Published online Nov 24, 2023. doi: 10.5306/wjco.v14.i11.504
Peer-review started: June 28, 2023
First decision: August 25, 2023
Revised: September 14, 2023
Accepted: October 26, 2023
Article in press: October 26, 2023
Published online: November 24, 2023
Processing time: 146 Days and 1.2 Hours
Pancreatic cancer is difficult to be diagnosed early clinically, while often leads to poor prognosis. If optimal personalized treatment plan can be provided to pancreatic cancer patient at an earlier stage, this can greatly improve overall survival (OS). Circulating tumor cells (CTCs) are a collective term for various types of tumor cells present in the peripheral blood (PB), which are formed by detachment during the development of solid tumor lesions. Most CTCs undergo apoptosis or are phagocytosed after entering the PB, whereas a few can escape and anchor at distal sites to develop metastasis, increasing the risk of death for patients with malignant tumors.
To investigate the significance of CTCs in predicting the prognosis of early pancreatic cancer patients.
The PubMed, EMBASE, Web of Science, Cochrane Library, China National Knowledge Infrastructure, China Biology Medicine, and ChinaInfo databases were searched for articles published through December 2022. Studies were considered qualified if they included patients with early pancreatic cancer, analyzed the prognostic value of CTCs, and were full papers reported in English or Chinese. Researches were selected and assessed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocol and the Newcastle-Ottawa Scale criteria. We used a funnel plot to assess publication bias.
From 1595 publications, we identified eight eligible studies that collectively enrolled 355 patients with pancreatic cancer. Among these original studies, two were carried out in China; three in the United States; and one each in Italy, Spain, and Norway. All eight studies analyzed the relevance between CTCs and the prognosis of patients with early-stage pancreatic cancer after surgery. A meta-analysis showed that the patients that were positive pre-treatment or post-treatment for CTCs were associated with decreased OS [hazard ratio (HR) = 1.93, 95% confidence interval (CI): 1.197-3.126, P = 0.007] and decreased relapse-free/disease-free/progression-free survival (HR = 1.27, 95%CI: 1.137-1.419, P < 0.001) in early-stage pancreatic cancer. Additionally, the results suggest no statistically noticeable publication bias for overall, disease-free, progression-free, and recurrence-free survival.
This pooled meta-analysis shows that CTCs, as biomarkers, can afford reliable prognostic information for patients with early-stage pancreatic cancer and help develop individualized treatment plans.
Core Tip: There is no consensus regarding the prognostic value of circulating tumor cells (CTCs) in early-stage pancreatic cancer after surgery. This is the first systematic review and meta-analysis to investigate the potential of CTCs in predicting survival time in early pancreatic cancer. We pooled the analyses of the relationship between CTCs and overall/disease-free/progression-free/relapse-free survival in related studies. Patients testing positive for CTCs pre- or intra-surgery may have worse prognoses, requiring more intense chemotherapy and closer follow-up.
- Citation: Zhang ZH, Bao YW, Zhao YJ, Wang JQ, Guo JT, Sun SY. Circulating tumor cells as potential prognostic biomarkers for early-stage pancreatic cancer: A systematic review and meta-analysis. World J Clin Oncol 2023; 14(11): 504-517
- URL: https://www.wjgnet.com/2218-4333/full/v14/i11/504.htm
- DOI: https://dx.doi.org/10.5306/wjco.v14.i11.504
Pancreatic cancer is a fatal disease with poor prognosis. In 2018, pancreatic cancer was the seventh leading cause of cancer-related mortality worldwide[1]. The number of annual diagnoses of and deaths related to pancreatic cancer in China have exceeded those in the United States[2]. The incidence of pancreatic cancer continues to increase at a rate of 0.5%-1.0% each year, and it is projected to be the second deadliest cancer by 2030 in Western countries[3]. Despite continuous advances in chemotherapy, radiation, and surgical techniques; the prognosis of patients with pancreatic cancer remains significantly poor. Approximately half of the patients experience reoccurrence within the first year, mainly due to metastatic disease occurrence after surgical resection. Consequently, one of the most important challenges is to identify a factor that can assess the survival outcome of patients with early pancreatic cancer before surgery, optimize treatment, and assist in the development of monitoring strategies.
Currently, technological innovations in imaging and endoscopy are being used to improve the diagnostic accuracy of pancreatic cancer. Computed tomography (CT) and magnetic resonance imaging (MRI) remain first-line diagnostic modalities for clinical suspicion. Endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration (EUS-FNA), also play important roles in its diagnosis. However, imaging features of early pancreatic cancer are subtle, and the general consensus is that it is difficult to detect early lesions[4]. Liquid biopsy is a new technology that detects biomarkers, such as carcinoembryonic antigen, carbohydrate antigen 19-9 (CA19-9), or CA125, from nonsolid biological tissues, such as blood[5], and has received increased attention because of its convenience and noninvasiveness[6]. However, these biomarkers all lack sufficient sensitivity and specificity for diagnostic purposes.
With the development of medical testing technologies, circulating tumor cells (CTCs) have emerged as a popular research topic over the last few decades. CTCs are a small number of tumor cells that detach from primary tumors, circulate through the bloodstream, and are the main source of its dissemination and metastasis[7]. Unlike traditional histopathological examinations, which are usually complex and risky for difficult-to-biopsy tumors such as pancreatic cancer, CTCs are noninvasive. CTCs can provide real-time and comprehensive information about the tumor because they are enriched in the bloodstream and originate from different regions of the original tumor or metastasis. Moreover, CTCs can present large-scale health information, such as the expression of genes and proteins and alteration of cellular contents and cell membranes, which are essential for improving diagnostic accuracy and developing individualized treatments[8]. Recently, an increasing number of studies have revealed that CTCs show promise for prognostic evaluation in several tumors, including lung[9], renal[10], breast[11], gastric[12], and colorectal cancers[13].
Nonetheless, the prognostic effect of CTCs in early pancreatic cancer remains ambiguous, mainly because of the increasing number of CTC isolation methods and different study designs. Therefore, this study aimed to perform a structural meta-analysis of currently available evidence on the prognostic value of CTCs in early-stage pancreatic cancer.
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, we conducted a comprehensive literature search of all studies published in database repositories before December 2022 that were related to the use of CTCs for the diagnosis of pancreatic cancer. The search was performed only in English and Chinese databases including PubMed, EMBASE, Web of Science, Cochrane Library, China National Knowledge Infrastructure (CNKI), China Biology Medicine (CBM), and ChinaInfo. Only studies published in Chinese and English were included in the analysis. Search terms included these keywords, MeSH (medical subject headings) terms, and their entry terms: “Pancreatic Neoplasms”(MeSH), “Neoplasm, Pancreatic,” “Pancreatic Neoplasm,” “Pancreas Neoplasms,” “Neoplasm, Pancreas,” “Neoplasms, Pancreas,” “Pancreas Neoplasm,” “Neoplasms, Pancreatic,” “Cancer of Pancreas,” “Pancreas Cancers,” “Pancreas Cancer,” “Cancer, Pancreas,” “Cancers, Pancreas,” “Pancreatic Cancer,” “Cancer, Pancreatic,” “Cancers, Pancreatic,” “Pancreatic Cancers,” “Cancer of the Pancreas,” “Neoplastic Cells, Circulating”(MeSH), ”Neoplasm Circulating Cells,” “Circulating Neoplastic Cells,” “Cell, Circulating Neoplastic,” “Cells, Circulating Neoplastic,” “Circulating Neoplastic Cell,” “Neoplastic Cell, Circulating,” “Circulating Tumor Cells,” ”Cell, Circulating Tumor,” “Cells, Circulating Tumor,” “Circulating Tumor Cell,” ”Tumor Cell, Circulating,” “Tumor Cells, Circulating,” “Cells, Neoplasm Circulating,” “Cell, Neoplasm Circulating,” “Neoplasm Circulating Cell,” “Circulating Cells, Neoplasm,” “Tumor Cells, Embolic,” “Cell, Embolic Tumor,” “Cells, Embolic Tumor,” “Embolic Tumor Cell,” ”Tumor Cell, Embolic,” “Embolic Tumor Cells,” “Embolism, Tumor,” “Embolisms, Tumor,” “Tumor Embolism,” “Tumor Embolisms,” “Prognostic,” “Prognosis,” “Prognos*”. These terms were supplemented by the logical operators “and” and “or.” To expand the literature, we reviewed and evaluated the references of the included studies.
Two independent investigators reviewed the article titles and abstracts according to inclusion and exclusion criteria to exclude irrelevant studies. Subsequently, the full texts of the included studies were analyzed to determine their suitability for meta-analysis. In case of any contradictions, a third reviewer was consulted for adjudication. The inclusion and exclusion criteria were formulated based on the PRISMA of Diagnostic Test Accuracy Studies guidelines. Inclusion criteria: (1) Studies included participants of any age with histologically or cytologically confirmed early pancreatic cancer (early pancreatic cancer was defined as pancreatic adenocarcinoma with a maximum tumor diameter of 4 cm, regional lymph node metastasis of no more than three nodes, and no distant metastasis); (2) studies that investigated pre- or intra-operative CTCs as a prognostic biomarker in blood for early pancreatic cancer patients’ survival results after surgery; (3) sufficient published data available for calculating hazard ratio (HR) and 95% confidence interval (CI) of overall survival (OS) and progression-free survival (PFS); (4) studies that were published in English or Chinese; and (5) studies that were reported as full paper publications. Exclusion criteria: (1) Patients were diagnosed with pancreatic cancer at advanced stages or studies that didn’t analyze the results of early pancreatic cancer patients independently; (2) studies that did not provide adequate data on the prognostic performance of CTCs for early pancreatic cancer patients; (3) patients didn’t receive surgical therapy; and (4) articles were published in languages other than English and Chinese. Overall, five non-human studies, case reports, comments, meta-analyses, reviews, and published clinical and treatment guidelines were excluded.
To decrease systematic errors during data collection, two reviewers independently selected the studies. Any conflicting cases were carefully reviewed, and a third reviewer was consulted to reach a consensus. Data extraction from the eligible papers included the following items: (1) General information about the article: first author, publication date, and country; (2) study information: number of patients, evidence of confirmed pancreatic cancer, CTCs separation solution, CTCs determination criteria, and follow-up time; and (3) data for the meta-analysis: HR with 95%CI for OS, PFS or disease-free survival (DFS), recurrence-free survival (RFS). For articles without HR and 95%CI, we used Engauge Digitizer 11.3 to calculate them based on the survival rate extracted from Kaplan-Meier curves.
The quality of the included studies was assessed based on the Newcastle-Ottawa Scale (NOS) criteria for non-randomized studies, which included three key domains covering “selection,” “comparability,” and “outcome.” A star rating system was used to semi-quantitatively evaluate study quality, and those who met the standards for each item were awarded one or two stars. Scores range from zero to nine. A score equal to or greater than seven indicates high quality. This tool objectively evaluates the risk of bias and assesses concerns regarding its applicability. The quality assessment was performed by two independent reviewers. Any disagreements were discussed until an agreement was reached. Publication bias was investigated using a funnel plot, with P < 0.05 indicating a significant publication bias.
Review Manager 5.3 software (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark) was used to assess the pooled HR effect size. Heterogeneity between studies was assessed using Cochran’s Q test and I2 statistics. According to Higgins and Thompson, I2 > 50% or P < 0.1 was viewed as consistent significant heterogeneity. A fixed-effect model was used when minor heterogeneity was observed. Otherwise, a random-effects model was used to calculate the pooled HR. Subgroup analyses based on ethnicity, separation solution, treatment, and follow-up time were performed to explore potential sources of heterogeneity. Finally, we conducted a sensitivity analysis to evaluate the effect of a single study on overall outcomes.
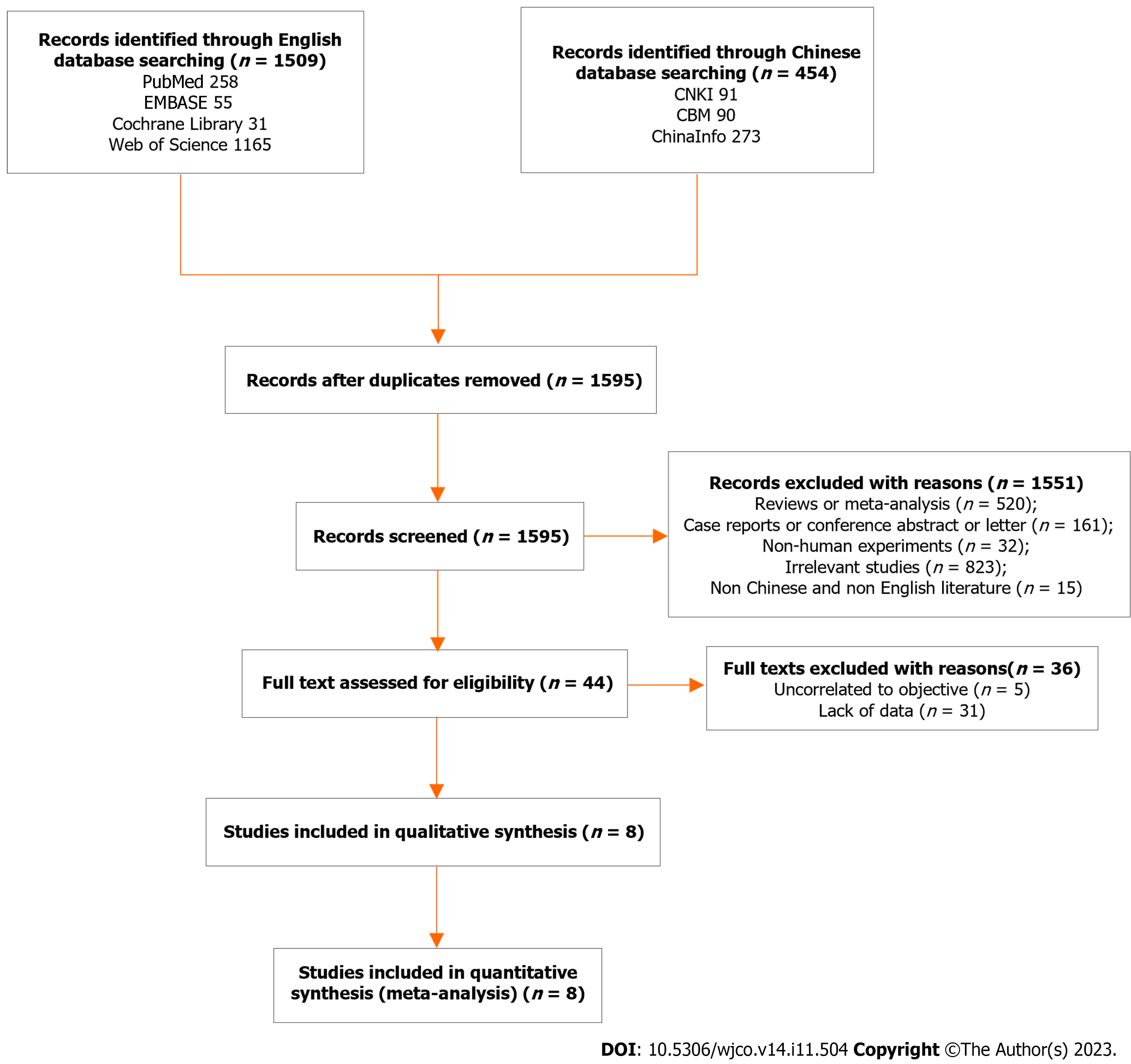
A total of 1963 articles were collected, including 91 from CNKI, 273 from ChianInfo, 90 from CBM, 258 from PubMed, 55 from EMBASE, 31 from Cochrane, and 1165 from Web of Science. After deleting 368 duplicate references, we screened the titles and abstracts of 1595 residual articles. In total, 1551 articles were excluded (520 meta-analyses or reviews; 32 non-human studies; 15 articles published in languages other than English and Chinese; 161 meeting abstracts, case reports, guidelines, or letters; and 823 irrelevant studies). According to the inclusion and exclusion criteria, we read the full text of the 44 remaining studies, of which 36 articles were ultimately excluded (5 irrelevant studies and 31 articles with data deficiency). Consequently, eight studies involving 355 patients with early-stage pancreatic cancer were included in our meta-analysis[14-21]. A flow diagram of the literature search and filtering process is shown in Figure 1.
The characteristics of these studies are summarized in Table 1. The overall research quality was rated as moderate by the NOS, with an average score of 6.75 (Table 2). Among these original studies, two were conducted in China; three in the United States; and one each in Italy, Spain, and Norway. The publication years were 2014 (n = 1), 2018 (n = 1), 2021 (n = 5), and 2022 (n = 1). All eight studies analyzed the correlation between CTCs and prognosis in patients with early-stage pancreatic cancer after surgery. While four studies included early pancreatic cancer patients only[14,19-21], the remaining four studies contained early and advanced pancreatic cancer patients with early pancreatic cancer patients accounting for at least 30% of the total patients[15-18]. In total, five studies detected CTCs in the peripheral blood (PB), two studies detected CTCs in the PB and portal venous blood (PVB), and one study detected CTCs in the central venous catheter and portal blood. Blood samples were collected solely before surgery in four studies[16,17,21], before and after surgery in three studies[14,15,18], and during surgery in only one study[19]. Only pre- and intra-operative CTC data were included in this meta-analysis. Although eight studies applied different CTC enrichment and separation methods, including commercially available CTC detection kits[14,17], dielectrophoresis-field flow fractionation (DEP-FFF)[15], and CTC isolation systems[18-21], most methods essentially rely on the density characteristics of CTCs as well as the epithelial and mesenchymal markers expressed by CTCs. Most studies applied DFS, OS, PFS, and RFS as survival outcomes, while only Hugenschmidt et al[20] adopted cancer-specific survival as an outcome indicator. Seven of the eight studies reported the adjusted HR and 95%CI for the association between DFS/PFS/RFS and positive CTCs. For the one that did not, we calculated these values according to the Kaplan-Meier curves provided in the articles. In addition, only three of the eight included studies reported the adjusted HR and 95%CI for the association between OS and positive CTCs while another two studies provided Kaplan-Meier curves for OS.
| Ref. | Year | Country | Sample | Separation Solution | Markers and expression level on PC CTC | Cases | No-positive | Treatment | Outcome | Follow-up time |
| Xing et al[14] | 2021 | China | Peripheral blood | SE-iFISH | CD44+ CTEC: DAPI +/CD45-/CD31 +/CD44 +/Vimentin (+ or -) with aneuploid CEP8 | 73 | Not reported | Surgery | OS/DFS | Median 10.8 mo (1.2-31.8 mo) |
| Semaan et al[15] | 2021 | United States | Peripheral blood | DEP-FFF | pEMT-CTC: CD45 -, EpCAM + and/or Pan-CK +, Vimentin +, DAPI + | 31 early stages (74 total) | 28 | Surgery/neoadjuvant treatment | OS/PFS | Median 15.4 mo (0-43.1 mo) |
| Padillo-Ruiz et al[21] | 2021 | Spain | Central venous catheter and portalblood | ICC | DAPI +/CK +/CD45 - | 35 | 35 | Surgery/chemotherapy | OS/DFS | 24 mo |
| Court et al[16] | 2018 | United States | Peripheral blood | NanoVelcro chip | DAPI +/CD45 -/CK +; CD45 positivity greater than 2 × background | 40 early stages (126 total) | 27 | Surgery/chemotherapy | OS/RFS | ≥ 24 mo |
| Cheng et al[17] | 2022 | China | Peripheral blood | LT-PCR | FR + CTC | 25 early stages (44 total) | 13 | Surgery/chemotherapy | OS/DFS | Median 20 mo (6-28 mo) |
| White et al[18] | 2021 | United States | Peripheral blood and portal venous blood | CellSearch | DAPI +/CK + /CD45 - | 33 early stages (34 total) | 21 | Surgery/neoadjuvant treatment | OS/RFS | Median 14.1 mo (0.86-1.97 mo) |
| Bissolati et al[19] | 2014 | Italy | Peripheral blood and portal venous blood | CellSearch | DAPI +/CK +/CD45 - | 20 | 9 | Surgery/chemotherapy | OS/PFS | Median 39.2 mo (36-45 mo) |
| Hugenschmidt et al[20] | 2021 | Norway | Peripheral blood | CellSearch | EpCAM +/DAPI +/CK +/CD45 - | 98 | 7 | Surgery | CSS/DFS | Median 96 mo (63-126 mo) |
| Ref. | Selection | Comparability | Outcome | Total stars | ||||||
| Representativeness | Selection of non-exposed | Ascertainment of exposure | Outcome not present at start | Comparability on most important factors | Comparability on other risk factors | Assessment of outcome | Long enough follow-up (median ≧ 24 mo) | Completeness of follow-up | ||
| Xing et al[14] | - | 1 | 1 | 1 | 1 | - | 1 | - | 1 | 6 |
| Semaan et al[15] | - | 1 | 1 | 1 | 1 | - | 1 | - | 1 | 6 |
| Padillo-Ruiz et al[21] | - | 1 | 1 | 1 | 1 | - | 1 | 1 | 1 | 7 |
| Court et al[16] | - | 1 | 1 | 1 | 1 | - | 1 | 1 | 1 | 7 |
| Cheng et al[17] | 1 | 1 | 1 | 1 | 1 | - | 1 | - | 1 | 7 |
| White et al[18] | - | 1 | 1 | 1 | 1 | - | 1 | - | 1 | 6 |
| Bissolati et al[19] | 1 | 1 | 1 | 1 | 1 | - | 1 | 1 | 1 | 8 |
| Hugenschmidt et al[20] | - | 1 | 1 | 1 | 1 | - | 1 | 1 | 1 | 7 |
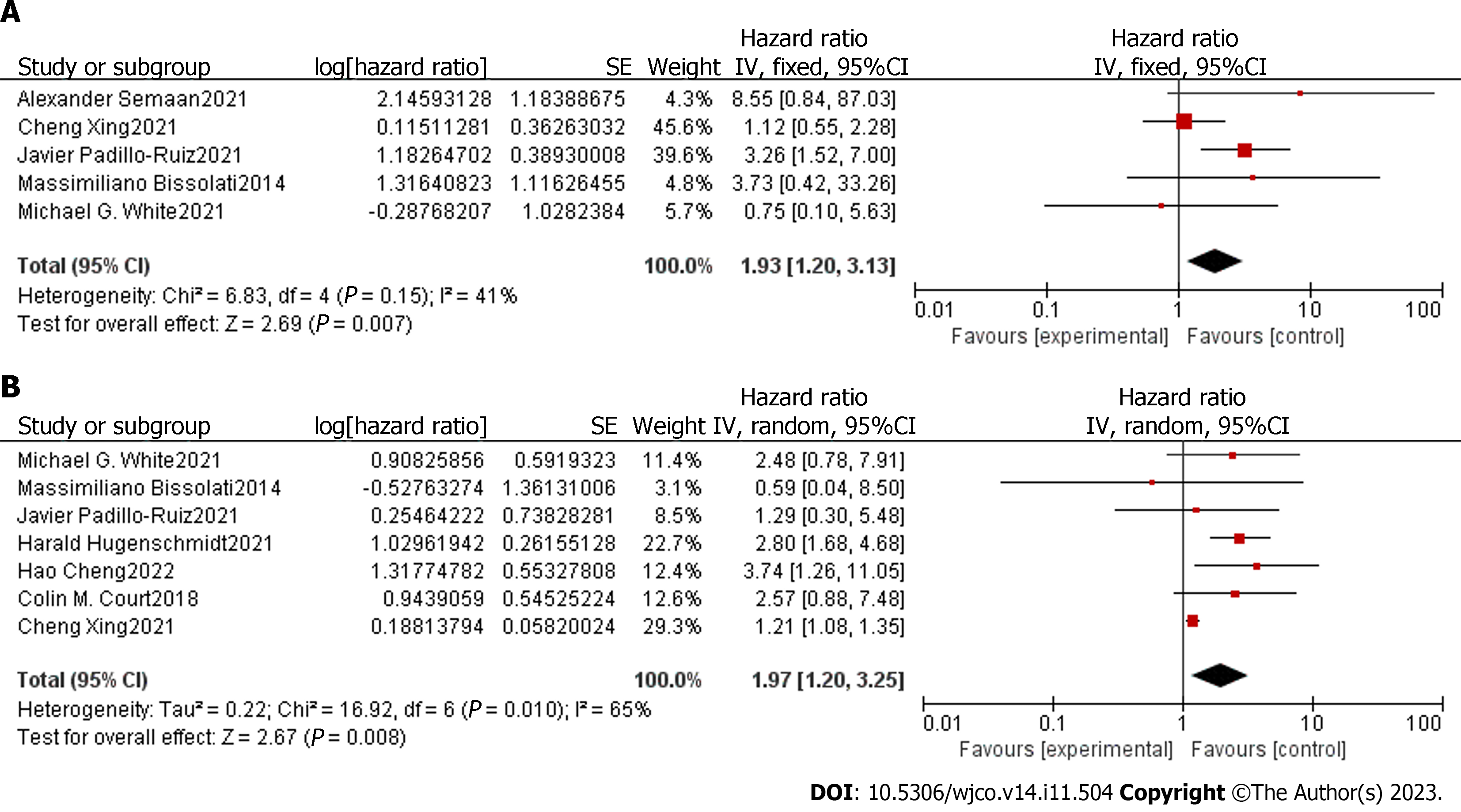
We conducted a meta-analysis to assess the association between CTCs detected in the blood samples of patients with early-stage pancreatic cancer and their prognosis after surgery. The degree of heterogeneity among the five studies that provided the adjusted HR and 95%CI for OS was low (I2 = 41%, P = 0.15); therefore, we chose a fixed-effect model for analysis. The pooled analyses of the five studies, containing 169 patients, showed that positive detection of pre-treatment or post-treatment CTCs was associated with decreased OS (HR = 1.93, 95%CI: 1.20-3.13) that was statistically significant (Z = 2.69, P = 0.007) (Figure 2A). There was a moderate degree of heterogeneity among the seven studies available for the adjusted HR and 95%CI of RFS/DFS/PFS (I2 = 65%, P = 0.01); therefore, we chose a random-effects model for analysis. The results showed that positive pre-treatment or post-treatment CTCs were associated with decreased DFS/PFS/RFS (HR = 1.97, 95%CI: 1.20-3.25) that were statistically significant (Z = 2.67, P = 0.008) (Figure 2B).
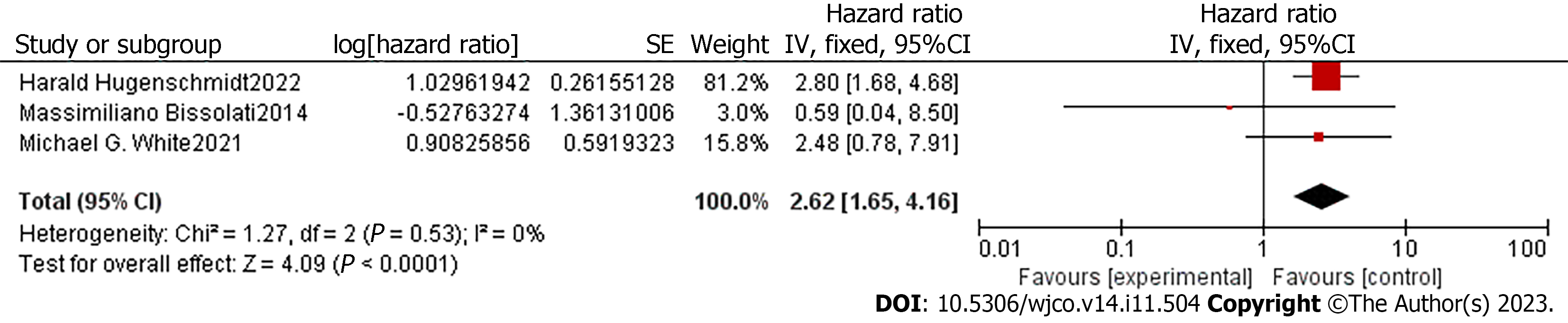
Because of the high heterogeneity in the DFS/PFS/RFS results, meta-regression was conducted to explore the sources of heterogeneity. No apparent deviation was found in the ethnicity, treatment, and follow-up time subgroups. Later analysis demonstrated that CTC-positive patients were associated with decreased DFS/PFS/RFS in subgroups that detected CTCs by the CellSearch system (HR = 2.62, 95%CI: 1.65-4.16, Z = 4.09, P < 0.001), and the heterogeneity between subgroups was low (P = 0.018) (Figure 3).
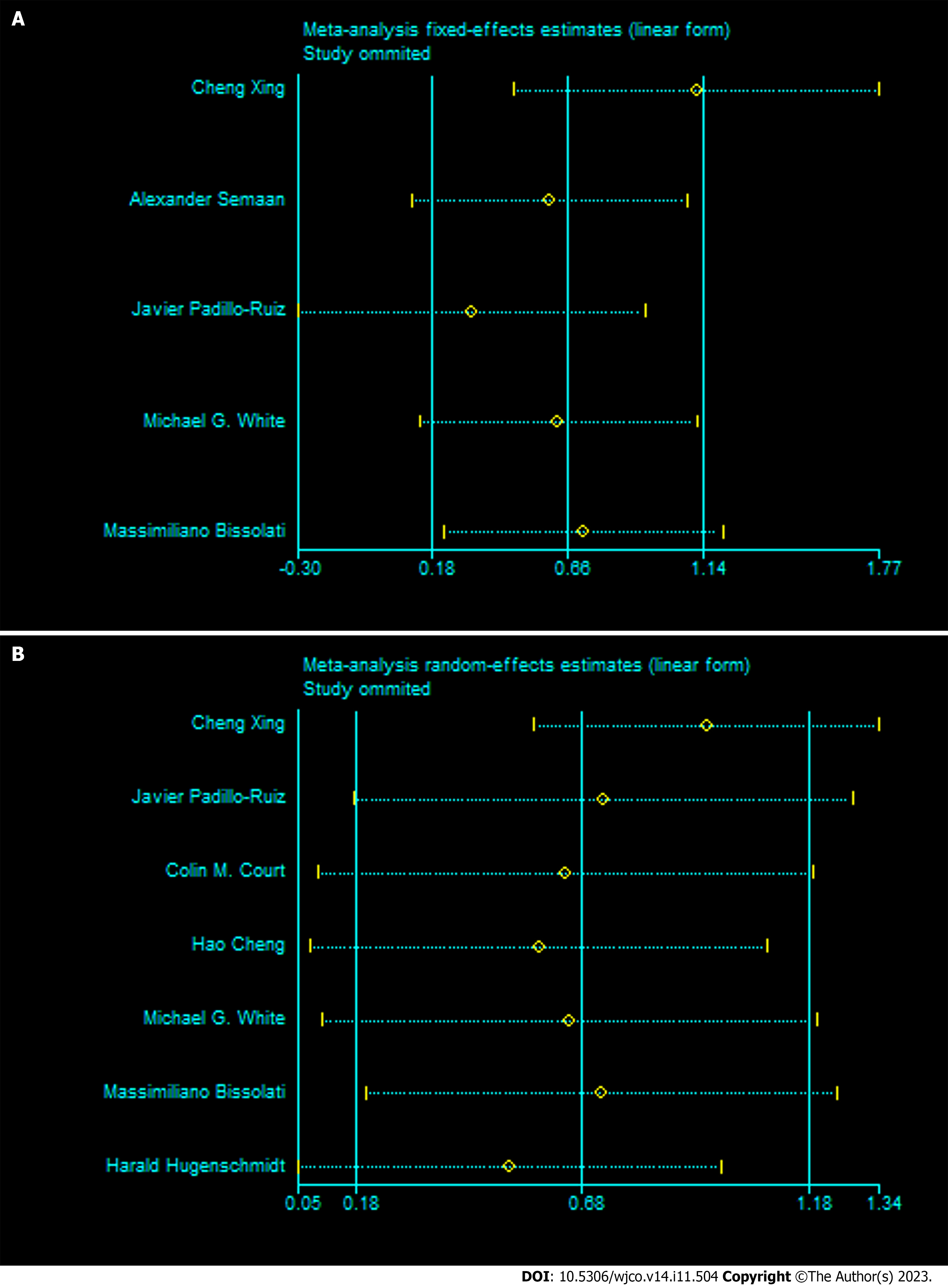
To explore the potential sources of this difference, we conducted sensitivity analysis by sequentially excluding each study. When the study by Xing et al[14] was excluded, the heterogeneity of the remaining studies was significantly reduced (OS: I2 = 0%, P = 0.44; DFS/PFS/RFS: I2 = 0%, P = 0.76), and the pooled results for both OS and DFS/PFS/RFS were increased (OS: HR = 3.06 95%CI: 1.59-5.86, Z = 3.36, P < 0.001; DFS/PFS/RFS: HR = 2.60 95%CI: 1.78-3.812, Z = 4.001, P < 0.001) (Figure 4). Nevertheless, the direction of the pooled results for the OS and DFS/PFS/RFS subgroups were not affected, indicating a negative association between CTC positivity and lower OS or DFS/PFS/RFS.
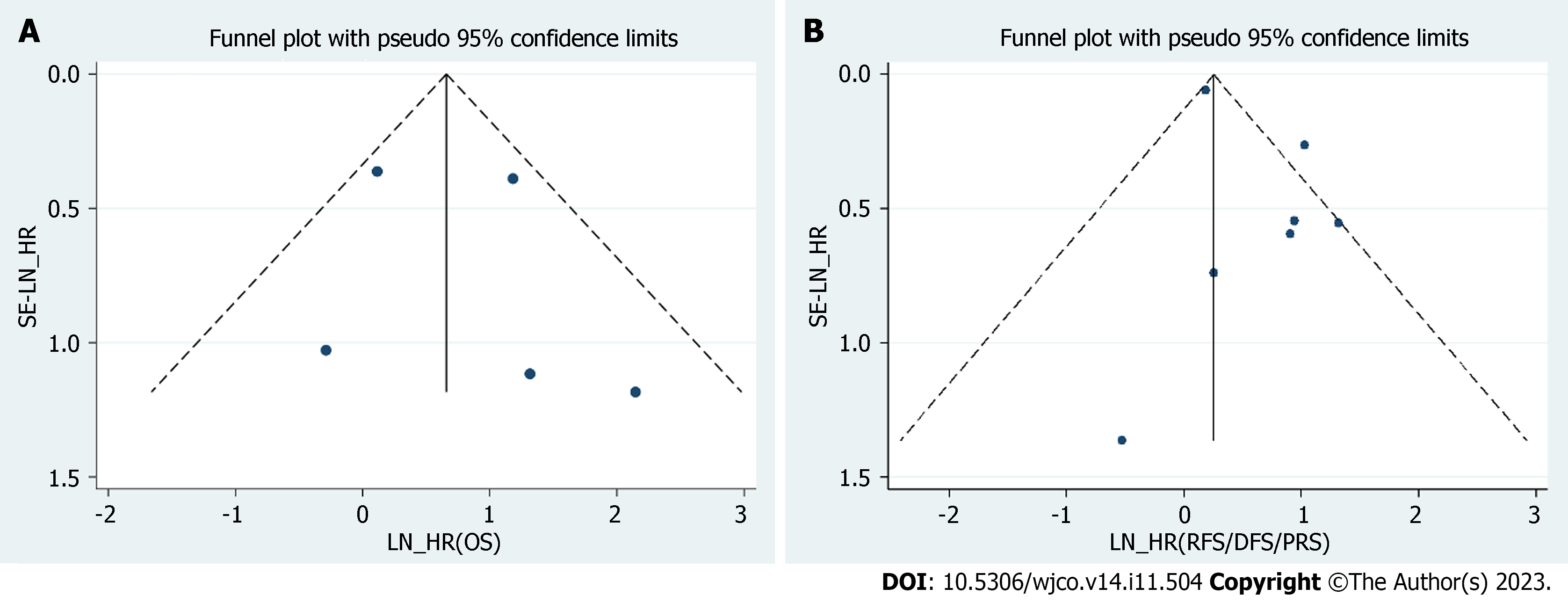
We used a funnel plot to assess publication bias in this meta-analysis. The results showed no publication bias for OS (P = 0.653) and DFS/PFS/RFS (P = 0.117) (Figure 5).
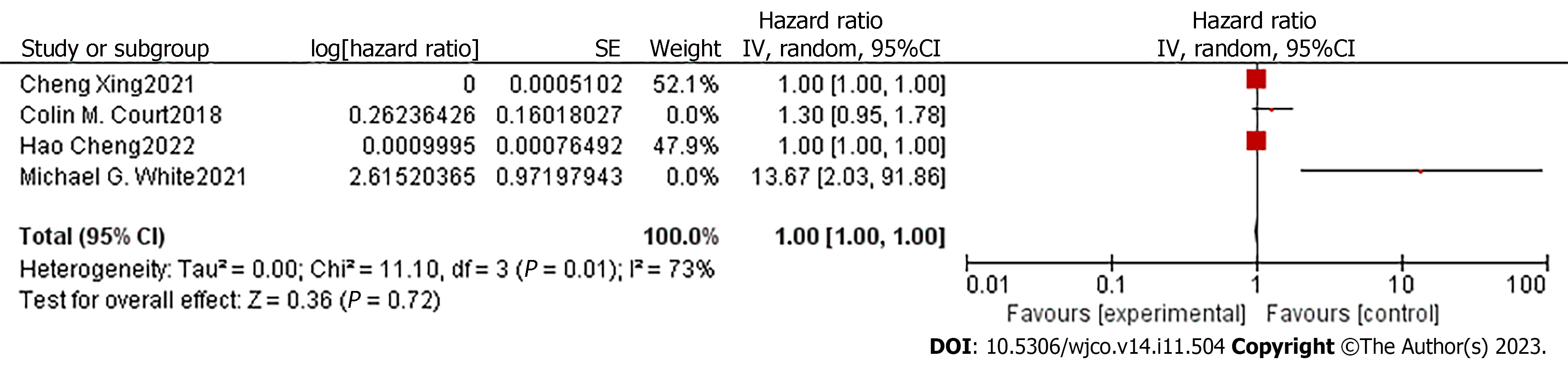
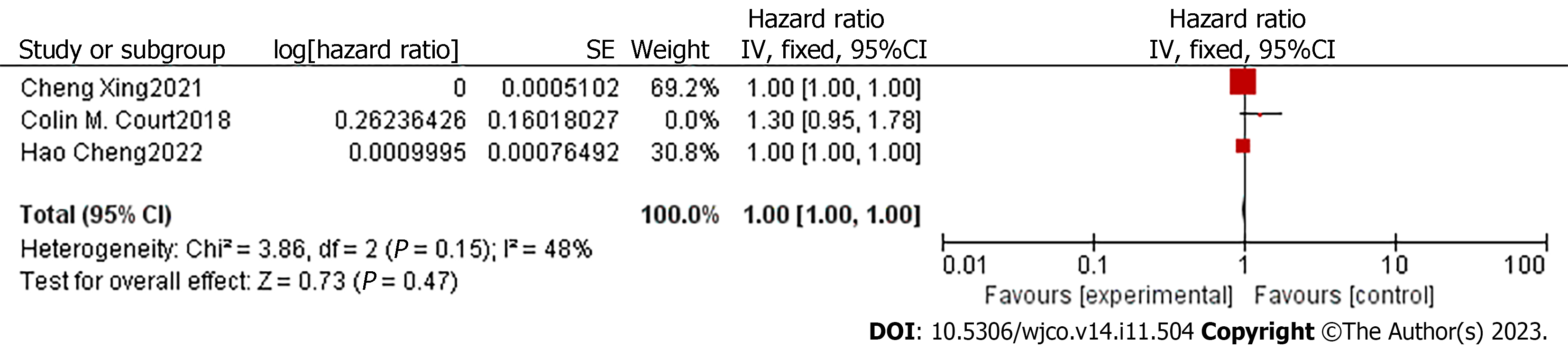
In order to compare the potential of CTC and CA19-9 in predicting patient prognosis, we extracted the adjusted HR and 95%CI of DFS related to CA19-9 in the included articles. Only four studies had the required data[14,16-18]. The degree of heterogeneity of the adjusted HR and 95%CI for DFS was high (I2 =73%, P = 0.01), so we used a random-effects model for the analysis. The pooled analyses of the four studies, containing 171 patients, showed that the CA19-9 level in the PVB of early-stage pancreatic cancer patients was not an independent predictor of a shorter time to recurrence (HR = 1, 95%CI: 1.00-1.00; Z = 0.36, P = 0.72) (Figure 6). Considering the impact of sampling time on the results, we excluded the study of White et al[18], which collected venous blood after resection. Though the heterogeneity of the remaining studies was significantly reduced (I2 = 48%, P = 0.15), the combined HR was still 1 (95%CI: 1.00-1.00; Z =2.20, P = 0.03) (Figure 7).
In this meta-analysis, we found that CTC detected in patients with early pancreatic cancer is negatively correlated with the prognosis time after surgery. After data collection and filtering, eight studies from 2014 to 2022, including 355 patients with early-stage pancreatic cancer, were included in our analysis. Through data sorting and meta-analysis, we demonstrated that testing positive pre- or intra-treatment for CTCs was associated with decreased OS and RFS/DFS/PFS in early-stage pancreatic cancer. Nevertheless, our meta-analysis showed a high degree of heterogeneity in DFS/PFS/RFS. To determine the potential sources of the heterogeneity, we conducted a subgroup analysis. The pooled results were not affected by ethnicity, treatment, or follow-up time, whereas subgroup analysis by CTCs separation solution decreased the heterogeneity between the groups, indicating that this might have caused the heterogeneity. Studies that enumerated CTCs by the CellSearch system demonstrated a more obvious correlation between CTC-positive patients and decreased DFS/PFS/RFS. However, except for this system, all other separation solutions contained only one study; therefore, we were unable to obtain pooled results. Sensitivity analysis was conducted by using the “leave-one-out method.” Notably, when a study by Xing et al[14] was removed, the heterogeneity of the remaining studies was significantly reduced, and the pooled results for both OS and DFS/PFS/RFS were relatively elevated. We infer that the differences present in this study may be derived from the separation solutions employed as well as the stemness markers used to identify the stem cell-like phenotype of CTCs. Importantly, the results demonstrating the survival-jeopardizing effects of CTCs were maintained. We suggest that patients testing positive for CTCs pre- or intra-treatment may have a worse prognosis and require more intense chemotherapy and closer follow-up. Furthermore, we collected data regarding CA19-9 in the included studies and four studies containing 171 patients, that met our requirements. Our meta-analysis showed that the preoperative CA19-9 level in the PVB of early-stage pancreatic cancer patients was not an independent predictor for shorter time of recurrence after resection.
The prevalence of pancreatic cancer has increased dramatically globally, and is expected to become a leading cause of cancer-related mortality[22]. Only 10%-15% of patients have localized pancreatic cancer suitable for surgery[3], which is the only potentially curative therapy known to date. Even for patients with a localized disease, a high proportion experience postoperative recurrence within 5 years, caused by local tumor recurrence or distant metastases. The 1- and 5-year survival rates are 63% and 17%, respectively[23]. Therefore, identifying high-risk populations for screening and prevention, early diagnosis, and establishing personalized treatment plans are currently the primary challenges[24].
Imaging, including EUS, CT and MRI, which can provide a convenient and noninvasive diagnosis, remains the first-line diagnostic modality for pancreatic cancer and is used to evaluate therapeutic efficacy in many organs[25-28]. However, cross-sectional imaging is limited in the visualization of small and metastatic tumors, which can frequently result in underestimation of the pancreatic cancer stage[29]. EUS-FNA or EUS-fine needle biopsy (EUS-FNB) can localize pancreatic lesions measuring < 3 cm, providing a minimally invasive tissue biopsy[30]. EUS-FNB is increasingly becoming a practical tool for diagnosing malignancy in various pancreatic solid lesions[31,32]. EUS-FNA combined with needle-based confocal laser endomicroscopy (nCLE) can achieve real-time imaging for in vivo tissue analysis[33]. However, these examinations requires anesthesia and may be accompanied by complications such as acute pancreatitis, tumor dissemination, and postoperative hemorrhage. Besides, nCLE is limited by the duration of the surgical time and the operability of the 19G FNA needle[33]. More recently, liquid biopsy has received a great deal of attention for its ability to assess a comprehensive cancer profile in a noninvasive and real-time manner[26]. Serum CA19-9 level is the only diagnostic biomarker approved by the United States Food and Drug Administration for pancreatic cancer. Moreover, CA19-9 can be an independent predictor of prognosis for pancreatic cancer patients[34]. An elevated CA19-9 level after resection or during chemotherapy predicts a high probability of tumor recurrence or progression[35]. However, the prognostic ability of preoperative CA19-9 is still disputed, since it is mostly detected in advanced stages, it is neither sensitive nor specific enough to identify early-stage patients or for the differential diagnosis of patients at different stages; additionally, it is also positive in many other benign and malignant pancreatic diseases such as pancreatitis, cholestasis, and gastric cancer[7]. In this meta-analysis, the pooled results of the four studies, that we examined, demonstrated that the prognostic effect of CA19-9 in peripheral venous blood on early postoperative recurrence of early-stage pancreatic cancer is not as obvious as that of CTCs.
In the past decade, an increasing number of studies have examined the prognostic value of CTCs in cancers of various organs, as the formation of tumor metastases relies heavily on the survival of CTCs and their ability to mediate angiogenesis in target organs[36]. CTCs are tumor cells shed from both primary and secondary foci and are found circulating in the bloodstream; therefore, they can provide valuable information about primary tumors and secondary deposits. In addition, isolation and in vivo cultures of animal xenografts provide deeper information on individual tumor characteristics[37]. Prospective observational studies have also revealed that CTC numbers rarely drop to zero even after complete resection in both chemo-naïve and post-neoadjuvant patients and can be observed longitudinally before disease recurrence[38]. However, the deficiency of relevant studies and different research designs make the clinical significance of CTCs in early pancreatic cancer prognosis a controversial topic. A thorough analysis of the prognostic performance of CTCs is critical for monitoring and developing treatment strategies for patients with pancreatic cancer.
CTCs in the bloodstream are difficult to capture and identify because their concentration in the circulatory system is extremely low (1-10 cells/10 mL) in most cases[39]. Furthermore, CTCs are scattered among an enormous number of erythrocytes and leukocytes, posing tremendous challenges for their complete collection and accurate detection. To improve this situation, several methods have been used for CTC enrichment, such as density centrifugation, immunomagnetic enrichment with anti-CD45 monoclonal antibodies, epithelial cell adhesion molecule (EpCAM), and cell filtration technology. Additionally, epithelial cell-specific markers, such as the cytokeratin (CK) family, and mesenchymal markers, such as N-cadherin and vimentin, have been used to identify epithelial and mesenchymal CTCs, respectively[40,41]. Numerous platforms based on these technologies have been developed. The CellSearch system is the only platform approved by the Food and Drug Administration for confirming the presence of CTCs in patients with pancreatic cancer. This system first enriches CTCs by taking advantage of their characteristic expression of the EpCAM on their membrane surface and then distinguishing different cells by immunostaining markers such as CD45, 4',6-diamidino-2-phenylindole (DAPI), and CK 8, 18, 19[42]. However, the detection rates are only 20% and 5%-42% for resectable and advanced pancreatic cancers, respectively [19,43]. This is because most CTCs undergo epithelial-mesenchymal transition (EMT) and thus lack or express low levels of epithelial markers that are generally used as the basis for the detection of these cells[44]. In addition, separation solutions based on these epithelial markers may exclude cells that play important roles in metastasis and chemoresistance[45]. In our included studies, Court et al[16] used the microfluidic NanoVelcro CTC chip to evaluate the presence and number of CTCs. This platform greatly improves CTC capture and identification by utilizing anti-EpCAM-coated 3D-nanosubstrates in conjunction with microfluidic chaotic mixers and by adding tumor identification markers, which enhance the synergistic effects of cell–substrate contact frequency as well as its affinity[46]. This platform also allows seamless integration with laser capture microdissection for single CTC isolation[47]; moreover, the separated cells can be subjected to downstream molecular analyses[48]. However, an important limitation of this assay is that it is similar to the CellSearch system. In addition, using membrane filtration may reduce specificity, as some CTCs are found to be equal or smaller in size than nucleated blood cells[49]. The combination of specific mesenchymal markers may be a future direction for these assays. Considering their failure to detect EpCAM- or CK-negative CTCs, Lin et al[50] developed an integrated tumor cell surface molecule-independent SE-iFISH platform in 2015. Using fluorescence in situ hybridization with a specific chromosome centromere probe, this system can detect aneuploidy in the PB, which is a common manifestation of chromosome instability and malignant solid tumors. Moreover, Semaan et al[15] used an antigen-independent approach called DEP-FFF, which utilizes the physical properties of cells and allows the isolation of phenotypically distinct CTCs. In doing so, they obtained not only epithelial and mesenchymal CTCs, but also intermediate-state CTCs, which may show greater invasiveness and therapeutic resistance. Another valid method to detect the molecular characterization of CTCs is PCR. This is most likely due to the detection of multiple tumor markers, which could downgrade the effect of the heterogeneity that exists in CTCs. Among the included studies, Cheng et al[17] used immunomagnetic depletion and ligand-targeted polymerase chain reaction to detect the expression rate of folate receptor + CTCs in patients with different stages of pancreatic cancer. Traditional genetic studies of CTCs are limited by the low specificity of the enrichment methods, which contributes to the presence of nuclear blood cells, necrotic cells, tumor-derived exosomes, and cellular fragments. Therefore, the obtained nucleic acids may not accurately reflect the hereditary properties of CTCs[42]. In recent years, single-cell separation and whole-genome amplification of CTCs have been developed to overcome this challenge. However, studies on pancreatic cancer are still lacking.
Although the pooled results of our meta-analysis indicated that CTCs were associated with shorter OS and DFS/PFS/RFS in patients with early pancreatic cancer after surgery, three of the eight included studies showed no significant correlation between the existence of CTCs and both OS and DFS/PFS/RFS. Colin et al[48] found that CTCs were independent predictors of RFS following surgery and could correctly identify patients with occult metastatic disease preoperatively. However, when the analysis was limited to the early-stage subset, CTC count was no longer associated with shorter RFS. One possibility is that they used only 4 mL of PB, which may be too small to detect CTCs in patients with early-stage pancreatic cancer. For example, White et al[18], the CTC number in the PB did not contribute to predicting the OS or RFS of patients after resection, and the CTC number in the PVB was not associated with RFS. However, their results showed complete collinearity between the number of CTCs detected in the PVB and the OS. They hypothesized that this was caused by pancreatic venous drainage, as well as the capture and dilution effects of the liver for CTCs in the portal circulation. Similarly, the research of Bissolati et al[19] showed no significant correlation between the number of CTCs and survival time. However, they found that patients with a positive intraoperative detection of CTCs in the PVB had a higher liver metastasis rate, indicating that CTCs are of great significance for guiding adjuvant chemotherapy and postoperative follow-up monitoring. Overall, these studies revealed that CTCs in the blood of patients with pancreatic cancer are difficult to detect, particularly in early-stage patients.
In contrast, despite the different separation technologies and biomarkers used, the prognostic value of CTCs as a survival indicator for patients with early-stage pancreatic cancer was demonstrated in the remaining five studies. Moreover, they indicated that CTCs could provide tumor characteristics. Xing et al[14] used CD44+ as a marker to detect CTCs, which represent a more stem-like phenotype and can cause tumor metastasis, promoting tumor growth, angiogenesis, and drug resistance. Semaan et al[15] detected the longitudinal characterization of CTC subtypes and described EMT-CTCs as a more aggressive phenotype that is more prone to therapeutic resistance. In addition, the preoperative CTCs level in the PVB of early-stage pancreatic cancer patients may be a better prognostic marker then the preoperative CA19-9 level. This may broaden the selectivity of biomarkers for pancreatic cancer.
Our study has several limitations. First, the small number of included studies as well as the numerous types of separation strategies and research designs limited the power of our analysis. Second, several studies did not provide HR and 95%CI directly; therefore, we estimated them based on Kaplan-Meier curves, which may cause deviations. Finally, four of the eight studies recruited patients with pancreatic cancer at all stages, and we only extracted data about patients with early-stage pancreatic cancer; hence, the information was not detailed.
This meta-analysis reveals the potential of CTCs as a prognostic biomarker for early-stage pancreatic cancer. Although we rigorously gathered and analyzed the data, the essential limitations of the included studies caused a high degree of heterogeneity and hindered deeper exploration, which reduced the confidence level of our study. To overcome these difficulties, large-scale multicenter cohort studies are urgently needed to explore the full potential of CTCs.
Pancreatic cancer is a terribly invasive and poorly prognosis disease with a five-year survival less than 10%. Recently, more and more studies demonstrated that circulating tumor cells (CTCs) can be a significant prognostic marker of pancreatic cancer.
In this study, we conducted a meta-analysis to analyse the prognostic role of CTCs in patients with pancreatic cancer and investigated whether CTCs can provide prognostic information and assist develop personalized treatment plans.
Our research aims at exploring the predictive effect of CTCs on survival indicators of pancreatic cancer patients in different studies.
A standardized literature search of databases was conducted for articles about CTCs published through December 2022. After screening based on inclusion and exclusion criteria, data relevant to prognosis were extracted for analysis. We used a fixed- or random-effect model to calculate the pooled hazard ratio (HR) and 95% confidence interval (CI) of overall survival (OS) and progression-free survival (PFS) according to the degree of heterogeneity.
Eight eligible studies with a total number of 355 patients with early-stage pancreatic cancer were included. This meta-analysis showed that positive pre-treatment or post-treatment CTCs was associated with shorter OS (HR = 1.93, 95%CI: 1.197-3.126, P = 0.007) and decreased relapse-free/disease-free/PFS (HR = 1.27, 95%CI: 1.137-1.419, P < 0.001) in patients with early-stage pancreatic cancer. While the CA19-9 level in the portal venous blood of early-stage pancreatic cancer patients showed no significant correlation with postoperative recurrence time of patients (HR = 1, 95%CI: 1.00-1.00, P = 0.03).
Our meta-analysis indicates that CTCs are closely related to the prognosis of early pancreatic cancer patients and can serve as a guiding indicator for developing patient important treatment plans.
Researchers should extend follow-up time to observe the relationship between CTC and OS. Besides, large-scale multicenter cohort studies are urgently needed to explore the full potential of CTCs.
We thank Professor Si-Yu Sun, for his support and guidance, as well as all other doctors who participated in this study.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kitamura K, Japan; Mizuno S, Japan S-Editor: Qu XL L-Editor: A P-Editor: Chen YX
| 1. | Park W, Chawla A, O'Reilly EM. Pancreatic Cancer: A Review. JAMA. 2021;326:851-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 798] [Cited by in RCA: 1089] [Article Influence: 272.3] [Reference Citation Analysis (0)] |
| 2. | Lin QJ, Yang F, Jin C, Fu DL. Current status and progress of pancreatic cancer in China. World J Gastroenterol. 2015;21:7988-8003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 205] [Cited by in RCA: 213] [Article Influence: 21.3] [Reference Citation Analysis (1)] |
| 3. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15320] [Article Influence: 3064.0] [Reference Citation Analysis (4)] |
| 4. | Kamyabi N, Bernard V, Maitra A. Liquid biopsies in pancreatic cancer. Expert Rev Anticancer Ther. 2019;19:869-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10:472-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1318] [Article Influence: 109.8] [Reference Citation Analysis (0)] |
| 6. | Marrugo-Ramírez J, Mir M, Samitier J. Blood-Based Cancer Biomarkers in Liquid Biopsy: A Promising Non-Invasive Alternative to Tissue Biopsy. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 284] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 7. | Hou J, Li X, Xie KP. Coupled liquid biopsy and bioinformatics for pancreatic cancer early detection and precision prognostication. Mol Cancer. 2021;20:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 8. | Gall TMH, Belete S, Khanderia E, Frampton AE, Jiao LR. Circulating Tumor Cells and Cell-Free DNA in Pancreatic Ductal Adenocarcinoma. Am J Pathol. 2019;189:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 9. | Kanayama M, Kuwata T, Mori M, Nemoto Y, Nishizawa N, Oyama R, Matsumiya H, Taira A, Shinohara S, Takenaka M, Yoneda K, Kuroda K, Ohnaga T, Tanaka F. Prognostic impact of circulating tumor cells detected with the microfluidic "universal CTC-chip" for primary lung cancer. Cancer Sci. 2022;113:1028-1037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 10. | Guan Y, Xu F, Tian J, Gao K, Wan Z, Wang Y, Gao M, Wang Z, Chong T. The prognostic value of circulating tumour cells (CTCs) and CTC white blood cell clusters in patients with renal cell carcinoma. BMC Cancer. 2021;21:826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Strati A, Nikolaou M, Georgoulias V, Lianidou ES. RNA-Based CTC Analysis Provides Prognostic Information in Metastatic Breast Cancer. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Zong W, Feng W, Jiang Y, Ju S, Cui M, Jing R. Evaluating the diagnostic and prognostic value of serum long non-coding RNA CTC-497E21.4 in gastric cancer. Clin Chem Lab Med. 2019;57:1063-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Cai D, Li N, Jin L, Qi X, Hua D, Wang T. High CTC-TRPC5 Expression Significantly Associated With Poor Prognosis in Radical Resected Colorectal Cancer Patients. Front Mol Biosci. 2021;8:727864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Xing C, Li Y, Ding C, Wang S, Zhang H, Chen L, Li P, Dai M. CD44+ Circulating Tumor Endothelial Cells Indicate Poor Prognosis in Pancreatic Ductal Adenocarcinoma After Radical Surgery: A Pilot Study. Cancer Manag Res. 2021;13:4417-4431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Semaan A, Bernard V, Kim DU, Lee JJ, Huang J, Kamyabi N, Stephens BM, Qiao W, Varadhachary GR, Katz MH, Shen Y, San Lucas FA, Gascoyne P, Alvarez HA, Maitra A, Guerrero PA. Characterisation of circulating tumour cell phenotypes identifies a partial-EMT sub-population for clinical stratification of pancreatic cancer. Br J Cancer. 2021;124:1970-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Court CM, Ankeny JS, Sho S, Winograd P, Hou S, Song M, Wainberg ZA, Girgis MD, Graeber TG, Agopian VG, Tseng HR, Tomlinson JS. Circulating Tumor Cells Predict Occult Metastatic Disease and Prognosis in Pancreatic Cancer. Ann Surg Oncol. 2018;25:1000-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 17. | Cheng H, Yang J, Fu X, Mao L, Chu X, Lu C, Li G, Qiu Y, He W. Folate receptor-positive circulating tumor cells predict survival and recurrence patterns in patients undergoing resection for pancreatic cancer. Front Oncol. 2022;12:1012609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | White MG, Lee A, Vicente D, Hall C, Kim MP, Katz MHG, Lee JE, Ikoma N, Lucci A, Tzeng CD. Measurement of Portal Vein Blood Circulating Tumor Cells is Safe and May Correlate With Outcomes in Resected Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol. 2021;28:4615-4622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Bissolati M, Sandri MT, Burtulo G, Zorzino L, Balzano G, Braga M. Portal vein-circulating tumor cells predict liver metastases in patients with resectable pancreatic cancer. Tumour Biol. 2015;36:991-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Hugenschmidt H, Labori KJ, Borgen E, Brunborg C, Schirmer CB, Seeberg LT, Naume B, Wiedswang G. Preoperative CTC-Detection by CellSearch((R)) Is Associated with Early Distant Metastasis and Impaired Survival in Resected Pancreatic Cancer. Cancers (Basel). 2021;13. [DOI] [Full Text] |
| 21. | Padillo-Ruiz J, Suarez G, Pereira S, Calero-Castro FJ, Tinoco J, Marin L, Bernal C, Cepeda-Franco C, Alamo JM, Almoguera F, Macher HC, Villanueva P, García-Fernandez FJ, Gallego I, Romero M, Gomez-Bravo MA, Denninghoff V, Serrano MJ. Circulating Tumor Cells Enumeration from the Portal Vein for Risk Stratification in Early Pancreatic Cancer Patients. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 695] [Article Influence: 173.8] [Reference Citation Analysis (0)] |
| 23. | Forgensen J. Resected adenocarcinoma of the pancreas--616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2001;5:681; author reply 681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15:333-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 805] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 25. | Liu P, Zhu H, Zhang X, Feng A, Zhu X, Sun Y. Predicting Survival for Hepatic Arterial Infusion Chemotherapy of Unresectable Colorectal Liver Metastases: Radiomics Analysis of Pretreatment Computed Tomography. J Transl Int Med. 2022;10:56-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 26. | Yang J, Xu R, Wang C, Qiu J, Ren B, You L. Early screening and diagnosis strategies of pancreatic cancer: a comprehensive review. Cancer Commun (Lond). 2021;41:1257-1274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 170] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 27. | Yin H, Yang X, Sun L, Pan P, Peng L, Li K, Zhang D, Cui F, Xia C, Huang H, Li Z. The value of artificial intelligence techniques in predicting pancreatic ductal adenocarcinoma with EUS images: A meta-analysis and systematic review. Endosc Ultrasound. 2023;12:50-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Sagami R, Hayasaka K, Ujihara T, Iwaki T, Katsuyama Y, Harada H, Ome Y, Honda G, Horiguchi SI, Murakami K, Amano Y. Role of EUS combined with a newly modified scoring system to detect pancreatic high-grade precancerous lesions. Endosc Ultrasound. 2023;12:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 29. | Bian Y, Jiang H, Zheng J, Shao C, Lu J. Basic Pancreatic Lesions: Radiologic-pathologic Correlation. J Transl Int Med. 2022;10:18-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 30. | Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75:319-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 510] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 31. | Facciorusso A, Gkolfakis P, Tziatzios G, Ramai D, Papanikolaou IS, Triantafyllou K, Lisotti A, Fusaroli P, Mangiavillano B, Chandan S, Mohan BP, Crinò SF. Comparison between EUS-guided fine-needle biopsy with or without rapid on-site evaluation for tissue sampling of solid pancreatic lesions: A systematic review and meta-analysis. Endosc Ultrasound. 2022;11:458-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 32. | Thomsen MM, Larsen MH, Di Caterino T, Hedegaard Jensen G, Mortensen MB, Detlefsen S. Accuracy and clinical outcomes of pancreatic EUS-guided fine-needle biopsy in a consecutive series of 852 specimens. Endosc Ultrasound. 2022;11:306-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 33. | Saghir SM, Dhindsa BS, Daid SGS, Mashiana HS, Dhaliwal A, Cross C, Singh S, Bhat I, Ohning GV, Adler DG. Efficacy of EUS-guided needle-based confocal laser endomicroscopy in the diagnosis of pancreatic lesions: A systematic review and meta-analysis. Endosc Ultrasound. 2022;11:275-282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 34. | Hartwig W, Strobel O, Hinz U, Fritz S, Hackert T, Roth C, Büchler MW, Werner J. CA19-9 in potentially resectable pancreatic cancer: perspective to adjust surgical and perioperative therapy. Ann Surg Oncol. 2013;20:2188-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 223] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 35. | Ferrone CR, Finkelstein DM, Thayer SP, Muzikansky A, Fernandez-delCastillo C, Warshaw AL. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol. 2006;24:2897-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 414] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 36. | Wang Y, Yu X, Hartmann D, Zhou J. Circulating tumor cells in peripheral blood of pancreatic cancer patients and their prognostic role: a systematic review and meta-analysis. HPB (Oxford). 2020;22:660-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 37. | Khoo BL, Grenci G, Lim YB, Lee SC, Han J, Lim CT. Expansion of patient-derived circulating tumor cells from liquid biopsies using a CTC microfluidic culture device. Nat Protoc. 2018;13:34-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 38. | Gemenetzis G, Groot VP, Yu J, Ding D, Teinor JA, Javed AA, Wood LD, Burkhart RA, Cameron JL, Makary MA, Weiss MJ, He J, Wolfgang CL. Circulating Tumor Cells Dynamics in Pancreatic Adenocarcinoma Correlate With Disease Status: Results of the Prospective CLUSTER Study. Ann Surg. 2018;268:408-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 39. | Li Y, Wu S, Bai F. Molecular characterization of circulating tumor cells-from bench to bedside. Semin Cell Dev Biol. 2018;75:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Pantel K, Alix-Panabières C. The clinical significance of circulating tumor cells. Nat Clin Pract Oncol. 2007;4:62-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Alix-Panabières C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14:623-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 917] [Cited by in RCA: 1013] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 42. | Cen P, Ni X, Yang J, Graham DY, Li M. Circulating tumor cells in the diagnosis and management of pancreatic cancer. Biochim Biophys Acta. 2012;1826:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Bidard FC, Huguet F, Louvet C, Mineur L, Bouché O, Chibaudel B, Artru P, Desseigne F, Bachet JB, Mathiot C, Pierga JY, Hammel P. Circulating tumor cells in locally advanced pancreatic adenocarcinoma: the ancillary CirCe 07 study to the LAP 07 trial. Ann Oncol. 2013;24:2057-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 44. | Pecot CV, Bischoff FZ, Mayer JA, Wong KL, Pham T, Bottsford-Miller J, Stone RL, Lin YG, Jaladurgam P, Roh JW, Goodman BW, Merritt WM, Pircher TJ, Mikolajczyk SD, Nick AM, Celestino J, Eng C, Ellis LM, Deavers MT, Sood AK. A novel platform for detection of CK+ and CK- CTCs. Cancer Discov. 2011;1:580-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 45. | Liu H, Sun B, Wang S, Liu C, Lu Y, Li D, Liu X. Circulating Tumor Cells as a Biomarker in Pancreatic Ductal Adenocarcinoma. Cell Physiol Biochem. 2017;42:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Wang S, Liu K, Liu J, Yu ZT, Xu X, Zhao L, Lee T, Lee EK, Reiss J, Lee YK, Chung LW, Huang J, Rettig M, Seligson D, Duraiswamy KN, Shen CK, Tseng HR. Highly efficient capture of circulating tumor cells by using nanostructured silicon substrates with integrated chaotic micromixers. Angew Chem Int Ed Engl. 2011;50:3084-3088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 477] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 47. | Hou S, Zhao L, Shen Q, Yu J, Ng C, Kong X, Wu D, Song M, Shi X, Xu X, OuYang WH, He R, Zhao XZ, Lee T, Brunicardi FC, Garcia MA, Ribas A, Lo RS, Tseng HR. Polymer nanofiber-embedded microchips for detection, isolation, and molecular analysis of single circulating melanoma cells. Angew Chem Int Ed Engl. 2013;52:3379-3383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 48. | Court CM, Ankeny JS, Sho S, Hou S, Li Q, Hsieh C, Song M, Liao X, Rochefort MM, Wainberg ZA, Graeber TG, Tseng HR, Tomlinson JS. Reality of Single Circulating Tumor Cell Sequencing for Molecular Diagnostics in Pancreatic Cancer. J Mol Diagn. 2016;18:688-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 49. | Zou D, Cui D. Advances in isolation and detection of circulating tumor cells based on microfluidics. Cancer Biol Med. 2018;15:335-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 50. | Lin PP. Integrated EpCAM-independent subtraction enrichment and iFISH strategies to detect and classify disseminated and circulating tumors cells. Clin Transl Med. 2015;4:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |