Published online Dec 24, 2023. doi: 10.5306/wjco.v14.i12.606
Peer-review started: June 1, 2023
First decision: August 16, 2023
Revised: September 5, 2023
Accepted: November 17, 2023
Article in press: November 17, 2023
Published online: December 24, 2023
Processing time: 203 Days and 19.4 Hours
High-dose methotrexate (HD-MTX) combined with other chemotherapeutic agents is an effective treatment for patients with newly diagnosed primary central nervous system lymphoma (PCNSL); however, some patients have adverse reactions.
To retrospectively evaluate disease outcomes and mutational profiles in newly diagnosed PCNSL patients treated with a zanubrutinib/HD-MTX combination regimen.
Nineteen newly diagnosed PCNSL patients were treated with zanubrutinib/HD-MTX until disease progression, intolerable toxicities, or physician/patient-directed withdrawal. Safety and efficacy were assessed per the CTCAE v5.0 and RECIST v1.1 criteria, respectively. The primary endpoint was the objective response rate (ORR), and the secondary endpoints were progression-free survival, overall survival (OS), and safety.
The median follow-up duration was 14.7 mo (range, 3.9–30 mo). The ORR for all patients was 84.2%, and 2-year progression-free- and OS rates were 75.6% and 94.1%, respectively. All patients completed the induction phase, and nine patients underwent autologous stem cell transplantation as consolidation therapy, resulting in an ORR of 88.9%. Ten patients received zanubrutinib as maintenance therapy and achieved an ORR of 80%. All patients showed an acceptable safety profile. The sequencing results for cerebrospinal fluid (CSF) and tumor tissue showed that PIM1 mutations were the most frequent genetic alterations. Circulating tumor DNA was correlated with disease relapse and response.
Our empirical observations demonstrated that the combination of zanubrutinib with HD-MTX yielded a marked clinical response and tolerability among newly diagnosed PCNSL patients. Non-invasive CSF liquid biopsy profiling may be feasible for evaluating treatment response and tumor burden.
Core Tip: Zanubrutinib combined with high-dose methotrexate provided a marked clinical response and tolerance in newly diagnosed primary central nervous system lymphoma patients. Additionally, the detection of circulating tumor DNA in cerebrospinal fluid played a significant part in disease surveillance and treatment response monitoring. However, given the small sample size and retrospective nature of this study, further research is required to validate our findings.
- Citation: Wang N, Chen FL, Pan L, Teng Y, Wei XJ, Guo HG, Jiang XM, Huang L, Liu SC, Liang ZL, Li WY. Clinical outcomes of newly diagnosed primary central nervous system lymphoma treated with zanubrutinib-based combination therapy. World J Clin Oncol 2023; 14(12): 606-619
- URL: https://www.wjgnet.com/2218-4333/full/v14/i12/606.htm
- DOI: https://dx.doi.org/10.5306/wjco.v14.i12.606
Primary central nervous system lymphoma (PCNSL) is an aggressive lymphoma that is confined to the brain, leptomeninges, eyes, cerebrospinal fluid (CSF), or spinal cord, without evidence of systemic disease[1,2]. Almost all PCNSLs constitute diffuse large B-cell lymphoma (DLBCL)[3]. However, the treatments for PCNSL and DLBCL differ. High-dose methotrexate (HD-MTX) is the primary treatment for PCNSL. HD-MTX (3.5 g/m2) combined with other chemotherapeutic agents is effective; however, some patients have adverse reactions[4,5]. Therefore, it is necessary to identify drugs that can be combined with HD-MTX to solve this issue.
Zanubrutinib, a novel oral inhibitor of Bruton’s tyrosine kinase (BTK), is a promising therapeutic intervention in B-cell antigen receptor (BCR) and Toll-like receptor (TLR) signaling. This signaling network integrates signals from the BCR and TLR pathways. The key players, BCR-associated protein CD79B and myeloid differentiation primary response 88 (MYD88), act as bridges linking interleukin-1 and TLRs with the potent nuclear factor kappa B pathway[6-9]. Activating mutations were observed in MYD88 and CD79B across various studies of PCNSL[6,10-13]. Studies have shown that BTK inhibitors can cross the blood-brain barrier and effectively modulate signaling cascades downstream of MYD88 and CD79B[14-17], demonstrating their potential efficacy in PCNSL. Recent studies on zanubrutinib-containing therapeutic regimens have highlighted their effectiveness in cases of DLBCL with CNS involvement[18]. However, despite these advancements, a critical gap remains: the absence of concrete clinical evidence supporting the use of zanubrutinib in PCNSL with CNS involvement. The BTK inhibitor, ibrutinib, combined with HD-MTX has demonstrated an objective response rate (ORR) of 80% with an acceptable safety profile in a phase Ib study[19]. Therefore, we retrospectively analyzed the clinicopathological characteristics, treatment outcomes, and adverse events in newly diagnosed PCNSL patients treated with combined HD-MTX and zanubrutinib. We also explored the next-generation sequencing of circulating tumor DNA (ctDNA) in CSF, both before and during treatment, as well as the safety profile, treatment response, and genomic biomarkers.
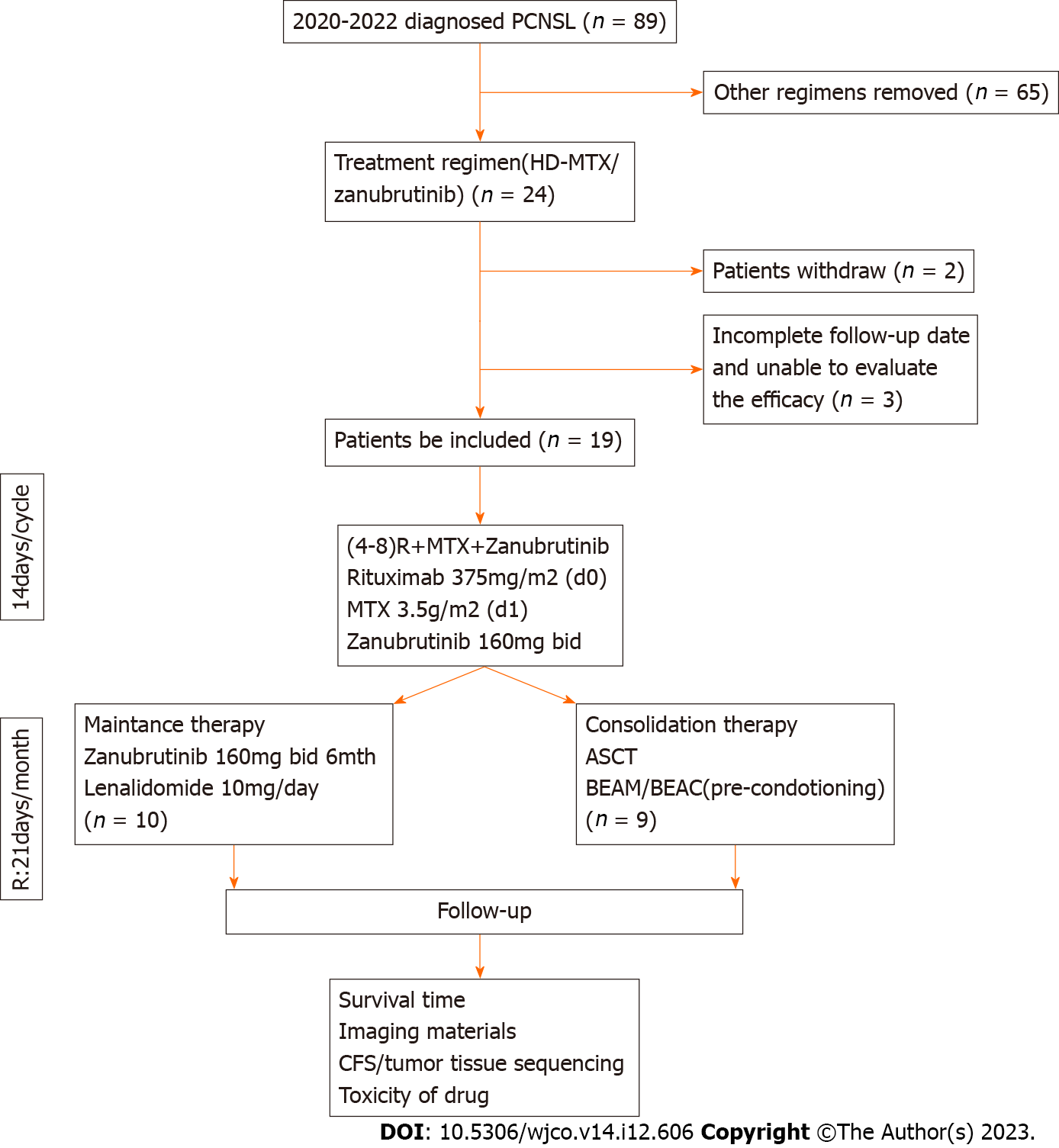
From May 2020 to April 2022, 19 eligible PCNSL patients from XX Hospital, China, were identified for inclusion in this study. The inclusion criteria were as follows: (1) Newly diagnosed pathologically confirmed PCNSL; (2) ≥ 18 years of age; (3) Treatment with HD-MTX and zanubrutinib combination therapy; and (4) Received at least two cycles of chemo
This study was approved by the Clinical and Research Ethics Committee of the Guangdong Provincial People’s Hospital, Guangzhou, China. All procedures in the present study that involved human participants were performed in accordance with the Declaration of Helsinki. All patients provided written informed consent to participate in this study.
The treatment regimen was designed to achieve optimal outcomes through induction therapy with combined HD-MTX and zanubrutinib. HD-MTX was administered at a dose of 3.5 g/m2, with a total of 4-8 doses planned. Zanubrutinib was prescribed at a dose of 160 mg orally (PO) twice daily (BID). Zanubrutinib administration was paused on the days of HD-MTX infusion to mitigate potential interactions and was resumed once HD-MTX clearance was achieved. Following induction therapy, zanubrutinib was administered as maintenance therapy until specific endpoints were reached, namely disease progression, intolerable toxicity, autologous stem cell transplantation (ASCT), or mortality.
Prior to ASCT, a comprehensive evaluation of each patient’s stem cell composition was performed. The ASCT process comprised the use of peripheral blood autologous hematopoietic stem cells. To prepare patients for ASCT, a pre-conditioning regimen was administered that comprised either carmustine, etoposide, cytarabine, and melphalan or carmustine, etoposide, cytarabine, and cyclophosphamide. This pre-conditioning aimed to optimize the transplantation environment. Subsequently, granulocyte colony-stimulating factor was administered to mobilize stem cells. The screening process involved monitoring cluster of differentiation 34-positive (CD34+) hematopoietic stem cells in peripheral blood using flow cytometry. The ideal threshold for peripheral blood CD34+ cells was set at ≥ 20 cells/μL. This monitoring enabled prediction of the required collection quantity and duration, with a minimum standard of CD34+ cells not at 2 × 106/kg. A desirable transplant condition was generally achieved when the final collection of CD34+ cells exceeded 5 × 106/kg.
Therapeutic response was evaluated in accordance with the international PCNSL Collaborative Group guidelines[1]. Response to treatment was assessed using magnetic resonance imaging and CSF evaluation every second cycle. In accordance with the guidelines, each patient’s best response to treatment was recorded to evaluate the ORR, including complete response (CR, no contrast enhancement on imaging) and partial response (≥ 50% decrease disease enhancement on imaging). Any new lesions were defined as progressive disease (PD), and any other conditions were defined as stable disease. Progression-free survival (PFS) was calculated from the start of treatment to the time of disease progression or death due to PCNSL. Overall survival (OS) was calculated from the date of diagnosis to the time of death from any cause.
CSF and peripheral blood samples were collected and stored at -80°C. Tumor biopsy specimens were obtained using formalin-fixed, paraffin-embedded tissues. Samples were analyzed using capture-based targeted next-generation sequencing in a central testing laboratory (Nanjing Geneseq Technology, Inc., Nanjing, China). This approach, as previously outlined, targets 102 lymphoma-associated genes, facilitating precise genetic characterization[20,21]. The DLBCL [non-germinal center B-cell (non-GCB) or germinal center B-cell (GCB)] subtype was determined using immunohistochemical staining in accordance with the Hans classification, in the Department of Pathology of the Guangdong People’s Hospital, Guangzhou, China.
GraphPad Prism 9 (version 9.0.2; GraphPad Software, Inc., San Diego, CA, United States) was used for the data analysis. Baseline characteristics were described using medians for continuous variables and percentages for categorical variables. PFS and OS were analyzed by the Kaplan–Meier method, P values were calculated using the log-rank test, and P < 0.05 indicated a significant difference.
Data for 19 patients with newly diagnosed PCNSL who were treated with HD-MTX plus zanubrutinib were retrospectively analyzed (Figure 1). The patients’ clinicopathological characteristics are summarized in Table 1. The patients’ median age was 57 years (range, 27-81 years), and five patients had an Eastern Cooperative Oncology Group performance score > 2 (Table 1). Ten patients were women, and 16 patients had lesions in deep areas, namely the periventricular tissue, corpus callosum, brainstem, basal ganglia, and/or cerebellum. Eleven patients had high CSF protein concentrations (> 450 mg/L), and only one patient had a high lactate dehydrogenase serum level (> 250 U/L). The International Extranodal Lymphoma Study Group risk score was low-grade in 3 patients, median-grade in 12 patients, and high-grade in 1 patient.
| Characteristic | |
| Age, yr | 57 (27–81) |
| Sex, n (%) | |
| Male | 9 (47.4) |
| Female | 10 (52.6) |
| ECOG-PS ≥ 2, n (%) | 5 (26.3) |
| Invasion of deep intracranial areas, n (%) | 16 (84.2) |
| High CSF protein concentration (> 450 mg/L), n (%) | 11 (68.75)1 |
| High LDH serum concentration (> 250 U/L), n (%) | 1 (5.3) |
| IELSG risk score, n (%) | |
| Low | 3 (18.75)1 |
| Intermediate | 12 (75)1 |
| High | 1 (6.25)1 |
| Follow-up time (mo) | 14.7 (3.9–30) |
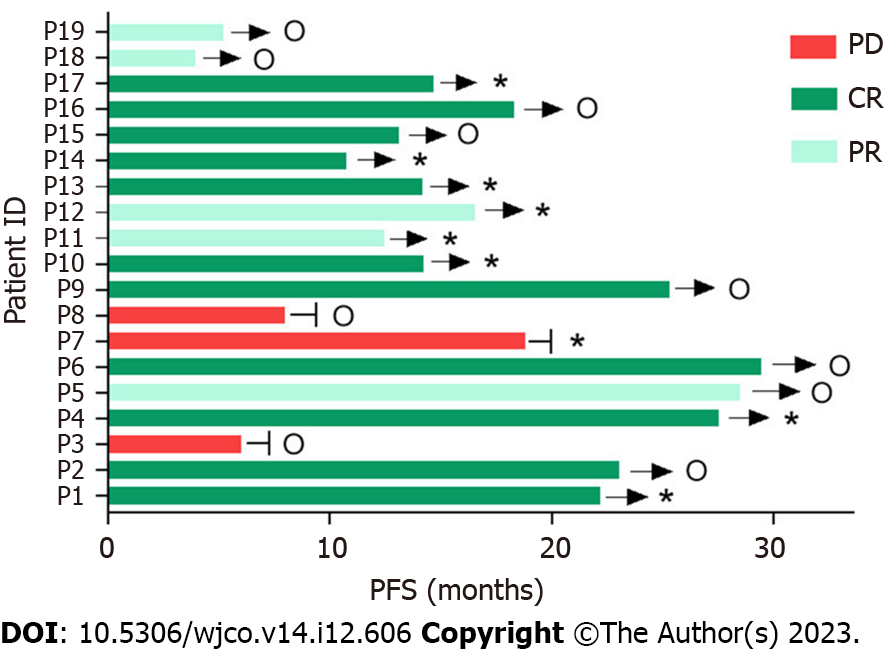
All 19 patients received 120 doses of induction therapy. ASCT was administered as consolidation therapy in nine patients. None of the patients received corticosteroid therapy. HD-MTX therapy was discontinued in one patient due to delayed HD-MTX excretion. Nine patients completed ASCT, with an ORR of 88.9% (CR/PR: 6/2). Eight patients were still in remission at the time of writing (Figure 2 and Table 2). Ten patients received maintenance therapy comprising zanubrutinib with lenalidomide for 6 mo, and zanubrutinib monotherapy was administered continuously until disease progression.
| Patient | ID | COO Subtype | Best response (mo) | MYDBB | CD79B | Ki-67 | Cyclin D1 | Other IHC results |
| p973624 | 1 | Non-GCB | CR (22.2)1 | L265P | Y196D | > 90%+ | NA | CD20(+++), CD79a(+++), CD3(−), CD5(−), CD21(−), CD23(−), Bcl6(>90%+), MUM1(>90%+), FOXP1(>90%+), Bcl2(60%+), c-Myc(40%+), CD30(−), ALK(ALK1)(−), CD138(−), P53(+), c-Met(−), PD-L1(22C3)(30%+) |
| p968283 | 2 | GCB | CR (23)1 | NA | NA | 100%+ | − | CD43(−), CD20(+++), CD3(−), CD79a(+++), CD5(−), CD23(−), CD10(95%+), CD21(−), CD30(−), ALK(ALK1)(−), Bcl6(90%+), CD138(−), MUM1(65%+), Bcl2(50%+), c-Myc(20%+), GFAP(−), Olig2(−) |
| p955842 | 3 | Non-GCB | SD (6.0) | NA | NA | 70%+ | − | LCA(+++), OCT-2(+++), CD20(+++), CD19(+++), CD10(−), Bcl6(70%+), MUM1(40%+), CD3(−), CD5(+), ALK(ALK1)(-), CD23(−), CD21(−), CD30(−), CD138(−), Bcl2(++), TdT(−), GECT1(+), FOXP1(+++), c-Myc(80%+), c-Met(−), P53(+++), GFAP(−), CK(−), EMA(−) |
| p241574 | 4 | Non-GCB | CR (27.5)1 | NA | K159Q, V223R | 90%+ | − | LCA(++), CD79a(++), CD43(−), CD20(+), CD3(−), CD5(−), CD23(−), CD10(−), CD21(−), CD30(−), ALK(ALK1)(−), Bcl6(90%++), CD138(−), MUM1(70%+), Bcl2(50%+), TdT(−), GECT1(30%+), FOXP1(+), c-Myc(70%+), c-Met(+), LMP-1(−), EBNA2(−), P53(+), PD-L1(22C3)(90%+) |
| p939668 | 5 | Non-GCB | PR (28.5)1 | NA | NA | NA | NA | NA |
| p932230 | 6 | GCB | CR (29.5)1 | S219C | NA | 98%+ | NA | CD20(+++), CD79a(+++), CD3(−), CD5(−), ALK(ALK1)(−), CD21(−), CD23(−), Bcl6(90%+), MUM1(20%+), CD10(100%+), CK(−), Vimentin(−), EMA(−), S100(−), GFAP(−), Bcl2(80%+), GECT1(35%+), FOXP1(80%+), c-Myc(45%+), C-MET(50%+), P53(4%+), PD-L1(22C3)(10%+) |
| p929763 | 7 | Non-GCB | CR (18.8) | L265P | C.553-2A>C | 90%+ | − | CD43(−), CD20(+++), CD3(−), CD79a(+++), CD5(−), CD23(−), CD10(−), CD19(+++), CD22(++), CD21(−), CD30(−), ALK(ALK1)(−), Bcl6(10%+), CD138(−), MUM1(20%+), Bcl2(70%+), TdT(−), c-Myc(5%+), GFAP(−) |
| p173185 | 8 | Non-GCB | PR (8.0) | L265P | NA | NA | NA | ERCC1(−), β-tubulin(+++), EGFR(+++), VEGF(+), ALK(−), CD56(−), CgA(−), Syn(−) |
| p651739 | 9 | GCB | CR (23.0) | NA | NA | 90%+ | − | CD43(+), CD20(+++), CD3(+), CD79a(++), CD5(−), CD23(−), CD10(+++), CD21(−), CD30(−), ALK(ALK1)(−), Bcl6(60%+), CD138(−), MUM1(++), Bcl2(−), TdT(−), c-Myc(20%+), GFAP(−), Olig2(−) |
| p1013138 | 10 | GCB | CR (12.5) | NA | NA | 90%+ | − | CD3(−), CD5(−), CD20(++), CD79a(++), CD30(−), ALK(ALK1)(−), SALL4(−), OCT3/4(−), AFP(−), GFAP(−), Olig2(−), MUM1(+), CD10(++), Bcl6(++), CD23(−), Bcl2(−), GCET-1(+), FOXP1(+), c-Myc(70%+), c-Met(−), P53(95%++), PD-L1(22C3)(TC <1%+, IC 70%+) |
| p2010722 | 11 | Non-GCB | PR (16.6) | NA | NA | 80%+ | − | CK(−), CD20 and CD79a(+), CD3(−), CD5(−), Bcl-2(80%+), MUM-1(+), CD10(−), Bcl6(−) |
| p998505 | 12 | Non-GCB | PR (22.2) | NA | NA | 70%+ | 2%+ | D3(+), CD5(++), CD20(+++), CD79a(+++), CD30(−), CD10(−), GFAP(−), Olig2(−), CK(−), CD43(++), CD23(−), CD21(−), ALK(ALK1)(−), Bcl6(20%+), CD138(−), MUM1(80%+), Bcl2(90%+++), TdT(−), GCET-1(−), FOXP1(+++), c-Myc(70%+), c-Met(−), P53(1%+), PD-L1(22C3)(70%+) |
| p1013897 | 13 | GCB | CR (14.2) | NA | NA | 85%+ | − | CD20(+++), CD3(−), CD79a(+++), CD5(−), CD30(<1%+), ALK(ALK1)(−), CD23(−), CD10(+++), CD21(−), Bcl6(70%+), CD138(+), MUM1(40%+), Bcl2(5%+), TdT(−), Cyclin D1(−), c-Myc(40%+), c-Met(60%+), P53(70%+), PD-L1(22C3)(40%+) |
| p2020811 | 14 | GCB | CR (10.8) | NA | NA | 90%+ | NA | CD3(+), CD5(+), CD79a(+), CD10(+), Bcl6(±), CD20(+++), Bcl6(80%+), MUM1(<5%+), CD10(+), Ki67(90%+), CD3(−), AE1/AE3(−), EMA(−), P40(−), CD3(−), GFAP(−) |
| P2003851 | 15 | GCB | CR (13.1) | NA | NA | 80%+ | − | EMA(−), S100(−), GFAP(+), Syn(+), CgA(−), Olig2(+), NeuN(+), CD34(+), CD3(+), CD5(+), CD79a(+), CD20(+), CD43(10%+), CD30(−), ALK(ALK1)(−), Bcl6(>90%+), Bcl2(90%+), TdT(−), GCET-1(90%+), FOXP1(>90%+), c-Myc(40%+), c-Met(−), LMP-1(−), EBNA2(−), P53(60%+), PD(−), L1(22C3)(30%+) |
| p996213 | 16 | GCB | CR (18.3) | NA | NA | NA | NA | NA |
| P1010986 | 17 | GCB | CR (14.7) | NA | NA | 95%+ | − | CD43(+), CD20(+++), CD3(−), CD79a(++), CD5(−), CD23(−), CD10(60%+), CD21(−), CD30(−), ALK(ALK1)(−), Bcl6(60%+), CD138(−), MUM1(90%+), Bcl2(15%+), TdT(−), c-Myc(30%+) |
| P2052819 | 18 | Non-GCB | PR (3.9) | NA | NA | 90%+ | − | GFAP(−), Olig2(−), CD20(+++), CD79a(++), CD3(−), CD5(−), CD21(−), CD23(−), CD10(−), Bcl6(60%++), MUM1(90%+++), CD138(−), Bcl2(90%+++), c-Myc(70%++), CD30(−), ALK(ALK1)(−) |
| P2045773 | 19 | GCB | PR (5.2) | NA | NA | 90%+ | − | CD19(+++), CD20(+++), CD3(−), CD5(−), GFAP(−), Olig2(−), CD23(+), CD10(−), CD21(−), CD30(−), ALK(ALK1)(−), Bcl6(70%+), CD138(−), MUM1(70%+), Bcl-2(95%+), TdT(−), c-Myc(40%+), c-Met(−) |
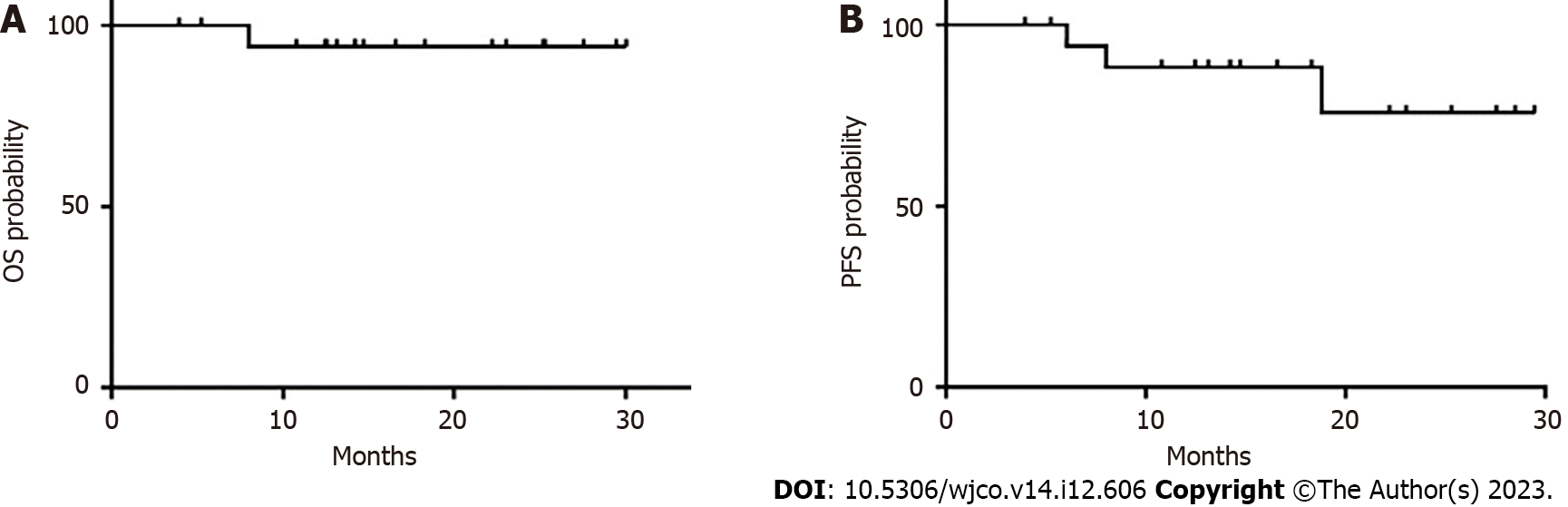
The median follow-up duration was 14.7 mo (range, 3.9-30 mo). All patients were evaluated for treatment response, which revealed CR in 11 patients, partial response in 5 patients, and PD in 3 patients. The ORR was 84.2%, and 2-year PFS and OS rates were 75.6% and 94.1%, respectively. The median PFS and median OS for the entire cohort were not reached (Table 3 and Figure 3).
| Parameter | N = 19 |
| OS rate (%) | - |
| 24-mo (95%CI) | 94.1% (83.6%-100%) |
| Median PFS | - |
| 24-mo (95%CI) | 75.6% (53.4%-100%) |
| ORR (%) | 84.2% |
| ASCT (consolidation therapy) | 88.9% |
| Zanubrutinib (maintenance therapy) | 80% |
| Median follow-up time (mo) | 14.7 |
| 95%CI | 3.9–30 |
The prevalent hematological toxicity in patients who received HD-MTX plus zanubrutinib treatment was anemia (100%), followed by lymphocytopenia (84.2%). The leading non-hematological toxicities were hypoalbuminemia (94.7%) and hypokalemia (78.9%) (Table 4). It is noteworthy that no grade 4 non-hematological toxicities were recorded, and the observed adverse effects of therapy were mild and required no additional therapeutic interventions. No treatment-related fatalities were observed.
| Adverse event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total (%) |
| Hematological toxicities | |||||
| Leukopenia | 3 | 7 | 1 | 11 (57.9) | |
| Neutropenia | 3 | 6 | 2 | 11 (57.9) | |
| Lymphocytopenia | 6 | 8 | 2 | 16 (84.2) | |
| Thrombocytopenia | 5 | 1 | 6 (31.6) | ||
| Anemia | 8 | 10 | 1 | 19 (100) | |
| Non-hematological toxicities | |||||
| Transaminase increase | 4 | 4 (21.1) | |||
| Creatinine increase | 2 | 1 | 3 (15.8) | ||
| Hypoalbuminemia | 16 | 2 | 18 (94.7) | ||
| Hypokalemia | 10 | 4 | 1 | 15 (78.9) | |
| Lung infection | NA | ||||
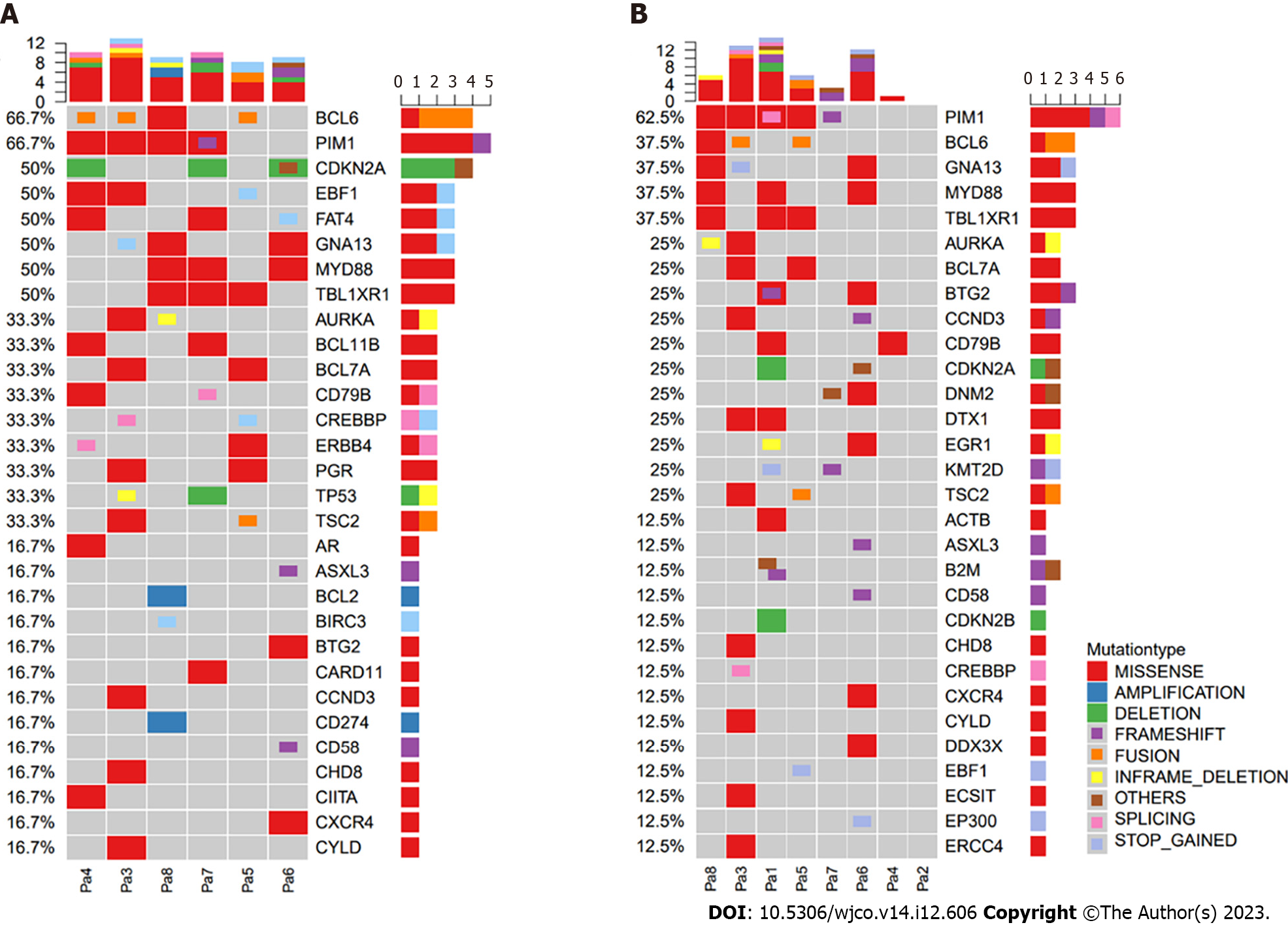
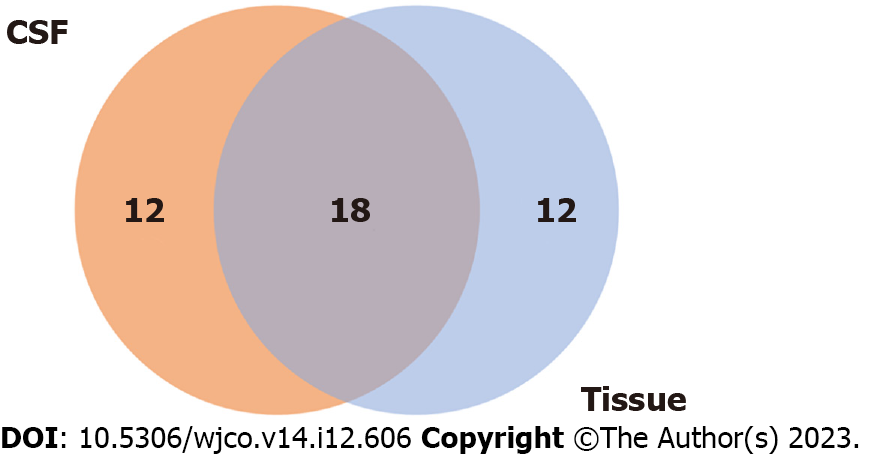
We also explored the association between treatment response and tumor genomic traits. CSF samples were available for eight patients, while six patients had baseline tumor biopsy samples available for genomic analysis (Figure 4). Forty-two genetic alterations were detected (tumor tissue samples: n = 30, CSF samples: n = 30), and 18 alterations were the same in the primary tumor tissue and CSF samples (Figure 5). The most common mutation detected in both CSF and primary tumor samples was PIM1, followed by alterations of B-cell lymphoma 6, MYD88, GNA13, and TBL1XR1.
Among the 19 patients, 9 had non-GCB disease, with 6 (66.7%) responding positively to the zanubrutinib-based regimen. The remaining 10 patients with GCB disease achieved a 100% response rate to the same regimen. Among the patients with MYD88 alterations, four achieved CR, constituting 50% of this subgroup, with the zanubrutinib-based regimen. In the subset of eight patients with alterations in key genes involved in the BCR pathway, such as CD79B and MYD88, the ORR reached 60%. The response rates for patients with alterations in MYD88 and CD79B were 50% (4/8) and 37.5% (3/8), respectively (Table 2). Two patients (P1 and P7) with alterations in both MYD88 and CD79B genes demonstrated a 50% ORR, with one achieving a CR, as shown in Table 2.
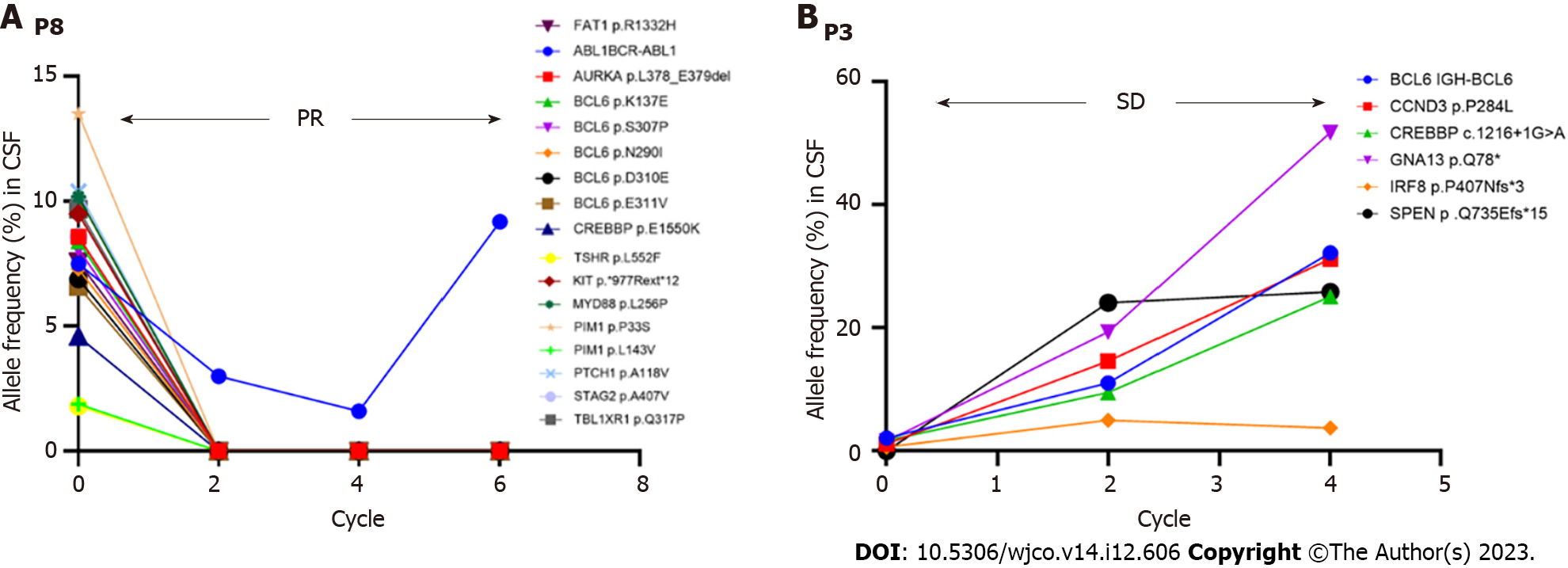
Eight CSF samples were collected during various treatment cycles, i.e., at baseline and just before cycles 3, 5, and 6. One exception was patient 6, who underwent assessments only after cycle 3 and cycle 6 for personal reasons. Six patients showed a robust radiographic response to treatment, resulting in a significant reduction in CSF mutant allele frequency. One patient (P3) showed a stable radiographic response, confirmed by magnetic resonance imaging, but the ctDNA levels remained unchanged in the CSF specimen (Figure 6). However, this patient experienced PD following completion of the zanubrutinib-based induction regimen. Therefore, whole-brain radiotherapy (30 GY) with temozolomide and zanubrutinib was initiated. This approach led to a favorable radiographic response, and the patient is presently continuing with this treatment. Another patient (P8) demonstrated a partial radiographic response. The mutant allele frequency in the CSF decreased markedly with treatment, excluding the gene fusion of BCR-ABL1 (Figure 6). However, this patient developed PD as peripheral lesions, and subsequently received rituximab, zanubrutinib, and lenalidomide (IR2) as second-line treatment.
The outcomes in our case series showed that combined therapy with zanubrutinib and HD-MTX was well-tolerated as a frontline therapeutic regimen for patients diagnosed with PCNSL. Nine patients transitioned to ASCT following the zanubrutinib and HD-MTX induction phase, while another 10 patients underwent maintenance therapy with zanubrutinib alone. Only three patients developed disease progression. Data for all 19 patients were included in the evaluation of PFS and OS. Only one patient (P6) discontinued HD-MTX therapy, owing to delayed HD-MTX excretion, and the regimen was changed to rituximab, zanubrutinib, and lenalidomide. No instances of treatment-related mortality were recorded throughout the study. However, limitations exist in our study. It is well recognized that the journey from the initiation of the oncogenic event to the point of clinical diagnosis is protracted, spanning approximately a decade. This extended timeline underscores the intricacies inherent in the development of neoplastic disorders, revealing that the characterization of “newly diagnosed” necessitates a more nuanced understanding-one that acknowledges the substantial span of disease evolution prior to medical recognition.
Our study emphasizes the importance of adopting novel therapeutic strategies to address the multifaceted nature of PCNSL. Historically, HD-MTX has played a pivotal role, serving as a cornerstone for first-line induction regimens in PCNSL. This is owing to its ability to penetrate the blood-brain barrier and achieve effective anti-tumor concentrations[22-24]. However, even with this treatment, approximately half of the patients experience relapse, and 5-year survival rates remain discouragingly low, at 30%-40%[3]. Studies have shown that HD-MTX-based first-line regimens result in an ORR of approximately 68% in PCNSL patients over the age of 60 years. In newly diagnosed PCNSL, the median PFS is 35 mo and 8 mo for patients younger and older than 60 years, respectively[22,25]. In this study, the combination of zanubrutinib with HD-MTX demonstrated robust anti-tumor activity, with an ORR of 84.2%, which is higher than that achieved by HD-MTX-based chemotherapy alone. Our results also identified a 2-year PFS of 75.6% and an OS of 94.1%. The median PFS and median OS for the entire cohort were not reached at the time of writing, even after a follow-up of 14.7 mo (range: 3.9-30 mo). Previous studies have shown that zanubrutinib exhibits greater selectivity in inhibiting BTK compared with the off-target effects observed with ibrutinib[26]. The profound BTK inhibition observed with zanubrutinib in both blood and lymph nodes is hypothesized to maximize the potential for deep and sustained remissions in conditions such as chronic lymphocytic leukemia and other hematological disorders. In the phase I BGB-3111-AU-003 study, which evaluated zanubrutinib monotherapy for chronic lymphocytic leukemia/small lymphocytic leukemia, efficacy was assessed in a cohort of 78 patients. This patient group included individuals with high-risk disease features, such as adverse cytogenetics (del(11q), 23.3%; del(17p) and/or TP53 mutation) at a rate of 19.1%[26]. After a median follow-up of 13.7 mo (range: 0.4-30.5 mo), the ORR was 96.2% (75/78) (95% confidence interval: 89.2-99.2). This ORR group included two patients (2.6%) who achieved CR, 63 (80.8%) who achieved PR, and 10 (12.8%) with PR with lymphocytosis[26].
In our study, nine patients completed ASCT after the induction phase of zanubrutinib-based combination therapy and achieved an ORR of 88.9% (CR/PR: 6/2), indicating the advantage of ASCT as a consolidation regimen. Furthermore, studies have shown that ASCT is an effective consolidation strategy in PCNSL[27,28]. Therefore, ASCT should be the first choice for suitable patients.
Lenalidomide is an immunomodulatory agent that shows good anti-tumor activity as a BTK inhibitor in DLBCL[27]. Lenalidomide combined with ibrutinib and rituximab shows promising anti-tumor activity in relapsed/refractory DLBCL[29]. In our study, one patient (P6) achieved CR after receiving an IR2-based regimen and zanubrutinib maintenance. In patients who do not tolerate HD-MTX and experience toxicity, IR2 may be a better choice. In our study, 8 of 10 patients achieved a therapeutic response with zanubrutinib maintenance. Therefore, for PCNSL patients who are unsuitable for ASCT, lenalidomide and zanubrutinib as maintenance therapy might be promising.
PCNSL patients are divided into three major molecular subtypes: A GCB subtype, an activated B-cell (ABC) subtype, and a type III subtype, whose cell origin is unidentified. The first two subtypes account for approximately 80% of all cases; ABC DLBCL patients have poorer outcomes[30]. To our knowledge, there are no reports of the results of zanubrutinib therapy for the GCB and ABC subtypes of PCNSL. Nine of the 19 patients in our study had non-GCB disease and 6 (66.7%) responded to the zanubrutinib-based regimen. Ten patients had GCB disease, and all (100%) responded to the zanubrutinib-based regimen. Zanubrutinib may have had a better effect on the ABC subtype in previous studies.
Previous studies have shown that next-generation sequencing may be used as a molecular diagnostic method prior to delivering targeted therapies, particularly BCR inhibitors, in the case of MYD88-mutated tumors[31]. In our study, CSF liquid biopsies were evaluated using next-generation sequencing in eight patients, while XX underwent radiological evaluation. Six patients had dramatically lower CSF mutant allele frequencies compared with patients 3 and 8. Patient 8 achieved a partial radiographic response during the induction treatment, while the CSF mutant allele frequency increased after cycle 4 (Figure 6). This patient developed PD while receiving the maintenance regimen. As shown in Figure 6, patient 3 had a stable radiographic response, with an increased level of ctDNA in the CSF specimen. This patient developed PD after completing the induction regimen.
Performing CSF liquid biopsy profiling with radiologic evaluation is feasible in PCNSL. Studies show frequent MYD88 and CD79B mutations in PCNSL[6,10-13]. On the basis of the genetic analysis of CSF in our study, we found frequent alterations of MYD88 and CD79B involved in the BCR pathway, and zanubrutinib combined with HD-MTX resulted in good anti-tumor activity. Therefore, CSF liquid biopsy profiling might be feasible for evaluating the response to a therapeutic protocol. However, the fleeting presence of ctDNA in the bloodstream poses a challenge to the reliability of the results of CSF liquid biopsy profiling[32].
An extensive safety analysis performed on pooled data from six zanubrutinib monotherapy trials revealed a notable trend toward favorable tolerability among patients diagnosed with various B-cell malignancies[29]. These conditions, which are often associated with symptoms such as diarrhea, thrombocytopenia, bleeding, atrial fibrillation, skin rash, and fatigue, respond well to zanubrutinib treatment[33]. The results of our study highlight the reassuring absence of grade 4 non-hematological toxicities. The reported side effects were characterized as mild and did not require further therapeutic intervention. No treatment-related mortality was observed, indicating a moderate safety profile for zanubrutinib combined with HD-MTX for patients with PCNSL.
While our findings provide valuable insights into the tolerability of zanubrutinib, this study has limitations. First, owing to the retrospective design and the small number of included patients, larger-scale prospective cohort studies and longer follow-up may be warranted to validate our results. Second, generally, regarding cellular origin in PCNSL, zanubrutinib may have a better effect on the ABC subtype. However, in this study, we were able to identify only the GCB and non-GCB phenotypes owing to the limited experimental conditions; ABC genotyping was not performed. Therefore, it is not possible to conduct a more detailed analysis.
Zanubrutinib combined with HD-MTX provided a good clinical response and was well tolerated in newly diagnosed PCNSL patients. Additionally, the detection of ctDNA in CSF was very useful in disease surveillance and treatment response monitoring. However, given the small sample size and retrospective study design, further research is required to validate our findings.
Primary central nervous system lymphoma (PCNSL) is an aggressive brain lymphoma with limited treatment options. The current standard treatment involves high-dose methotrexate (HD-MTX), but there is a need for effective combination therapies to address adverse reactions. Zanubrutinib, a Bruton’s tyrosine kinase inhibitor, shows promise owing to its potential to modulate B-cell receptor and Toll-like receptor signaling, which are associated with PCNSL.
This study aimed to evaluate the efficacy and safety of combining zanubrutinib with HD-MTX for newly diagnosed PCNSL patients. Additionally, the study explored the use of circulating tumor DNA (ctDNA) in cerebrospinal fluid (CSF) as a monitoring tool for treatment response.
The main objectives were to assess the treatment outcomes, adverse events, and genomic characteristics of PCNSL patients treated with HD-MTX and zanubrutinib combination therapy, and to investigate the potential of CSF ctDNA in disease surveillance.
Nineteen eligible PCNSL patients were included in the study and received HD-MTX and zanubrutinib combination therapy. Clinical responses were evaluated, and ctDNA in CSF was analyzed using next-generation sequencing. Safety, treatment duration, and response were assessed.
The study demonstrated an overall response rate of 84.2% with the combination therapy, including complete and partial responses. Adverse events were mild and manageable. ctDNA levels in CSF were monitored and correlated with treatment response.
Zanubrutinib combined with HD-MTX resulted in effective clinical responses in newly diagnosed PCNSL patients. The study highlighted the potential of CSF ctDNA for monitoring treatment response and disease surveillance. This combination therapy demonstrated promising safety and efficacy profiles.
While the study results are promising, further research with larger patient cohorts and longer follow-up periods is needed to confirm the findings. The potential of zanubrutinib in different molecular subtypes of PCNSL and its long-term effects need to be explored. The clinical use of CSF ctDNA requires further investigation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Mehdipour P, Iran S-Editor: Fan JR L-Editor: Webster JR P-Editor: Yu HG
| 1. | Ferreri AJM. Therapy of primary CNS lymphoma: role of intensity, radiation, and novel agents. Hematology Am Soc Hematol Educ Program. 2017;2017:565-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Batchelor TT. Primary central nervous system lymphoma: A curable disease. Hematol Oncol. 2019;37 Suppl 1:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 3. | Schaff LR, Grommes C. Primary central nervous system lymphoma. Blood. 2022;140:971-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 110] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 4. | Langner-Lemercier S, Houillier C, Soussain C, Ghesquières H, Chinot O, Taillandier L, Soubeyran P, Lamy T, Morschhauser F, Benouaich-Amiel A, Ahle G, Moles-Moreau MP, Moluçon-Chabrot C, Bourquard P, Damaj G, Jardin F, Larrieu D, Gyan E, Gressin R, Jaccard A, Choquet S, Brion A, Casasnovas O, Colin P, Reman O, Tempescul A, Marolleau JP, Fabbro M, Naudet F, Hoang-Xuan K, Houot R. Primary CNS lymphoma at first relapse/progression: characteristics, management, and outcome of 256 patients from the French LOC network. Neuro Oncol. 2016;18:1297-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 145] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 5. | Ferreri AJ, Reni M, Foppoli M, Martelli M, Pangalis GA, Frezzato M, Cabras MG, Fabbri A, Corazzelli G, Ilariucci F, Rossi G, Soffietti R, Stelitano C, Vallisa D, Zaja F, Zoppegno L, Aondio GM, Avvisati G, Balzarotti M, Brandes AA, Fajardo J, Gomez H, Guarini A, Pinotti G, Rigacci L, Uhlmann C, Picozzi P, Vezzulli P, Ponzoni M, Zucca E, Caligaris-Cappio F, Cavalli F; International Extranodal Lymphoma Study Group (IELSG). High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet. 2009;374:1512-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 476] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 6. | Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH, Kohlhammer H, Xu W, Yang Y, Zhao H, Shaffer AL, Romesser P, Wright G, Powell J, Rosenwald A, Muller-Hermelink HK, Ott G, Gascoyne RD, Connors JM, Rimsza LM, Campo E, Jaffe ES, Delabie J, Smeland EB, Fisher RI, Braziel RM, Tubbs RR, Cook JR, Weisenburger DD, Chan WC, Staudt LM. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1041] [Cited by in RCA: 1177] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 7. | Jost PJ, Ruland J. Aberrant NF-kappaB signaling in lymphoma: mechanisms, consequences, and therapeutic implications. Blood. 2007;109:2700-2707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 319] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 8. | Eluard B, Nuan-Aliman S, Faumont N, Collares D, Bordereaux D, Montagne A, Martins I, Cagnard N, Caly M, Taoui O, Lordello L, Lehmann-Che J, Tesson B, Martinez-Climent JA, Copie-Bergman C, Haioun C, Tilly H, Bonsang B, Vincent-Salomon A, Jais JP, Jardin F, Leroy K, Maiuri MC, Kroemer G, Molina TJ, Feuillard J, Baud V. The alternative RelB NF-κB subunit is a novel critical player in diffuse large B-cell lymphoma. Blood. 2022;139:384-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Xinshuang Z, Lei S, Hailong Y, Haiping LI, Mengheng W, Wanci S, Laichun L, Hezhen WU, Yanfang Y, Junfeng Z, Yanwen L, Hanxiong D, Qiang Y, Pengtao Y. Compound Gaoziban tablet alleviates depression toll-like receptor 4/myeloid differentiation factor 88/nuclear factor-kappa B pathway. J Tradit Chin Med. 2022;42:956-964. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Braggio E, Van Wier S, Ojha J, McPhail E, Asmann YW, Egan J, da Silva JA, Schiff D, Lopes MB, Decker PA, Valdez R, Tibes R, Eckloff B, Witzig TE, Stewart AK, Fonseca R, O'Neill BP. Genome-Wide Analysis Uncovers Novel Recurrent Alterations in Primary Central Nervous System Lymphomas. Clin Cancer Res. 2015;21:3986-3994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 174] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 11. | Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, Roulland S, Kasbekar M, Young RM, Shaffer AL, Hodson DJ, Xiao W, Yu X, Yang Y, Zhao H, Xu W, Liu X, Zhou B, Du W, Chan WC, Jaffe ES, Gascoyne RD, Connors JM, Campo E, Lopez-Guillermo A, Rosenwald A, Ott G, Delabie J, Rimsza LM, Tay Kuang Wei K, Zelenetz AD, Leonard JP, Bartlett NL, Tran B, Shetty J, Zhao Y, Soppet DR, Pittaluga S, Wilson WH, Staudt LM. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N Engl J Med. 2018;378:1396-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1316] [Cited by in RCA: 1549] [Article Influence: 221.3] [Reference Citation Analysis (0)] |
| 12. | Roschewski M, Phelan JD. Sorting biologic subtypes of primary CNS lymphoma. Blood. 2021;137:1436-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Nakamura T, Tateishi K, Niwa T, Matsushita Y, Tamura K, Kinoshita M, Tanaka K, Fukushima S, Takami H, Arita H, Kubo A, Shuto T, Ohno M, Miyakita Y, Kocialkowski S, Sasayama T, Hashimoto N, Maehara T, Shibui S, Ushijima T, Kawahara N, Narita Y, Ichimura K. Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol Appl Neurobiol. 2016;42:279-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 14. | Soussain C, Choquet S, Blonski M, Leclercq D, Houillier C, Rezai K, Bijou F, Houot R, Boyle E, Gressin R, Nicolas-Virelizier E, Barrie M, Moluçon-Chabrot C, Lelez ML, Clavert A, Coisy S, Leruez S, Touitou V, Cassoux N, Daniau M, Ertault de la Bretonnière M, El Yamani A, Ghesquières H, Hoang-Xuan K. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase II 'proof-of-concept' iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer. 2019;117:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 15. | Nayyar N, White MD, Gill CM, Lastrapes M, Bertalan M, Kaplan A, D'Andrea MR, Bihun I, Kaneb A, Dietrich J, Ferry JA, Martinez-Lage M, Giobbie-Hurder A, Borger DR, Rodriguez FJ, Frosch MP, Batchelor E, Hoang K, Kuter B, Fortin S, Holdhoff M, Cahill DP, Carter S, Brastianos PK, Batchelor TT. MYD88 L265P mutation and CDKN2A loss are early mutational events in primary central nervous system diffuse large B-cell lymphomas. Blood Adv. 2019;3:375-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 16. | Lionakis MS, Dunleavy K, Roschewski M, Widemann BC, Butman JA, Schmitz R, Yang Y, Cole DE, Melani C, Higham CS, Desai JV, Ceribelli M, Chen L, Thomas CJ, Little RF, Gea-Banacloche J, Bhaumik S, Stetler-Stevenson M, Pittaluga S, Jaffe ES, Heiss J, Lucas N, Steinberg SM, Staudt LM, Wilson WH. Inhibition of B Cell Receptor Signaling by Ibrutinib in Primary CNS Lymphoma. Cancer Cell. 2017;31:833-843.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 386] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 17. | Grommes C, Pastore A, Palaskas N, Tang SS, Campos C, Schartz D, Codega P, Nichol D, Clark O, Hsieh WY, Rohle D, Rosenblum M, Viale A, Tabar VS, Brennan CW, Gavrilovic IT, Kaley TJ, Nolan CP, Omuro A, Pentsova E, Thomas AA, Tsyvkin E, Noy A, Palomba ML, Hamlin P, Sauter CS, Moskowitz CH, Wolfe J, Dogan A, Won M, Glass J, Peak S, Lallana EC, Hatzoglou V, Reiner AS, Gutin PH, Huse JT, Panageas KS, Graeber TG, Schultz N, DeAngelis LM, Mellinghoff IK. Ibrutinib Unmasks Critical Role of Bruton Tyrosine Kinase in Primary CNS Lymphoma. Cancer Discov. 2017;7:1018-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 304] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 18. | Zhang Y, Li Y, Zhuang Z, Wang W, Wei C, Zhao D, Zhou D, Zhang W. Preliminary Evaluation of Zanubrutinib-Containing Regimens in DLBCL and the Cerebrospinal Fluid Distribution of Zanubrutinib: A 13-Case Series. Front Oncol. 2021;11:760405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Grommes C, Tang SS, Wolfe J, Kaley TJ, Daras M, Pentsova EI, Piotrowski AF, Stone J, Lin A, Nolan CP, Manne M, Codega P, Campos C, Viale A, Thomas AA, Berger MF, Hatzoglou V, Reiner AS, Panageas KS, DeAngelis LM, Mellinghoff IK. Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. Blood. 2019;133:436-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 178] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 20. | Shu Y, Wu X, Tong X, Wang X, Chang Z, Mao Y, Chen X, Sun J, Wang Z, Hong Z, Zhu L, Zhu C, Chen J, Liang Y, Shao H, Shao YW. Circulating Tumor DNA Mutation Profiling by Targeted Next Generation Sequencing Provides Guidance for Personalized Treatments in Multiple Cancer Types. Sci Rep. 2017;7:583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 21. | Yang Z, Yang N, Ou Q, Xiang Y, Jiang T, Wu X, Bao H, Tong X, Wang X, Shao YW, Liu Y, Wang Y, Zhou C. Investigating Novel Resistance Mechanisms to Third-Generation EGFR Tyrosine Kinase Inhibitor Osimertinib in Non-Small Cell Lung Cancer Patients. Clin Cancer Res. 2018;24:3097-3107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 390] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 22. | Kasenda B, Ferreri AJ, Marturano E, Forst D, Bromberg J, Ghesquieres H, Ferlay C, Blay JY, Hoang-Xuan K, Pulczynski EJ, Fosså A, Okoshi Y, Chiba S, Fritsch K, Omuro A, O'Neill BP, Bairey O, Schandelmaier S, Gloy V, Bhatnagar N, Haug S, Rahner S, Batchelor TT, Illerhaus G, Briel M. First-line treatment and outcome of elderly patients with primary central nervous system lymphoma (PCNSL)--a systematic review and individual patient data meta-analysis. Ann Oncol. 2015;26:1305-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 23. | Li Y, Sun Z, Liu B, Shan Y, Zhao L, Jia L. Tumor-suppressive miR-26a and miR-26b inhibit cell aggressiveness by regulating FUT4 in colorectal cancer. Cell Death Dis. 2017;8:e2892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 24. | Han CH, Batchelor TT. Diagnosis and management of primary central nervous system lymphoma. Cancer. 2017;123:4314-4324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 130] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 25. | Mendez JS, Grommes C. Treatment of Primary Central Nervous System Lymphoma: From Chemotherapy to Small Molecules. Am Soc Clin Oncol Educ Book. 2018;38:604-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D, Harrup R, Johnston PB, Marlton P, Munoz J, Seymour JF, Simpson D, Tedeschi A, Elstrom R, Yu Y, Tang Z, Han L, Huang J, Novotny W, Wang L, Roberts AW. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 287] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 27. | Ferreri AJ, Illerhaus G. The role of autologous stem cell transplantation in primary central nervous system lymphoma. Blood. 2016;127:1642-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 28. | Houillier C, Taillandier L, Dureau S, Lamy T, Laadhari M, Chinot O, Moluçon-Chabrot C, Soubeyran P, Gressin R, Choquet S, Damaj G, Thyss A, Abraham J, Delwail V, Gyan E, Sanhes L, Cornillon J, Garidi R, Delmer A, Tanguy ML, Al Jijakli A, Morel P, Bourquard P, Moles MP, Chauchet A, Gastinne T, Constans JM, Langer A, Martin A, Moisson P, Lacomblez L, Martin-Duverneuil N, Delgadillo D, Turbiez I, Feuvret L, Cassoux N, Touitou V, Ricard D, Hoang-Xuan K, Soussain C; Intergroupe GOELAMS–ANOCEF and the LOC Network for CNS Lymphoma. Radiotherapy or Autologous Stem-Cell Transplantation for Primary CNS Lymphoma in Patients 60 Years of Age and Younger: Results of the Intergroup ANOCEF-GOELAMS Randomized Phase II PRECIS Study. J Clin Oncol. 2019;37:823-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 29. | Morabito F, Skafi M, Recchia AG, Kashkeesh A, Hindiyeh M, Sabatleen A, Morabito L, Alijanazreh H, Hamamreh Y, Gentile M. Lenalidomide for the treatment of mantle cell lymphoma. Expert Opin Pharmacother. 2019;20:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Dunleavy K, Erdmann T, Lenz G. Targeting the B-cell receptor pathway in diffuse large B-cell lymphoma. Cancer Treat Rev. 2018;65:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Fontanilles M, Marguet F, Bohers É, Viailly PJ, Dubois S, Bertrand P, Camus V, Mareschal S, Ruminy P, Maingonnat C, Lepretre S, Veresezan EL, Derrey S, Tilly H, Picquenot JM, Laquerrière A, Jardin F. Non-invasive detection of somatic mutations using next-generation sequencing in primary central nervous system lymphoma. Oncotarget. 2017;8:48157-48168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 32. | Pan S, Sun S, Liu B, Hou Y. Pan-cancer Landscape of the RUNX Protein Family Reveals their Potential as Carcinogenic Biomarkers and the Mechanisms Underlying their Action. J Transl Int Med. 2022;10:156-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 33. | Yang Y, Shaffer AL 3rd, Emre NC, Ceribelli M, Zhang M, Wright G, Xiao W, Powell J, Platig J, Kohlhammer H, Young RM, Zhao H, Yang Y, Xu W, Buggy JJ, Balasubramanian S, Mathews LA, Shinn P, Guha R, Ferrer M, Thomas C, Waldmann TA, Staudt LM. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell. 2012;21:723-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 420] [Article Influence: 32.3] [Reference Citation Analysis (0)] |