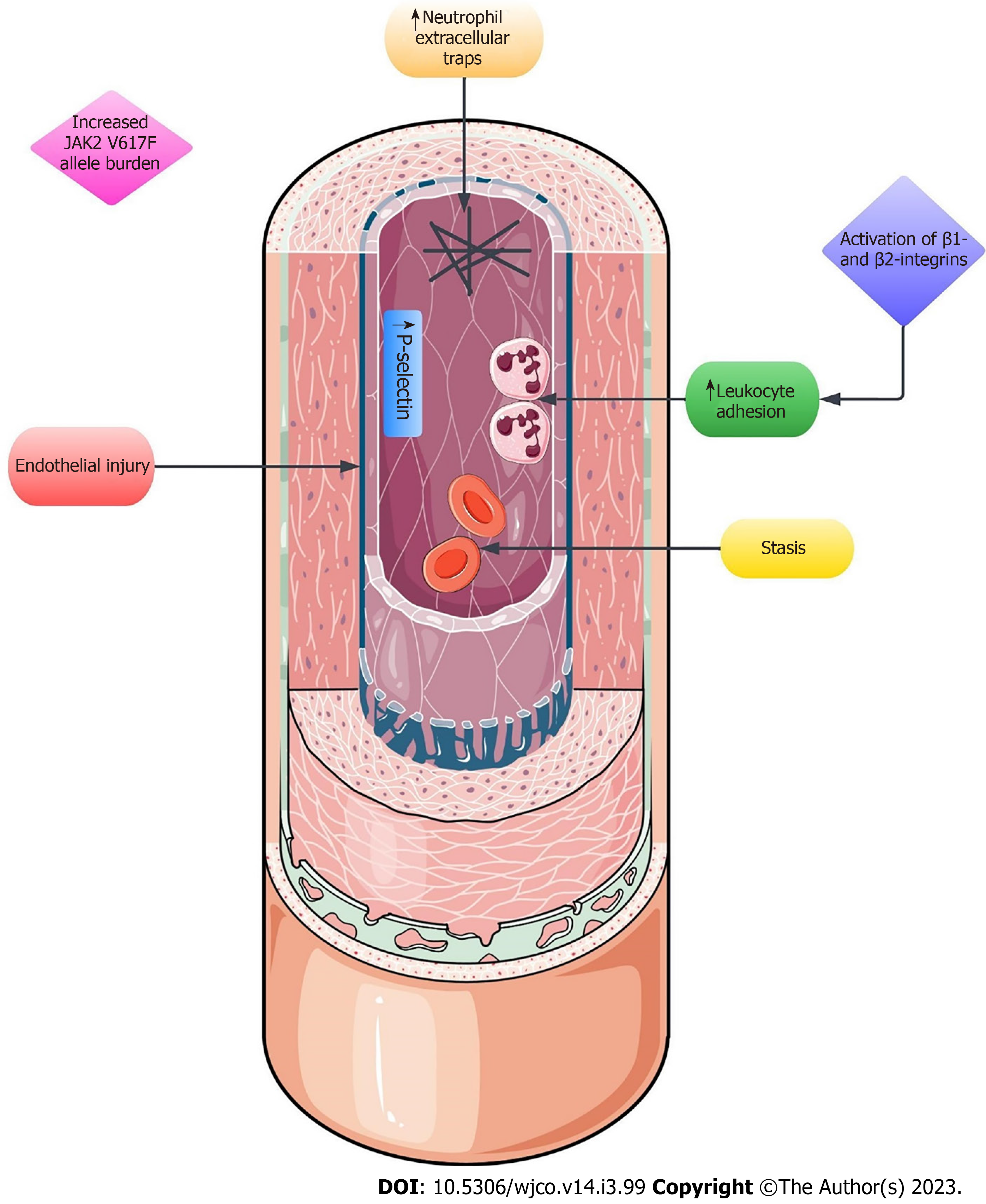
Myeloproliferative neoplasms (MPNs) are defined as clonal disorders of the hematopoietic stem cell in which an exaggerated production of terminally differentiated myeloid cells occurs[1]. Classical, Philadelphia-negative MPNs include polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis, whereas chronic myeloid leukemia (CML) is the hallmark Philadelphia-positive MPN[1,2]. Philadelphia-negative MPNs exhibit a propensity towards the development of thrombotic complications[2,3]. In MPNs, thrombosis can occur in unusual sites, e.g., portal, splanchnic or hepatic veins, the placenta or cerebral sinuses[4]. The pathogenesis of thrombotic events in MPNs is complex and requires an intricate mechanism involving endothelial injury, stasis, elevated leukocyte adhesion, integrins, neutrophil extracellular traps (NETs), somatic mutations (e.g., the V617F point mutation in the JAK2 gene), microparticles, circulating endothelial cells, and other factors, to name a few (Figure 1)[4,5]. Herein, we review the available data on Budd-Chiari syndrome (BCS) in Philadelphia-negative MPNs, with a particular focus on its epidemiology, pathogenesis, histopathology, risk factors, classification, clinical presentation, diagnosis, and management. MPNs lead to an increased risk of thrombosis through various mechanisms. This includes increased P-selectin expression, activation of integrins causing leukocyte adhesion, and the novel mechanism of NETs formation.
BCS is a heterogeneous group of disorders characterized by hepatic venous outflow tract obstruction, ranging from the small hepatic veins, the three suprahepatic veins and all the way to the junction of the inferior vena cava (IVC) and right atrium. This classification eliminates hepatic blood flow impairments caused by cardiac illness, pericardial disease, or sinusoidal obstruction syndrome (porto-sinusoidal vascular disorder)[6-9]. Primary BCS is the obstruction due to a predominantly venous process (thrombosis or phlebitis), whereas secondary BCS denominates the compression or invasion of the hepatic veins and/or IVC by a lesion that originates from outside of the vein (most commonly malignancy, abscess, or lymphadenopathy)[7,10,11]. It is a typical example of post-sinusoidal portal hypertension[6,8].
BCS is a rare condition across the globe. Expectedly, there is limited epidemiologic data on this entity. A recent meta-analysis involving data from Asian and European studies highlighted a pooled incidence of BCS at 1 case per million people and a prevalence of 11 cases per million people[12]. This report found significant heterogeneity among the analyzed assessments due to differences in study designs (diagnostic criteria, population characteristics, population sizes), as well as generally limited data on the topic from the Americas or Africa. The investigations were conducted on populations from Japan, South Korea, Denmark, Sweden, Italy, and France. Earlier studies from China depicted the incidence of BCS at 0.88 per million and a prevalence ranging from 6.40 to 7.69 per million[13,14]. It is important to note, though, that most of these epidemiologic studies included both primary and secondary BCS. One study from France reported a median age of patients with primary BCS at 46.9 years. In their cohort, 30.6% were male and 69.4% female. Oral contraceptive use and pregnancy are gender-specific risk factors of BCS which may contribute to the female predominance[15]. In fact, hepatic vein thrombosis leading to BCS was more common in females, whereas obstruction of both the hepatic veins and IVC was more common in males[16]. There seems to be a geographic distribution of MPNs-related BCS cases. For example, Qi et al[17] reported that of their cohort of 246 cases of BCS diagnosed over nearly 12 years in China, only 5 cases were attributable to MPNs.
BCS, by definition, is the obstruction of the hepatic veins and/or outflow tract into the IVC. This entity is distinguished from portal vein thrombosis (PVT) and splanchnic vein thrombosis (SVT), which can coexist with BCS though are distinct pathologic processes[18]. Prior literature even reports presence of PVT in BCS at 10%-20%, which suggests a relatively poor prognosis[19]. Generally, the etiology of BCS is categorized as thrombotic or non-thrombotic. Thrombotic obstruction is the most common cause of BCS, and this is referred to as primary BCS. There are numerous conditions associated with primary BCS, including inherited thrombophilia, thalassemia, paroxysmal nocturnal hemoglobinuria, MPNs, pregnancy, oral contraceptive use, or even inflammatory conditions, e.g., Behcet’s disease, celiac disease, and ulcerative colitis[20-22]. Non-thrombotic causes of BCS, referred to as secondary BCS, typically involve a mass lesion involving the hepatic veins or compression by adjacent structures. There are many case reports describing unique causes of secondary BCS, e.g., polycystic kidney disease, liver abscess, hydatid cysts, and cardiac myxoma; however, this etiology is uncommon[23-26]. MPNs are the most common cause of BCS, and the prevalence of BCS in the setting of MPN ranges from 32.9% up to 49.5%[27,28]. MPNs represent a malignant proliferation of myeloid cell lines, with the most common blood cancers classified in this category being CML, PV, ET and PMF. Thrombotic complications, such as BCS, are of particular concern in the setting of PV and ET, as these conditions carry significantly greater risk of such thrombotic processes[28]. A meta-analysis found that PV was the most common of MPNs to be diagnosed in the setting of BCS, even more than in subjects with PVT[28]. There is also evidence pointing towards increased thrombotic risk in MPNs carrying JAK2 gene mutations[29] and about 41% of individuals with BCS exhibit genetic changes in the aforementioned gene[30,31].
Histopathological features of BCS are studied in great detail and the histopathology of BCS is well established in the existing body of evidence[32-35]. Sinusoidal dilatation and congestion, centrilobular inflammation and necrosis, regenerative hyperplasia, macrovesicular steatosis, cholestasis, glycogenated nuclei, and perivenular fibrosis are the common histological features seen in this condition. Regenerative nodules, even though seen in both BCS and cardiac cirrhosis, is more common in BCS. Sinusoidal dilatation is the hallmark microscopic finding which can be appreciated in the initial stages of the disease[36]. However, this finding is not unique to BCS and can be seen in other conditions[37]. Having said that, in the presence of prominent sinusoidal dilatation, hepatic outflow obstruction should be ruled out as an important differential. Centrilobular necrosis is another important pathological feature that is more commonly seen in BCS compared to cardiac cirrhosis. This is attributed to the fact that hepatic hypoxia preferentially affects the centrilobular hepatocytes[38,39]. To the best of our knowledge, there is no literature on variation of the histopathological patterns in BCS secondary to MPNs. Moreover, a prognostic grading system for the same also does not exist and is a potential area for further studies.
Smalberg et al[28] have depicted in their meta-analysis a strong relation between MPNs and SVT. This was confirmed by the high prevalence of JAK2V617F in BCS. MPNs and JAK2V617F are more commonly associated with BCS compared to PVT. This may be due to focal inflammatory insult to the portal venous system which is required for PVT[40]. There is a considerable difference between PVT and BCS with regard to the subtypes of MPNs. PV is more commonly associated with BCS than with PVT. There is a high pro-thrombotic effect as the hematocrit increases. In these situations, low-shear venous circulation is impacted more by the increased blood viscosity[41-43]. The interaction between adhesion molecules and red cells may be responsible for this mechanism. However, it was observed that PVT is more common in PMF compared to BCS[28]. This may be due to the fact that splenomegaly in PMF causes compression of the portal system leading to stasis of blood. In the same study, JAK2V617F-positive MPNs were found to be associated with PVT more frequently than BCS[28]. However, the exact reason is not known. In terms of CALR gene mutations, Li et al[45] highlighted that 1.41% of BCS cases exhibit genetic alterations in the CALR gene. In JAK2V617F-negative MPNs-related BCS cases, CALR gene mutations were detected in 17.22% of the examined individuals [44].Mutations in other genes, i.e., MPL or TET2, have rarely been depicted in BCS. However, the detection of a somatic gene mutation and especially of JAK2V617F in BCS should alert the clinician to screen for MPNs, including at follow-up if the diagnosis of overt MPNs is not established. In addition, work-up for hereditary thrombophilia should be performed as part of the molecular-driven diagnosis of BCS.
BCS can be classified based on three factors: (1) Origin of obstructive lesion (endoluminal/primary and extraluminal/secondary); (2) Site of obstruction; (3) Onset of disease pathology (fulminant, subacute, acute, and chronic) (Table 1). Primary BCS refers to occlusion resulting from endoluminal venous pathologies such as thrombosis, stenosis, endophlebitis, and webs, while secondary BCS is extraluminal in origin with compression being caused extrinsically by neighboring structures, i.e., cysts, abscess, hyperplastic nodules, or invasive tumors[18]. Three classifications can be presented based on the site of the obstructive lesion (Table 2). As highlighted by Patil et al veno-occlusive disease (type III presented by Chaubal et al[46]) results when sinusoidal endothelial cells are primarily injured, and so it may be regarded as a separate entity known as the sinusoidal obstruction syndrome[46-48].
| Ref. | Type | Site of obstruction |
| Chaubal et al[46] | I | Obstruction of IVC with or without secondary hepatic vein occlusion |
| II | Obstruction of major hepatic veins | |
| III | Obstruction of small centrilobular veins (considered by some as veno-occlusive disease) | |
| Patil et al[48] | I | Lesions of the IVC |
| IIa | Short segment (< 4 cm) lesion of the hepatic vein | |
| IIb | Diffuse lesion of the hepatic vein | |
| III | Mixed type with lesions of IVC and the hepatic vein | |
| Bansal et al[47] | I | Hepatic vein obstruction or thrombosis without IVC obstruction or compression |
| II | Hepatic vein obstruction or thrombosis with IVC obstruction or thrombosis | |
| III | Isolated hepatic venous webs | |
| IV | Isolated IVC webs |
| Type | Description of pathology |
| A | Acute hepatic vein thrombosis or stent block precipitates ACLF in a BCS |
| B | Non-thrombotic acute insult precipitates ACLF in a chronic BCS |
| C | Acute hepatic vein thrombosis precipitates ACLF in a non-vascular chronic |
| Liver disease |
The clinical presentation of BCS varies widely depending upon the extent as well as the site and rapidity of hepatic venous outflow obstruction. This causes varied degrees of liver involvement, resulting in about 20% of the patients having little to no symptoms at all[49,50]. Owing to the development of intrahepatic, extrahepatic, or portosystemic collaterals, these patients do not show any discernible signs of venous obstruction. In contrast, patients with symptomatic hepatic vein obstruction present with symptoms of portal hypertension such as ascites, upper gastrointestinal bleeding, and hepatic encephalopathy with right upper quadrant abdominal pain. Abdominal examination may further reveal a tender hepatomegaly with splenomegaly. Therefore, a classical triad of abdominal pain, ascites, and hepatomegaly should raise a clinical suspicion of BCS. In patients with obstruction of the IVC, the signs and symptoms greatly vary. As a result, some authors refer to the hepatic complications of IVC obstruction as “obliterative hepatocavopathy”[51]. These patients may have signs of caval obstruction such as pedal edema, varicocele, lower limb ulcers, and/or dilated subcutaneous veins in the abdomen, chest, and back[52]. The rapidity of venous obstruction may give rise to varied degrees and forms of presentation, i.e., fulminant, acute, subacute, and chronic. Patients with fulminant disease have a hyperacute onset of disease pathology (≤ 2 month), which is manifested as acute hepatic failure with ascites, hyperbilirubinemia, tender hepatomegaly, and renal failure secondary to renal outflow compromise resulting from hepatic vein obstruction[8,53]. Particularly, the development of hepatic encephalopathy within 2 months of onset of jaundice is regarded as fulminant disease[54]. Fulminant disease requires acute obstruction of all three hepatic veins and so its recorded incidence is quite low [55]. Acute BCS has a short duration of onset which is usually within a month while the onset of subacute ranges from one to six months[54]. Interestingly, there is data to suggest geographical variation in the incidence of various types of BCS based on onset of pathology. In the eastern geographic regions, chronic presentations are more prevalent with onset ranging from 6 months to 30 years[54,56]. In the western geographic region, acute presentation is encountered relatively more frequently[56]. Esophageal bleeding, ascites, and hepatic necrosis may be absent in patients of subacute BCS[8]. Finally, the chronic form may take more than 6 months to develop and is characterized by progressive abdominal distention without jaundice. These patients may have signs of portal hypertension including variceal bleeding as well as splenomegaly. Renal impairment may not be seen in 50% of these patients with chronic BCS[57]. These symptoms may or may not be accompanied by a wide range of nonspecific symptoms. Though a plethora of differential diagnosis may be present at this point-and though it is true that BCS is generally a rare disease-clinicians must not exclude the possibility of BCS and as discussed by Aydinli and Bayraktar, clinical suspicion of BCS should escalate in the following scenarios: Acute onset ascites with tender hepatomegaly, massive ascites with relatively preserved liver functions, fulminant hepatic failure associated with hepatomegaly and ascites, unexplained chronic liver disease, liver disease with thrombogenic disorder, and sinusoidal dilation on liver biopsy without heart disease[18].
Acute liver failure is a sequelae of BCS that is infrequent in its occurrence. According to two case series, BCS accounts for 0.9% to 15% of the cases of acute liver failure. Majority of the patients, in the larger case series reporting 20 cases of BCS in 2344 patients of acute liver failure, were middle aged Caucasian women with PV. Acute-on-chronic manifestation, however, is relatively more frequent as compared to acute liver failure. To direct appropriate management, the Asian Pacific Association for the Study of the Liver has further classified the acute-on-chronic manifestation of BCS into three types based on underlying liver disease and acute insult (Table 2)[56]. Table 3 depicts the clinical characteristics of BCS based on geographic region of the studies population.
| Clinical characteristics | Percentage in various geographic regions | ||||||
| Egypt | France | Sweden | China | Japan | United States | Algeria | |
| Ascites/Abdominal distension | 82 | 74.4 | 88 | 51.9 | 31.2 | 29.9 | 74.8 |
| Abdominal fullness | - | - | - | - | 26.1 | - | - |
| Abdominal discomfort | - | - | - | - | 17.8 | - | - |
| Abdominal pain | 96 | 72.4 | 81 | - | 2.5 | - | 42.6 |
| Cirrhosis | - | - | - | - | - | 18.6 | - |
| Hepatomegaly | 50 | 70.1 | 72 | - | 54.7 | - | - |
| Splenomegaly | 42 | 49.1 | 66 | - | - | - | - |
| Esophageal varices | - | 54.8 | 67 | - | - | 7.4 | - |
| Hematemesis/Variceal bleed | 36 | - | 9 | 16 | 8.3 | 3.2 | - |
| Melena | - | - | - | - | 2.5 | - | - |
| Jaundice | 16 | 20.3 | 29 | - | 5.7 | - | 13.9 |
| Fever | - | 20.1 | 27 | - | - | - | 15.7 |
| Hydrothorax | - | 13.0 | - | - | - | - | - |
| Acute kidney injury | - | - | - | - | - | 18.8 | - |
| History of recurrent abortions | 6 | - | - | - | - | - | - |
| History of previous thrombosis | 10 | - | - | - | - | 7 | - |
| Recurrent orogenital ulcers | 4 | - | - | - | - | - | - |
| Leg ulcers | 2 | - | - | - | 3.8 | - | - |
| Lower limb edema | 68 | - | - | 58.9 | 31.8 | - | 13 |
| Dilated abdominal veins | 40 | - | - | 57 | 27.3 | - | - |
| Hepatic encephalopathy | 36 | - | 12 | 0.7 | - | 9.5 | 5.2 |
| Ileus | - | - | 21 | - | - | - | - |
| General malaise | - | - | - | - | 12.1 | - | - |
| Acute respiratory failure | - | - | - | - | - | 7 | - |
Although BCS is considered a rare disease, it has the potential to rapidly deteriorate a patient's health. Therefore, the need to obtain a correct diagnosis, followed by rapid specific treatment is urgent and extremely important.
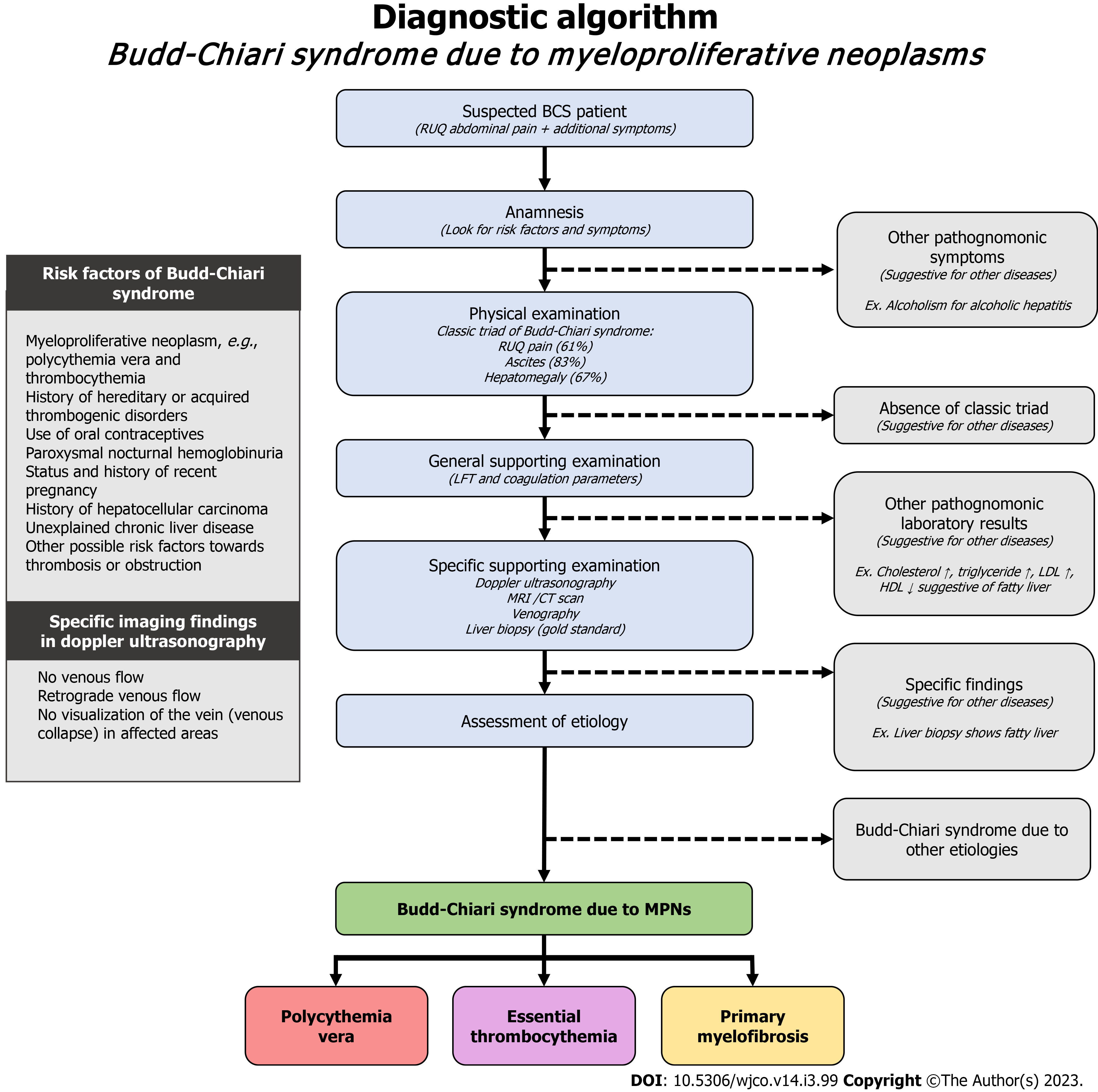
As BCS may be classified as primary (endoluminal lesion-like thrombosis) or secondary (extra-venous system causes), assessment of medical history plays a key role in identifying predisposing factors towards BCS[95]. Knowledge about these key points might be suggestive, not sufficient, of BCS diagnosis. Treatments administered by clinicians must put into account not only the obstruction by itself, but also its possible underlying causes: MPNs, e.g., PV, ET, PMF[63]; History of hereditary or acquired thrombogenic disorders[64]; Use of oral contraceptives[65,66]; Paroxysmal nocturnal hemoglobinuria[67]; Status and history of recent pregnancy[68,69]; History of hepatocellular carcinoma[70]; Chronic liver disease, remained unexplained after exclusion of alcoholism, chronic viral hepatitis B or C, autoimmunity, iron overload, Wilson’s disease and alpha-1 antitrypsin deficiency[71]; and other possible risk factors towards thrombosis or obstruction.
Majority of BCS patients present with the classic triad of abdominal pain especially in the upper right quadrant presenting in 61% of cases, ascites presenting in 83% of cases, and hepatomegaly presenting in 67% of cases[6,72]. The classic triad may form suddenly in acute cases (< 6 months), or progressively in chronic cases (> 6 months)[73]. These symptoms may or may not be accompanied by a wide range of nonspecific symptoms. Though a plethora of differential diagnosis may be present at this point-and though it is true that BCS is generally a rare disease – clinicians must not exclude the possibility of BCS.
Routine laboratory examinations might help aid clinicians to further pursue the presumptive diagnosis of BCS. Results that suggest the possibility of BCS include[6,73]: Diagnosis of PV, ET or PMF; normal or increase in liver function tests (alanine aminotransferase; aspartate aminotransferase); or findings which indicate thrombosis.
If a patient presents with the suspected risk factors, clinical presentation, and general supporting examination, clinicians must continue the diagnostic work-up with a high index of suspicion towards BCS. Specific supporting examination lies all around imaging modalities. These are the investigation methods useful towards diagnosing BCS, in an arranged order from the first line to the last[71]: Doppler ultrasonography; Magnetic resonance imaging (MRI) or computed tomography (CT) scan; Venography; Liver biopsy. Doppler ultrasonography is regarded as the initial technique of choice, offering a pooled sensitivity of 89% and specificity of 68% across multiple studies[74]. Doppler ultrasonography will show findings of no venous flow, retrograde venous flow, no visualization of the vein (possibly due to venous collapse) in the affected areas[75]. These findings might indicate BCS, though still overlapped by advanced cirrhosis[71]. However, in conditions where sonographic examination is inadequate to evaluate BCS, or in conditions where the distinct characteristics of BCS were not found, imaging through MRI or CT scan may be used in place to evaluate the presumptive diagnosis of BCS. MRI scan offers a pooled sensitivity of 93% and specificity of 55%, while CT scan offers a pooled sensitivity of 89% and specificity of 72%[74]. These modalities usually offer a clear diagnosis, though there might be uncertainty in patients with advanced cirrhosis[71]. Venography, and especially liver biopsy might be the last, most invasive, yet the gold standard of diagnosis. However, biopsy provides the best explanation towards the damage and specific etiologies contributing to the disease.
The assessment of BCS etiology should definitely actively search for potential MPNs. A meta-analysis found that distribution of MPN subtypes in BCS were as follows: PV (52.9%), ET (24.6%), PMF (6.7%), and unclassifiable MPNs (17.0%)[28]. A presumption about the etiology of BCS might be estimated from the results of general supporting examination. We propose a diagnostic algorithm for this instance as reported in Figure 2.
The treatment of BCS in MPNs requires a stepwise approach that may require a complete interdisciplinary team to adequately manage[54]. The goals of treatment aim at relieving obstruction, correcting the underlying conditions, and lastly to monitor for any liver deterioration[72]. The level of liver dysfunction can affect the coagulopathy of the patient and make anticoagulation difficult to predict in patients with BCS[54]. Despite this, the first line treatment for BCS due to MPNs still consists of anticoagulation therapy in order to relieve any obstruction. Furthermore, it is important to note that antiplatelet therapy should be initiated in patients as soon as possible once a diagnosis is established. Currently, the consensus when it comes to anticoagulation therapy is to treat with low molecular weight heparin (LMWH) and target an international normalized ratio (INR). In addition to the LMWH, patients should be started on an oral vitamin K antagonists (VKAs) (i.e., warfarin). Once the INR is between 2 and 3, the LMWH can be discontinued, however, the oral vitamin K antagonist should be continued life-long[6]. Although anticoagulation is the first line therapy for patients with BCS in MPNs, unfortunately, only 15%-20% of patients will respond to anticoagulation, as a result, other interventions may also need to be implemented in 80-85% of cases[54]. When looking at acute BCS it is important to consider methods that will restore patency of the thrombosed veins[76]. Treatment may start with thrombolytic therapy in select patients who have had symptoms for a few weeks and who have a well-established clot[54]. However, it is important to note that pharmacological thrombolysis has only been demon
Although angioplasty and stenting has started to play a major role and is a staple in the treatment of BCS it is not without risks. The primary risk associated with stenting is the risk of reocclusion. One intervention that has shown some promise in preventing reocclusion is the placement of a metal stent following angioplasty. However, it is important to also note that increased outcomes for this particular method is limited to some small case studies[40]. For BCS patients, inclusive of the individuals waiting for orthotopic liver transplantation, transjugular intrahepatic portosystemic shunt (TIPS) is suggested as a safer and very successful treatment strategy. TIPS is one of the major treatment options available for the treatment of BCS[81]. The Baveno IV consensus has resulted in a fairly uniform course of care for the patients of BCS, with prior consideration for the medical therapy alongside anticoagulation among all patients with no contraindications. This has been the case for more than 20 years of TIPS usage in BCS[82]. Because of the technical difficulty in sustaining venous patency for a longer period of time, TIPS should be specifically considered for the patients with Rotterdam class III, acute liver failure, or in those individuals who have failed medical therapy, diffuse hepatic vein thrombosis or prior hepatic venous stenting. Ascites is typically the most prevalent symptom, followed by variceal or gastrointestinal hemorrhage, with ascites rates in the trials under review reaching 100% and variceal bleeding rates reaching up to 30.9%. Since it has not been found as a potential risk factor in the occurrence of post-TIPS hepatic encephalopathy, prior hepatic encephalopathy need not be regarded as a contraindication to TIPS in BCS[81]. Additionally, pre-procedure jaundice is not regarded as a contraindication for TIPS in BCS patients, despite the fact that this is the case in patients with end-stage liver disease due to the higher mortality in that cohort, with the reason for the difference being hypothesized to be related to the absence of hepatocyte necrosis in the BCS patients[83]. Although there is no universal agreement across the studies regarding the ideal timing to perform TIPS, patients presenting with refractory ascites, hepatic failure, or the gastrointestinal hemorrhage should have access to this right away[84]. BCS patients have a greater rate of shunt dysfunction than other cirrhotic patients receiving TIPS (about 50% vs 80% within 1 year), which is likely related to the higher incidence of underlying thrombophilia[85]. Development of stents coated with polytetrafluoroethylene to be used during TIPS in the management of BCS has improved patients’ prognosis, mainly due to the decrease in the need for re-interventions and due to the tripling of the shunt patency[86]. Hence, TIPS could be a safer and effective treatment option for managing BCS in MPNs.
Over time, transplantation results have significantly improved and liver transplant outcomes are not harmed by prior TIPS. For patients with BCS, establishing venous outflow after liver transplantation is very essential and necessitates a variety of surgical procedures. These patients' outcomes exhibit various issues, such as vascular thrombosis and biliary difficulties[87]. Anticoagulation therapy, angioplasty, and TIPS fail in the 10% to 20% of BCS patients treated with a step-by-step management method, either due to technical failure or due to subpar clinical outcomes of a technically successful procedure necessitating rescue transplantation. Additionally, patients with fulminant liver failure and those with extremely advanced liver cirrhosis may benefit most from liver transplantation as treatment[88]. In the case that a medical therapy does not succeed, interventional revascularization and TIPS are recommended. The only guaranteed alternative for treating BCS is liver transplantation, if the presumptive medications and procedures are ineffective. Liver transplantation may be recommended as a last resort or in fulminant situations, with promising and favorable outcomes. Ibach et al[89] analyzed 46 cases of BCS of whom 22 suffered from MPNs and reported that individuals with BCS who were subjected to liver transplantation experienced a median survival of approximately 24 years. Mortality rates were higher in patients with BCS and MPNs (RR=3.44, P = 0.05), however, two patients diagnosed with these blood cancers died due to secondary acute myeloid leukemia and extramedullary hematopoiesis in the spleen with consequent organ rupture, events which can occur during disease evolution irrespective of the use of liver transplantation in these cases. Considering 5-year survival rates for PV and ET are of about 80% and of about 50% for PMF, the use of liver transplantation for MPNs-linked BCS is satisfactory in terms of survival prolongation[90]. Patients with BCS and Philadelphia-negative MPNs receive the same care as those without MPNs during the acute phase. LMWH or unfractionated heparin should be administered as soon as possible, followed by VKAs. It is advised to proceed gradually. A second-line approach based on invasive treatments, such as angioplasty with or without stenting, TIPS, or surgical portosystemic shunt, should be taken into consideration in the event that clinical deterioration persists despite anticoagulation [14,40,59]. While catheter-directed thrombolysis may be helpful for the treatment of acute and partially occlusive thrombosis, systemic thrombolytic therapy with tissue plasminogen activator is not very successful[78,91,92]. TIPS has recently been suggested as the preferred course of care for individuals with BCS who exhibit symptoms of portal hypertension. If TIPS is ineffective or inappropriate, angioplasty/stenting should be the second line of treatment for the subset of individuals. When TIPS and angioplasty/stenting are ineffective or inappropriate, surgical shunts ought to be the first line of treatment[93]. Consider liver transplantation as a curative measure[3,40,93,94].
An improved prognosis was introduced in the 1980s with the systematic use of VKAs in BCS patients[71,95], while the impact of oral anticoagulation on the survival of the most severe patients is debatable[6]. Although the ideal time frame for VKA is uncertain, lifelong medication is generally advised for BCS[40,94,96]. Only 5 (8%) of the 163 patients in the comprehensive survey-of whom the majority (86%) were getting VKA-experienced non-fatal variceal hemorrhage[97]. The rate of both recurrent thrombosis and bleeding complications was 11% in a different study on patients with BCS who underwent liver transplantation and received VKAs afterwards, but the mortality rate related to recurrence is higher than that related to bleeding (4.4% and 0.8% of patients, respectively)[98]. There are few specific studies on the effectiveness and safety of VKAs treatment in individuals with MPN-related BCS, and the majority of the studies refer to SVT as a whole. In total, 49 of the 604 patients with SVT in the aforementioned multicenter prospective cohort (55 had BCS) had MPNs and had a 9-fold increased risk of recurrent thrombosis during follow-up[99]. The presence of JAK2 gene mutations was substantially correlated with liver-related thrombotic problems in a series of 36 BCS patients with recurrent thrombosis following liver transplantation in 42% of instances (15/36). Moreover, 11 of the 12 patients who experienced post-transplant thrombotic events and 10 of the 24 patients who did not (P = 0.005) both exhibited JAK2V617F. Additionally, an increased incidence of thrombosis at any site was linked to a JAK2 gene mutation (14/15 vs 7/21, P = 0.005). Liver-related thrombotic problems were more common in people with overt MPNs (9/12 vs 8/24, P = 0.03)[100]. An investigation of 181 patients suffering from MPNs who had their first episode of SVT was conducted retrospectively. In total, 31 (17.1%) and 109 (60.3%) patients, respectively, had BCS and extra-hepatic portal vein obstruction diagnosis; isolated thrombosis of the mesenteric or splenic veins was found in 18 and 23 cases, respectively. Following this index occurrence, the subjects were observed for 735 patient-years, and during that time, 31 recurrences occurred, representing an incidence rate of 4.2 per 100 patient-years. The recurrence rate was 3.9 per 100 patient-years in the 85% of patients who received VKAs, compared to 7.2 per 100 patient-years in the small portion (15%) of patients who did not. Compared to those who had thrombosis at the portal or other abdominal sites, patients with BCS had an incidence rate of new events that was significantly higher at 8.0 per 100 patient-years. In contrast, there was no difference in the rate of new arterial thrombosis between the two groups. Of note, patients with BCS had a 3-fold higher risk of recurrent SVT than those with other index SVT[101]. This difference was caused by an increased rate of venous events in BCS patients. Nine individuals with BCS (4 without and 5 with liver cirrhosis) were included in a survey on the use of direct oral anticoagulants (DOACs) in 94 patients with SVT, but no information was provided regarding the presence of MPNs as the underlying cause of SVT[102]. Anecdotally, it has been mentioned that a patient with PV and BCS used the direct factor Xa oral inhibitor rivaroxaban[103]. Semmler et al[104] analyzed the potential efficacy of DOACs, specifically edoxaban, apixaban, rivaroxaban and dabigatran, in the management of BCS. Their sample size consisted of 47 BCS subjects: 22 (of whom 10 had MPNs) were put on DOACs, whereas 21 (of whom 9 had MPNs) received LMWHs or VKAs. Complete response was noted in >60% of the BCS subjects who were prescribed DOACs. Complications during DOAC use included major spontaneous or surgery-related hemorrhage (n = 4 and n = 1, respectively) and minor hemorrhages (n = 7), whereas transplant-free survival at 5 years exceeded 90% and at 10 years exceeded 80%. JAK2V617F-negative MPNs experienced better treatment responses to DOACs as compared to JAK2V617F-positive individuals. Nevertheless, further research needs to assess the benefits of DOAC use in MPN-related BCS as the sample of the aforementioned investigation was too small to draw pertinent conclusions.
Cytoreduction is necessary in MPN patients who have experienced thrombosis in the past[105]. It is unknown whether it is appropriate to administer cytoreduction to SVT patients with JAK2V617F but without an explicit MPN diagnosis in accordance with the WHO criteria. Given the lack of evidence, care must be taken when prescribing cytoreductive regimens to JAK2V617F-positive SVT patients because approximately half of them will not develop MPN during the follow-up[106]. On the other hand, the JAK2V617F mutation increases the incidence of recurrent thrombosis in both BCS patients who have undergone liver transplantation[100] and SVT patients generally[107,108]. Therefore, it seems sensible to utilize medications to slow the growth of the mutant clone. Only one of the 17 MPN patients with BCS in a small retrospective cohort who received hydroxyurea and aspirin after liver donation developed recurrent extrahepatic portal vein obstruction (EHPVO)[109]. The rate of recurrence was 22% (4/18) in another small series of 18 MPN individuals with BCS; all new thrombotic events occurred in patients who were not receiving cytoreductive therapy[110]. In a pooled cohort of 1500 patients with MPNs and thrombosis, the multivariable analysis limited to the patients with first arterial thrombosis showed that recurrent arterial thrombosis was prevented by antiplatelet agents and by hydroxyurea and only partially by VKAs; on the contrary, in patients with the first venous thrombosis, venous recurr
Patients with BCS who experience failure of the aforementioned therapies are candidates for orthotopic liver transplantation in the range of 10 to 20 percent of cases[6]. Following liver transplantation, the 1-year and 5-year survival rates in a group of 36 BCS patients were 84% and 69%, respectively; the presence of a molecular characteristic for MPNs had no bearing on these survival rates[100]. The mortality rate following liver transplantation in a different series of 25 BCS patients was comparable in MPN (3/18, 16.7%) and non-MPN patients (1/7, 14.3%)[110]. In a retrospective cohort of 78 BCS patients, the 5-year survival was 78% vs 76%, and the 10-year survival was 68% vs 73%, respectively. Long-term survival following liver transplantation was similar in MPN (n = 41) and non-MPN patients (n = 37), with P values of 0.81 and 0.66, respectively. Twelve of the 41 MPN patients (or 29%) passed away within the first three years following liver transplantation, but only one death with recurrent BCS was attributable to the hematologic condition[146]. Following liver transplantation, progression to myelofibrosis or acute leukemia was not noted in 17 cases with a follow-up period of up to 20 years[109], nor in 78 cases in a mean follow-up period of 12.4 years (range 3-28.4 years)[113] in two series of BCS patients. While there are many treatment options available for BCS, the availability of many creates obstacles in maintaining a standard treatment plan. It is usual for clinicians to use anticoagulation as the first line of treatment for tackling BCS[114]; however, in cases when the condition cannot be controlled by medical treatment alone, several trials have shown encouraging outcomes with the use of TIPS in BCS patients as an alternative to shunt surgery or liver transplantation[115,116].
Anticoagulant therapy has been an accepted standard of treatment in BCS[117]; however, this standard remains controversial amidst clinicians. Emergent anticoagulation may not significantly improve clinical outcomes for individuals with acute ischemic stroke, according to several clinical investigations[118-120]. The current mode of treatment also selects LT as a de-facto last resort when it requires for a complex venous outflow reconstruction that would be difficult to acquire in medically underserved areas[6,121]. We have previously established that TIPS is the equivocally accepted and proven form of treatment for most BCS patients; in fact, the 10-year survival of the procedure is 69%[122]. It naturally becomes a concern if the current chronology requires a deeper revision. For instance, if anticoagulants truly seem to have no significant impact on the pathophysiology of the condition, could we derive a better medicinal first line of treatment for the condition? Could TIPS become the first mode of treatment if more non-invasive mechanisms are innovated to perform it? BCS is a rare disease, and it is important to remember that time is an invaluable resource in situations as such. Resorting to ineffective treatment not only delays medical management, but it deters the patient’s condition. If the first line of treatment leads a patient to the second line in the longer run, maybe it is a scope for physicians to rethink the chronology of treatment. Constant revisions of guidelines will allow us to not only discard what is counterintuitive, but it will promote clinicians to adapt to newer and more effective modes of treatment.
Hepatic vein obstruction in BCS may lead to abdominal pain, ascites, jaundice, and hepatomegaly. Given the significant overlap of these symptoms with other hepatic pathologies the differential diagnosis remains broad. PVT is distinct from BCS and directly involves the liver vasculature. MPNs are the most common cause of noncirrhotic, nonmalignant PVT[28]. Given the similar presentation of both PVT and BCS and its high prevalence in MPNs, diagnostic tests and imaging modalities should be utilized in differentiating both conditions. In BCS, Doppler ultrasound is effective in visualizing occlusion of the hepatic vein[123]. The absence of hepatic vein thrombosis and presence of reduced or absent flow in the portal veins with duplex ultrasound points towards PVT as the primary differential diagnosis[10]. Other differential diagnoses that should be given consideration include granulomatous liver disease, hemochromatosis, and alcoholic liver disease. A retrospective study across three centers in Europe studied the prognostic factors associated with BCS in MPNs. Their results indicated poorer baseline prognostic features, earlier hepatic decompression procedures, but no effect on 5-year survival. However, the presence of MPNs was associated with event free survival in BCS[124]. Generally, the determinants of prognosis in BCS are age, serum creatinine, Child-Pugh score, and ascites. A higher Child-Pugh score, older age, refractory ascites to diuretics, and higher serum creatinine are all factors pointing towards a poor prognosis[125]. In recent years 5-year survival rates have improved in BCS. Improved survival is largely attributed to the improved management of hypercoagulable states, and endovascular intervention[95]. Although rarely performed now, surgical portosystemic shunting improved survival in BCS patients who were determined to have a poor prognosis[126]. In a retrospective analysis of 78 BCS patients both with and without MPNs similar outcomes were measured after liver transplantation[113]. Janssen et al studied 172 patients with EHPVO, 24 of which carried a diagnosis of MPN. The five-year survival rates were similar between both groups (92% vs 53%, P = 0.18)[127]. Significant consideration must also be given to the role of VKAs in the prognosis of BCS. De Stefano et al[128] performed a retrospective analysis of 94 patients with MPNs (PV or ET), significant reduction of re-thrombosis was independently achieved with VKAs (HR 0.32; 95%CI: 0.15–0.64) and antiplatelet agents (HR 0.42; 95%CI: 0.22–0.77). DOACs may improve outcomes in patients with BCS. Semmler et al[104] in 2022 performed a retrospective analysis of 46 patients across three Australian centers with BCS. Six patients were managed with DOACs and 16 were switched to DOACs from LMWHs (n = 12) or VKAs (n = 4). In total, 4 major and 7 minor bleeding events were reported. Larger prospective studies need to be conducted assessing the safety and prognosis of VKAs vs DOACs in patients with BCS. Based on these previous studies it is determined that identification of BCS in patients with MPNs should be promptly treated, thereby improving prognosis. Complications secondary to BCS can be determined based on the varying degree of ensuing hepatic injury and dysfunction. When untreated BCS can progress to fulminant liver failure, hepatorenal syndrome, hepatocellular carcinoma, and hepatic encephalopathy amongst other complications. In 2021, Asl et al[129] retrospectively reported on complications associated with liver transplantation (LT) in 4225 patients. 108 patients had BCS and were matched with a non-BCS group of 108 patients. One-, 3-, 5-, and 10- year survival rates were the same in both groups (82%, 78%, 76%, and 76% vs 83%, 83%, 83%, and 76%, P = 0.556). No differences were noted in the 6-month follow-up after LT. However, at a later period vascular thrombosis was more prevalent in the BCS group. In 2016, Ki et al[130] conducted a population-based study in South Korea identifying a total of 423 BCS patients from 2009-2013. Among them, 10.3% developed hepatic malignancy, and 3.3% underwent LT. The annual-case fatality rate was 2.8%. Hayek et al[131] performed a retrospective analysis on the long-term safety of patients with BCS who underwent TIPS. In total, 54 patients were identified, 34 (52%) of which suffered from MPNs. TIPS dysfunction was associated with MPNs (HR, 8.18; 95%CI: 1.45-46.18; P = 0.017).
Although BCS and MPNs are rare disorders, BCS can develop in the setting of MPNs. In this patient population, individualized, distinctive counseling and multidisciplinary surveillance and treatment strategies are crucial in achieving better possible outcomes. Individuals with MPNs should be managed in accordance with the most recent guidelines to avoid the occurrence of BCS, whereas a diagnosis of BCS should warrant an active search for the potential diagnosis of MPNs.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Guo X, China; Manesis EK, Greece; Qi XS, China S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Nangalia J, Green AR. Myeloproliferative neoplasms: from origins to outcomes. Blood. 2017;130:2475-2483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 2. | Barbui T, Thiele J, Gisslinger H, Kvasnicka HM, Vannucchi AM, Guglielmelli P, Orazi A, Tefferi A. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J. 2018;8:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 412] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 3. | Greenfield G, McMullin MF, Mills K. Molecular pathogenesis of the myeloproliferative neoplasms. J Hematol Oncol. 2021;14:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 4. | Moliterno AR, Ginzburg YZ, Hoffman R. Clinical insights into the origins of thrombosis in myeloproliferative neoplasms. Blood. 2021;137:1145-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 5. | Găman MA, Cozma MA, Dobrică EC, Crețoiu SM, Găman AM, Diaconu CC. Liquid biopsy and potential liquid biopsy-based biomarkers in philadelphia-negative classical myeloproliferative neoplasms: A systematic review. Life. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 6. | Darwish Murad S, Plessier A, Hernandez-Guerra M, Fabris F, Eapen CE, Bahr MJ, Trebicka J, Morard I, Lasser L, Heller J, Hadengue A, Langlet P, Miranda H, Primignani M, Elias E, Leebeek FW, Rosendaal FR, Garcia-Pagan JC, Valla DC, Janssen HL; EN-Vie (European Network for Vascular Disorders of the Liver). Etiology, management, and outcome of the Budd-Chiari syndrome. Ann Intern Med. 2009;151:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 338] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 7. | Faraoun SA, Boudjella Mel A, Debzi N, Afredj N, Guerrache Y, Benidir N, Bouzid C, Bentabak K, Soyer P, Bendib SE. Budd-Chiari syndrome: a prospective analysis of hepatic vein obstruction on ultrasonography, multidetector-row computed tomography and MR imaging. Abdom Imaging. 2015;40:1500-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Ferral H, Behrens G, Lopera J. Budd-Chiari syndrome. AJR Am J Roentgenol. 2012;199:737-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Menon KVN, Shah V, Kamath PS. The Budd–Chiari Syndrome. N Engl J Med. 2004;350:578-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 325] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 10. | Ponziani FR, Zocco MA, Campanale C, Rinninella E, Tortora A, Di Maurizio L, Bombardieri G, De Cristofaro R, De Gaetano AM, Landolfi R, Gasbarrini A. Portal vein thrombosis: insight into physiopathology, diagnosis, and treatment. World J Gastroenterol. 2010;16:143-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 227] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (2)] |
| 11. | Plessier A, Valla DC. Budd-Chiari syndrome. Semin Liver Dis. 2008;28:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Li Y, De Stefano V, Li H, Zheng K, Bai Z, Guo X, Qi X. Epidemiology of Budd-Chiari syndrome: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2019;43:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Wang ZG, Zhang FJ, Yi MQ, Qiang LX. Evolution of management for Budd-Chiari syndrome: a team's view from 2564 patients. ANZ J Surg. 2005;75:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Zhang W, Qi X, Zhang X, Su H, Zhong H, Shi J, Xu K. Budd-Chiari Syndrome in China: A Systematic Analysis of Epidemiological Features Based on the Chinese Literature Survey. Gastroenterol Res Pract. 2015;2015:738548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 15. | Ollivier-Hourmand I, Allaire M, Goutte N, Morello R, Chagneau-Derrode C, Goria O, Dumortier J, Cervoni JP, Dharancy S, Ganne-Carrié N, Bureau C, Carbonell N, Abergel A, Nousbaum JB, Anty R, Barraud H, Ripault MP, De Ledinghen V, Minello A, Oberti F, Radenne S, Bendersky N, Farges O, Archambeaud I, Guillygomarc'h A, Ecochard M, Ozenne V, Hilleret MN, Nguyen-Khac E, Dauvois B, Perarnau JM, Lefilliatre P, Raabe JJ, Doffoel M, Becquart JP, Saillard E, Valla D, Dao T, Plessier A; French Network for Vascular Disorders of the Liver. The epidemiology of Budd-Chiari syndrome in France. Dig Liver Dis. 2018;50:931-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Shin N, Kim YH, Xu H, Shi HB, Zhang QQ, Colon Pons JP, Kim D, Xu Y, Wu FY, Han S, Lee BB, Li LS. Redefining Budd-Chiari syndrome: A systematic review. World J Hepatol. 2016;8:691-702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Qi X, Wu F, Ren W, He C, Yin Z, Niu J, Bai M, Yang Z, Wu K, Fan D, Han G. Thrombotic risk factors in Chinese Budd-Chiari syndrome patients. An observational study with a systematic review of the literature. Thromb Haemost. 2013;109:878-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Aydinli M, Bayraktar Y. Budd-Chiari syndrome: etiology, pathogenesis and diagnosis. World J Gastroenterol. 2007;13:2693-2696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Mahmoud AE, Helmy AS, Billingham L, Elias E. Poor prognosis and limited therapeutic options in patients with Budd-Chiari syndrome and portal venous system thrombosis. Eur J Gastroenterol Hepatol. 1997;9:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Dacha S, Devidi M, Osmundson E. Budd-Chiari syndrome in a patient with ulcerative colitis and no inherited coagulopathy. World J Hepatol. 2011;3:164-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Meena DS, Sonwal VS, Bohra GK, Balesa J, Rohila AK. Celiac disease with Budd-Chiari syndrome: A rare association. SAGE Open Med Case Rep. 2019;7:2050313X19842697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Bayraktar Y, Balkanci F, Bayraktar M, Calguneri M. Budd-Chiari syndrome: A common complication of Behçet’s disease. Am J Gastroenterol. 1997;92:858-862. [PubMed] |
| 23. | Anagnostopoulos GK, Margantinis G, Kostopoulos P, Papadopoulou G, Roulias A, Sakorafas G, Liassis N. Budd-Chiari syndrome and portal vein thrombosis due to right atrial myxoma. Ann Thorac Surg. 2004;78:333-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Neelakantan S, Babu AA, Anandarajan R, Philip B. Hepatic hydatid disease presenting as secondary Budd-Chiari syndrome. BMJ Case Rep. 2016;2016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 25. | de Menezes Neves PDM, Balbo BEP, Watanabe EH, Rocha-Santos V, Andraus W, D'Albuquerque LAC, Onuchic LF. Functional Budd-Chiari Syndrome Associated With Severe Polycystic Liver Disease. Clin Med Insights Gastroenterol. 2017;10:1179552217713003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Karadag O, Akinci D, Aksoy DY, Bayraktar Y. Acute Budd-Chiari syndrome resulting from a pyogenic liver abscess. Hepatogastroenterology. 2005;52:1554-1556. [PubMed] |
| 27. | Mahmoud AE, Mendoza A, Meshikhes AN, Olliff S, West R, Neuberger J, Buckels J, Wilde J, Elias E. Clinical spectrum, investigations and treatment of Budd-Chiari syndrome. QJM. 1996;89:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Smalberg JH, Arends LR, Valla DC, Kiladjian JJ, Janssen HL, Leebeek FW. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: a meta-analysis. Blood. 2012;120:4921-4928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 251] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 29. | Palandri F, Latagliata R, Polverelli N, Tieghi A, Crugnola M, Martino B, Perricone M, Breccia M, Ottaviani E, Testoni N, Merli F, Aversa F, Alimena G, Cavo M, Martinelli G, Catani L, Baccarani M, Vianelli N. Mutations and long-term outcome of 217 young patients with essential thrombocythemia or early primary myelofibrosis. Leukemia. 2015;29:1344-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2647] [Cited by in RCA: 2747] [Article Influence: 137.4] [Reference Citation Analysis (0)] |
| 31. | Smalberg JH, Darwish Murad S, Braakman E, Valk PJ, Janssen HL, Leebeek FW. Myeloproliferative disease in the pathogenesis and survival of Budd-Chiari syndrome. Haematologica. 2006;91:1712-1713. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 32. | Ibarrola C, Castellano VM, Colina F. Focal hyperplastic hepatocellular nodules in hepatic venous outflow obstruction: a clinicopathological study of four patients and 24 nodules. Histopathology. 2004;44:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Tanaka M, Wanless IR. Pathology of the liver in Budd-Chiari syndrome: portal vein thrombosis and the histogenesis of veno-centric cirrhosis, veno-portal cirrhosis, and large regenerative nodules. Hepatology. 1998;27:488-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 146] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Lefkowitch JH, Mendez L. Morphologic features of hepatic injury in cardiac disease and shock. J Hepatol. 1986;2:313-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 62] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Cazals-Hatem D, Vilgrain V, Genin P, Denninger MH, Durand F, Belghiti J, Valla D, Degott C. Arterial and portal circulation and parenchymal changes in Budd-Chiari syndrome: a study in 17 explanted livers. Hepatology. 2003;37:510-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Gonzalez RS, Gilger MA, Huh WJ, Washington MK. The Spectrum of Histologic Findings in Hepatic Outflow Obstruction. Arch Pathol Lab Med. 2017;141:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Brunt EM, Gouw AS, Hubscher SG, Tiniakos DG, Bedossa P, Burt AD, Callea F, Clouston AD, Dienes HP, Goodman ZD, Roberts EA, Roskams T, Terracciano L, Torbenson MS, Wanless IR. Pathology of the liver sinusoids. Histopathology. 2014;64:907-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Arcidi JM, Moore GW, Hutchins GM. Hepatic morphology in cardiac dysfunction. A clinicopathologic study of 1000 subjects at autopsy. Am J Pathol. 1981;104:159-166. [PubMed] |
| 39. | Ghaferi AA, Hutchins GM. Progression of liver pathology in patients undergoing the Fontan procedure: Chronic passive congestion, cardiac cirrhosis, hepatic adenoma, and hepatocellular carcinoma. J Thorac Cardiovasc Surg. 2005;129:1348-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 257] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 40. | DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study Liver Diseases. Vascular disorders of the liver. Hepatology. 2009;49:1729-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 650] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 41. | Landolfi R, Rocca B, Patrono C. Bleeding and thrombosis in myeloproliferative disorders: mechanisms and treatment. Crit Rev Oncol Hematol. 1995;20:203-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Landolfi R, Di Gennaro L, Falanga A. Thrombosis in myeloproliferative disorders: pathogenetic facts and speculation. Leukemia. 2008;22:2020-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Pearson TC, Wetherley-Mein G. Vascular occlusive episodes and venous haematocrit in primary proliferative polycythaemia. Lancet. 1978;2:1219-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 275] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 44. | Li M, De Stefano V, Song T, Zhou X, Guo Z, Zhu J, Qi X. Prevalence of CALR mutations in splanchnic vein thrombosis: A systematic review and meta-analysis. Thromb Res. 2018;167:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | De Stefano V, Qi X, Betti S, Rossi E. Splanchnic vein thrombosis and myeloproliferative neoplasms: molecular-driven diagnosis and long-term treatment. Thromb Haemost. 2016;115:240-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 46. | Chaubal N, Dighe M, Hanchate V, Thakkar H, Deshmukh H, Rathod K. Sonography in Budd-Chiari syndrome. J Ultrasound Med. 2006;25:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Bansal V, Gupta P, Sinha S, Dhaka N, Kalra N, Vijayvergiya R, Dutta U, Kochhar R. Budd-Chiari syndrome: imaging review. Br J Radiol. 2018;91:20180441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 48. | Patil P, Deshmukh H, Popat B, Rathod K. Spectrum of imaging in Budd Chiari syndrome. J Med Imaging Radiat Oncol. 2012;56:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Liu L, Qi XS, Zhao Y, Chen H, Meng XC, Han GH. Budd-Chiari syndrome: Current perspectives and controversies. Eur Rev Med Pharmacol Sci. 2016;20:3273-3281. [PubMed] |
| 50. | Falcão CK, Fagundes GC, Lamos GC, Felipe-Silva A, Lovisolo SM, Martines JA, de Campos FP. Budd-Chiari Syndrome: an unnoticed diagnosis. Autops Case Rep. 2015;5:17-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 51. | Okuda K. Obliterative hepatocavopathy-inferior vena cava thrombosis at its hepatic portion. Hepatobiliary Pancreat Dis Int. 2002;1:499-509. [PubMed] |
| 52. | Sharma A, Keshava SN, Eapen A, Elias E, Eapen CE. An Update on the Management of Budd-Chiari Syndrome. Dig Dis Sci. 2021;66:1780-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 53. | Lupasco I, Dumbrava VT. Diagnosis and therapy of Budd Chiari syndrome. Med Pharm Rep. 2021;94:S68-S71. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 54. | Gavriilidis P, Marangoni G, Ahmad J, Azoulay D. State of the Art, Current Perspectives, and Controversies of Budd-Chiari Syndrome: A Review. J Clin Med Res. 2022;14:147-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Parekh J, Matei VM, Canas-Coto A, Friedman D, Lee WM; Acute Liver Failure Study Group. Budd-chiari syndrome causing acute liver failure: A multicenter case series. Liver Transpl. 2017;23:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 56. | Shukla A, Shreshtha A, Mukund A, Bihari C, Eapen CE, Han G, Deshmukh H, Cua IHY, Lesmana CRA, Al Meshtab M, Kage M, Chaiteeraki R, Treeprasertsuk S, Giri S, Punamiya S, Paradis V, Qi X, Sugawara Y, Abbas Z, Sarin SK. Budd-Chiari syndrome: consensus guidance of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int. 2021;15:531-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 57. | Valla DC. Hepatic vein thrombosis (Budd-Chiari syndrome). Semin Liver Dis. 2002;22:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Abdel Hameed MR, Elbeih EAS, Abd El-Aziz HM, Afifi OA, Khalaf LMR, Ali Abu Rahma MZ, Sabry A. Epidemiological Characteristics and Etiology of Budd-Chiari Syndrome in Upper Egypt. J Blood Med. 2020;11:515-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Zhang W, Wang QZ, Chen XW, Zhong HS, Zhang XT, Chen XD, Xu K. Budd-Chiari syndrome in China: A 30-year retrospective study on survival from a single center. World J Gastroenterol. 2018;24:1134-1143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 60. | Okuda H, Yamagata H, Obata H, Iwata H, Sasaki R, Imai F, Okudaira M, Ohbu M, Okuda K. Epidemiological and clinical features of Budd-Chiari syndrome in Japan. J Hepatol. 1995;22:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 144] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 61. | Alukal JJ, Zhang T, Thuluvath PJ. A Nationwide Analysis of Budd-Chiari Syndrome in the United States. J Clin Exp Hepatol. 2021;11:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 62. | Afredj N, Guessab N, Nani A, Faraoun SA, Ouled Cheikh I, Kerbouche R, Hannoun D, Amir ZC, Ait Kaci H, Bentabak K, Plessier A, Valla DC, Cazals-Hatem V, Denninger MH, Boucekkine T, Debzi N. Aetiological factors of Budd-Chiari syndrome in Algeria. World J Hepatol. 2015;7:903-909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 63. | Vannucchi AM. Insights into the pathogenesis and management of thrombosis in polycythemia vera and essential thrombocythemia. Intern Emerg Med. 2010;5:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 64. | Denninger MH, Chaït Y, Casadevall N, Hillaire S, Guillin MC, Bezeaud A, Erlinger S, Briere J, Valla D. Cause of portal or hepatic venous thrombosis in adults: the role of multiple concurrent factors. Hepatology. 2000;31:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 450] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 65. | Ponnatapura J, Kielar A, Burke LMB, Lockhart ME, Abualruz AR, Tappouni R, Lalwani N. Hepatic complications of oral contraceptive pills and estrogen on MRI: Controversies and update - Adenoma and beyond. Magn Reson Imaging. 2019;60:110-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Valla D, Le MG, Poynard T, Zucman N, Rueff B, Benhamou JP. Risk of hepatic vein thrombosis in relation to recent use of oral contraceptives. A case-control study. Gastroenterology. 1986;90:807-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 90] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Valla D, Dhumeaux D, Babany G, Hillon P, Rueff B, Rochant H, Benhamou JP. Hepatic vein thrombosis in paroxysmal nocturnal hemoglobinuria. A spectrum from asymptomatic occlusion of hepatic venules to fatal Budd-Chiari syndrome. Gastroenterology. 1987;93:569-575. [PubMed] [DOI] [Full Text] |
| 68. | Altunayoglu V, Turedi S, Gunduz A, Karaca Y, Akdogan RA. Cerebral venous thrombosis and hepatic venous thrombosis during pregnancy. J Obstet Gynaecol Res. 2007;33:78-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 69. | Khuroo MS, Datta DV. Budd-Chiari syndrome following pregnancy. Report of 16 cases, with roentgenologic, hemodynamic and histologic studies of the hepatic outflow tract. Am J Med. 1980;68:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 87] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 70. | Ren W, Qi X, Yang Z, Han G, Fan D. Prevalence and risk factors of hepatocellular carcinoma in Budd-Chiari syndrome: a systematic review. Eur J Gastroenterol Hepatol. 2013;25:830-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 71. | Janssen HL, Garcia-Pagan JC, Elias E, Mentha G, Hadengue A, Valla DC; European Group for the Study of Vascular Disorders of the Liver. Budd-Chiari syndrome: a review by an expert panel. J Hepatol. 2003;38:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 327] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 72. | Hitawala AA, Gupta V. Budd Chiari Syndrome. 2022 Feb 9. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. [PubMed] |
| 73. | Niknam R, Hajizadegan N, Mohammadkarimi V, Mahmoudi L. A study of the different parameters in acute and chronic Budd–Chiari syndrome. Egypt Liver J. 2020;10: 1-8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 74. | Gupta P, Bansal V, Kumar-M P, Sinha SK, Samanta J, Mandavdhare H, Sharma V, Dutta U, Kochhar R. Diagnostic accuracy of Doppler ultrasound, CT and MRI in Budd Chiari syndrome: systematic review and meta-analysis. Br J Radiol. 2020;93:20190847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 75. | Millener P, Grant EG, Rose S, Duerinckx A, Schiller VL, Tessler FN, Perrella RR, Ragavendra N. Color Doppler imaging findings in patients with Budd-Chiari syndrome: correlation with venographic findings. AJR Am J Roentgenol. 1993;161:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Mukund A, Gamanagatti S. Imaging and interventions in Budd-Chiari syndrome. World J Radiol. 2011;3:169-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 77. | Raju GS, Felver M, Olin JW, Satti SD. Thrombolysis for acute Budd-Chiari syndrome: Case report and literature review. Am J Gastroenterol. 1996;91:1262-1263. [PubMed] |
| 78. | Sharma S, Texeira A, Texeira P, Elias E, Wilde J, Olliff SP. Pharmacological thrombolysis in Budd Chiari syndrome: a single centre experience and review of the literature. J Hepatol. 2004;40:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 79. | Fisher NC, McCafferty I, Dolapci M, Wali M, Buckels JA, Olliff SP, Elias E. Managing Budd-Chiari syndrome: a retrospective review of percutaneous hepatic vein angioplasty and surgical shunting. Gut. 1999;44:568-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 80. | Eapen CE, Velissaris D, Heydtmann M, Gunson B, Olliff S, Elias E. Favourable medium term outcome following hepatic vein recanalisation and/or transjugular intrahepatic portosystemic shunt for Budd Chiari syndrome. Gut. 2006;55:878-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 81. | Khan F, Mehrzad H, Tripathi D. Timing of Transjugular Intrahepatic Portosystemic Stent-shunt in Budd-Chiari Syndrome: A UK Hepatologist's Perspective. J Transl Int Med. 2018;6:97-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 82. | Seijo S, Plessier A, Hoekstra J, Dell'era A, Mandair D, Rifai K, Trebicka J, Morard I, Lasser L, Abraldes JG, Darwish Murad S, Heller J, Hadengue A, Primignani M, Elias E, Janssen HL, Valla DC, Garcia-Pagan JC; European Network for Vascular Disorders of the Liver. Good long-term outcome of Budd-Chiari syndrome with a step-wise management. Hepatology. 2013;57:1962-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 219] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 83. | Tripathi D, Macnicholas R, Kothari C, Sunderraj L, Al-Hilou H, Rangarajan B, Chen F, Mangat K, Elias E, Olliff S. Good clinical outcomes following transjugular intrahepatic portosystemic stent-shunts in Budd-Chiari syndrome. Aliment Pharmacol Ther. 2014;39:864-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 84. | Fitsiori K, Tsitskari M, Kelekis A, Filippiadis D, Triantafyllou K, Brountzos E. Transjugular intrahepatic portosystemic shunt for the treatment of Budd-Chiari syndrome patients: results from a single center. Cardiovasc Intervent Radiol. 2014;37:691-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 85. | Qi X, Yang M, Fan D, Han G. Transjugular intrahepatic portosystemic shunt in the treatment of Budd-Chiari syndrome: a critical review of literatures. Scand J Gastroenterol. 2013;48:771-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 86. | Sonavane AD, Amarapurkar DN, Rathod KR, Punamiya SJ. Long Term Survival of Patients Undergoing TIPS in Budd-Chiari Syndrome. J Clin Exp Hepatol. 2019;9:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 87. | Aktas H, Ozer A, Yilmaz TU, Keceoglu S, Can MG, Emiroglu R. Liver transplantation for Budd-Chiari syndrome: A challenging but handable procedure. Asian J Surg. 2022;45:1396-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 88. | Akamatsu N, Sugawara Y, Kokudo N. Budd-Chiari syndrome and liver transplantation. Intractable Rare Dis Res. 2015;4:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 89. | Ibach M, Eurich D, Dobrindt E, Lurje G, Schöning W, Öllinger R, Pratschke J, Globke B. Orthotopic Liver Transplantation for Budd-Chiari Syndrome: Observations from a 30-Year Liver Transplant Program. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 90. | Verstovsek S, Yu J, Scherber RM, Verma S, Dieyi C, Chen CC, Parasuraman S. Changes in the incidence and overall survival of patients with myeloproliferative neoplasms between 2002 and 2016 in the United States. Leuk Lymphoma. 2022;63:694-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 91. | Smalberg JH, Spaander MV, Jie KS, Pattynama PM, van Buuren HR, van den Berg B, Janssen HL, Leebeek FW. Risks and benefits of transcatheter thrombolytic therapy in patients with splanchnic venous thrombosis. Thromb Haemost. 2008;100:1084-1088. [PubMed] |
| 92. | Zhang Q, Xu H, Zu M, Gu Y, Wei N, Wang W, Gao Z, Shen B. Catheter-directed thrombolytic therapy combined with angioplasty for hepatic vein obstruction in Budd-Chiari syndrome complicated by thrombosis. Exp Ther Med. 2013;6:1015-1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 93. | Mancuso A. An update on the management of Budd-Chiari syndrome: the issues of timing and choice of treatment. Eur J Gastroenterol Hepatol. 2015;27:200-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 94. | Senzolo M, Riggio O, Primignani M; Italian Association for the Study of the Liver (AISF) ad hoc. Vascular disorders of the liver: recommendations from the Italian Association for the Study of the Liver (AISF) ad hoc committee. Dig Liver Dis. 2011;43:503-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 95. | Zeitoun G, Escolano S, Hadengue A, Azar N, El Younsi M, Mallet A, Boudet MJ, Hay JM, Erlinger S, Benhamou JP, Belghiti J, Valla D. Outcome of Budd-Chiari syndrome: a multivariate analysis of factors related to survival including surgical portosystemic shunting. Hepatology. 1999;30:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 160] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 96. | de Franchis R, Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2293] [Article Influence: 229.3] [Reference Citation Analysis (3)] |
| 97. | Hadengue A, Poliquin M, Vilgrain V, Belghiti J, Degott C, Erlinger S, Benhamou JP. The changing scene of hepatic vein thrombosis: recognition of asymptomatic cases. Gastroenterology. 1994;106:1042-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 131] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 98. | Mentha G, Giostra E, Majno PE, Bechstein WO, Neuhaus P, O'Grady J, Praseedom RK, Burroughs AK, Le Treut YP, Kirkegaard P, Rogiers X, Ericzon BG, Hockerstedt K, Adam R, Klempnauer J. Liver transplantation for Budd-Chiari syndrome: A European study on 248 patients from 51 centres. J Hepatol. 2006;44:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 99. | Ageno W, Riva N, Schulman S, Beyer-Westendorf J, Bang SM, Senzolo M, Grandone E, Pasca S, Di Minno MN, Duce R, Malato A, Santoro R, Poli D, Verhamme P, Martinelli I, Kamphuisen P, Oh D, D'Amico E, Becattini C, De Stefano V, Vidili G, Vaccarino A, Nardo B, Di Nisio M, Dentali F. Long-term Clinical Outcomes of Splanchnic Vein Thrombosis: Results of an International Registry. JAMA Intern Med. 2015;175:1474-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 100. | Westbrook RH, Lea NC, Mohamedali AM, Smith AE, Orr DW, Roberts LN, Heaton ND, Wendon JA, O'Grady JG, Heneghan MA, Mufti GJ. Prevalence and clinical outcomes of the 46/1 haplotype, Janus kinase 2 mutations, and ten-eleven translocation 2 mutations in Budd-Chiari syndrome and their impact on thrombotic complications post liver transplantation. Liver Transpl. 2012;18:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 101. | De Stefano V, Vannucchi AM, Ruggeri M, Cervantes F, Alvarez-Larrán A, Iurlo A, Randi ML, Pieri L, Rossi E, Guglielmelli P, Betti S, Elli E, Finazzi MC, Finazzi G, Zetterberg E, Vianelli N, Gaidano G, Nichele I, Cattaneo D, Palova M, Ellis MH, Cacciola E, Tieghi A, Hernandez-Boluda JC, Pungolino E, Specchia G, Rapezzi D, Forcina A, Musolino C, Carobbio A, Griesshammer M, Barbui T. Splanchnic vein thrombosis in myeloproliferative neoplasms: risk factors for recurrences in a cohort of 181 patients. Blood Cancer J. 2016;6:e493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 102. | De Gottardi A, Trebicka J, Klinger C, Plessier A, Seijo S, Terziroli B, Magenta L, Semela D, Buscarini E, Langlet P, Görtzen J, Puente A, Müllhaupt B, Navascuès C, Nery F, Deltenre P, Turon F, Engelmann C, Arya R, Caca K, Peck-Radosavljevic M, Leebeek FWG, Valla D, Garcia-Pagan JC; VALDIG Investigators. Antithrombotic treatment with direct-acting oral anticoagulants in patients with splanchnic vein thrombosis and cirrhosis. Liver Int. 2017;37:694-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 176] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 103. | Jones C, Levy Y, Tong AW. Elevated serum erythropoietin in a patient with polycythaemia vera presenting with Budd-Chiari syndrome. BMJ Case Rep. 2014;2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 104. | Semmler G, Lindorfer A, Schäfer B, Bartl S, Hametner-Schreil S, Gensluckner S, Balcar L, Pomej K, Lampichler K, Trauner M, Aigner E, Datz C, Zoller H, Hofer H, Schöfl R, Mandorfer M, Reiberger T, Scheiner B. Outcome of Budd-Chiari Syndrome Patients Treated With Direct Oral Anticoagulants: An Austrian Multicenter Study. Clin Gastroenterol Hepatol. 2022;S1542-3565(22)00449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 105. | Barbui T, Tefferi A, Vannucchi AM, Passamonti F, Silver RT, Hoffman R, Verstovsek S, Mesa R, Kiladjian JJ, Hehlmann R, Reiter A, Cervantes F, Harrison C, Mc Mullin MF, Hasselbalch HC, Koschmieder S, Marchetti M, Bacigalupo A, Finazzi G, Kroeger N, Griesshammer M, Birgegard G, Barosi G. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32:1057-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 418] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 106. | Dentali F, Squizzato A, Brivio L, Appio L, Campiotti L, Crowther M, Grandi AM, Ageno W. JAK2V617F mutation for the early diagnosis of Ph- myeloproliferative neoplasms in patients with venous thromboembolism: a meta-analysis. Blood. 2009;113:5617-5623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 107. | De Stefano V, Ruggeri M, Cervantes F, Alvarez-Larrán A, Iurlo A, Randi ML, Elli E, Finazzi MC, Finazzi G, Zetterberg E, Vianelli N, Gaidano G, Rossi E, Betti S, Nichele I, Cattaneo D, Palova M, Ellis MH, Cacciola R, Tieghi A, Hernandez-Boluda JC, Pungolino E, Specchia G, Rapezzi D, Forcina A, Musolino C, Carobbio A, Griesshammer M, Sant'Antonio E, Vannucchi AM, Barbui T. High rate of recurrent venous thromboembolism in patients with myeloproliferative neoplasms and effect of prophylaxis with vitamin K antagonists. Leukemia. 2016;30:2032-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 108. | Colaizzo D, Amitrano L, Guardascione MA, Tiscia GL, D'Andrea G, Longo VA, Grandone E, Margaglione M. Outcome of patients with splanchnic venous thrombosis presenting without overt MPN: a role for the JAK2 V617F mutation re-evaluation. Thromb Res. 2013;132:e99-e104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 109. | Chinnakotla S, Klintmalm GB, Kim P, Tomiyama K, Klintmalm E, Davis GL, Trotter JF, Saad R, Landaverde C, Levy MF, Goldstein RM, Stone MJ. Long-term follow-up of liver transplantation for Budd-Chiari syndrome with antithrombotic therapy based on the etiology. Transplantation. 2011;92:341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 110. | Oldakowska-Jedynak U, Ziarkiewicz M, Ziarkiewicz-Wróblewska B, Dwilewicz-Trojaczek J, Górnicka B, Nyckowski P, Paluszkiewicz R, Wróblewski T, Zieniewicz K, Patkowski W, Pączek L, Jedrzejczak WW, Krawczyk M. Myeloproliferative neoplasms and recurrent thrombotic events in patients undergoing liver transplantation for Budd-Chiari syndrome: a single-center experience. Ann Transplant. 2014;19:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 111. | De Stefano V, Rossi E, Carobbio A, Ghirardi A, Betti S, Finazzi G, Vannucchi AM, Barbui T. Hydroxyurea prevents arterial and late venous thrombotic recurrences in patients with myeloproliferative neoplasms but fails in the splanchnic venous district. Pooled analysis of 1500 cases. Blood Cancer J. 2018;8:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 112. | Chait Y, Condat B, Cazals-Hatem D, Rufat P, Atmani S, Chaoui D, Guilmin F, Kiladjian JJ, Plessier A, Denninger MH, Casadevall N, Valla D, Brière JB. Relevance of the criteria commonly used to diagnose myeloproliferative disorder in patients with splanchnic vein thrombosis. Br J Haematol. 2005;129:553-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 113. | Potthoff A, Attia D, Pischke S, Mederacke I, Beutel G, Rifai K, Deterding K, Heiringhoff K, Klempnauer J, Strassburg CP, Manns MP, Bahr MJ. Long-term outcome of liver transplant patients with Budd-Chiari syndrome secondary to myeloproliferative neoplasms. Liver Int. 2015;35:2042-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 114. | Payancé A, Plessier A. Anticoagulation for budd-chiari syndrome. Budd-Chiari Syndr. 2019;131-145. [DOI] [Full Text] |
| 115. | Mancuso A, Fung K, Mela M, Tibballs J, Watkinson A, Burroughs AK, Patch D. TIPS for acute and chronic Budd-Chiari syndrome: a single-centre experience. J Hepatol. 2003;38:751-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 116. | Tavill AS, Wood EJ, Kreel L, Jones EA, Gregory M, Sherlock S. The Budd-Chiari syndrome: correlation between hepatic scintigraphy and the clinical, radiological, and pathological findings in nineteen cases of hepatic venous outflow obstruction. Gastroenterology. 1975;68:509-518. [PubMed] [DOI] [Full Text] |
| 117. | Adams HP Jr. Emergent use of anticoagulation for treatment of patients with ischemic stroke. Stroke. 2002;33:856-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 118. | Sandercock PAG, Counsell C, Gubitz GJ, Tseng MC. Antiplatelet therapy for acute ischaemic stroke. Cochrane Database Syst Rev. 2018;16:CD000029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 119. | Pullicino P, Thompson JL, Barton B, Levin B, Graham S, Freudenberger RS; WARCEF Investigators. Warfarin vs aspirin in patients with reduced cardiac ejection fraction (WARCEF): rationale, objectives, and design. J Card Fail. 2006;12:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 120. | Adams HP Jr, Bendixen BH, Leira E, Chang KC, Davis PH, Woolson RF, Clarke WR, Hansen MD. Antithrombotic treatment of ischemic stroke among patients with occlusion or severe stenosis of the internal carotid artery: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999;53:122-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 121. | Deltenre P, Denninger MH, Hillaire S, Guillin MC, Casadevall N, Brière J, Erlinger S, Valla DC. Factor V Leiden related Budd-Chiari syndrome. Gut. 2001;48:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 122. | Hernández-Guerra M, Turnes J, Rubinstein P, Olliff S, Elias E, Bosch J, García-Pagán JC. PTFE-covered stents improve TIPS patency in Budd-Chiari syndrome. Hepatology. 2004;40:1197-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 123. | Iliescu L, Toma L, Mercan-Stanciu A, Grumeza M, Dodot M, Isac T, Ioanitescu S. Budd-Chiari syndrome - various etiologies and imagistic findings. A pictorial review. Med Ultrason. 2019;21:344-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 124. | Kiladjian JJ, Cervantes F, Leebeek FW, Marzac C, Cassinat B, Chevret S, Cazals-Hatem D, Plessier A, Garcia-Pagan JC, Darwish Murad S, Raffa S, Janssen HL, Gardin C, Cereja S, Tonetti C, Giraudier S, Condat B, Casadevall N, Fenaux P, Valla DC. The impact of JAK2 and MPL mutations on diagnosis and prognosis of splanchnic vein thrombosis: a report on 241 cases. Blood. 2008;111:4922-4929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 221] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 125. | Langlet P, Escolano S, Valla D, Coste-Zeitoun D, Denie C, Mallet A, Levy VG, Franco D, Vinel JP, Belghiti J, Lebrec D, Hay JM, Zeitoun G. Clinicopathological forms and prognostic index in Budd-Chiari syndrome. J Hepatol. 2003;39:496-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 126. | Darwish Murad S, Valla DC, de Groen PC, Zeitoun G, Hopmans JA, Haagsma EB, van Hoek B, Hansen BE, Rosendaal FR, Janssen HL. Determinants of survival and the effect of portosystemic shunting in patients with Budd-Chiari syndrome. Hepatology. 2004;39:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 224] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 127. | Janssen HL, Wijnhoud A, Haagsma EB, van Uum SH, van Nieuwkerk CM, Adang RP, Chamuleau RA, van Hattum J, Vleggaar FP, Hansen BE, Rosendaal FR, van Hoek B. Extrahepatic portal vein thrombosis: aetiology and determinants of survival. Gut. 2001;49:720-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 219] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 128. | De Stefano V, Za T, Rossi E, Vannucchi AM, Ruggeri M, Elli E, Micò C, Tieghi A, Cacciola RR, Santoro C, Gerli G, Vianelli N, Guglielmelli P, Pieri L, Scognamiglio F, Rodeghiero F, Pogliani EM, Finazzi G, Gugliotta L, Marchioli R, Leone G, Barbui T; GIMEMA CMD-Working Party. Recurrent thrombosis in patients with polycythemia vera and essential thrombocythemia: incidence, risk factors, and effect of treatments. Haematologica. 2008;93:372-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 248] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 129. | Asl AA, Lankarani KB, Nikeghbalian S, Kazemi K, Shamsaieefar A, Alizade-Naini M, Fattahi MR, Taghavi SA, Niknam R, Ejtehadi F, Dehghan M, Sivandzadeh G, Ghahramani S, Malek-Hosseini SA. Post liver transplant complications of Budd-Chiari syndrome. Indian J Gastroenterol. 2021;40:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 130. | Ki M, Choi HY, Kim KA, Kim BH, Jang ES, Jeong SH. Incidence, prevalence and complications of Budd-Chiari syndrome in South Korea: a nationwide, population-based study. Liver Int. 2016;36:1067-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 131. | Hayek G, Ronot M, Plessier A, Sibert A, Abdel-Rehim M, Zappa M, Rautou PE, Valla D, Vilgrain V. Long-term Outcome and Analysis of Dysfunction of Transjugular Intrahepatic Portosystemic Shunt Placement in Chronic Primary Budd-Chiari Syndrome. Radiology. 2017;283:280-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |