Published online Nov 24, 2023. doi: 10.5306/wjco.v14.i11.459
Peer-review started: September 4, 2023
First decision: September 19, 2023
Revised: September 28, 2023
Accepted: October 16, 2023
Article in press: October 16, 2023
Published online: November 24, 2023
Processing time: 78 Days and 13.7 Hours
Liver cancer resection, especially in patients with hemihepatectomy or extended hemihepatectomy, often leads to poor prognosis, such as liver insufficiency and even liver failure and death, because the standard residual liver volume (SRLV) cannot be fully compensated after surgery.
To explore the risk factors of poor prognosis after hemihepatectomy for hepatocellular carcinoma and evaluate the application value of related prognostic approaches.
The clinical data of 35 patients with primary liver cancer in Nantong Third People's Hospital from February 2016 to July 2020 were retrospectively analyzed. The receiver operating characteristic curve was created using medcac19.0.4 to compare the critical values of the SRLV in different stages of liver fibrosis after hemihepatectomy with those of liver dysfunction after hemihepatectomy. It was constructed by combining the Child-Pugh score to evaluate its application value in predicting liver function compensation.
The liver stiffness measure (LSM) value and SRLV were associated with liver dysfunction after hemihepatectomy. Logistic regression analysis showed that an LSM value ≥ 25 kPa [odds ratio (OR) = 6.254, P < 0.05] and SRLV ≤ 0.290 L/m2 (OR = 5.686, P < 0.05) were independent risk factors for postoperative liver dysfunction. The accuracy of the new liver reserve evaluation model for predicting postoperative liver function was higher than that of the Child-Pugh score (P < 0.05).
SRLV and LSM values can be used to evaluate the safety of hemihepatectomy. The new liver reserve evaluation model has good application potential in the evaluation of liver reserve function after hemihepatectomy.
Core Tip: To explore the risk factors and predictive methods of poor prognosis after hemihepatectomy for hepatocellular carcinoma and evaluate its application value. The clinical data of 35 patients with primary liver cancer were retrospectively analyzed. The critical values of standard residual liver volume (SRLV) in different stages of liver fibrosis after hemihepatectomy were compared with those of liver dysfunction after hemihepatectomy. We found that SRLV and liver stiffness measure values can be used to evaluate the safety of hemihepatectomy.
- Citation: Yue ZQ, Zhang P, Yan S, Ju LL, Wang HX, Yuan LX, Chen L, Wu JZ, Cao YL. Clinical study of standard residual liver volume and transient elastography in predicting poor prognosis of patients after hemihepatectomy. World J Clin Oncol 2023; 14(11): 459-470
- URL: https://www.wjgnet.com/2218-4333/full/v14/i11/459.htm
- DOI: https://dx.doi.org/10.5306/wjco.v14.i11.459
Liver cancer is a malignant tumor associated with high mortality worldwide[1-3]. Hepatocellular carcinoma (HCC), one of the main types of liver cancer, is often found in advanced stages and cannot be cured[4-6]. As a highly heterogeneous disease, HCC mostly develops as a result of hepatitis B cirrhosis[7]. China has the largest number of hepatitis B virus infections in the world; therefore, the number of HCC patients accounts for more than half of the total number of HCCs worldwide[8]. To date, surgical resection and liver transplantation are still effective treatments for HCC; however, due to the shortage of liver sources, the main treatment for HCC is surgery[9]. Liver cancer resection, especially in patients with hemihepatectomy or extended hemihepatectomy, often leads to poor prognosis, such as liver insufficiency and even liver failure and death, because the standard residual liver volume (SRLV) cannot be fully compensated after surgery[10]. Research suggests that preoperative liver fibrosis and cirrhosis are the main causes[11]. In recent years, an increasing number of studies have shown that the liver stiffness measure (LSM) value is significantly related to the degree of cirrhosis, which can reflect the degree of liver inflammation and fibrosis[12,13]. Accordingly, the purpose of this study was to investigate the risk factors and predictive methods of poor prognosis after hemihepatectomy for HCC and verify whether the changes in liver structure can be reflected by the LSM value and SRLV to assess the liver's compensatory capacity. Finally, we established a liver reserve function evaluation model by combining the LSM value and Child-Pugh scores and evaluated its application value.
The clinical case data were obtained from 35 HCC patients undergoing hemihepatectomy in the Nantong Third People’s Hospital between February 2016 and July 2020, and all patients met the inclusion criteria for this study. The study was approved by the Ethics Committee of the Nantong Third People's Hospital Affiliated with Nantong University. Written informed consent was obtained from all patients before being enrolled in the study. The inclusion criteria were as follows: (1) According to the China liver cancer staging for the diagnosis and treatment standard of primary liver cancer (2019 edition), the stage of liver cancer was stage Ia, Ib or IIa, the tumor was located only in the left or right half of the liver, and hemihepatectomy was needed; (2) all patients were positive for HBsAg before the operation, and HCC was confirmed by pathology after surgery; (3) liver enhancement computed tomography (CT) was performed before the operation; (4) the LSM value was detected by transient elastography (Fibro Touch) before the operation; and (5) patients had more complete clinical case data. The exclusion criteria were as follows: (1) The patient did not have a standard hemihepatectomy; (2) the postoperative pathology of the patients was confirmed as cholangiocarcinoma or metastatic carcinoma; (3) the patient had a preoperative intervention, ablation, or chemoradiotherapy; (4) secondary operation; (5) other complications affecting liver function before the operation, such as hepatic encephalopathy, abdominal dropsy, and other conditions; and (6) the presence of other malignant tumors or serious diseases.
The patient was placed in a supine position with a soft pad on the high right lumbar back (no pad height was required for left hemihepatectomy), an oblique incision was made at the right abdominal costal margin, approximately 30 cm in length, layer by layer into the abdominal cavity; adhesions were separated, and each connective tissue and ligament of the liver were cut to fully expose the liver. The texture and morphology of the liver and spleen were observed, the completely free left or right lobe of the liver was selected according to the location of the tumor, the lesions that had not been detected before the operation were examined by intraoperative ultrasound, and the abdominal cavity was explored for the presence or absence of tumor implantation and metastasis. Subsequently, the liver hilum was selectively blocked, and the left or right hemiliver was resected, along with the gallbladder removal and extended hemihepatectomy according to the preoperative conditions, with surgical margins generally larger than 1 cm from the tumor margin. The operation area was carefully checked for the presence or absence of bleeding and biliary fistula, an abdominal drainage tube was placed, and each layer of the abdominal wall was closed layer by layer.
Preoperatively, Philips brilliance CT was used to perform a routine double-phase scan of the patient's liver with a thickness of 1.25 mm. Then, portal vein stage tomography was selected, and rapid liver volume measurement software was used to draw the liver boundary layer-by-layer (the inferior vena cava and gallbladder were avoided) and calculate the total liver volume (TLV). The volume of half of the liver was measured by drainage after the liver was isolated. The body surface area (BSA) was calculated according to the literature[14,15]. Finally, postoperative remnant liver volume (RLV) = TLV-the volume of half of the liver, and SRLV = RLV/BSA.
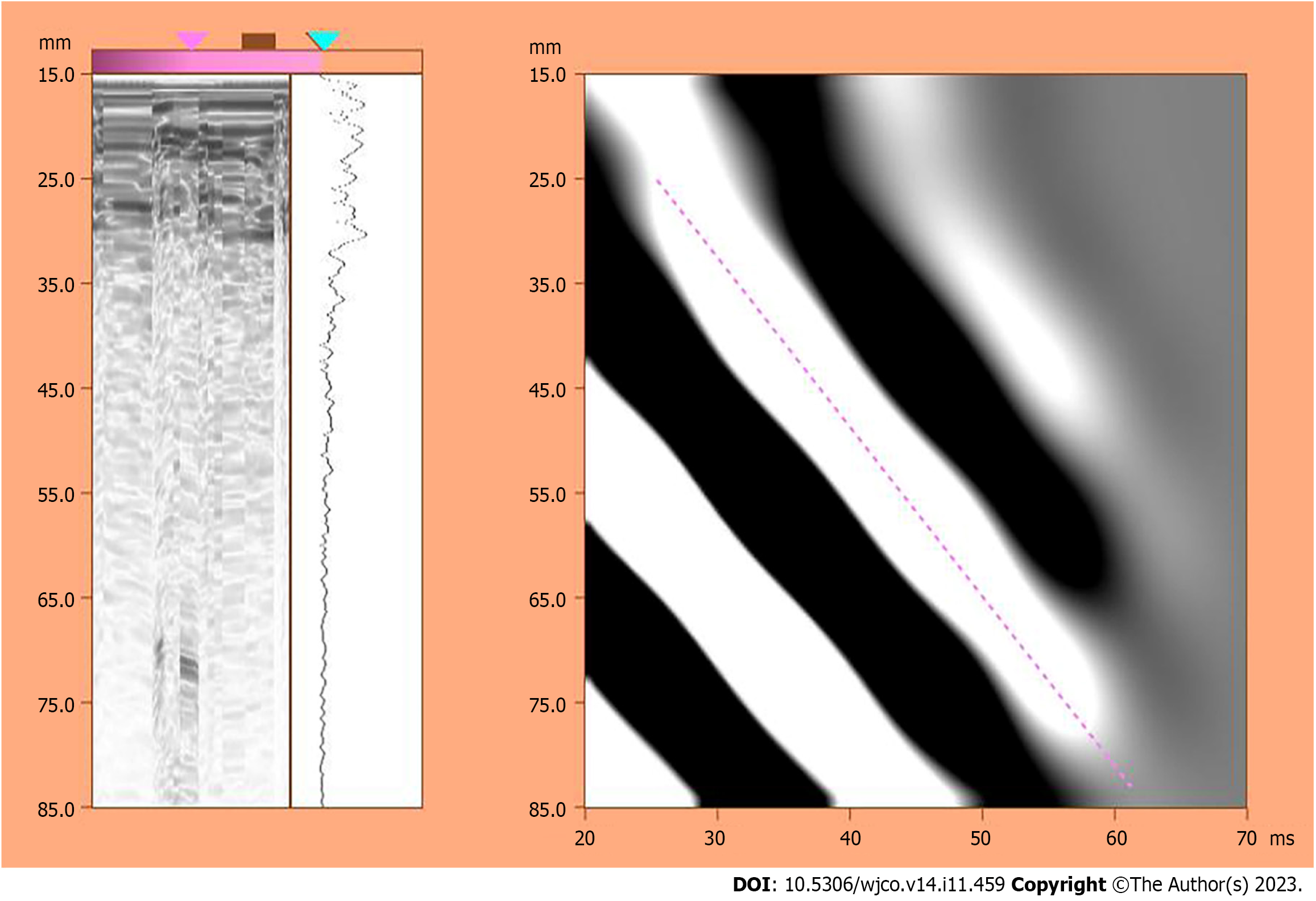
According to the measurement requirements of the American Association for the Study of Liver Diseases[16], we measured the LSM value using a liver FibroTouch (FT) device developed by Haskell Medical Technology Company. In detail, the LSM value was measured 10 consecutive times for each patient, the quartile spacing was specified to be less than 30% as the effective measurement, and the median was chosen as the LSM value. All operations were performed by the same physician with extensive experience in the diagnosis of hepatobiliary diseases using ultrasound. A schematic illustration of the measurement results is shown in Figure 1. Then, a new model of liver reserve assessment was constructed according to the combination of the Child-Pugh score and the LSM value (Table 1).
| Score | 1 | 2 | 3 |
| HE | No | 1-2 | 3-4 |
| ABD | No | Mild | Moderate to severe |
| TIBL (μmol/L) | < 34 | 34-51 | > 51 |
| ALB (g/L) | > 35 | 28-35 | > 28 |
| Prothrombin Time (secprolonged) | < 15 | 15-17 | > 17 |
| LSM (kPa) | < 15 | 15-25 | > 25 |
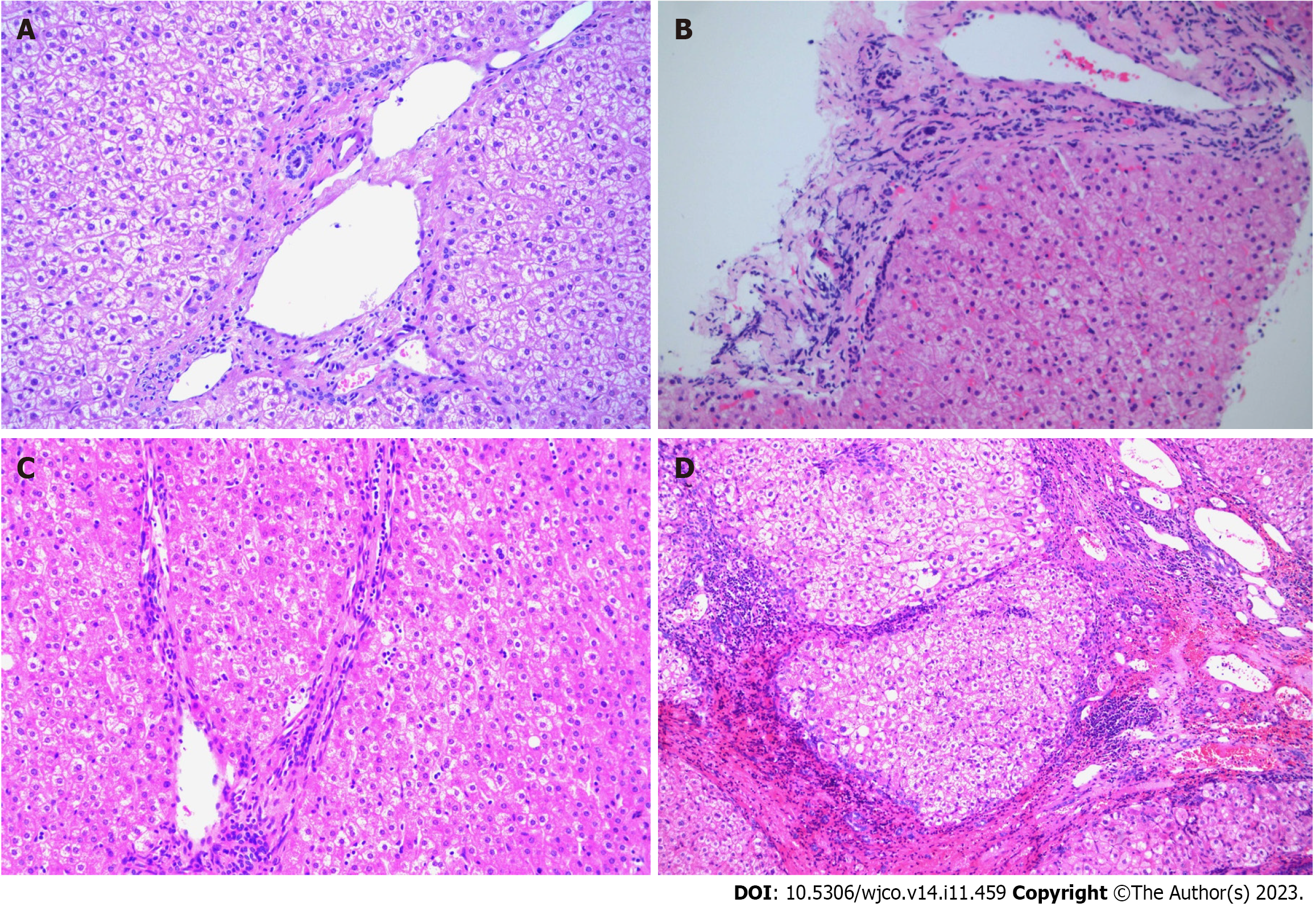
By observing the paraffin sections of the liver under the microscope, we phased fibrosis according to the Scheuer scoring system as follows[17]: S0 stage, no liver fibrosis; S1 stage, liver fibrosis limited to the portal region; S2 stage, liver fibrosis extending to the portal region or portal interval, but the vascular relationship was normal; S3 stage, liver fibrosis with structural changes but not obvious cirrhosis; and S4 stage, cirrhosis (Figure 2).
According to the definition of liver dysfunction after hepatectomy from the International Study Group of Liver Surgery[18], we defined liver dysfunction as the results of a 5-d laboratory examination after hepatectomy that showed elevated international normalized ratio (INR) and total bilirubin (INR > 1.5; total bilirubin > 20.5 mmol/L); additionally, the patient was assessed for liver function, kidney function, respiratory function and the need for special assessment and special clinical treatment.
All statistical analyses were performed using IBM SPSS Statistics 25.0, and the measurement data were compared using the t test or single-factor analysis of variance (ANOVA). The Wilcoxon rank-sum test was used when the variance was uneven, and the Chi square test was used for counting data. Analysis of independent risk factors was completed using unconditional logistic regression. We used medcalc19.0.4 to draw the receiver operating characteristic (ROC) curve of the subjects and analyzed the area under the ROC curve under different factors. P < 0.05 was considered statistically significant.
The 35 patients in this study were grouped according to the presence or absence of liver dysfunction after surgery as follows: 12 patients had postoperative liver dysfunction, and 23 patients had no liver dysfunction. Then, the following 25 factors were analyzed. The results showed that the preoperative LSM value and SRLV were correlated with liver dysfunction after hemihepatectomy in HCC patients (P < 0.05, Table 2).
| Variables | Total | Postoperative liver function | P value | |
| No liver dysfunction | Liver dysfunction | |||
| Sex | ||||
| Male | 19 | 12 | 7 | 1.000 |
| Female | 16 | 11 | 5 | |
| Age (yr) | ||||
| < 60 | 20 | 14 | 6 | 0.721 |
| ≥ 60 | 15 | 9 | 6 | |
| BMI | ||||
| < 24 kg/m2 | 21 | 13 | 8 | 0.721 |
| > 24 kg/m2 | 14 | 10 | 4 | |
| BSA (m2) | 1.71 ± 0.18 | 1.75 ± 0.16 | 0.514 | |
| WBC (× 109/L) | 5.51 ± 2.39 | 5.19 ± 1.55 | 0.672 | |
| RBC (1012/L) | 4.30 ± 0.65 | 4.61 ± 0.44 | 0.146 | |
| PLT (× 109/L) | 126.17 ± 53.74 | 149.33 ± 79.83 | 0.314 | |
| ALB (g/L) | 40.71 ± 4.23 | 39.63 ± 4.18 | 0.478 | |
| Scr (μmol/L) | 76.98 ± 39.07 | 64.87 ± 10.63 | 0.303 | |
| ALT [U/L, M (QR)] | 67.26 ± 94.41 | 46.58 ± 30.40 | 0.468 | |
| AST [U/L, M (QR)] | 45.61 ± 23.63 | 58.00 ± 68.68 | 0.436 | |
| TB [umol/L, M (QR)] | 15.63 ± 7.20 | 20.99 ± 8.36 | 0.056 | |
| GGT [U/L, M (QR)] | 115.17 ± 112.78 | 124.00 ± 145.78 | 0.844 | |
| AFP [ng/mL, M (QR)] | 9999.23 ± 25773.40 | 8545.66 ± 15372.77 | 0.859 | |
| PIVKA-II [μg/L, M (QR)] | 1799.54 ± 5017.78 | 3574.73 ± 6543.79 | 0.378 | |
| PT (s) | 12.59 ± 1.46 | 12.39 ± 1.04 | 0.682 | |
| INR (s) | 1.10 ± 0.12 | 1.05 ± 0.11 | 0.227 | |
| LSM value (kPa) | 20.34 ± 4.89 | 25.78 ± 5.38 | 0.005a | |
| ICG R15 (%) | 7.99 ± 5.13 | 11.96 ± 6.43 | 0.055 | |
| SRLV (L/m2) | 0.349 ± 0.075 | 0.276 ± 0.036 | 0.003a | |
| Tumor-localizing | ||||
| Left half liver | 15 | 10 | 5 | 1.000 |
| Right half liver | 20 | 13 | 7 | |
| Tumor diameter [cm, M (QR)] | 6.63 ± 3.86 | 7.81 ± 4.91 | 0.436 | |
| Time of hepatic portal occlusion [min, M (QR)] | 14.65 ± 19.42 | 15.75 ± 14.10 | 0.864 | |
| Intraoperative bleeding [mL, M (QR)] | 908.70 ± 818.76 | 1541.67 ± 1612.57 | 0.130 | |
| Operation time (min) | 176.30 ± 49.98 | 185.83 ± 72.89 | 0.651 | |
The preoperative LSM value and SRLV were selected as independent variables, and regardless of whether liver dysfunction was selected as the dependent variable, a logistic regression model was developed for analysis. The results showed that the preoperative LSM value and SRLV were independent risk factors for liver dysfunction after hemihepatectomy (P < 0.05, Table 3).
| Independent variables | P value | OR | 95%CI |
| LSM ≥ 25 kPa | 0.032 | 6.254 | 1.172-33.374 |
| SRLV ≤ 290 ML/m2 | 0.048 | 5.686 | 1.017-31.793 |
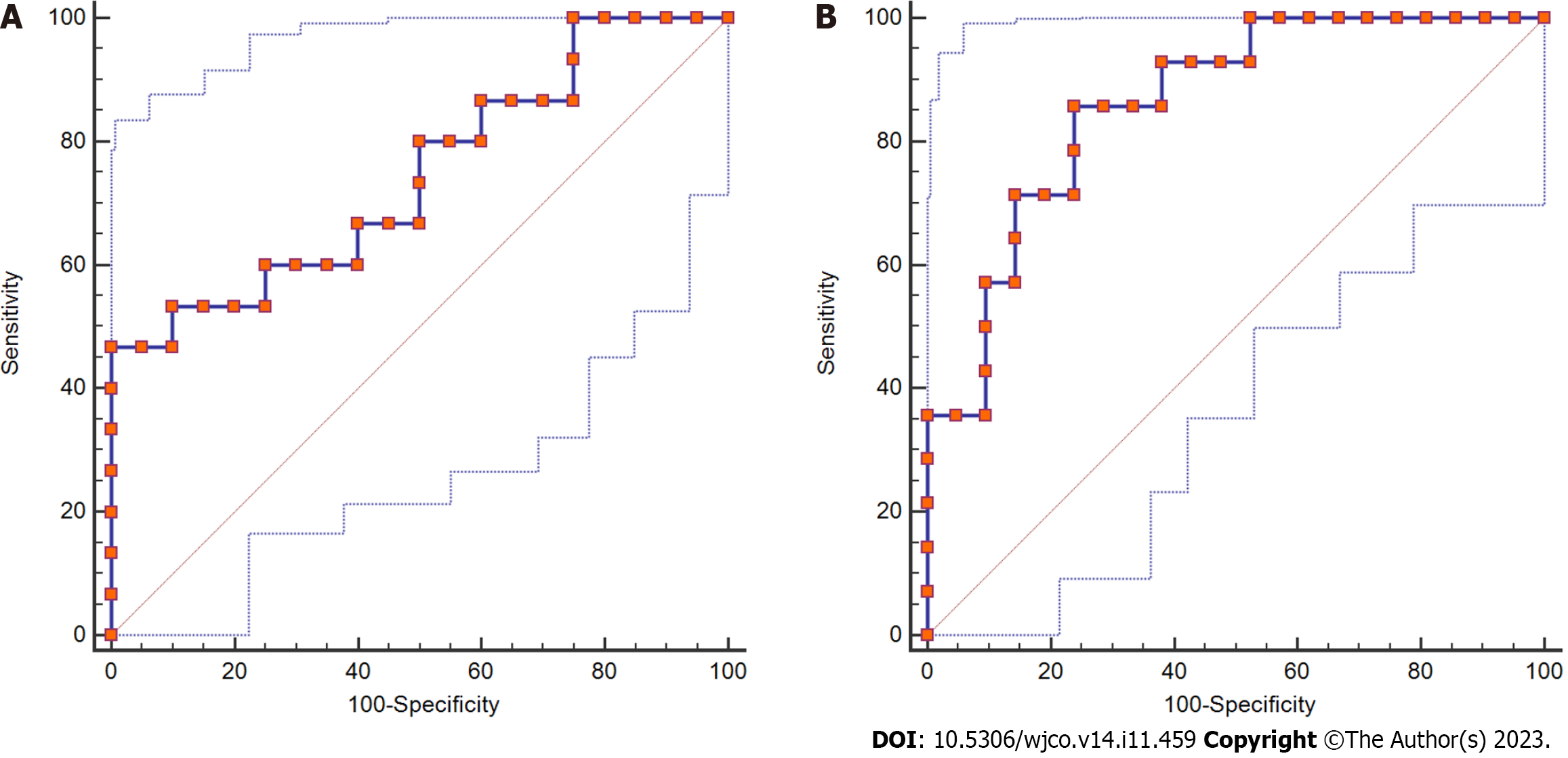
The staging results of postoperative liver fibrosis showed 0 cases in the S0 stage, 6 cases in the S1 stage, 14 cases in the S2-S3 stage, and 15 cases in the S4 stage. Then, we compared and analyzed the critical values of SRLV for different stages, and the results showed that the difference in SRLV among the three phases was statistically significant (P < 0.05, Table 4). ROC curve analysis showed that the area under the curve for the S2-S3 stage was 0.743, the sensitivity was 0.467, the specificity was 0.100, and the critical value of SRLV was 0.257 L/m2; the area under the curve for the S4 phase was 0.861, the sensitivity was 0.857, the specificity was 0.762, and the critical value of SRLV was 0.311 L/m2 (Figure 3).
| Liver fibrosis stage | Number | SRLV (L/m2) | F value | P value |
| S1 | 6 | 289.43 ± 22.36 | 8.164 | 0.001 |
| S2-S3 | 15 | 290.33 ± 56.70 | ||
| S4 | 14 | 375.53 ± 72.24 |
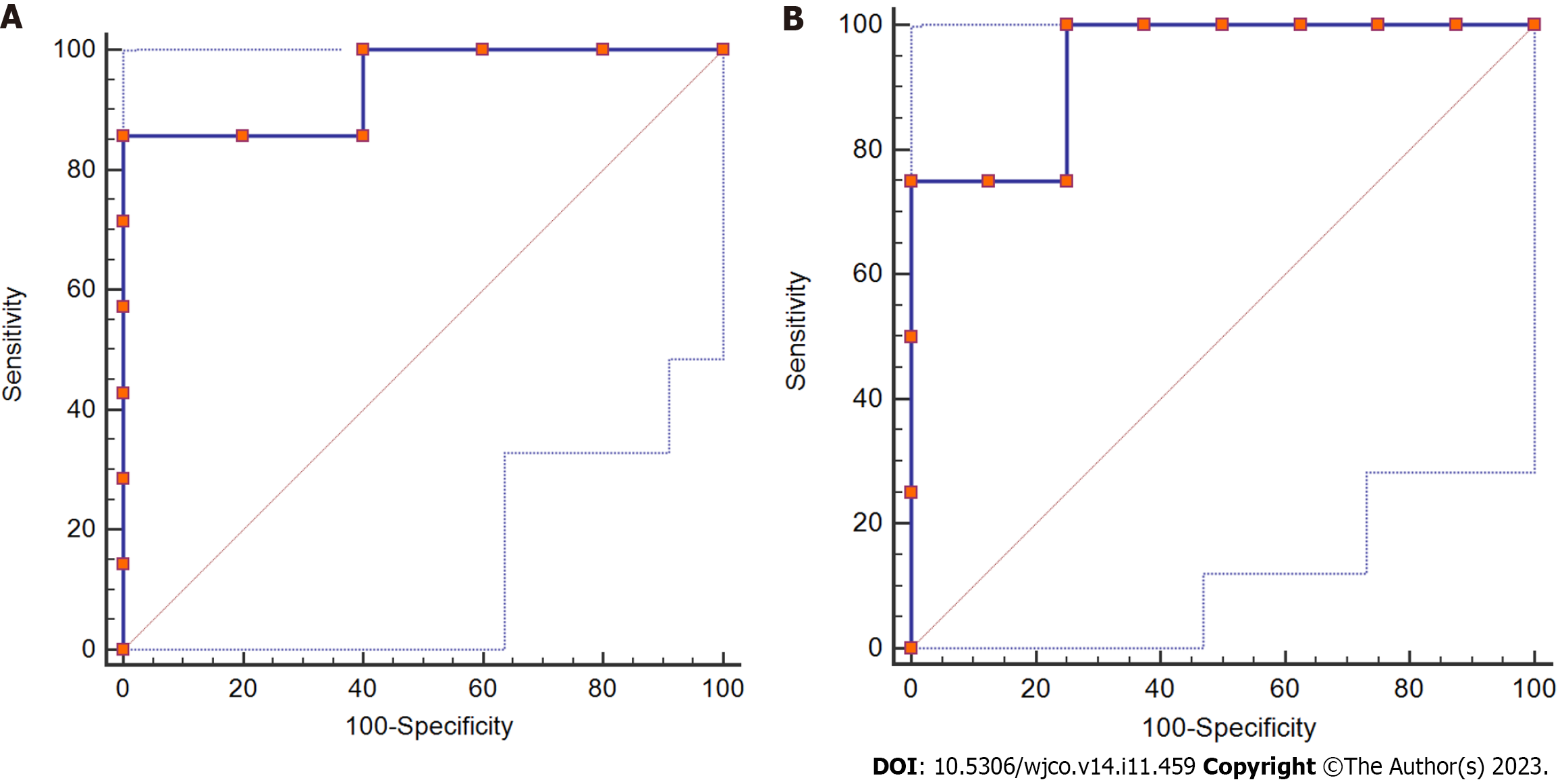
In 12 patients with postoperative liver dysfunction, the staging results of postoperative liver fibrosis showed 0 cases in the S0 stage, 1 case in the S1 stage, 7 cases in the S2-S3 stage, and 4 cases in the S4 stage. Additionally, the corresponding SRLVs were compared and analyzed, and the results showed that the difference in SRLV among the three phases was statistically significant (P < 0.05, Table 5). ROC curve analysis showed that the area under the curve for stage S2-S3 was 0.943, the sensitivity was 0.857, the specificity was 0.100, and the safety-critical value for SRLV was 0.285 L/m2; the area under the curve for stage S4 was 0.938, the sensitivity was 0.100, the specificity was 0.750, and the safety-critical value of SRLV was 0.285 L/m2 (Figure 4).
| Liver fibrosis stage | Number | SRLV (L/m2) | F value | P value |
| S1 | 1 | 234.20 | 4.768 | 0.039 |
| S2-S3 | 7 | 263.14 ± 31.28 | ||
| S4 | 4 | 308.98 ± 18.02 |
We reviewed and analyzed the clinical data of 35 patients in this study and followed up with the patients. The results showed that there were no postoperative deaths, and all patients were discharged within 3 wk after the operation. Statistical analysis showed that with Child-Pugh score was grade A, the accuracy rate of predicting postoperative liver function compensation was 54.8%; the accuracy rate of grade B was 25.0%. The new model was classified as grade I, and the accuracy rate of predicting postoperative liver function compensation was 100.0%, which was higher than that of the Child-Pugh score (χ2 = 7.452, P = 0.007). Similarly, that of grade II was 91.3%, which was higher than the Child-Pugh score (χ2 = 9.928, P = 0.013). There was a significant difference between the two models in evaluating the prognosis after hemihepatectomy (P < 0.05, Table 6).
HCC is one of the most common malignant tumors. With the improvement of the technical level of hepatectomy, the mortality rate after HCC resection has decreased significantly[19-21]. However, the mortality rate is still 5%-8%, especially in patients with hemihepatectomy[21]. The main cause of death after hemihepatectomy is liver failure[22]. The surgical resection range is so large such that the postoperative remnant liver cannot meet the needs of the body; more importantly, doctors lack a comprehensive understanding of the liver reserve function of patients before surgery. As a single evaluation indicator, indocyanine green (ICG) is better than many biochemical indicators. When many conventional liver function indicators have not yet become abnormal in value, the ICG retention rate at 15 min (ICG R15) can reflect liver function damage or occult liver disease in a timely manner[23]. However, ICG has certain limitations and is easily interfered with by factors such as the patient's cooperation ability, liver cell uptake capacity, liver blood flow, bile duct obstruction, bilirubin, etc[24,25]. SRLV is a reliable index of preoperative liver reserve function at home and abroad[26,27]. However, considering that HCC patients often have varying degrees of liver fibrosis before the operation, the liver reserve and regeneration function in such patients may vary depending on the extent of liver fibrosis, even if the SRLV is the same; therefore, it is not satisfactory to evaluate liver reserve function only in terms of liver volume. The diagnosis of preoperative liver fibrosis mainly depends on liver histopathological examination; however, because of invasive examination, a low positive rate, difficulty in follow-up, and dynamic detection, the need to consider the wishes of patients and other factors, scholars at home and abroad have explored the use of elastic techniques instead of liver biopsy to assess the extent of liver fibrosis or cirrhosis by measuring the LSM value[17]. Therefore, it is very important to evaluate the safety of hemihepatectomy by correctly staging the degree of liver fibrosis before surgery.
First, in this study, the factors that may be related to liver dysfunction in HCC patients after hemihepatectomy were statistically analyzed. The results showed that preoperative LSM and SRLV were associated with liver dysfunction in HCC patients after hemihepatectomy (P < 0.05). Multivariate logistic regression analysis showed that preoperative LSM and SRLV were independent risk factors for liver dysfunction in HCC patients after hemihepatectomy.
Then, according to the Scheuer score standard, we observed the degree of liver fibrosis using microscopy and analyzed the SRLV critical value of different stages of liver fibrosis in all patients and the SRLV critical value of different stages of liver fibrosis in postoperative liver insufficiency cases by ROC curve analysis. The results showed that the critical values of SRLV were 0.257 L/m2 and 0.310 L/m2 in patients with liver fibrosis in stages S2-S3 and S4, respectively, and 0.285 L/m2 in patients with postoperative liver dysfunction. SRLV critical values were similar in both cases, suggesting that it is safe and feasible to predict the SRLV threshold of HCC patients undergoing hemihepatectomy by pathological stages of liver fibrosis. It is suggested that the operation is safe if SRLV > 0.310 L/m2.
At present, the elastic technique has been used to evaluate the degree of liver fibrosis or cirrhosis. It has been widely accepted because of its simplicity, repeatability, noninvasiveness, low cost, and other factors. At present, studies have reported that the sensitivity and specificity of the LSM value to predict the degree of hepatitis cirrhosis are high, and the LSM value is confirmed to be related to complications after partial hepatectomy in patients[28]. However, there is no uniform standard for the patient's disease background, and the operation is limited to only partial or segmental hepatectomy. There is no study on the application of transient elastography to predict the degree of liver fibrosis and cirrhosis in hemihepatectomy, and there is no study on the LSM value in evaluating liver function reserve before hemihepatectomy. In addition, recent studies have shown that transient elastography cannot be used to accurately assess patients with obstructive jaundice. Therefore, more rigorous inclusion and exclusion criteria were adopted in this study. We used Fibro Touch elastic imaging equipment (FT-3.5R50) developed by Haskell Medical Technology Company and a two-dimensional ultrasonic probe to avoid the influence of liver tumors and large blood vessels inside and outside the liver on the measurement results. The measured LSM value was 22.20 ± 5.63 kPa, which is similar to that reported at home and abroad[12]. We established a new liver reserve assessment model based on the Child-Pugh score combined with the LSM value and observed its application in the evaluation of liver reserve function in patients with HCC undergoing hemihepatectomy. The results showed that the accuracy of the new evaluation model in predicting postoperative liver function compensation was 100.0% (P < 0.05), and the accuracy rate of predicting mildly poor liver function compensation after the operation was 91.3% (P < 0.05), which was higher than that of the Child-Pugh score. Therefore, we believe that the new liver reserve assessment model can provide a reference for preoperative safety assessment of patients with liver cancer undergoing hemihepatectomy, which can increase patient safety during the perioperative period and reduce the incidence of liver failure after the operation. Additionally, it can provide a reference for patients with liver cancer who are expected to receive hemihepatectomy or extended hemihepatectomy.
In summary, through this study, we found that for patients with moderate or severe liver fibrosis, when the predicted SRLV is greater than 0.310 L/m2, the new evaluation model of liver function reserve predicts that the postoperative liver function compensation is good before the operation, and hemihepatectomy is safe; when the predicted SRLV is less than 0.285 L/m2, the new liver reserve assessment model predicts poor liver function compensation after hepatectomy, and the probability of liver dysfunction after hemihepatectomy is higher. A blind operation should be avoided, and the operation should be evaluated after full liver protection. Patients in whom severe liver dysfunction is expected after surgery need to undergo antiviral treatment and undergo portal vein embolization or associated life partition and portal vein ligation for staged hepatectomy, and the values of SRLV and LSM should be reevaluated after liver regeneration. After contralateral liver regeneration, the SRLV and LSM values are reevaluated. It is expected that hemihepatectomy is still feasible for patients with well-compensated liver function. The LSM value combined with SRLV is safe and reliable.
However, the sample size involved in this study is too small and has no statistical significance in theory; nevertheless, the author believes that the LSM value and SRLV are useful safety indices for the evaluation of HCC hemihepatectomy. The new liver reserve evaluation model based on the Child-Pugh score combined with the LSM value can improve on the Child-Pugh score; it has important clinical guiding importance for the evaluation of liver reserve function in HCC patients with hemihepatectomy and provides a theoretical basis for further investigations conducted by our research group.
Liver cancer resection often leads to poor prognosis, because the standard residual liver volume (SRLV) cannot be fully compensated after surgery.
Hemihepatectomy or extended hemihepatectomy often leads to liver insufficiency and even liver failure.
This study aimed to explore the risk factors of poor prognosis after hemihepatectomy for hepatocellular carcinoma and evaluate the application value of related prognostic approaches.
The clinical data of 35 patients with primary liver cancer were retrospectively analyzed. The critical values of SRLV in different stages of liver fibrosis after hemihepatectomy were compared with those of liver dysfunction after hemihepatectomy.
Logistic regression analysis showed that the liver stiffness measure (LSM) value ≥ 25 kPa [odds ratio (OR) = 6.254, P < 0.05)] and SRLV ≤ 0.290 L/m2 (OR = 5.686, P < 0.05) were independent risk factors for postoperative liver dysfunction. The accuracy of the new liver reserve evaluation model for predicting postoperative liver function was higher than that of the Child-Pugh score (P < 0.05).
LSM values and SRLV can be used to evaluate the safety of hemihepatectomy.
The new liver reserve evaluation model has good application potential in the evaluation of liver reserve function after hemihepatectomy.
We appreciate teachers and classmates support and guidance through this process. Express respect and gratitude to patients who provide medical records.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sahin TT, Turkey S-Editor: Qu XL L-Editor: A P-Editor: Zhang XD
| 1. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3232] [Article Influence: 461.7] [Reference Citation Analysis (1)] |
| 2. | Liu Z, Suo C, Mao X, Jiang Y, Jin L, Zhang T, Chen X. Global incidence trends in primary liver cancer by age at diagnosis, sex, region, and etiology, 1990-2017. Cancer. 2020;126:2267-2278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 3. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55774] [Article Influence: 7967.7] [Reference Citation Analysis (132)] |
| 4. | Shen S, Qiu Y, Yang X, Wang W. Remnant Liver-to-Standard Liver Volume Ratio Below 40% is Safe in Ex Vivo Liver Resection and Autotransplantation. J Gastrointest Surg. 2019;23:1964-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15468] [Article Influence: 2578.0] [Reference Citation Analysis (2)] |
| 6. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64442] [Article Influence: 16110.5] [Reference Citation Analysis (176)] |
| 7. | Liu PJ, Harris JM, Marchi E, D'Arienzo V, Michler T, Wing PAC, Magri A, Ortega-Prieto AM, van de Klundert M, Wettengel J, Durantel D, Dorner M, Klenerman P, Protzer U, Giotis ES, McKeating JA. Hypoxic gene expression in chronic hepatitis B virus infected patients is not observed in state-of-the-art in vitro and mouse infection models. Sci Rep. 2020;10:14101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Zheng Y, Wu J, Ding C, Xu K, Yang S, Li L. Disease burden of chronic hepatitis B and complications in China from 2006 to 2050: an individual-based modeling study. Virol J. 2020;17:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 9. | Salimi S, Pandya K, Sastry V, West C, Virtue S, Wells M, Crawford M, Pulitano C, McCaughan GW, Majumdar A, Strasser SI, Liu K. Impact of Having a Planned Additional Operation at Time of Liver Transplant on Graft and Patient Outcomes. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Mai RY, Lu HZ, Bai T, Liang R, Lin Y, Ma L, Xiang BD, Wu GB, Li LQ, Ye JZ. Artificial neural network model for preoperative prediction of severe liver failure after hemihepatectomy in patients with hepatocellular carcinoma. Surgery. 2020;168:643-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Möller K, Safai Zadeh E, Görg C, Dong Y, Cui X, Lim A, de Molo C, Serra C, Martín Algíbez A, Berzigotti A, Piscaglia F, Faiss S, Dietrich CF. Focal Liver Lesions other than Hepatocellular Carcinoma in Cirrhosis: Diagnostic Challenges. J Transl Int Med. 2022;10:308-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Reference Citation Analysis (0)] |
| 12. | Hwang JY, Yoon HM, Kim JR, Lee JS, Jung AY, Kim KM, Cho YA. Diagnostic Performance of Transient Elastography for Liver Fibrosis in Children: A Systematic Review and Meta-Analysis. AJR Am J Roentgenol. 2018;211:W257-W266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Zhang Y, Wang C, Li H, Ding Y. Decreased liver stiffness by transient elastography indicates lower incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Medicine (Baltimore). 2019;98:e13929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Feng LM, Wang PQ, Yu H, Chen RT, Wang J, Sheng X, Yuan ZL, Shi PM, Xie WF, Zeng X. New formula for predicting standard liver volume in Chinese adults. World J Gastroenterol. 2017;23:4968-4977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | van Wissen J, Bakker N, Doodeman HJ, Jansma EP, Bonjer HJ, Houdijk AP. Preoperative Methods to Reduce Liver Volume in Bariatric Surgery: a Systematic Review. Obes Surg. 2016;26:251-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Furlan A, Tublin ME, Yu L, Chopra KB, Lippello A, Behari J. Comparison of 2D Shear Wave Elastography, Transient Elastography, and MR Elastography for the Diagnosis of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. AJR Am J Roentgenol. 2020;214:W20-W26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 17. | Taneja S, Tohra S, Duseja A, Dhiman RK, Chawla YK. Noninvasive Assessment of Liver Fibrosis By Transient Elastography and FIB4/APRI for Prediction of Treatment Response in Chronic Hepatitis C-An Experience from a Tertiary Care Hospital. J Clin Exp Hepatol. 2016;6:282-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Maruyama M, Yoshizako T, Araki H, Yoshida R, Ando S, Nakamura M, Kitagaki H. Future Liver Remnant Indocyanine Green Plasma Clearance Rate as a Predictor of Post-hepatectomy Liver Failure After Portal Vein Embolization. Cardiovasc Intervent Radiol. 2018;41:1877-1884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Bozkurt B, Emek E, Arikan T, Ceyhan O, Yazici P, Sahin T, Mammadov E, Serin A, Gurcan NI, Yuzer Y, Tokat Y. Liver Graft Volume Estimation by Manual Volumetry and Software-Aided Interactive Volumetry: Which is Better? Transplant Proc. 2019;51:2387-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Endo Y, Ohta M, Tada K, Nakanuma H, Saga K, Masuda T, Hirashita T, Iwashita Y, Ozeki Y, Masaki T, Inomata M. Improvement of non-alcoholic fatty liver disease after laparoscopic sleeve gastrectomy in Japanese obese patients. Ann Gastroenterol Surg. 2019;3:285-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Gong WF, Zhong JH, Lu Z, Zhang QM, Zhang ZY, Chen CZ, Liu X, Ma L, Zhang ZM, Xiang BD, Li LQ. Evaluation of liver regeneration and post-hepatectomy liver failure after hemihepatectomy in patients with hepatocellular carcinoma. Biosci Rep. 2019;39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Xue C, Chu Q, Li L. Research Progress on the Role of Probiotics in Acute Liver Failure. J Transl Int Med. 2022;10:83-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 23. | Zhang D, Pan Y, Yang Z, Zeng H, Wang X, Chen J, Wang J, Zhang Y, Zhou Z, Chen M, Hu D. A Nomogram Based on Preoperative Lab Tests, BMI, ICG-R15, and EHBF for the Prediction of Post-Hepatectomy Liver Failure in Patients with Hepatocellular Carcinoma. J Clin Med. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Allaire M, Goumard C, Lim C, Le Cleach A, Wagner M, Scatton O. New frontiers in liver resection for hepatocellular carcinoma. JHEP Rep. 2020;2:100134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 25. | Kokudo T, Hasegawa K, Shirata C, Tanimoto M, Ishizawa T, Kaneko J, Akamatsu N, Arita J, Demartines N, Uldry E, Kokudo N, Halkic N. Assessment of Preoperative Liver Function for Surgical Decision Making in Patients with Hepatocellular Carcinoma. Liver Cancer. 2019;8:447-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 26. | Fitzpatrick JA, Kim JU, Cobbold JF, McPhail MJ, Crossey MM, Bak-Bol AA, Zaky A, Taylor-Robinson SD. Changes in Liver Volume in Patients with Chronic Hepatitis C Undergoing Antiviral Therapy. J Clin Exp Hepatol. 2016;6:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Yang X, Yang JD, Lee S, Hwang HP, Ahn S, Yu HC, You H. Estimation of Standard Liver Volume Using CT Volume, Body Composition, and Abdominal Geometry Measurements. Yonsei Med J. 2018;59:546-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Serra-Burriel M, Graupera I, Torán P, Thiele M, Roulot D, Wai-Sun Wong V, Neil Guha I, Fabrellas N, Arslanow A, Expósito C, Hernández R, Lai-Hung Wong G, Harman D, Darwish Murad S, Krag A, Pera G, Angeli P, Galle P, Aithal GP, Caballeria L, Castera L, Ginès P, Lammert F; investigators of the LiverScreen Consortium. Transient elastography for screening of liver fibrosis: Cost-effectiveness analysis from six prospective cohorts in Europe and Asia. J Hepatol. 2019;71:1141-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |